Abstract
Ischemic stroke is a neurological disorder resulting from localized brain injury due to focal cerebral ischemia, typically caused by the blockage of one or, in some cases, a few cerebral arteries. This arterial obstruction leads to hypoxia and energy failure, culminating in primary brain damage. Although reperfusion is critical to salvage viable tissue, it often intensifies injury through oxidative stress, inflammation, and cell death—a phenomenon called ischemia–reperfusion (I/R) injury. Milk fat globule-EGF factor 8 (MFG-E8), a multifunctional glycoprotein secreted by stem and immune cells, is a key regulator of inflammation and tissue repair. By modulating microglial activation, attenuating proinflammatory cytokine releases, and preserving neuronal integrity, MFG-E8 mitigates ischemia–reperfusion injury and emerges as a novel therapeutic target for ischemic stroke.
1. Introduction
Ischemic stroke is the second leading cause of death and a major cause of disability worldwide, causing significant social and economic burdens [1,2,3,4]. It accounts for approximately 87% [5] of all stroke cases and is predominantly caused by such focal arterial obstructions [6]. Extensive cellular necrosis occurs in the ischemic core, while in the surrounding penumbra region [7]. Complex secondary damage ensues, including the activation of reactive glial cells and the breakdown of the blood–brain barrier (BBB) [8]. Current standard treatments include intravenous thrombolysis with recombinant tissue plasminogen activator (r-tPA), which must be administered within a narrow 4.5 h therapeutic window after symptom onset, and mechanical thrombectomy, which can be performed in selected patients up to 24 h after onset. Despite these advances, the restricted therapeutic windows still limit the proportion of patients who can benefit from these interventions [9,10,11,12,13,14]. Furthermore, despite advancements in medical infrastructure and administration of treatment within the recommended time frame [15], clinical improvement often falls short of expectations, with low reperfusion success rates of approximately 35% [16,17].
Serious side effects, such as intracranial hemorrhage, reperfusion injury, and long-term neurological deficits, can occur [18,19,20]. The severe limitations of current ischemic stroke interventions underscore the urgent need for novel therapeutic strategies with longer treatment windows and neuroprotective effects that ultimately improve prognosis [21,22,23,24]. Milk fat globule-EGF factor 8 (MFG-E8) has emerged as a promising biomolecule in stroke research [25,26,27]. MFG-E8, also known as lactadherin, is a multifunctional glycoprotein originally identified in milk fat globules and is constitutively expressed in various tissues, including the brain, liver, kidneys, and immune cells [28]. Initially recognized as a “bridging” protein mediating the phagocytic clearance of apoptotic cells [29,30,31,32], MFG-E8 has multifaceted physiological functions, including inflammation suppression, tissue homeostasis maintenance, and tissue repair promotion [33,34]. Specifically, in ischemic brain injury models, MFG-E8 expression has been reported to increase primarily in the ischemic penumbra rather than in the infarct core [25]. Cheyuo et al. [27] demonstrated that intravenous administration of exogenous recombinant human MFG-E8 (rhMFG-E8) one hour after ischemia resulted in reduced infarct volume and improved neurological function at 24 and 48 h post-ischemia. Histological analysis confirmed that rhMFG-E8 protected neurons from necrosis in the ischemic penumbra. Additionally, Choi et al. [25] reported that animals treated with MFG-E8 showed a significant decrease in Iba-1-positive cells and an increase in RECA-1-positive cells in the peri-infarct region. Furthermore, the number of 5-Bromo-2′-deoxyuridine (BrdU)- and Doublecortin (DCX)-positive cells in the subventricular zone was significantly higher in the MFG-E8-treated group compared to controls. These findings suggest that MFG-E8 reduces inflammation, promotes angiogenesis, and enhances neurogenesis in ischemic models.
It exerts neuroprotective effects through various pathophysiological mechanisms, including enhanced phagocytosis by macrophages and microglia, modulation of reactive astrocytes [35,36,37], upregulation of angiogenesis [38], and enhanced neuroregeneration [25,26,27,28]. These multifaceted mechanisms demonstrate the potential of MFG-E8 as a next-generation multifunctional therapeutic that can target multiple pathways simultaneously.
Although several original research articles have investigated MFG-E8 in the context of ischemic stroke, most existing review articles have either focused on its general biological functions or explored other pathological conditions. Comprehensive reviews that emphasize ischemic stroke specifically, while integrating molecular structural biology with mechanistic insights into stroke pathology, remain limited. This review aims to fill this gap by providing an in-depth analysis of MFG-E8’s structural characteristics, its diverse mechanistic roles in ischemic brain injury, and the therapeutic potential that arises from these insights. By thoroughly examining the inflammatory responses and secondary damage mechanisms underlying ischemic stroke pathology, we propose new therapeutic strategies targeting MFG-E8 that may overcome current treatment limitations and ultimately improve patient outcomes.
2. Pathophysiological Mechanisms and Signaling Pathways of Ischemic Stroke
Ischemic stroke (IS) arises from diverse mechanisms, including cardioembolism, focal arterial thrombosis in an atherosclerotic plaque, and artery-to-artery embolism originating from such thrombi. Additionally, small artery disease, arterial dissection, vasculitis, prothrombotic states, and hemodynamic disturbances contribute to IS pathogenesis. These events cause localized infarctions in the brain, spinal cord, or retina, leading to neurological deficits [39]. The most common clinical manifestations observed in approximately 80% of patients are motor impairments involving the face, arm, and leg [40,41]. Current therapeutic options include intravenous administration of r-tPA within 4.5 h of symptom onset [42,43,44] and mechanical thrombectomy (MT) for large vessel occlusions [45,46]. However, only a limited number of patients receive early intervention, partly due to restricted access and narrow therapeutic windows [10,11,12,13,14].
Ischemic brain injury progresses through a temporally defined sequence of overlapping phases, spanning from hyperacute to chronic stages [47]. During the hyperacute phase (≤12 h), excitotoxicity and oxidative stress rapidly develop and peak, driving early neuronal damage. The acute phase (12–24 h) follows, characterized by progressively intensifying inflammatory cascades and microvascular dysfunction, which exacerbate tissue injury. In the subacute phase (2 days–2 weeks), injured cerebral tissue releases cytokines, chemokines, adhesion molecules, and matrix metalloproteases (MMPs), leading to increased blood–brain barrier (BBB) permeability and recruitment of peripheral leukocytes, thereby amplifying the inflammatory response. Apoptotic cell death also becomes increasingly prominent, while reparative processes such as angiogenesis and neuroplasticity gradually emerge [48]. Finally, the chronic phase (2 weeks–2 months) involves resolution of inflammation and initiation of tissue repair, with a gradual re-establishment of homeostasis and suppression of inflammatory signaling. However, endogenous repair mechanisms alone are often insufficient to achieve full functional recovery in stroke patients [49,50,51]. The temporal ordering and partial overlap of these injury and repair processes are summarized in Figure 1 [47,48,49,50,51].
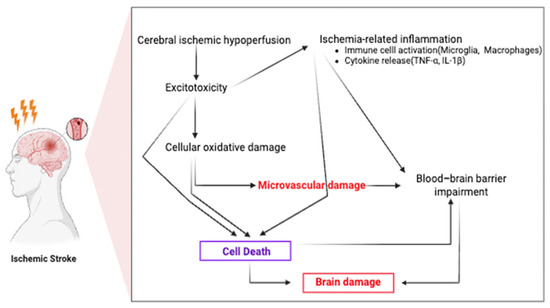
Figure 1.
Pathophysiological mechanisms of ischemic stroke. Cerebral ischemia induces hypoperfusion, excitotoxicity, oxidative damage, microvascular dysfunction, and blood–brain barrier breakdown, which contribute to brain injury and inflammation.
Despite advances in reperfusion therapies, including intravenous thrombolysis and MT, clinical application is limited by narrow therapeutic windows and strict eligibility criteria, leaving many patients without effective treatment options. Furthermore, even after successful reperfusion, I/R injury can exacerbate infarct size and worsen clinical outcomes [52]. Without timely and appropriate intervention, the ischemic cascade culminates in irreversible neuronal death and structural brain damage, often resulting in permanent disability [47,48,49,50,51,52,53,54].
These challenges underscore the urgent need for a deeper understanding of the sequential pathophysiological mechanisms underlying ischemic injury, which is essential for developing more effective therapeutic strategies and improving long-term patient outcomes. In this context, MFG-E8 emerges as a key regulator, linking critical injury-related processes such as efferocytosis and microglial polarization. Recent evidence suggests that MFG-E8 can modulate these core pathophysiological pathways, thereby influencing the progression of ischemic injury. These mechanistic insights provide a conceptual bridge between the pathophysiological framework described in this section and the mechanistic and therapeutic perspectives elaborated in Section 3, Section 4 and Section 5, highlighting MFG-E8 as a potential therapeutic target.
2.1. Signaling Pathways in Ischemic Stroke: Excitotoxicity
Ischemic stroke is initiated by a sudden reduction in cerebral blood flow, causing oxygen and glucose deprivation, and, ultimately, neuronal energy depletion and impaired Adenosine Triphosphate (ATP) production [55]. Sodium–potassium adenosine triphosphatase (Na+/K+-ATPase) dysfunction disrupts ionic gradients and induces neuronal depolarization. Consequently, ischemic neurons and surrounding cells release an excessive amount of glutamate into the synaptic cleft, leading to its accumulation in the extracellular space [52,56]. This elevated extracellular glutamate overstimulates ionotropic glutamate receptors on postsynaptic neurons, including N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors [53,57,58]. Overactivation of NMDARs causes a pathological influx of calcium ions (Ca2+) into the postsynaptic neurons. This excessive intracellular calcium overload triggers excitotoxicity characterized by mitochondrial dysfunction, reactive oxygen species (ROS) generation, activation of calpains, and initiation of downstream cell death pathways [57,59].
NMDARs exhibit dual functions in neuronal survival and death, depending on their subunit composition and subcellular localization. Synaptic NMDARs containing the GluN2A subunit promote neuronal survival by mediating Ca2+ influx and activating prosurvival signaling pathways, such as Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (Akt), Extracellular signal-regulated kinases (ERK), and cAMP response element-binding protein (CREB) [59,60,61]. Subsequently, these pathways induce the expression of anti-apoptotic genes, including brain-derived neurotrophic factor (BDNF) and B-cell lymphoma 2 (Bcl-2) [62]. In contrast, extrasynaptic NMDAR predominantly composed of GluN2B subunits are associated with neurotoxicity. Their activation induces CREB deactivation, proapoptotic gene upregulation, and mitochondrial dysfunction, thereby facilitating neuronal cell death [63,64].
Ischemic conditions promote pathological changes, such as increased extracellular potassium (K+) concentrations, reduced pH, and widespread ionic imbalance, further enhancing glutamate release. Furthermore, impaired intercellular communication—such as dysregulated gap junction activity—facilitates the propagation of neuronal injury across adjacent cells and tissues [65].
The prosurvival signaling cascade mediated by synaptic NMDARs is critical in neuronal protection [57]. The PI3K/Akt pathway is activated using calcium/calmodulin, 3-Phosphoinositide-dependent protein kinase-1 (PDK1), and Insulin receptor substrate-1 (IRS-1), leading to Akt phosphorylation and activation [66,67]. Activated Akt subsequently inhibits key proapoptotic factors, including glycogen synthase kinase 3 (GSK3β), apoptosis signal-regulating kinase 1 (ASK1), and p53, thereby preventing cell death [47,68,69,70,71]. Concurrently, CREB phosphorylation using the Ras-ERK-CREB pathway induces BDNF expression, which supports neuronal function and enhances resistance to ischemic injury [72,73,74,75,76,77]. Ischemia experimental models have demonstrated that these signaling pathways provide robust neuroprotective mechanisms against excitotoxic damage [78,79].
In contrast, extrasynaptic NMDARs serve as central mediators of pathological signaling. GluN2B-containing NMDARs are associated with pro-death pathways involving phosphate and tensin homolog (PTEN), death-associated protein kinase 1 (DAPK1), and the Postsynaptic density protein 95 (PSD95)/neuronal nitric oxide synthase (nNOS) complex. These pathways inhibit the PI3K/Akt survival cascade while promoting nitric oxide (NO) production, oxidative stress, and DNA damage, thereby facilitating neuronal cell death. Notably, DAPK1 interferes with prosurvival signaling by sequestering ERK in the cytoplasm, whereas the PSD95/nNOS complex intensifies injury by enhancing calcium influx and NO generation [80,81,82,83,84,85].
Therapeutic strategies targeting GluN2B-containing or specifically extrasynaptic NMDARs have been proposed to counteract these deleterious processes [86]. One such agent is memantine, a low-affinity, noncompetitive NMDAR antagonist that preferentially inhibits extrasynaptic receptors without significantly affecting physiological synaptic transmission [87]. However, the binary distinction between synaptic and extrasynaptic NMDARs in terms of function and localization is an oversimplification. Therefore, future therapeutic approaches should precisely target the structural and spatial characteristics of NMDARs to achieve selective neuroprotection.
2.2. Signaling Pathways in Ischemic Stroke: Mitochondrial Dysfunction
Mitochondria are intracytoplasmic double-membraned organelles crucial for ATP production and calcium signaling, regulating their shape and function through fusion and fission. By generating ATP via glucose- and oxygen-dependent oxidative phosphorylation, they serve as metabolic hubs essential for cell survival and function. During ischemic stroke, mitochondrial dysfunction promotes cell death, making mitochondria key targets for neuronal survival and neuroprotective therapy [88]. However, under pathological conditions such as ischemic stroke, where energy supply is abruptly interrupted, mitochondrial function is severely compromised, causing various forms of cellular stress [89]. Inhibiting ATP production diminishes the mitochondrial membrane potential and induces excessive accumulation of intracellular Ca2+, which in turn induces the opening of the mitochondrial permeability transition pore [90,91,92]. This facilitates the release of cytochrome c into the cytosol, activating the intrinsic apoptotic pathways [93,94].
