Brain Endothelial Cells in Blood–Brain Barrier Regulation and Neurological Therapy
Abstract
1. Introduction
2. Cellular Origin of Brain Endothelial Cells
3. Morphology and Structure of Brain Endothelial Cells
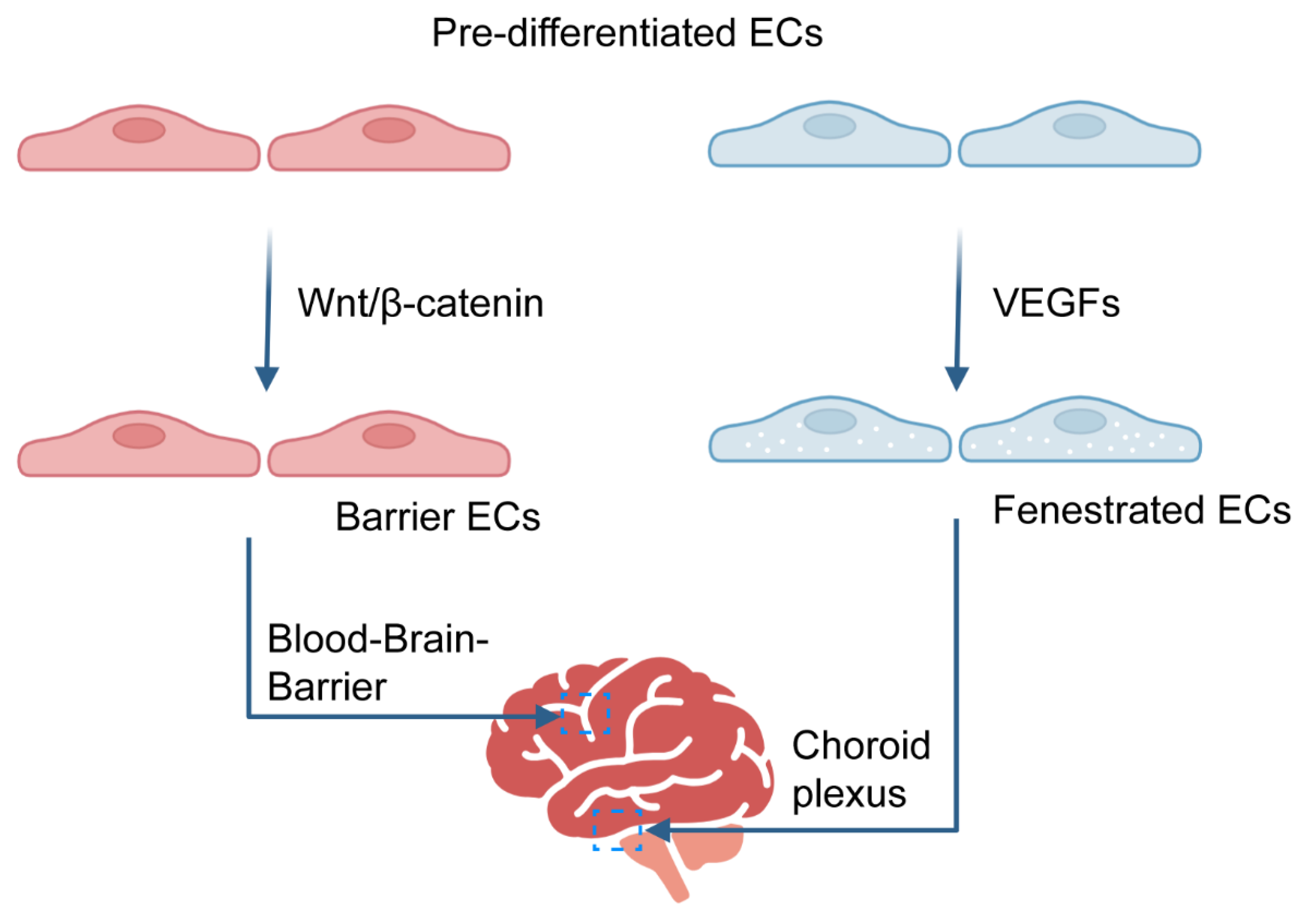
3.1. Development and Maintenance of the BBB
3.2. Development and Maintenance of Hypertonic Vasculature
4. Brain Endothelial Cells and Neurological Disorders
4.1. Alzheimer’s Disease
4.2. Multiple Sclerosis
4.3. Stroke and Vascular Lesions
4.4. Glioblastoma
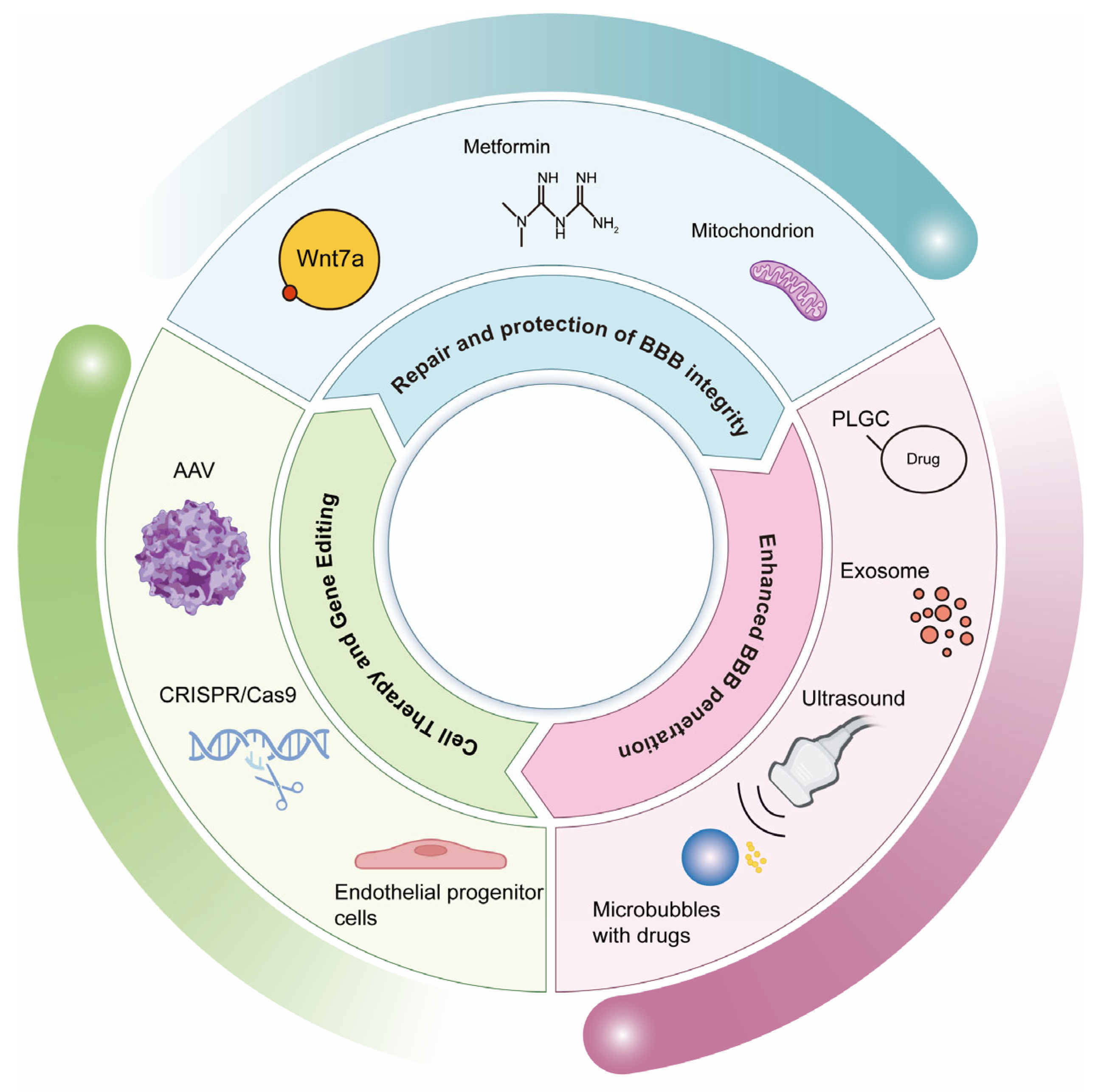
5. Therapeutic Strategies for Targeting Brain Endothelial Cells
5.1. Repair and Protection of BBB Integrity
5.1.1. Wnt/β-Catenin Pathway Agonists
5.1.2. Anti-Inflammatory Factors
5.1.3. Anti-Oxidative Stress
5.2. Enhanced BBB Penetration (Drug Delivery Strategy)
5.2.1. Carrier-Mediated Delivery Systems
Nanoparticles (NPs)
Exosome Engineering
5.2.2. Physical/Chemical Methods for the Instantaneous Opening of the BBB
Focused Ultrasound Combined with Microbubbles
Mannitol Shrinks Endothelial Cells
5.3. Cell Therapy and Gene Editing
5.3.1. Transplantation of Endothelial Progenitor Cells (EPCs)
5.3.2. Gene Editing Techniques
CRISPR-Cas9 Corrects Transporter Defects
AAV Delivery
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-alpha and IL-1beta Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-beta Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.C.S.; Dando, S.J.; White, A.R.; Oikari, L.E. Blood-brain barrier transporters: An overview of function, dysfunction in Alzheimer’s disease and strategies for treatment. Biochim. Biophys. Acta Mol. Basis. Dis. 2024, 1870, 166967. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Q.; Ruan, Y.; Xia, Y.; Fang, Z. Caveolae-Mediated Transcytosis and Its Role in Neurological Disorders. Biomolecules 2025, 15, 456. [Google Scholar] [CrossRef]
- Torices, S.; Teglas, T.; Naranjo, O.; Fattakhov, N.; Frydlova, K.; Cabrera, R.; Osborne, O.M.; Sun, E.; Kluttz, A.; Toborek, M. Occludin Regulates HIV-1 Infection by Modulation of the Interferon Stimulated OAS Gene Family. Mol. Neurobiol. 2023, 60, 4966–4982. [Google Scholar] [CrossRef]