Furthermore, the ischemic environment promotes pathological ROS accumulation, intensifying oxidative stress [95]. This effect is further intensified during reperfusion, contributing to persistent cytotoxicity and secondary tissue damage. Thus, mitochondrial dysfunction is a critical nexus in the pathophysiological cascade of ischemic stroke that links energy failure to cell death mechanisms and long-term neurological impairment [96].
Mitochondrial dysfunction interacts with various intracellular signaling pathways to modulate ischemic brain injury progression [97]. One key pathway involves hypoxia-inducible factor-1 alpha (HIF-1α), which facilitates metabolic adaptation by upregulating glycolytic genes under hypoxic conditions [98,99]. However, elevated ROS levels stabilize HIF-1α, creating a positive feedback loop intensifying oxidative stress [100].
In contrast, nuclear factor erythroid 2-related factor 2 (Nrf2) exerts a protective role by inducing the expression of antioxidant response element (ARE)-driven genes, thereby promoting ROS detoxification and enhancing cellular defense mechanisms [101]. Nrf2 activation is further supported by upstream signaling cascades, such as PI3K/Akt and ERK/mitogen-activated protein kinase (MAPK), which amplify its antioxidant and cytoprotective effects. The dynamic interplay among these pathways critically influences neuronal survival and the extent of ischemia-induced damage [102].
Casein kinase 2 (CK2) performs a dual role in the context of ischemic injury. It promotes antioxidant defense and angiogenic responses by modulating downstream targets, such as Rac Family Small GTPase 1 (Rac1), Signal transducer and activator of transcription 3 (STAT3), and Nuclear factor kappa B (NF-κB). Paradoxically, under overactivation conditions, CK2 facilitates cell death by promoting the release of apoptosis-inducing factors from mitochondria, highlighting its context-dependent functionality [102,103,104].
Meanwhile, mitophagy, which is specifically mediated by PTEN-induced kinase 1/Parkin and Bcl2-interacting protein 3, is a crucial process in maintaining cellular homeostasis that selectively removes damaged mitochondria [105]. This process prevents excessive ROS accumulation and mitigates mitochondrial-driven toxicity. Mitophagy is especially important during I/R injury, where the efficient clearance of dysfunctional mitochondria can significantly reduce secondary damage and promote neuronal survival [106].
In conclusion, mitochondrial dysfunction and its intricate interplay with multiple signaling pathways orchestrate the pathophysiology of ischemic stroke. These pathways constitute a critical molecular axis that governs the balance between neuronal survival and death. Elucidating and modulating these pathological mechanisms is essential for the development of effective therapeutic strategies. Future research should explore integrated, signaling-targeted approaches to preserving mitochondrial function and mitigating oxidative stress. Such strategies would significantly enhance neuronal survival and facilitate long-term recovery following cerebral ischemia.
2.3. Signaling Pathways in Ischemic Stroke: Autophagy
Autophagy is the process by which cells engulf cytoplasmic proteins and damaged organelles, delivering them to lysosomes for degradation. It serves as a key mechanism for removing old components and provides nutrients and energy during stress conditions [107]. Autophagy is an evolutionary conserved self-protective mechanism that is crucial to maintaining cellular homeostasis. It facilitates the degradation of long-lived proteins, misfolded protein aggregates, and damaged organelles, thereby regulating energy balance and enabling cellular adaptation to stress. Recent studies have investigated the activation of autophagy in various cell types, such as neurons, astrocytes, and endothelial cells, following ischemic stroke [107,108].
Moderate levels of autophagy promote neuronal survival by removing toxic protein aggregates and damaged cellular components. However, excessive or dysregulated autophagy facilitates a pathological process that promotes cell death. This dual nature underscores the context-dependent role of autophagy in ischemic injury [109].
Mechanistic target of rapamycin (mTOR) is a central regulator of autophagy that senses intracellular nutrient availability and stress levels, primarily suppressing the initiation of autophagy under normal or nutrient-rich conditions. In ischemic injury, two major pathways, PI3K/Akt/mTOR and AMP-activated protein kinase (AMPK)/mTOR, exert opposing influences on autophagy; the former inhibits autophagy, while the latter promotes it [102]. AMPK is activated in response to energy depletion, leading to the inhibition of mechanistic/mammalian target of rapamycin complex 1 (mTORC1) and stimulation of autophagy through calcium-dependent calmodulin signaling [48,110,111].
The MAPK/ERK pathway also demonstrates context-dependent effects, specifically, either suppressing or promoting autophagy by activating or inhibiting mTOR, respectively, using ERK-mediated mechanisms [102]. In parallel, Beclin1 is crucial in the formation of autophagosomes, with its interaction with the anti-apoptotic protein Bcl-2 as a key regulatory node. In ischemic stroke, Bcl-2 phosphorylation or peroxisome proliferator-activated receptor gamma (PPAR-γ) activation can disrupt or modulate the Bcl-2–Beclin1 interaction, thereby influencing autophagy induction and progression [112,113].
2.4. Signaling Pathways in Ischemic Stroke: Cell Death
Ischemic stroke, caused by a disruption of blood flow to the brain, can lead to cell death through various programmed cell death pathways. These pathways include apoptosis, necroptosis, and pyroptosis, all of which contribute to the progression of ischemic damage. In ischemic stroke, energy depletion and disruption of oxygen and nutrient supply lead to extensive neuronal death, primarily through programmed cell death known as apoptosis. Apoptosis is a regulated, genetically controlled process distinct from necrotic cell death caused by cytotoxic injury. It proceeds via two main pathways: the extrinsic (death receptor-mediated) and intrinsic (mitochondria-mediated) pathways [114,115].
When tumor necrosis factor alpha (TNFα) binds to tumor necrosis factor receptor 1 (TNF-R1), TNF-R1 forms a trimer and recruits complex I, which consists of TNF receptor-associated death domain (TRADD), cellular inhibitor of apoptosis proteins (cIAPs), receptor interacting protein 1 (RIP1), and TNF receptor-associated factors 2 and 5 (TRAF2/5). Complex I then forms complex II by recruiting Fas-associated protein with a death domain (FADD), receptor interacting protein 3 (RIP3), and procaspase-8. Within complex II, procaspase-8 (pro-Casp8) is cleaved to active caspase-8 (Casp8). Casp8 cleaves BID into truncated BID (tBID), which activates Bcl-2-associated X protein (Bax) to form pores in the mitochondrial outer membrane, inducing the release of cytochrome c (CYT C) and promoting apoptosis [116].
In contrast, the intrinsic pathway is triggered by intracellular stress signals, such as calcium overload and oxidative stress, which cause mitochondrial outer membrane permeabilization [114]. This induces the release of proapoptotic factors, such as cytochrome c, into the cytosol, where they bind to Apoptotic protease activating factor 1 (Apaf-1) and procaspase-9 (pro-Casp9) to form the apoptosome, thereby activating caspase-9 (Casp9) and downstream effector caspases (e.g., caspase-3) [117,118]. Together, these cascades amplify neuronal death in the ischemic brain and significantly contribute to infarct progression and neurological dysfunction.
The tumor suppressor protein p53 plays a pivotal role in neuronal apoptosis in ischemic stroke. Activated by DNA damage, hypoxia, and oxidative stress, p53 modulates the transcription of numerous proapoptotic genes, including Bax, in the ischemic region, thereby promoting mitochondrial-dependent apoptosis [119,120,121]. Furthermore, p53 interacts with multiple signaling cascades, including the Notch pathway, where crosstalk among Notch1, p53, NF-κB, and HIF-1α forms a complex regulatory network contributing to neuronal death [122,123]. Inhibition of Notch signaling by γ-secretase inhibitors attenuates microglial activation and confers neuroprotection, highlighting its therapeutic relevance in stroke [123].
Beyond classical apoptosis, nonapoptotic forms of cell death contribute significantly to ischemic brain injury [124]. Pyroptosis is a form of proinflammatory programmed cell death driven by inflammasome formation and caspase-1 activation, leading to the release of inflammatory cytokines, such as Interleukin-1 beta (IL-1β) and Interleukin-18 (IL-18) [125]. Meanwhile, ferroptosis, a unique form of regulated cell death driven by iron-dependent lipid peroxidation, is triggered by intracellular iron overload, glutathione depletion, and impaired glutathione peroxidase 4 (GPX4) activity [126]. Notably, BBB disruption in stroke enhances neuronal iron influx, thereby accelerating ferroptosis. Calcium signaling and the Kelch-like ECH-associated protein 1 (Keap1)–Nrf2 antioxidant pathway regulate this process, which further implicates redox imbalance in cell death [127].
Collectively, these diverse cell death mechanisms reflect the multifaceted nature of ischemic brain injury and underscore the potential of therapeutic interventions targeting pathways such as p53, Notch, pyroptosis, and ferroptosis.
2.5. Signaling Pathways in Ischemic Stroke: Neuroinflammation and Microglia
Tumor necrosis factor alpha (TNF-α) is an extensively studied proinflammatory cytokine in ischemic stroke and a critical mediator of neuroinflammatory responses. TNF-α exists in two biologically active forms: soluble TNF (solTNF) and transmembrane TNF (tmTNF), which primarily signal through TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2), respectively [128]. The solTNF–TNFR1 axis is associated with proapoptotic and proinflammatory signaling, thereby exacerbating neuronal injury and BBB breakdown. In contrast, the tmTNF–TNFR2 pathway promotes cell survival, neuroregeneration, and anti-inflammatory responses, indicating functional divergence between the two receptors [129].
Microglia are the principal source of TNF-α in the ischemic brain. Experimental studies have shown that inhibiting solTNF signaling can mitigate ischemic brain damage, whereas promoting tmTNF signaling may confer neuroprotective effects. This dichotomy underscores the importance of the selective targeting of TNF-α pathways for therapeutic purposes [130].
Other cytokines, such as the interleukin-1 (IL-1) and interleukin-6 (IL-6) families, also exhibit pleiotropic effects during stroke. IL-1 β levels rapidly increase in the acute phase of ischemia, amplifying the inflammatory cascade and exacerbating neuronal damage (Figure 2). Conversely, interleukin-10 (IL-10), a key anti-inflammatory cytokine, exerts neuroprotection by suppressing inflammation, reducing apoptosis, and improving poststroke outcomes. These findings highlight the complex, context-dependent roles of cytokine signaling in ischemic pathophysiology and provide a foundation for the development of cytokine-targeted interventions in stroke therapy [131,132].
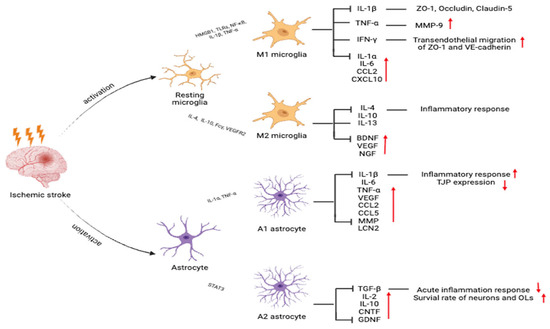
Figure 2.
Activation states of microglia and astrocytes after ischemic stroke that affect neuroinflammation and repair. Microglia differentiate into the proinflammatory M1 or anti-inflammatory M2 phenotypes. Astrocytes are polarized into neurotoxic A1 or neuroprotective A2 subtypes. Red upward arrows (↑) indicate an increase in the corresponding cytokines, chemokines, or functional outcomes after ischemic stroke, while red downward arrows (↓) indicate a decrease.
Chemokines are key players in orchestrating immune cell recruitment and amplifying neuroinflammatory responses following ischemic stroke. C-C motif chemokine ligand 2 (CCL2) and its receptor chemokine (C-C motif) receptor 2 (CCR2) are particularly well characterized. The CCL2/CCR2 axis facilitates the adhesion and transmigration of monocytes and other leukocytes across the BBB, thereby promoting their infiltration into the ischemic brain parenchyma. Elevated CCL2/CCR2 signaling is closely associated with increased infarct volume, enhanced neuroinflammation, and worsened neurological outcomes [133,134].
Furthermore, other chemokines, such as C-C motif chemokine ligand 3 (CCL3), C-C motif chemokine ligand 5 (CCL5), chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-X-C motif) ligand 2 (CXCL2), and chemokine (C-X-C motif) ligand 8 (CXCL8), contribute to stroke pathology by promoting neutrophil and Th1-polarized T cell infiltration. These chemokines are rapidly upregulated in response to ischemic injury and are critical in shaping the inflammatory milieu of the poststroke brain. Their concerted actions intensify tissue injury, disrupt the BBB, and perpetuate secondary damage [135].
Therefore, targeting chemokine signaling pathways is a promising therapeutic strategy for mitigating immune cell-mediated injury and limiting postischemic inflammation.
High mobility group box 1 (HMGB1), a key mediator of neuroinflammatory signaling, is markedly upregulated in the ischemic brain and plays a critical role in poststroke immune activation. Once released from necrotic or stressed neurons, HMGB1 binds to pattern recognition receptors, such as Toll-like receptors 2 and 4 (TLR2 and TLR4), leading to the activation of the NF-κB pathway and subsequently transcription of proinflammatory cytokines. This signaling cascade contributes to peripheral immune cell recruitment and neuroinflammation amplification [102,136].
TLR signaling also exhibits a dual role, depending on the timing and severity of the injury. Notably, TLR4 mediates the intensity of inflammatory responses in association with circulating lipopolysaccharide (LPS) levels, functioning as a critical regulator of immune homeostasis and damage propagation [136,137]. In parallel, MAPK pathways, including ERK, c-Jun N-terminal kinases (JNK), and p38, are rapidly activated in early-phase ischemia. These kinases promote the expression of inflammatory mediators and matrix metalloproteinases (MMPs), particularly MMP-9, which diminishes BBB integrity. The disruption of the BBB facilitates immune cell infiltration and intensifies cerebral edema and neuronal injury [138,139,140].
Targeting the HMGB1/TLR/NF-κB and MAPK/MMP signaling axes presents a promising therapeutic approach to alleviating neuroinflammation and preserving BBB function in ischemic stroke [141,142].
Microglia function critically in maintaining neural homeostasis following ischemic injury by clearing dead cells and cellular debris through phagocytosis. This process is primarily mediated by transmembrane protein 16F (TMEM16F) and triggering receptor expressed on myeloid cells 2 (TREM2) signaling pathways. While microglial phagocytosis is essential for limiting secondary damage and facilitating tissue repair, excessive or dysregulated phagocytic activity may facilitate synaptic pruning and neuronal loss, ultimately impairing cognitive function [143,144].
The complement system, particularly C1q and C3 activation, enhances microglial phagocytosis by tagging apoptotic cells and synapses for elimination. These complement components accumulate in the ischemic brain, where they interact with microglial receptors and promote phagocytosis [145,146]. The participation of both beneficial and detrimental phagocytic responses signifies that microglial activation and its regulation using TMEM16F, TREM2, and complement signaling constitute a complex and dynamic component of ischemic stroke pathology and recovery.
3. Structural and Functional Characteristics of MFG-E8
MFG-E8, also known as lactadherin, is a multifunctional glycoprotein that was first identified in milk fat globules and is expressed in various tissues [147], including the brain, liver, and kidneys, as well as in immune cells [26,30,148,149]. MFG-E8 contains an N-terminal EGF-like domain that includes an Arg-Gly-Asp (RGD) motif [149] responsible for binding integrins αvβ3 and αvβ5 [150,151]. Its C-terminal region consists of two discoidin domains (C1 and C2) [152,153,154,155], which mediate binding to phosphatidylserine (PS) exposed on the surface of apoptotic cells. Additionally, integrins, located on the outer membrane of phagocytes, act as receptors that recognize and bind to these discoidin domains, facilitating the engulfment of apoptotic cells [156,157,158,159].
This dual-binding capacity allows MFG-E8 to function as a bridging molecule between apoptotic cells and phagocytes, thereby facilitating the noninflammatory clearance of dying cells [150,155,160,161]. By interacting with integrins and PS, MFG-E8 activates downstream signaling pathways, including PI3K/Akt and ERK [161,162], which promote anti-inflammatory responses, cell survival, and tissue repair [152,163,164,165,166].
Unlike Annexin V, MFG-E8 binds to PS in a calcium-independent manner [167,168] and demonstrates high sensitivity, even at low PS concentrations [169,170,171,172]. Alternative splicing variants enriched in proline/threonine residues can modulate binding affinity and phagocytic activity [159,173], adding an extra layer of regulatory complexity [174,175]. These features position MFG-E8 as a crucial modulator in mitigating inflammation and maintaining tissue homeostasis [170,171,172,174,175].
3.1. Structural Features of MFG-E8: Domain Characteristics
MFG-E8 is a secreted glycoprotein first identified by Stubbs et al. [156] in 1990 as a novel cDNA sequence isolated from Mus musculus mammary epithelial cells. MFG-E8 was named for its high abundance in milk fat globules and its structural similarity to EGF and blood coagulation factors V and VIII [148,149]. MFG-E8 is a secreted protein 46–66 kDa in length that contains a signal peptide at the N-terminus [176], followed by two EGF-like domains (EGF1 and EGF2) and two C-terminal discoidin-like domains (C1 and C2) [152,153,154,155].
MFG-E8 typically contains a single EGF-like domain in humans, whereas two EGF-like domains are present in mice and bovines [31,176]. These EGF domains share homology with structural motifs found in Notch receptors and are implicated in cell growth and differentiation regulation [159,177]. The C1 and C2 discoidin-like domains are essential for binding to PS on the surface of apoptotic cells [152,153,155,159]. Specifically, the C1 domain mediates direct interaction with PS, whereas the C2 domain facilitates membrane association and facilitates cell adhesion and migration (Figure 3).
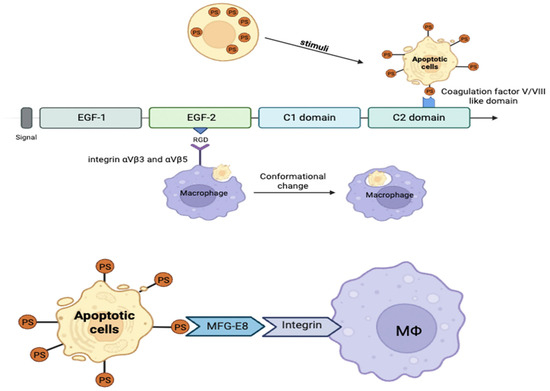
Figure 3.
Structural domains of milk fat globule-EGF factor 8 (MFG-E8) and its functional mechanism. MFG-E8 binds phosphatidylserine on the surface of apoptotic cells using the C2 domain and integrins αvβ3/β5 using its RGD motif in the EGF-like domain to facilitate macrophage phagocytosis.
This domain configuration enables MFG-E8 to link apoptotic cells with phagocytes, thereby expediting the efficient clearance of cellular debris and contributing to anti-inflammatory and tissue repair processes [153,155,177].
3.2. Structural Characteristics of MFG-E8: Integrin-Binding Domain
The second EGF-like domain of MFG-E8 contains a conserved RGD motif, which specifically binds to αvβ3 and αvβ5 integrin receptors [149]. This interaction serves as a crucial molecular bridge that facilitates the recognition by macrophages of apoptotic cells, facilitating phagocytosis [156,157,158,159]. Through integrin binding, MFG-E8 activates multiple downstream signaling pathways, including AMPK/proto-oncogene tyrosine-protein kinase Src (Src) [178], Rac1 [165], and PI3K/Akt/mTOR [161], which collectively regulate phagocytosis, anti-inflammatory responses, angiogenesis, cell survival, and cell migration [163,164,165,177].
MFG-E8 exists in two splice variants. The longer isoform contains a proline/threonine-rich domain [159,173], which enhances the affinity of MFG-E8 for PS and potentiates phagocytic efficiency. In contrast, the shorter isoform lacks this domain and exhibits lower affinity for PS, resulting in reduced phagocytic activity [174,175]. This domain variation further modulates the functional capacity of MFG-E8 in immune regulation and tissue remodeling [168,179,180,181].
3.3. Structural Characteristics of MFG-E8: PS Binding Capacity and Therapeutic Potential
MFG-E8 exhibits a remarkably high affinity for PS [174,175], responding sensitively even to very low PS levels [169,170,171,172]. This high-affinity binding is distinct from that of Annexin V, which requires Ca2+ for PS interaction [168,182]. The calcium-independent PS binding by MFG-E8 not only differentiates it mechanistically from Annexin V but also indicates its broader role as a sensor of cellular stress beyond mere apoptotic cell recognition [165,183,184].
MFG-E8 binds preferentially to extracellular vesicles (EVs) characterized by highly curved membrane structures and PS exposure, indicating its involvement in EV function modulation [185]. These unique structural properties enable MFG-E8 to regulate several physiological processes, including cell adhesion, migration, immune modulation, and tissue homeostasis [35,161,165,178].
These multifaceted roles signify the considerable therapeutic potential of MFG-E8 as a target in diverse pathological conditions, from inflammatory diseases to tissue injury and repair. Its high sensitivity for PS detection and binding demonstrates its potential future clinical applications in modulating cell clearance, immune responses, and intercellular communication.
4. Functional Roles of MFG-E8 in the Central Nervous System
4.1. Phagocytic Function of MFG-E8 and Apoptotic Cell Clearance Mechanism
MFG-E8 is a critical bridging molecule that mediates the phagocytic clearance of apoptotic cells by recognizing PS as a lipid that is exposed on the surface of dying cells during apoptosis [150,155,160,161]. The F5/8C domain on the C-terminus of MFG-E8 binds specifically to PS [148], whereas the RGD motif in the N-terminal EGF-like domain interacts with αvβ3 and αvβ5 integrins expressed on phagocytes, thereby facilitating the efficient engulfment of apoptotic cells [149,150,151].
During apoptosis, exposed PS serves as a phagocytic “eat-me” signal to attract phagocytes, which are further guided by “find-me” signals released from dying cells [186]. This coordinated signaling ensures the rapid and precise clearance of cellular debris, thereby preventing secondary necrosis and subsequent inflammation [187]. Furthermore, chemokines such as chemokine (C-X3-C motif) ligand 1 (CX3CL1) upregulate MFG-E8 expression, thereby enhancing phagocytic activity [187]. MFG-E8 also cooperates with other receptors, such as T-cell membrane protein 4 (Tim-4), to orchestrate a two-step apoptotic cell removal process [188].
Conversely, structural alterations or domain deletions in MFG-E8 impair its PS binding and integrin-mediated phagocytic functions, leading to defective apoptotic cell clearance and exacerbated inflammatory response (Figure 4). Overall, MFG-E8 is an indispensable factor in maintaining tissue homeostasis and immune regulation through effective apoptotic cell clearance [152,189].
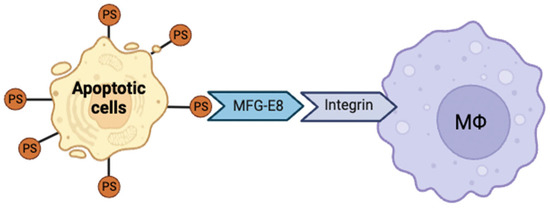
Figure 4.
Mechanism of milk fat globule-EGF factor 8 (MFG-E8)–mediated apoptotic cell clearance. MFG-E8 bridges apoptotic cells and macrophages upon binding to phosphatidylserine (PS) and integrins, triggering signaling cascades that promote efferocytosis and inflammation resolution.
4.2. Anti-Inflammatory Mechanisms and Immunomodulatory Roles of MFG-E8
The efficient clearance of apoptotic cells is a noninflammatory process; however, delayed removal leads to secondary necrosis, causing the release of intracellular contents and damage-associated molecular patterns that activate immune cells and promote proinflammatory cytokine generation [150,155,160,161,187]. During this process, macrophages secrete inflammatory mediators, such as NO, prostaglandin E2, ROS, and cytokines (TNF-α, IL-1β, and IL-6), thereby amplifying inflammatory responses [190,191].
MFG-E8 attenuates inflammation by suppressing key signaling pathways, including NF-κB and MAPK, and modulates cytokine production using the suppressor of cytokine signaling-3 (SOCS3) [31] and STAT3 pathways [163]. Notably, MFG-E8 inhibits LPS-induced macrophage activation and competitively blocks integrin receptor binding of damage-associated molecules, such as HMGB1, thereby mitigating tissue injury [180,181].
MFG-E8 prevents excessive inflammatory activation and fosters an anti-inflammatory microenvironment conducive to tissue repair [179]. The anti-inflammatory effects of MFG-E8 have been demonstrated across various inflammatory disease models, establishing it as a critical immunoregulatory factor in alleviating chronic inflammation and tissue damage.
4.3. Regulation of Microglial Activation by MFG-E8
Microglia are the primary immune surveillance cells in the central nervous system that polarize into distinct phenotypes in response to inflammatory stimuli: the proinflammatory M1 phenotype or the anti-inflammatory, tissue-reparative M2 phenotype. MFG-E8 promotes microglial polarization toward the M2 phenotype, enhancing the secretion of anti-inflammatory cytokines and activating phagocytosis of damaged neural tissue, thereby fostering a neuroprotective and neuroregenerative environment [192,193].
While M1 microglia secrete proinflammatory cytokines that exacerbate neuronal injury, MFG-E8-mediated M2 activation counteracts this effect and helps restore neuroinflammatory balance. Furthermore, MFG-E8 restores the phagocytic capacity of microglia, facilitating the clearance of apoptotic debris and suppressing proinflammatory signaling pathways, thereby mitigating chronic inflammation [193,194].
Thus, MFG-E8 functions as a critical regulator of microglial functional states by modulating neuroinflammation and promoting neural repair processes.
4.4. Regulation of Astrocyte Reactivity by MFG-E8
Astrocytes not only provide structural support within the nervous system but are also key players in the regulation of neuroinflammatory responses. Upon inflammatory stimuli, astrocytes polarize into two major phenotypes: neurotoxic A1 and neuroprotective A2. A1 astrocytes intensify neuronal injury through the secretion of proinflammatory cytokines, whereas A2 astrocytes promote tissue repair and neuroprotection by releasing anti-inflammatory cytokines and neurotrophic factors [195,196,197].
MFG-E8 promotes the activation of the A2 astrocytic phenotype, thereby fostering an anti-inflammatory environment and facilitating the recovery of damaged neural tissue. Furthermore, MFG-E8 interacts with microglia to regulate neuroinflammation and neuroregenerative processes, highlighting its integrative role in central nervous system homeostasis [187,198].
These functions indicate the potential of MFG-E8 as a therapeutic target for modulating astrocyte-mediated neuroinflammatory responses and enhancing neural repair mechanisms following central nervous system injury and disease.