- Zapata-Acevedo, J.F.; Mantilla-Galindo, A.; Vargas-Sanchez, K.; Gonzalez-Reyes, R.E. Blood-brain barrier biomarkers. Adv. Clin. Chem. 2024, 121, 1–88. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Ge, Y.; Chen, J.; Ma, J.; Wang, C.; Sun, M.; Wang, L.; Yao, S.; Yao, C. beta-amyloid protein induces mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN pathway leading to blood-brain barrier destruction in Alzheimer’s disease. Cell Biosci. 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Cashion, J.M.; Young, K.M.; Sutherland, B.A. How does neurovascular unit dysfunction contribute to multiple sclerosis? Neurobiol. Dis. 2023, 178, 106028. [Google Scholar] [CrossRef]
- Stone, O.A.; Zhou, B.; Red-Horse, K.; Stainier, D.Y.R. Endothelial ontogeny and the establishment of vascular heterogeneity. Bioessays 2021, 43, e2100036. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.L.; Stainier, D.Y.R. Recent insights into vascular development from studies in zebrafish. Curr. Opin. Hematol. 2018, 25, 204–211. [Google Scholar] [CrossRef]
- Mosimann, C.; Panakova, D.; Werdich, A.A.; Musso, G.; Burger, A.; Lawson, K.L.; Carr, L.A.; Nevis, K.R.; Sabeh, M.K.; Zhou, Y.; et al. Chamber identity programs drive early functional partitioning of the heart. Nat. Commun. 2015, 6, 8146. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Hollway, G.E.; Sonntag, C.; Miles, L.B.; Hall, T.E.; Berger, S.; Fernandez, K.J.; Gurevich, D.B.; Cole, N.J.; Alaei, S.; et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 2014, 512, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Pardanaud, L.; Luton, D.; Prigent, M.; Bourcheix, L.M.; Catala, M.; Dieterlen-Lievre, F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 1996, 122, 1363–1371. [Google Scholar] [CrossRef]