4.5. Regulatory Mechanisms of MFG-E8 Expression
The expression of MFG-E8 is tightly regulated by multiple transcriptional and post-transcriptional mechanisms. The chemokine CX3CL1 has been identified as a key upstream regulator that induces MFG-E8 transcription in microglia, with its activity closely linked to inflammatory transcription factors such as NF-κB and STAT3 [163,187]. Beyond transcriptional regulation, recent studies have demonstrated that nanoparticle-mediated microRNA (miRNA) delivery can modulate MFG-E8 expression in macrophages. For example, gold-core nanoparticles coated with miR-99b suppressed MFG-E8 protein levels in gut macrophages, thereby influencing enterocyte migration and local immune responses. In addition, macrophage-like nanoparticles have been developed to absorb endotoxins and proinflammatory cytokines, providing mechanistic insights into the functional networks of MFG-E8 and its role in macrophage-mediated inflammatory regulation. These findings underscore the importance of post-transcriptional regulation and tissue-specific contexts in shaping MFG-E8 function. Future studies should expand on these insights by applying transcriptomic profiling in ischemic stroke models, with particular attention to promoter regulation, splicing variants, and the interplay between transcription factors and miRNA [199,200].
At the protein level, MFG-E8 executes its biological functions by binding PS exposed on apoptotic cells while simultaneously engaging αvβ3 and αvβ5 integrins on phagocytes [150,155,160,161]. This bridging interaction activates downstream signaling cascades, including ERK1/2, p38 MAPK, and JNK, which collectively suppress proinflammatory cytokine production and enhance anti-inflammatory responses, thereby establishing a homeostatic feedback loop [201]. Conversely, reduced expression or disruption of MFG-E8 signaling impairs apoptotic cell clearance, leading to the accumulation of apoptotic debris, chronic inflammation, and subsequent tissue damage. Thus, delineating the precise molecular regulation of MFG-E8 expression and signaling remains critical for the development of targeted therapeutic strategies for inflammatory and neurodegenerative diseases.
In parallel, dysregulated MFG-E8 expression has been observed in tumor biology. Acting as a molecular bridge between PS and integrins, MFG-E8 is frequently overexpressed in a range of cancers and has been implicated in diverse processes, including attenuation of inflammation, induction of regulatory T cells (Tregs), promotion of efferocytosis, angiogenesis, allograft tolerance, and metastatic progression. Notably, MFG-E8 overexpression correlates with poor prognosis in breast, colorectal, and esophageal cancers [202,203,204]. Yamada et al. [205] further demonstrated that MFG-E8 promotes angiogenesis by upregulating VEGF and endothelin-1 (ET-1) in bone marrow-derived mesenchymal stromal cells, thereby facilitating melanoma progression. Moreover, MFG-E8 fosters M2 polarization of macrophages, while its blockade enhances antitumor effector T-cell responses and suppresses Tregs, resulting in tumor regression.
Taken together, these findings indicate that MFG-E8 exerts dual roles in cancer development and progression, with its effects shaped by cancer type, tumor microenvironment, and interactions between malignant and immune cells. However, its systemic impact on antitumor immunity remains incompletely understood, underscoring the need for more comprehensive mechanistic studies and rigorous safety evaluations to inform the clinical translation of MFG-E8-based interventions.
4.6. MFG-E8-Related Biomarkers: Potential Indicators and Clinical Applicability
Research on MFG-E8-related biomarkers remains in its early stages, and clinical validation is currently limited. Despite this, recent studies have identified several promising candidates that may serve as indicators of disease progression and therapeutic response. Concentrations of MFG-E8 in plasma and cerebrospinal fluid (CSF) have been shown to vary depending on the pathological context. For example, CSF MFG-E8 levels are decreased in patients with cerebral amyloid angiopathy (CAA) or Alzheimer’s disease, whereas plasma levels may be elevated in certain cancers. These context-dependent variations suggest that plasma and CSF MFG-E8 levels could provide valuable information for prognosis and the development of targeted therapeutic strategies [206,207].
MFG-E8 also interacts with PS-exposing cells and EVs, promoting efferocytosis and modulating immune responses. EV-associated MFG-E8 may serve as a surrogate marker for macrophage and microglial activation, reflecting therapeutic efficacy in neurological disorders [208]. In addition, MFG-E8 has been shown to influence microglial polarization, shifting cells from the proinflammatory M1 phenotype to the anti-inflammatory M2 phenotype. This shift is accompanied by reductions in M1-associated cytokines such as TNF-α, IL-1β, and IL-6, along with increases in M2 markers including Arg-1, IL-10, and CD206. Such modulation of inflammatory responses underscores the therapeutic potential of MFG-E8 in a variety of neuroinflammatory conditions [209].
To establish the clinical utility of these biomarkers, future preclinical and clinical studies are needed to systematically assess plasma and CSF MFG-E8 levels, PS-exposing EVs, and microglial activation markers. These investigations will be critical for patient stratification and for monitoring the effectiveness of MFG-E8-based therapeutic interventions.
5. MFG-E8 Expression in Ischemic Stroke Models
Ischemic stroke triggers a complex cascade of events, including cell death [210], inflammation [211], energy depletion [212], ionic imbalance [213], excitotoxicity [214], and oxidative stress [215], which collectively initiate inflammatory and immune responses [216]. These processes ultimately result in irreversible brain damage. Following successful reperfusion, blood flow restoration to the ischemic brain can paradoxically induce secondary reperfusion injury [217]. Reperfusion promotes ROS generation, amplifies inflammatory and immune responses, and induces excessive neuronal death, BBB disruption, and innate and adaptive immune system activation, thereby exacerbating brain injury [218]. These complex pathological processes contribute to the progression of ischemic brain injury. To counteract these detrimental mechanisms, various biomolecules and signaling pathways that promote neuroprotection and inflammation regulation have been extensively investigated [210]. MFG-E8, which is expressed by astrocytes and microglia in the brain, is crucial in attenuating secondary injury after cell death by mitigating inflammatory responses and tissue damage [219]. Notably, MFG-E8 binds to PS exposed on the surface of apoptotic cells and activates macrophage αvβ3/5 integrin receptors, thereby facilitating efficient efferocytosis [161,166,187]. Moreover, MFG-E8 is an important neuroinflammation and neuronal apoptosis modulator following ischemic injury [220,221]. MFG-E8 suppresses inflammatory responses and exerts tissue-protective effects, not only in ischemic stroke but also in intestinal ischemia [198] and Alzheimer’s disease models [139]. These findings demonstrate the significant neuroprotective potential of MFG-E8 in ischemic stroke, highlighting its promise as a novel therapeutic target for stroke treatment (Table 1).
Preclinical ischemic stroke models indicate that MFG-E8, primarily expressed by astrocytes and microglia, is upregulated in peri-infarct regions, coinciding with apoptotic cell accumulation and microglial activation. MFG-E8 binds PS on apoptotic cells and αvβ3/αvβ5 integrins on phagocytes, facilitating selective efferocytosis while suppressing proinflammatory signaling [221]. Comparative analyses with other PS-binding proteins reveal distinct advantages: Annexin V and PS-targeting antibodies inhibit phagocytosis but may exacerbate inflammation and delay tissue repair; Gas6 and Protein S activate TAM receptors to promote clearance and anti-inflammatory signaling but risk pathological angiogenesis and immunosuppression. In contrast, MFG-E8 coordinates apoptotic cell removal with anti-inflammatory responses, enhances neuronal survival, supports vascular remodeling, and demonstrates favorable stability and CNS targeting via exosome-based or intranasal delivery. These properties position MFG-E8 as a promising therapeutic candidate for ischemic stroke, though further investigation into potential adverse effects and drug resistance is warranted [221,222].

Table 1.
Functional roles of MFG-E8 after ischemic stroke in animal models.
Table 1.
Functional roles of MFG-E8 after ischemic stroke in animal models.
| Function | Potential Mechanism | Reference |
|---|---|---|
| Inflammation modulation | Suppresses cytokine release and promotes M2 macrophage polarization | [35] |
| Anti-apoptosis | Regulates Bax/Bcl-2 expression and caspase-3 inhibition | [27] |
| Efferocytosis promotion | Enhances efferocytosis by αvβ3 integrin binding | [186,213] |
| Neuroprotection | Promotes tissue repair and reduces infarct volume | [27,113] |
| Neurogenesis | Stimulates neural stem cell proliferation and migration | [223] |
Administration of rhMFG-E8 at 160 μg/kg via intravenous injection into the tail vein significantly reduced infarct volume in the ischemic penumbra to 25% at 24 h post-surgery in adult male Sprague–Dawley rats subjected to permanent middle cerebral artery occlusion (MCAO), a well-established rodent model of focal cerebral ischemia [27]. rhMFG-E8 markedly suppressed apoptosis markers, including cleaved caspase-3, Bcl-2, and Bax, and attenuated proinflammatory cytokines such as IL-6 (decreased by 39.6%) and TNF-α, while increasing the Bcl-2/Bax ratio by 51.9% compared to vehicle-treated animals [27]. Functionally, rhMFG-E8 improved neurological outcomes, reflected by significantly lower modified Neurological Severity Scores (mNSS) at days 4, 8, 11, and 15 post-stroke. Immunofluorescence analysis revealed increased RECA-1-positive endothelial cells in the peri-infarct region and higher numbers of BrdU- and DCX-positive cells in the subventricular zone (SVZ), indicating both neuroprotective and neuroregenerative effects [25]. These findings were obtained from controlled, randomized preclinical studies in rats, providing mechanistic evidence for rhMFG-E8-mediated suppression of apoptosis and inflammation following ischemic stroke.
These findings demonstrate the significant potential of MFG-E8 as a novel therapeutic target for ischemic stroke. Therapies that harness the neuroprotective and anti-inflammatory effects of MFG-E8 may offer an innovative approach to stroke treatment.
However, most studies involved small sample sizes and lacked blinding and randomization, limiting the interpretability of results. Additionally, species-specific differences in MFG-E8 domain structure warrant caution in extrapolating animal data to humans. Future research should provide quantitative comparisons across diverse models, dosages, and time points.
6. MFG-E8 in Ischemic Stroke Therapy: Limitations
Tag-free rhMFG-E8 can be lyophilized for long-term storage while retaining full biological activity upon reconstitution. Preclinical studies indicate favorable pharmacokinetics and safety: administration of up to 2 mg/kg in mice over 28 days showed no toxicity, inflammation, or body weight changes, and endotoxin levels were significantly below industry standards. Nevertheless, several limitations remain that should be considered in interpreting current findings and designing future studies [224].
Most preclinical investigations involve small sample sizes and often lack comprehensive pharmacokinetic, pharmacodynamic, and tissue distribution data. Assessments of BBB permeability and systemic toxicology are limited, leaving unresolved concerns regarding potential adverse effects, including immune dysregulation, tumor promotion via angiogenesis, and interference with normal apoptotic cell clearance [225,226]. Differences in MFG-E8 domain structure across species, lack of blinding and randomization, and variability in experimental models further constrain the generalizability of findings. Moreover, clinical data remain scarce, limiting translational potential.
To date, validated quantitative ranges of MFG-E8 in plasma or CSF of human ischemic stroke patients have not been established. Consequently, no standardized reference ranges are currently available for use in stroke cohorts. However, MFG-E8 levels reported in other neurological disorders may provide contextual insight. For instance, CSF MFG-E8 levels in patients with CAA were significantly lower than those in healthy controls (p = 0.01) and AD patients (p < 0.001) [227], suggesting that MFG-E8 concentrations may vary according to the type of neurological disorder. In summary, while MFG-E8 levels appear to differ across neurological conditions, validated quantitative ranges in ischemic stroke patients have not yet been reported.
Sex-specific differences in stroke pathophysiology and therapeutic responses represent another critical consideration. Women often present with atypical symptoms, experience delayed hospital arrival, and may have a higher risk of severe stroke compared to men [228]. Current data on MFG-E8 expression, signaling, and therapeutic efficacy across sexes are limited. Future studies should comprehensively evaluate immune responses, neuroprotection, and inflammatory biomarkers to inform sex-specific therapeutic strategies.
Finally, the context-dependent risks of MFG-E8-mediated angiogenesis should be addressed. The integrin-binding motif within MFG-E8′s EGF-like domain promotes cell adhesion, angiogenesis, and vascular remodeling [150,155,160,161]. While supporting tissue repair and apoptotic cell clearance, overexpression has been associated with tumor progression and an immunosuppressive microenvironment in certain cancers, whereas inhibition enhances antitumor T cell activity [229].
However, its systemic impact on antitumor immunity remains incompletely understood, underscoring the need for more comprehensive mechanistic studies and rigorous safety evaluations to inform the clinical translation of MFG-E8-based interventions.
These properties may theoretically exacerbate hemorrhagic transformation in stroke or tumor progression in individuals with latent neoplasia, emphasizing the need for careful evaluation of safety alongside therapeutic efficacy [202,204].
7. MFG-E8 in Ischemic Stroke Therapy: Prospects for Improvement
Advanced delivery strategies are under active investigation to overcome the aforementioned limitations. MFG-E8 can cross the BBB via exosome-mediated transport, supporting intranasal or intravenous exosome delivery as a promising strategy for sustained therapy. Small peptide derivatives, such as MSP68, may also penetrate the BBB, although their effects on neural stem cells (NSCs) require further evaluation. Engineering EVs with fusion proteins, such as the PS-binding domain of MFG-E8 linked to an RGD-4C peptide, has been shown to enhance targeted delivery to the ischemic brain by approximately 2.5-fold compared to naïve EVs [230,231].
Hypoxia-preconditioned human embryonic stem cell-derived EVs (hESC-HypoxEVs) exhibit enhanced glutathione redox capacity and potentiate senotherapeutic effects on NSCs. Administration of hESC-HypoxEVs precoated with MFG-E8 significantly increased NSC and newborn neuron populations in the subventricular zone and improved sensorimotor recovery in a rat ischemic stroke model [166,185].
Collectively, these findings highlight the potential of MFG-E8-based delivery systems to improve stability, targeting, and functional efficacy. Future research should provide quantitative comparisons across diverse models, dosages, and time points to enable robust evaluation of therapeutic outcomes. To establish the clinical utility of these biomarkers, future preclinical and clinical studies are needed to systematically assess plasma and CSF MFG-E8 levels, PS-exposing EVs, and microglial activation markers. These investigations will be critical for patient stratification and for monitoring the effectiveness of MFG-E8-based therapeutic interventions.
Future studies should also focus on optimizing dosing, administration routes, and combination strategies while rigorously addressing safety, pharmacokinetics, and long-term efficacy, ultimately facilitating clinical translation of MFG-E8-related therapeutics.
8. Discussion
With the rapid progression of global population aging, the incidence and socioeconomic burden of ischemic stroke are expected to rise substantially [232]. Aging is associated with vascular endothelial dysfunction, chronic low-grade inflammation, and increased vulnerability to cerebrovascular injury, complicating stroke pathophysiology and limiting the efficacy of current reperfusion-based therapies [233,234]. Against this backdrop, MFG-E8 has emerged as a promising therapeutic candidate due to its dual roles in apoptotic cell clearance and inflammation resolution. Positioned at the interface of immune regulation and tissue remodeling, MFG-E8 has demonstrated consistent preclinical efficacy in reducing infarct volume, attenuating neuroinflammation, and improving neurological and functional outcomes.
MFG-E8 shows potential not only as a monotherapy but also in combination with thrombolytic, neurorestorative, or advanced cell-based therapies. Its ability to promote long-term neuroregeneration further underscores the need for evaluation in chronic stroke models, where functional recovery remains a major clinical challenge.
To facilitate clinical translation, a structured and stepwise research roadmap is essential. Key priorities include the following: evaluating safety, pharmacokinetics, long-term functional recovery, and neuroregenerative effects in large-animal models; conducting preclinical and early-phase clinical trials to assess both monotherapy and combination strategies; validating translational biomarkers, such as plasma or cerebrospinal fluid MFG-E8 levels, phosphatidylserine-exposing extracellular vesicles, and microglial activation markers, for patient stratification and monitoring therapeutic responses; and developing stable formulations with efficient central nervous system-targeted delivery systems. Through these integrated approaches, MFG-E8-based therapeutics have the potential to advance from preclinical models to clinical application, offering a next-generation strategy for the treatment of ischemic stroke.
9.Methods
This review synthesizes the current knowledge on the functions of MFG-E8 in ischemic stroke and its regulatory mechanisms in neuroinflammation. A systematic search of the literature published from 1990 to 2025 was conducted on the PubMed, Scopus, and Web of Science databases. Keywords such as “ischemic stroke”, “MFG-E8”, “neuroinflammation”, “immune response” and “apoptotic cells” were used for the search and combined using Boolean operators (AND, OR, and NOT). The inclusion criteria were peer-reviewed English studies that investigated the relationship between MFG-E8 and ischemic stroke or elucidated the experimental/clinical mechanisms of inflammation regulation. Selected studies were thematically analyzed to evaluate the neuroprotective and anti-inflammatory roles of MFG-E8 in ischemic stroke, identify knowledge gaps, and determine future research directions.
10. Conclusions and Future Directions
The rising prevalence of ischemic stroke and limitations of current therapies highlight the need for novel interventions. MFG-E8, with its multifaceted roles in modulating inflammation, enhancing efferocytosis, and promoting tissue repair, emerges as a promising therapeutic candidate. Key actionable priorities include the following:
- (1)
- Standardize preclinical protocols: Optimize dosing, administration routes, and pharmacokinetic/pharmacodynamic (PK/PD) readouts in large animal stroke models.
- (2)
- Validate biomarkers: Quantify blood/CSF MFG-E8 and EV-associated markers as reliable pharmacodynamic and response indicators.
- (3)
- Define safety margins: Assess risks of hemorrhagic transformation and potential tumor-related effects with prospectively specified monitoring.
- (4)
- Conduct sex-stratified analyses: Evaluate efficacy and safety separately in male and female preclinical models.
- (5)
- Establish first-in-human criteria: Determine optimal timing relative to reperfusion therapy and compatibility with MT/rt-PA.
These priorities provide a structured framework to guide the translation of MFG-E8-based therapies from preclinical studies to clinical application and ultimately reduce the neurological and societal burden of ischemic stroke.
Author Contributions
Conceptualization, Y.-J.H.; writing—original draft preparation, Y.-J.H. and D.-H.P.; writing—review and editing, Y.-J.H., D.-H.G., J.-H.K. and D.-H.P.; visualization, Y.-J.H. and H.-J.L.; supervision, D.-H.P.; project administration, D.-H.G., J.-H.K. and D.-H.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT as well as the Ministry of Health & Welfare) (22A0204L1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Details are available from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Akt | Protein Kinase B |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPK | AMP-activated protein kinase |
| Apaf-1 | Apoptotic protease activating factor 1 |
| ARE | antioxidant response element |
| ASK1 | apoptosis signal-regulating kinase 1 |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2-associated X protein |
| BBB | blood–brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| BrdU | 5-Bromo-2′-deoxyuridine |
| C1, C2 | two C-terminal discoidin-like domains |
| Ca2+ | calcium ions |
| CAA | cerebral amyloid angiopathy |
| Casp8 | caspase-8 |
| Casp9 | caspase-9 |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL3 | C-C motif chemokine ligand 3 |
| CCL5 | C-C motif chemokine ligand 5 |
| CCR2 | chemokine (C-C motif) receptor 2 |
| CXCL1 | chemokine (C-X-C motif) ligand 1 |
| CXCL2 | chemokine (C-X-C motif) ligand 2 |
| CXCL8 | chemokine (C-X-C motif) ligand 8 |
| cIAPs | cellular inhibitor of apoptosis proteins |
| CK2 | Casein kinase 2 |
| CREB | cAMP response element-binding protein |
| CSF | cerebrospinal fluid |
| CX3CL1 | chemokines such as chemokine (C-X3-C motif) ligand 1 |
| CYT C | cytochrome c |
| DAPK1 | death-associated protein kinase 1 |
| DCX | Doublecortin |
| EGF1, EGF2 | EGF-like domains |
| ERK | Extracellular signal-regulated kinases |
| ET-1 | endothelin-1 |
| EVs | extracellular vesicles |
| FADD | Fas-associated protein with a death domain |
| GPX4 | impaired glutathione peroxidase 4 |
| GSK3β | glycogen synthase kinase 3 |
| hESC-HypoxEVs | human embryonic stem cell-derived EVs |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| HMGB1 | High mobility group box 1 |
| I/R | Ischemia–reperfusion |
| IL-1 | interleukin-1 |
| IL-1 β | interleukin-1β |
| IL-10 | interleukin-10 |
| IL-6 | interleukin-6 |
| IRS-1 | Insulin receptor substrate-1 |
| IS | Ischemic stroke |
| JNK | c-Jun N-terminal kinases |
| K | potassium |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCAO | middle cerebral artery occlusion |
| MEM16F | transmembrane protein 16F |
| MFG-E8 | Milk fat globule-EGF factor 8 |
| miRNA | microRNA |
| MMPs | matrix metalloproteinases |
| MT | mechanical thrombectomy |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | mechanistic/mammalian target of rapamycin complex 1 |
| Na+/K+-ATPase | sodium–potassium adenosine triphosphatase |
| NF-κB | Nuclear factor kappa B |
| NMDARs | N-methyl-D-aspartate receptors |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSCs | neural stem cells |
| PDK1 | 3-Phosphoinositide-dependent protein kinase-1 |
| PI3K | Phosphoinositide 3-Kinase |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| pro-Casp8 | procaspase-8 |
| pro-Casp9 | procaspase-9 |
| PS | phosphatidylserine |
| PSD95 | Postsynaptic density protein 95 |
| PTEN | Phosphatase and tensin homolog |
| r-tPA | recombinant tissue plasminogen activator |
| Rac1 | Rac Family Small GTPase 1 |
| RGD | Arg-Gly-Asp |
| rhMFG-E8 | recombinant human MFG-E8 |
| RIP1 | receptor interacting protein 1 |
| RIP3 | receptor interacting protein 3 |
| ROS | reactive oxygen species |
| SOCS3 | suppressor of cytokine signaling-3 |
| solTNF | soluble TNF |
| Src | proto-oncogene tyrosine-protein kinase Src |
| STAT3 | Signal transducer and activator of transcription 3 |
| SVZ | subventricular zone |
| tBID | truncated BID |
| Tim-4 | T-cell membrane protein 4 |
| TLR2, TLR4 | Toll-like receptors 2 and 4 |
| tmTNF | transmembrane TNF |
| TNF-R1 | tumor necrosis factor receptor 1 |
| TNFR1 | through TNF receptor 1 |
| TNFR2 | TNF receptor 2 |
| TNFα | tumor necrosis factor alpha |
| TRADD | TNF receptor-associated death domain |
| TRAF2/5 | TNF receptor-associated factors 2 and 5 |
| Tregs | regulatory T cells |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidhrence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Hur, J.W.; Kim, M.S.; Oh, S.Y.; Kang, H.Y.; Bae, J.; Kim, H.; Lee, H.; Lee, S.W.; Park, D.H. Label-free quantitative proteome profiling of cerebrospinal fluid from a rat stroke model with stem cell therapy. Cell Transplant. 2021, 30, 9636897211023474. [Google Scholar] [CrossRef] [PubMed]
- Sevick, L.K.; Ghali, S.; Hill, M.D.; Danthurebandara, V.; Lorenzetti, D.L.; Noseworthy, T.; Spackman, E.; Clement, F. Systematic review of the cost and cost-effectiveness of rapid endovascular therapy for acute ischemic stroke. Stroke 2017, 48, 2519–2526. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Abou-Hamden, A. Neurosurgery in Ischemic Stroke. In PanVascular Medicine; Lanzer, P., Ed.; 2013; pp. 1–32. [Google Scholar] [CrossRef]
- Bandera, E.; Botteri, M.; Minelli, C.; Sutton, A.; Abrams, K.R.; Latronico, N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: A systematic review. Stroke 2006, 37, 1334–1339. [Google Scholar] [CrossRef]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front. Physiol. 2020, 11, 605398. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Massaro, A.R. Standard strategies for acute ischemic stroke within the rtPA therapeutic window: Brazil. Neurol. Clin. Pract. 2013, 3, 210–213. [Google Scholar] [CrossRef]
- Campbell, B.C. Thrombolysis and thrombectomy for acute ischemic stroke: Strengths and synergies. Semin. Thromb. Hemost. 2017, 43, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cabral, N.L.; Conforto, A.; Magalhaes, P.S.C.; Longo, A.L.; Moro, C.H.C.; Appel, H.; Wille, P.; Nagel, V.; Venancio, V.; Garcia, A.C.; et al. Intravenous rtPA versus mechanical thrombectomy in acute ischemic stroke: A historical cohort in Joinville, Brazil. eNeurologicalSci 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balami, J.S.; White, P.M.; McMeekin, P.J.; Ford, G.A.; Buchan, A.M. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int. J. Stroke 2018, 13, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Pilgram-Pastor, S.M.; Piechowiak, E.I.; Dobrocky, T.; Kaesmacher, J.; Den Hollander, J.; Gralla, J.; Mordasini, P. Stroke thrombectomy complication management. J. Neurointerv. Surg. 2021, 13, 912–917. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Hamed, Y.; Seddeek, M.I.; Ahmed, A.M.; Dawa, T.A.; Hashem, H.; Othman, A.M.; Fayed, A.-G.I.; Elbazzar, N.; Metwally, R.A.; El Sayed, M.E.S.A.E.; et al. Factors predicting functional outcome after rtPA for patients with acute ischemic stroke. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 17. [Google Scholar] [CrossRef]
- González, R.G.; Furie, K.L.; Goldmacher, G.V.; Smith, W.S.; Kamalian, S.; Payabvash, S.; Harris, G.J.; Halpern, E.F.; Koroshetz, W.J.; Camargo, E.C.; et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke 2013, 44, 3109–3113. [Google Scholar] [CrossRef]
- Huang, Y.; Han, Z.; Shen, T.; Zheng, Y.; Yang, Z.; Fan, J.; Wang, R.; Yan, F.; Tao, Z.; Luo, Y.; et al. Neutrophil migration participates in the side effect of recombinant human tissue plasminogen activator. CNS Neurosci. Ther. 2024, 30, e14825. [Google Scholar] [CrossRef]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischaemic stroke-renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Irisa, K.; Shichita, T. Neural repair mechanisms after ischemic stroke. Inflamm. Regen. 2025, 45, 7. [Google Scholar] [CrossRef]
- Liaw, N.; Liebeskind, D. Emerging therapies in acute ischemic stroke. F1000Res 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. tPA helpers in the treatment of acute ischemic stroke: Are they ready for clinical use? J. Stroke 2019, 21, 160–174. [Google Scholar] [CrossRef]
- Lo, E.H. A new penumbra: Transitioning from injury into repair after stroke. Nat. Med. 2008, 14, 497–500. [Google Scholar] [CrossRef]
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective strategies for ischemic stroke-future perspectives. Int. J. Mol. Sci. 2023, 24, 4334. [Google Scholar] [CrossRef]
- Choi, J.I.; Kang, H.Y.; Han, C.; Woo, D.H.; Kim, J.H.; Park, D.H. Milk fat globule-epidermal growth factor VIII ameliorates brain injury in the subacute phase of cerebral ischemia in an animal model. J. Korean Neurosurg. Soc. 2020, 63, 163–170. [Google Scholar] [CrossRef]
- Kim, D.; Cho, G.S.; Han, C.; Park, D.H.; Park, H.K.; Woo, D.H.; Kim, J.H. Current understanding of stem cell and secretome therapies in liver diseases. Tissue Eng. Regen. Med. 2017, 14, 653–665. [Google Scholar] [CrossRef]
- Cheyuo, C.; Jacob, A.; Wu, R.; Zhou, M.; Qi, L.; Dong, W.; Ji, Y.; Chaung, W.W.; Wang, H.; Nicastro, J.; et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology 2012, 62, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S.-I. Protective Effect of MFG-E8 after Cutaneous Ischemia–Reperfusion Injury. J. Investig. Dermatol. 2015, 135, 1157–1165. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Fujimoto, T.; Nakamura, S.; Ohmura, K.; Mimori, T.; Matsuda, F.; Nagata, S. Aberrant splicing of the milk fat globule-EGF factor 8 (MFG-E8) gene in human systemic lupus erythematosus. Eur. J. Immunol. 2010, 40, 1778–1785. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional role of milk fat globule-epidermal growth factor VIII in macrophage-mediated inflammatory responses and inflammatory/autoimmune diseases. Mediat. Inflamm. 2016, 2016, 5628486. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Jacob, A.; Matsuda, A.; Wang, P. Review: Milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis 2011, 16, 1077–1086. [Google Scholar] [CrossRef]
- Li, B.Z.; Zhang, H.Y.; Pan, H.F.; Ye, D.Q. Identification of MFG-E8 as a novel therapeutic target for diseases. Expert Opin. Ther. Targets 2013, 17, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Liu, X.; Lu, W.; Mao, Y.; Mao, Y.; Ma, Y.; Wang, R.; Li, Q. Bioactive milk-derived nutrient MFG-E8 ameliorates skeletal muscle atrophy induced by mitochondria damage in aging rats via activating the MAPK/ERK signaling pathway. J. Dairy Sci. 2025, 108, 1182–1197. [Google Scholar] [CrossRef]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020, 6, 84. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, W.; Zhang, L.; Zhang, J.; Bi, J.; Wang, T.; Wang, M.; Du, Z.; Wang, Y.; Zhang, L.; et al. Milk fat globule EGF factor 8 restores mitochondrial function via integrin-mediated activation of the FAK-STAT3 signaling pathway in acute pancreatitis. Clin. Transl. Med. 2021, 11, e295. [Google Scholar] [CrossRef]
- Li, E.; Noda, M.; Doi, Y.; Parajuli, B.; Kawanokuchi, J.; Sonobe, Y.; Takeuchi, H.; Mizuno, T.; Suzumura, A. The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid β toxicity. J. Neuroinflamm. 2012, 9, 148. [Google Scholar] [CrossRef]
- Franchi, A.; Bocca, S.; Anderson, S.; Riggs, R.; Oehninger, S. Expression of milk fat globule EGF-factor 8 (MFG-E8) mRNA and protein in the human endometrium and its regulation by prolactin. Mol. Hum. Reprod. 2011, 17, 360–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanayama, R.; Nagata, S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc. Natl. Acad. Sci. USA 2005, 102, 16886–16891. [Google Scholar] [CrossRef]
- Lui, F.; Khan Suheb, M.Z.; Patti, L. Ischemic stroke. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Siniscalchi, A.; Gallelli, L.; Labate, A.; Malferrari, G.; Palleria, C.; Sarro, G.D. Post-stroke movement disorders: Clinical manifestations and pharmacological management. Curr. Neuropharmacol. 2012, 10, 254–262. [Google Scholar] [CrossRef]
- Bansil, S.; Prakash, N.; Kaye, J.; Wrigley, S.; Manata, C.; Stevens-Haas, C.; Kurlan, R. Movement disorders after stroke in adults: A review. Tremor Other Hyperkinet. Mov. 2012, 2, tre-02. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Catanese, L.; Tarsia, J.; Fisher, M. Acute ischemic stroke therapy overview. Circ. Res. 2017, 120, 541–558. [Google Scholar] [CrossRef]
- Frey, J.L. Recombinant tissue plasminogen activator (rtPA) for stroke. The perspective at 8 years. Neurologist 2005, 11, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Hill, M.D.; Shobha, N.; Menon, B.; Bal, S.; Kochar, P.; Watson, T.; Goyal, M.; Demchuk, A.M. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: Real-world experience and a call for action. Stroke 2010, 41, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Rama, R.; García, J.C. Excitotoxicity and Oxidative Stress in Acute Stroke. In Ischemic Stroke—Updates; Schaller, B., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Rehman, S.; Nadeem, A.; Akram, U.; Sarwar, A.; Quraishi, A.; Siddiqui, H.; Malik, M.A.J.; Nabi, M.; Ul Haq, I.; Cho, A.; et al. Molecular Mechanisms of Ischemic Stroke: A Review Integrating Clinical Imaging and Therapeutic Perspectives. Biomedicines 2024, 12, 812. [Google Scholar] [CrossRef]
- Akihiko, Y.; Minako, I. Resolution of inflammation and repair after ischemic brain injury. Neuroimmunol. Neuroinflamm. 2020, 7, 264–276. [Google Scholar] [CrossRef]
- Levinson, S.; Pulli, B.; Heit, J.J. Neuroinflammation and acute ischemic stroke: Impact on translational research and clinical care. Front. Surg. 2025, 12, 1501359. [Google Scholar] [CrossRef]
- Gauberti, M.; Martinez de Lizarrondo, S.; Vivien, D. Thrombolytic strategies for ischemic stroke in the thrombectomy era. J. Thromb. Haemost. 2021, 19, 1618–1628. [Google Scholar] [CrossRef]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, X.; Wang, Q.; Zheng, Y.; Cheng, J.; Han, J.; Li, C.; Cheng, F.; Wang, X. Decoding ischemic stroke: Perspectives on the endoplasmic reticulum, mitochondria, and their crosstalk. Redox Biol. 2025, 82, 103622. [Google Scholar] [CrossRef]
- Stankowski, J.N.; Gupta, R. Therapeutic targets for neuroprotection in acute ischemic stroke: Lost in translation? Antioxid. Redox Signal. 2011, 14, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Prescott, K.; Münch, A.E.; Brahms, E.; Weigel, M.K.; Inoue, K.; Buckwalter, M.S.; Liddelow, S.A.; Peterson, T.C. Blocking of microglia-astrocyte proinflammatory signaling is beneficial following stroke. Front. Mol. Neurosci. 2023, 16, 1305949. [Google Scholar] [CrossRef]
- Aronowski, J.; Strong, R.; Grotta, J.C. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 1048–1056. [Google Scholar] [CrossRef]
- Dietrich, W.D. Morphological manifestations of reperfusion injury in brain. Ann. N. Y. Acad. Sci. 1994, 723, 15–24. [Google Scholar] [CrossRef]
- Kuroda, S.; Siesjö, B.K. Reperfusion damage following focal ischemia: Pathophysiology and therapeutic windows. Clin. Neurosci. 1997, 4, 199–212. [Google Scholar] [PubMed]
- Garbuzova-Davis, S.; Rodrigues, M.C.; Hernandez-Ontiveros, D.G.; Tajiri, N.; Frisina-Deyo, A.; Boffeli, S.M.; Abraham, J.V.; Pabon, M.; Wagner, A.; Ishikawa, H.; et al. Blood-brain barrier alterations provide evidence of subacute diaschisis in an ischemic stroke rat model. PLoS ONE 2013, 8, e63553. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Li, K.; Wohlford-Lenane, C.; Perlman, S.; Zhao, J.; Jewell, A.K.; Reznikov, L.R.; Gibson-Corley, K.N.; Meyerholz, D.K.; McCray, P.B., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016, 213, 712–722. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef] [PubMed]
- Ludhiadch, A.; Sharma, R.; Muriki, A.; Munshi, A. Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Tymianski, M. Targeting NMDA receptors in stroke: New hope in neuroprotection. Mol. Brain 2018, 11, 15. [Google Scholar] [CrossRef]
- Dhawan, J.; Benveniste, H.; Luo, Z.; Nawrocky, M.; Smith, S.D.; Biegon, A. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol. 2011, 6, 823–834. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Pourkhodadad, S.; Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, Q.; Chen, Z.; Yun, H.; Wang, H. Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J. Biol. Chem. 2013, 288, 24151–24159. [Google Scholar] [CrossRef]
- Ladagu, A.D.; Olopade, F.E.; Adejare, A.; Olopade, J.O. GluN2A and GluN2B N-Methyl-D-aspartate receptor (NMDARs) subunits: Their roles and therapeutic antagonists in neurological diseases. Pharmaceuticals 2023, 16, 1535. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, G.; Bao, X. Extracellular vesicles as a potential therapy for stroke. Int. J. Mol. Sci. 2025, 26, 3130. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Q.; Li, Y.; Li, R.; Feng, J.; Chen, W.; Ahmed, W.; Soufiany, I.; Huang, S.; Long, J.; et al. The PI3K/AKT pathway-The potential key mechanisms of traditional Chinese medicine for stroke. Front. Med. 2022, 9, 900809. [Google Scholar] [CrossRef]
- Ding, P.F.; Zhang, H.S.; Wang, J.; Gao, Y.Y.; Mao, J.N.; Hang, C.H.; Li, W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front. Endocrinol. 2022, 13, 1092431. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.X.; Papadia, S.; Hofmann, F.; Hardingham, N.R.; Bading, H.; Hardingham, G.E. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 2006, 26, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Tamatani, M.; Matsuzaki, H.; Namikawa, K.; Kiyama, H.; Vitek, M.P.; Mitsuda, N.; Tohyama, M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 2001, 276, 5256–5264. [Google Scholar] [CrossRef]
- Downward, J. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1999, 1, E33–E35. [Google Scholar] [CrossRef]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef]
- Jo, H.; Mondal, S.; Tan, D.; Nagata, E.; Takizawa, S.; Sharma, A.K.; Hou, Q.; Shanmugasundaram, K.; Prasad, A.; Tung, J.K.; et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, 10581–10586. [Google Scholar] [CrossRef]
- Wu, G.Y.; Deisseroth, K.; Tsien, R.W. Activity-dependent CREB phosphorylation: Convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 2808–2813. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Ginty, D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Impey, S.; Fong, A.L.; Wang, Y.; Cardinaux, J.R.; Fass, D.M.; Obrietan, K.; Wayman, G.A.; Storm, D.R.; Soderling, T.R.; Goodman, R.H. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 2002, 34, 235–244. [Google Scholar] [CrossRef]
- Favaron, M.; Manev, R.M.; Rimland, J.M.; Candeo, P.; Beccaro, M.; Manev, H. NMDA-stimulated expression of BDNF mRNA in cultured cerebellar granule neurones. Neuroreport 1993, 4, 1171–1174. [Google Scholar]
- Hansen, H.H.; Briem, T.; Dzietko, M.; Sifringer, M.; Voss, A.; Rzeski, W.; Zdzisinska, B.; Thor, F.; Heumann, R.; Stepulak, A.; et al. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol. Dis. 2004, 16, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.B.; Hu, S.C.; Bobb, K.; Timmusk, T.; Ghosh, A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 1998, 20, 727–740. [Google Scholar] [CrossRef]
- Comelli, M.C.; Seren, M.S.; Guidolin, D.; Manev, R.M.; Favaron, M.; Rimland, J.M.; Canella, R.; Negro, A.; Manev, H. Photochemical stroke and brain-derived neurotrophic factor (BDNF) mRNA expression. Neuroreport 1992, 3, 473–476. [Google Scholar] [CrossRef]
- Chen, M.; Lu, T.J.; Chen, X.J.; Zhou, Y.; Chen, Q.; Feng, X.Y.; Xu, L.; Duan, W.H.; Xiong, Z.Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, P.L.; Panahian, N.; Dalkara, T.; Fishman, M.C.; Moskowitz, M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994, 265, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Lonobile, C.; Di Nubila, A.; Simone, R.; Hushi, M.; Barbieri, S.S. The Mitochondrial Permeability Transition Pore in Platelets: Mechanisms, Physiological Roles, and Therapeutic Perspectives. Antioxidants 2025, 14, 923. [Google Scholar] [CrossRef]
- Ning, K.; Pei, L.; Liao, M.; Liu, B.; Zhang, Y.; Jiang, W.; Mielke, J.G.; Li, L.; Chen, Y.; El-Hayek, Y.H.; et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J. Neurosci. 2004, 24, 4052–4060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Taghibiglou, C.; Girling, K.; Dong, Z.; Lin, S.Z.; Lee, W.; Shyu, W.C.; Wang, Y.T. Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J. Neurosci. 2013, 33, 7997–8008. [Google Scholar] [CrossRef]
- Deiss, L.P.; Feinstein, E.; Berissi, H.; Cohen, O.; Kimchi, A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995, 9, 15–30. [Google Scholar] [CrossRef]
- Kissil, J.L.; Feinstein, E.; Cohen, O.; Jones, P.A.; Tsai, Y.C.; Knowles, M.A.; Eydmann, M.E.; Kimchi, A. DAP-kinase loss of expression in various carcinoma and B-cell lymphoma cell lines: Possible implications for role as tumor suppressor gene. Oncogene 1997, 15, 403–407. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Mi, K.; Yang, S.; Xu, Y.; Li, J.; Chen, J.; Zhang, X. GluN2B-containing NMDA receptor attenuated neuronal apoptosis in the mouse model of HIBD through inhibiting endoplasmic reticulum stress-activated PERK/eIF2α signaling pathway. Front. Mol. Neurosci. 2024, 17, 1375843. [Google Scholar] [CrossRef]
- Lapchak, P.A. Memantine, an uncompetitive low affinity NMDA open-channel antagonist improves clinical rating scores in a multiple infarct embolic stroke model in rabbits. Brain Res. 2006, 1088, 141–147. [Google Scholar] [CrossRef]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in ischemic stroke: New insight and implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Vosler, P.S.; Graham, S.H.; Wechsler, L.R.; Chen, J. Mitochondrial targets for stroke: Focusing basic science research toward development of clinically translatable therapeutics. Stroke 2009, 40, 3149–3155. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Yao, Z.M.; Sun, X.R.; Tong, X.H.; Dong, S.Y. The role of mitochondrial dynamics in cerebral ischemia-reperfusion injury. Biomed. Pharmacother. 2023, 162, 114671. [Google Scholar] [CrossRef]
- Monsour, M.; Gordon, J.; Lockard, G.; Alayli, A.; Borlongan, C.V. Mitochondria in cell-based therapy for stroke. Antioxidants 2023, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Guan, S.; Wang, S.; Yu, Z.; Liu, T.; Du, S.; Zhu, C. Intercellular mitochondrial transfer in the brain, a new perspective for targeted treatment of central nervous system diseases. CNS Neurosci. Ther. 2023, 29, 3121–3135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial transfer/transplantation: An emerging therapeutic approach for multiple diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Feng, S.; Yang, M.; Liu, S.; He, Y.; Deng, S.; Gong, Y. Oxidative stress as a bridge between age and stroke: A narrative review. J. Intensive Med. 2023, 3, 313–319. [Google Scholar] [CrossRef]
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic edema: Mechanisms of pathological cell swelling. Neurosurg. Focus 2007, 22, E2. [Google Scholar] [CrossRef]
- Gao, L.; Peng, L.; Wang, J.; Zhang, J.H.; Xia, Y. Mitochondrial stress: A key role of neuroinflammation in stroke. J. Neuroinflamm. 2024, 21, 44. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Savyuk, M.O.; Ponimaskin, E.; Vedunova, M.V. Hypoxia-inducible factor (HIF) in ischemic stroke and neurodegenerative disease. Front. Cell Dev. Biol. 2021, 9, 703084. [Google Scholar] [CrossRef]
- Pan, Z.; Ma, G.; Kong, L.; Du, G. Hypoxia-inducible factor-1: Regulatory mechanisms and drug development in stroke. Pharmacol. Res. 2021, 170, 105742. [Google Scholar] [CrossRef]
- Shi, H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 2009, 16, 4593–4600. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 regulates oxidative stress and its role in cerebral ischemic stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.; Bhowmik, A.; Chatterjee, A.; Chatterjee, U.; Chatterjee, S.; Ghosh, M.K. Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2-protein phosphatase 2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell. Signal. 2014, 26, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, G.S.; Chan, P.H. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke 2011, 42, 3574–3579. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Zhou, X.; Lu, Y.; Ge, Y.; Zhang, X. Targeting neuronal mitophagy in ischemic stroke: An update. Burn. Trauma 2023, 11, tkad018. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.J.; Yang, X.S.; Zhou, H.; Xie, B.R.; Liu, W.W.; Li, Y. Role of mitophagy in the pathogenesis of stroke: From mechanism to therapy. Oxid. Med. Cell. Longev. 2022, 2022, 6232902. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Sun, Y.Y.; Liu, K.Y. Autophagy and inflammation in ischemic stroke. Neural. Regen. Res. 2020, 15, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Y.; Huang, Q.; Xu, P.; Lenahan, C.; Lu, J.; Zheng, J.; Dong, X.; Shao, A.; Zhang, J. An updated review of autophagy in ischemic stroke: From mechanisms to therapies. Exp. Neurol. 2021, 340, 113684. [Google Scholar] [CrossRef]
- Kazmi, S.; Farokhi-Sisakht, F.; Davoody, S.; Bahlakeh, G.; Abbaszadeh, F.; Rahbarghazi, R.; Shekarchi, A.; Karimipour, M. Role of autophagy in neurotoxic protein’s clearance following post-ischemic stroke: Where we are and what we know? Mol. Brain 2025, 18, 60. [Google Scholar] [CrossRef]
- Xu, D.; Kong, T.; Zhang, S.; Cheng, B.; Chen, J.; Wang, C. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cell. Signal. 2021, 79, 109839. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Melanis, K.; Stefanou, M.I.; Themistoklis, K.M.; Papasilekas, T. mTOR pathway—A potential therapeutic target in stroke. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231187770. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, J.; Cui, H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed. Pharmacother. 2018, 99, 583–590. [Google Scholar] [CrossRef]
- Xu, F.; Li, J.; Ni, W.; Shen, Y.W.; Zhang, X.P. Peroxisome proliferator-activated receptor-γ agonist 15d-prostaglandin J2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS ONE 2013, 8, e55080. [Google Scholar] [CrossRef]
- Duan, W.L.; Gu, L.H.; Guo, A.; Wang, X.J.; Ding, Y.Y.; Zhang, P.; Zhang, B.G.; Li, Q.; Yang, L.X. Molecular mechanisms of programmed cell death and potential targeted pharmacotherapy in ischemic stroke (Review). Int. J. Mol. Med. 2025, 56, 5544. [Google Scholar] [CrossRef]
- Shu, J.; Yang, L.; Wei, W.; Zhang, L. Identification of programmed cell death-related gene signature and associated regulatory axis in cerebral ischemia/reperfusion injury. Front. Genet. 2022, 13, 934154. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Swerdlow, R.H. TNFα in cerebral ischemia: Another stroke against you? J. Neurochem. 2015, 132, 369–372. [Google Scholar] [CrossRef]
- Ho, A.T.; Li, Q.H.; Hakem, R.; Mak, T.W.; Zacksenhaus, E. Coupling of caspase-9 to Apaf1 in response to loss of pRb or cytotoxic drugs is cell-type-specific. EMBO J. 2004, 23, 460–472. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhu, Q.; Gu, X.; Zhu, Y.Z. The discovery of a novel inhibitor of apoptotic protease activating factor-1 (Apaf-1) for ischemic heart: Synthesis, activity and target identification. Sci. Rep. 2016, 6, 29820. [Google Scholar] [CrossRef]
- Nakano, K.; Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Wolff, S.; Erster, S.; Palacios, G.; Moll, U.M. p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Z.; Zhao, X.Y.; Zhang, H.L. p53-mediated neuronal cell death in ischemic brain injury. Neurosci. Bull. 2010, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Balaganapathy, P.; Baik, S.H.; Mallilankaraman, K.; Sobey, C.G.; Jo, D.G.; Arumugam, T.V. Interplay between Notch and p53 promotes neuronal cell death in ischemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.V.; Baik, S.H.; Balaganapathy, P.; Sobey, C.G.; Mattson, M.P.; Jo, D.G. Notch signaling and neuronal death in stroke. Prog. Neurobiol. 2018, 165–167, 103–116. [Google Scholar] [CrossRef]
- Green, D.R. Nonapoptotic cell death pathways. Cold Spring Harb. Perspect. Biol. 2022, 14, a041079. [Google Scholar]
- Hao, W.; Feng, C. Research progress on pyroptosis and its effect on the central nervous system. Neurobiol. Dis. 2023, 188, 106333. [Google Scholar] [CrossRef]
- Wei, C. The role of glutathione peroxidase 4 in neuronal ferroptosis and its therapeutic potential in ischemic and hemorrhagic stroke. Brain Res. Bull. 2024, 217, 111065. [Google Scholar] [CrossRef]
- Alfieri, A.; Srivastava, S.; Siow, R.C.; Modo, M.; Fraser, P.A.; Mann, G.E. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J. Physiol. 2011, 589, 4125–4136. [Google Scholar] [CrossRef]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflamm. 2008, 5, 45. [Google Scholar] [CrossRef]
- Yli-Karjanmaa, M.; Clausen, B.H.; Degn, M.; Novrup, H.G.; Ellman, D.G.; Toft-Jensen, P.; Szymkowski, D.E.; Stensballe, A.; Meyer, M.; Brambilla, R.; et al. Topical administration of a soluble TNF inhibitor reduces infarct volume after focal cerebral ischemia in mice. Front. Neurosci. 2019, 13, 781. [Google Scholar] [CrossRef]
- Lu, W.; Chen, Z.; Wen, J. Flavonoids and ischemic stroke-induced neuroinflammation: Focus on the glial cells. Biomed. Pharmacother. 2024, 170, 115847. [Google Scholar] [CrossRef]
- Doll, D.N.; Barr, T.L.; Simpkins, J.W. Cytokines: Their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014, 5, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Sharma, P.; Nagar, P.; Medhi, B.; HariKrishnaReddy, D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 2024, 78, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Chen, L.; Tang, J.; Chen, Y.; Wang, L. The role of CCL2/CCR2 axis in cerebral ischemia-reperfusion injury and treatment: From animal experiments to clinical trials. Int. J. Mol. Sci. 2022, 23, 3485. [Google Scholar] [CrossRef]
- Koizumi, T.; Herckenrath, E.M.; Taguchi, K.; Mizuta, I.; Mizuno, T.; Tanaka, M. CCL2/CCR2 signaling-mediated microglial migration leads to cerebral small vessel dysfunction in chronic hypertension model rats. Exp. Neurol. 2025, 387, 115192. [Google Scholar] [CrossRef]
- Xu, H.; Lin, S.; Zhou, Z.; Li, D.; Zhang, X.; Yu, M.; Zhao, R.; Wang, Y.; Qian, J.; Li, X.; et al. New genetic and epigenetic insights into the chemokine system: The latest discoveries aiding progression toward precision medicine. Cell Mol Immunol 2023, 20, 739–776. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nishibori, M. Current status and future prospects in HMGB1 and receptor researches. Nihon Rinsho. 2016, 74, 703–711. [Google Scholar] [PubMed]
- Vartanian, K.B.; Stevens, S.L.; Marsh, B.J.; Williams-Karnesky, R.; Lessov, N.S.; Stenzel-Poore, M.P. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J. Neuroinflamm. 2011, 8, 140. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nan, G. The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. J. Mol. Neurosci. 2016, 59, 90–98. [Google Scholar] [CrossRef]
- Pfefferkorn, T.; Rosenberg, G.A. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke 2003, 34, 2025–2030. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Yang, Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus 2007, 22, E4. [Google Scholar] [CrossRef]
- Chi, O.Z.; Mellender, S.J.; Barsoum, S.; Liu, X.; Weiss, H.R. Hypoxic preconditioning increases blood-brain barrier disruption in the early stages of cerebral ischemia. Curr. Neurovasc. Res. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Li, X.; Wu, J.; Xue, T.; Wu, J.; Shen, H.; Li, X.; Shen, M.; Chen, G. TMEM16F aggravates neuronal loss by mediating microglial phagocytosis of neurons in a rat experimental cerebral ischemia and reperfusion model. Front. Immunol. 2020, 11, 1144. [Google Scholar] [CrossRef]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef]
- van Lookeren Campagne, M.; Wiesmann, C.; Brown, E.J. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 2007, 9, 2095–2102. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Zhang, Z.; Yang, G.Y. Significance of complement system in ischemic stroke: A comprehensive review. Aging Dis. 2019, 10, 429–462. [Google Scholar] [CrossRef]
- Stubbs, J.D.; Lekutis, C.; Singer, K.L.; Bui, A.; Yuzuki, D.; Srinivasan, U.; Parry, G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. USA 1990, 87, 8417–8421. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Atabai, K.; Fernandez, R.; Huang, X.; Ueki, I.; Kline, A.; Li, Y.; Sadatmansoori, S.; Smith-Steinhart, C.; Zhu, W.; Pytela, R.; et al. Mfge8 is critical for mammary gland remodeling during involution. Mol. Biol. Cell 2005, 16, 5528–5537. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Anand, M.; Almeida, D.; Atabai, K.; Sheppard, D.; Finnemann, S.C. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12005–12010. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Finnemann, S.C. Lack of alphavbeta5 integrin receptor or its ligand MFG-E8: Distinct effects on retinal function. Ophthal. Res. 2008, 40, 120–123. [Google Scholar] [CrossRef]
- Oshima, K.; Yasueda, T.; Nishio, S.; Matsuda, T. MFG-E8: Origin, structure, expression, functions and regulation. In MFG-E8 and Inflammation; Wang, P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Raymond, A.; Ensslin, M.A.; Shur, B.D. SED1/MFG-E8: A bimotif protein that orchestrates diverse cellular interactions. J. Cell. Biochem. 2009, 106, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, M.; Vogel, T.; Calvete, J.J.; Thole, H.H.; Schmidtke, J.; Matsuda, T.; Töpfer-Petersen, E. Molecular cloning and characterization of P47, a novel boar sperm-associated zona pellucida-binding protein homologous to a family of mammalian secretory proteins. Biol. Reprod. 1998, 58, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.S.; Shur, B.D. A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium. J. Cell Sci. 2009, 122, 849–858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.; Rongchun, W.; Lu, W.; Ma, Y. Exploring the potential of MFG-E8 in neurodegenerative diseases. Crit Rev Food Sci Nutr 2024, 1–15. [Google Scholar] [CrossRef]
- Suwatthee, T.; Kerr, D.; Maltseva, S.; Dulberger, C.L.; Hwang, L.H.; Slaw, B.R.; Bu, W.; Lin, B.; Adams, E.J.; Lee, K.Y.C. MFG-E8: A model of multiple binding modes associated with ps-binding proteins. Eur. Phys. J. E 2023, 46, 114. [Google Scholar] [CrossRef]
- Castellanos, E.R.; Ciferri, C.; Phung, W.; Sandoval, W.; Matsumoto, M.L. Expression, purification, and characterization of recombinant human and murine milk fat globule-epidermal growth factor-factor 8. Protein Expr. Purif. 2016, 124, 10–22. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Chen, H.; Wang, R.; Ma, Y. The structure and properties of MFG-E8 and the In vitro assessment of its toxic effects on myoblast cells. Protein Expr. Purif. 2021, 178, 105720. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, M.A.; Shur, B.D. The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Ma, Y.; Zhou, S.; Xiao, R. Milk fat globule membrane protein promotes C(2)C(12) cell proliferation through the PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2018, 114, 1305–1314. [Google Scholar] [CrossRef]
- Xu, X.; Cai, X.; Zhu, Y.; He, W.; Wu, Q.; Shi, X.; Fang, Y.; Pei, Z. MFG-E8 inhibits Aβ-induced microglial production of cathelicidin-related antimicrobial peptide: A suitable target against Alzheimer’s disease. Cell. Immunol. 2018, 331, 59–66. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 70. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Akakura, S.; Singh, S.; Spataro, M.; Akakura, R.; Kim, J.I.; Albert, M.L.; Birge, R.B. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. Cell Res. 2004, 292, 403–416. [Google Scholar] [CrossRef]
- Lee, J.; Geum, D.; Park, D.-H.; Kim, J.-H. Molecular targeting of ischemic stroke: The promise of naïve and engineered extracellular vesicles. Pharmaceutics 2024, 16, 1492. [Google Scholar] [CrossRef]
- Gidon-Jeangirard, C.; Solito, E.; Hofmann, A.; Russo-Marie, F.; Freyssinet, J.M.; Martínez, M.C. Annexin V counteracts apoptosis while inducing Ca2+ influx in human lymphocytic T cells. Biochem. Biophys. Res. Commun. 1999, 265, 709–715. [Google Scholar] [CrossRef]
- Swairjo, M.A.; Concha, N.O.; Kaetzel, M.A.; Dedman, J.R.; Seaton, B.A. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 1995, 2, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Heegaard, C.W.; Rasmussen, J.T.; Gilbert, G.E. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim. Biophys. Acta 2004, 1667, 82–90. [Google Scholar] [CrossRef]
- Otzen, D.E.; Blans, K.; Wang, H.; Gilbert, G.E.; Rasmussen, J.T. Lactadherin binds to phosphatidylserine-containing vesicles in a two-step mechanism sensitive to vesicle size and composition. Biochim. Biophys. Acta 2012, 1818, 1019–1027. [Google Scholar] [CrossRef]
- Ye, H.; Li, B.; Subramanian, V.; Choi, B.H.; Liang, Y.; Harikishore, A.; Chakraborty, G.; Baek, K.; Yoon, H.S. NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim. Biophys. Acta 2013, 1828, 1083–1093. [Google Scholar] [CrossRef]
- Fairn, G.D.; Schieber, N.L.; Ariotti, N.; Murphy, S.; Kuerschner, L.; Webb, R.I.; Grinstein, S.; Parton, R.G. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 2011, 194, 257–275. [Google Scholar] [CrossRef]
- Andersen, M.H.; Berglund, L.; Rasmussen, J.T.; Petersen, T.E. Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry 1997, 36, 5441–5446. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Patton, S.; Hamosh, M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate 1998, 74, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Graversen, H.; Fedosov, S.N.; Petersen, T.E.; Rasmussen, J.T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 2000, 39, 6200–6206. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Aoki, N.; Kato, T.; Kitajima, K.; Matsuda, T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 2002, 269, 1209–1218. [Google Scholar] [CrossRef]
- Cai, X.; Li, Y.; Zheng, X.; Hu, R.; Li, Y.; Xiao, L.; Wang, Z. Propofol suppresses microglial phagocytosis through the downregulation of MFG-E8. J. Neuroinflamm. 2021, 18, 18. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Wang, X.; Bu, H.F.; Zhong, W.; Asai, A.; Zhou, Z.; Tan, X.D. MFG-E8 and HMGB1 are involved in the mechanism underlying alcohol-induced impairment of macrophage efferocytosis. Mol. Med. 2013, 19, 170–182. [Google Scholar] [CrossRef]
- Qiu, J.; Li, W.; Mu, R.; Wang, L.; Guo, L.; Ma, L. MFGE8 decreased neuronal apoptosis and neuroinflammation to ameliorate early brain injury induced by subarachnoid hemorrhage through the inhibition of HMGB1. Hum. Exp. Toxicol. 2022, 41, 9603271221093635. [Google Scholar] [CrossRef]
- Furuta, Y.; Pena-Ramos, O.; Li, Z.; Chiao, L.; Zhou, Z. Calcium ions trigger the exposure of phosphatidylserine on the surface of necrotic cells. PLoS Genet. 2021, 17, e1009066. [Google Scholar] [CrossRef] [PubMed]
- Kenis, H.; van Genderen, H.; Deckers, N.M.; Lux, P.A.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp. Cell Res. 2006, 312, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Heit, B.; Dubuisson, J.F.; Fairn, G.D.; Chiu, B.; Inman, R.; Kapus, A.; Swanson, M.; Grinstein, S. Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. J. Cell Biol. 2009, 185, 917–928. [Google Scholar] [CrossRef]
- Fuller, A.D.; Van Eldik, L.J. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 2008, 3, 246–256. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, J.; Cho, S.; Lee, H.J.; Geum, D.; Park, D.H.; Kim, J.H. hESC-derived extracellular vesicles enriched with MFGE-8 and the GSH redox system act as senotherapeutics for neural stem cells in ischemic stroke. Free Radic. Biol. Med. 2025, 229, 333–349. [Google Scholar] [CrossRef]
- Moon, B.; Yang, S.; Moon, H.; Lee, J.; Park, D. After cell death: The molecular machinery of efferocytosis. Exp. Mol. Med. 2023, 55, 1644–1651. [Google Scholar] [CrossRef]
- Miksa, M.; Wu, R.; Dong, W.; Komura, H.; Amin, D.; Ji, Y.; Wang, Z.; Wang, H.; Ravikumar, T.S.; Tracey, K.J.; et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J. Immunol. 2009, 183, 5983–5990. [Google Scholar] [CrossRef]
- Hanayama, R. Autoimmune diseases and the role of MFG-E8. In MFG-E8 and Inflammation; Wang, P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 97–117. [Google Scholar]
- Asano, K.; Miwa, M.; Miwa, K.; Hanayama, R.; Nagase, H.; Nagata, S.; Tanaka, M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 2004, 200, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef]
- Yu, T.; Yi, Y.S.; Yang, Y.; Oh, J.; Jeong, D.; Cho, J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediat. Inflamm. 2012, 2012, 979105. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Du, R.H.; Sun, H.B.; Hu, Z.L.; Lu, M.; Ding, J.H.; Hu, G. Kir6.1/K-ATP channel modulates microglia phenotypes: Implication in Parkinson’s disease. Cell Death Dis. 2018, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Taylor, X.; Cisternas, P.; Jury, N.; Martinez, P.; Huang, X.; You, Y.; Redding-Ochoa, J.; Vidal, R.; Zhang, J.; Troncoso, J.; et al. Activated endothelial cells induce a distinct type of astrocytic reactivity. Commun. Biol. 2022, 5, 282. [Google Scholar] [CrossRef]
- Ding, Z.B.; Song, L.J.; Wang, Q.; Kumar, G.; Yan, Y.Q.; Ma, C.G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural. Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of microglia and astrocytes in the neurovascular unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Lauber, K.; Keppeler, H.; Munoz, L.E.; Koppe, U.; Schröder, K.; Yamaguchi, H.; Krönke, G.; Uderhardt, S.; Wesselborg, S.; Belka, C.; et al. Milk fat globule-EGF factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ. 2013, 20, 1230–1240. [Google Scholar] [CrossRef]
- He, X.; Tan, S.; Shao, Z.; Wang, X. Latitudinal and longitudinal regulation of tissue macrophages in inflammatory diseases. Genes Dis. 2022, 9, 1194–1207. [Google Scholar] [CrossRef]
- Singh, K.; Pal, D.; Sinha, M.; Ghatak, S.; Gnyawali, S.C.; Khanna, S.; Roy, S.; Sen, C.K. Epigenetic modification of microRNA-200b contributes to diabetic vasculopathy. Mol. Ther. 2017, 25, 2689–2704. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Amin, D.; Wu, R.; Ravikumar, T.S.; Wang, P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol. Med. 2007, 13, 553–560. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, L.; Jia, Z.; Bi, J.; Wei, Q.; Song, X.; Jiang, L.; Lin, S.; Wei, M. MFG-E8 overexpression is associated with poor prognosis in breast cancer patients. Pathol. Res. Pract. 2019, 215, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Sato, M.; Kanamoto, A.; Itoh, A.; Nagai, S.; Koyasu, S.; Dranoff, G.; Tahara, H. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J. Exp. Med. 2009, 206, 1317–1326. [Google Scholar] [CrossRef]
- Oba, J.; Moroi, Y.; Nakahara, T.; Abe, T.; Hagihara, A.; Furue, M. Expression of milk fat globule epidermal growth factor-VIII may be an indicator of poor prognosis in malignant melanoma. Br. J. Dermatol. 2011, 165, 506–512. [Google Scholar] [CrossRef]
- Marazuela, P.; Solé, M.; Bonaterra-Pastra, A.; Pizarro, J.; Camacho, J.; Martínez-Sáez, E.; Kuiperij, H.B.; Verbeek, M.M.; de Kort, A.M.; Schreuder, F.; et al. MFG-E8 (LACTADHERIN): A novel marker associated with cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell-Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Mizote, Y.; Inoue, T.; Akazawa, T.; Kunimasa, K.; Tamiya, M.; Kumamoto, Y.; Tsuda, A.; Yoshida, S.; Tatsumi, K.; Ekawa, T.; et al. Potent CTLs can be induced against tumor cells in an environment of lower levels of systemic MFG-E8. Cancer Sci. 2024, 115, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Shimagaki, T.; Yoshio, S.; Kawai, H.; Sakamoto, Y.; Doi, H.; Matsuda, M.; Mori, T.; Osawa, Y.; Fukai, M.; Yoshida, T.; et al. Serum milk fat globule-EGF factor 8 (MFG-E8) as a diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Sci. Rep. 2019, 9, 15788. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, W.; Wang, R.; Ma, Y. MFG-E8 in Microglial Regulation: A Review of Basic Research in the Central Nervous System. J. Food Sci. 2025, 90, e70235. [Google Scholar] [CrossRef]
- Truman, L.A.; Ford, C.A.; Pasikowska, M.; Pound, J.D.; Wilkinson, S.J.; Dumitriu, I.E.; Melville, L.; Melrose, L.A.; Ogden, C.A.; Nibbs, R.; et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 2008, 112, 5026–5036. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, W.H.; Suk, K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 2016, 103, 1–16. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miyasaka, K.; Aozasa, K.; Koike, M.; Uchiyama, Y.; Nagata, S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef]
- Sokolova, D.; Ghansah, S.A.; Puletti, F.; Georgiades, T.; De Schepper, S.; Zheng, Y.; Crowley, G.; Wu, L.; Rueda-Carrasco, J.; Koutsiouroumpa, A.; et al. Astrocyte-derived MFG-E8 facilitates microglial synapse elimination in Alzheimer’s disease mouse models. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, E.Y.; Chen, H.S.; Wu, M.C.; Yang, Y.L.; Wang, H.L.; Liu, C.W.; Lai, T.W. Microglia through MFG-E8 signaling decrease the density of degenerating neurons and protect the brain from the development of cortical infarction after stroke. PLoS ONE 2024, 19, e0308464. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, A.; Zhu, Y.; He, W.; Di, W.; Fang, Y.; Shi, X. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J. Cell. Physiol. 2018, 234, 904–914. [Google Scholar] [CrossRef]
- Wang, X.; Bu, H.F.; Liu, S.X.; De Plaen, I.G.; Tan, X.D. Molecular mechanisms underlying the regulation of the MFG-E8 gene promoter activity in physiological and inflammatory conditions. J. Cell. Biochem. 2015, 116, 1867–1879. [Google Scholar] [CrossRef]
- Bu, H.F.; Subramanian, S.; Geng, H.; Wang, X.; Liu, F.; Chou, P.M.; Du, C.; De Plaen, I.G.; Tan, X.D. MFG-E8 plays an important role in attenuating cerulein-induced acute pancreatitis in mice. Cells 2021, 10, 728. [Google Scholar] [CrossRef]
- Gao, L.; Manaenko, A.; Zeng, F.; Li, J.; Liu, L.; Xie, R.; Zhang, X.; Zhang, J.H.; Mei, Q.; Tang, J.; et al. Efferocytosis: A new therapeutic target for stroke. Chin. Med. J. 2024, 137, 2843–2850. [Google Scholar] [CrossRef]
- Lam, A.L.; Heit, B. Having an Old Friend for Dinner: The Interplay between Apoptotic Cells and Efferocytes. Cells 2021, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Cheyuo, C.; Aziz, M.; Yang, W.L.; Jacob, A.; Zhou, M.; Wang, P. Milk fat globule-EGF factor VIII attenuates CNS injury by promoting neural stem cell proliferation and migration after cerebral ischemia. PLoS ONE 2015, 10, e0122833. [Google Scholar] [CrossRef] [PubMed]
- Chaung, W.; Ma, G.; Jacob, A.; Brenner, M.; Wang, P. Human cell-expressed tag-free rhMFG-E8 as an effective radiation mitigator. Sci. Rep. 2023, 13, 22186. [Google Scholar] [CrossRef]
- Polson, A.G.; Fuji, R.N. The successes and limitations of preclinical studies in predicting the pharmacodynamics and safety of cell-surface-targeted biological agents in patients. Br. J. Pharmacol. 2012, 166, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).