- Couly, G.; Coltey, P.; Eichmann, A.; Le Douarin, N.M. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech. Dev. 1995, 53, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Wilting, J.; Brand-Saberi, B.; Huang, R.; Zhi, Q.; Kontges, G.; Ordahl, C.P.; Christ, B. Angiogenic potential of the avian somite. Dev. Dyn. 1995, 202, 165–171. [Google Scholar] [CrossRef]
- Wasteson, P.; Johansson, B.R.; Jukkola, T.; Breuer, S.; Akyurek, L.M.; Partanen, J.; Lindahl, P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development 2008, 135, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, D.A.; Zhao, J.; Merrell, A.; Haldar, M.; Kardon, G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 2009, 23, 997–1013. [Google Scholar] [CrossRef]
- Stone, O.A.; Stainier, D.Y.R. Paraxial Mesoderm Is the Major Source of Lymphatic Endothelium. Dev. Cell 2019, 50, 247–255.e3. [Google Scholar] [CrossRef]
- Gore, A.V.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular development in the zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Plein, A.; Fantin, A.; Denti, L.; Pollard, J.W.; Ruhrberg, C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 2018, 562, 223–228. [Google Scholar] [CrossRef]
- Feng, T.; Gao, Z.; Kou, S.; Huang, X.; Jiang, Z.; Lu, Z.; Meng, J.; Lin, C.P.; Zhang, H. No Evidence for Erythro-Myeloid Progenitor-Derived Vascular Endothelial Cells in Multiple Organs. Circ. Res. 2020, 127, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Vogeli, K.M.; Jin, S.W.; Martin, G.R.; Stainier, D.Y. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 2006, 443, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Bai, L.; Zong, W.; Wang, X.; Bu, Y.; Xiong, C.; Zheng, J.; Li, J.; Gao, W.; Feng, Z.; et al. Light-sheet fluorescence imaging charts the gastrula origin of vascular endothelial cells in early zebrafish embryos. Cell. Discov. 2020, 6, 74. [Google Scholar] [CrossRef]
- Proulx, K.; Lu, A.; Sumanas, S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev. Biol. 2010, 348, 34–46. [Google Scholar] [CrossRef]
- Nag, S. Morphology and properties of brain endothelial cells. Methods Mol. Biol. 2011, 686, 3–47. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Yamaguchi, H.; Katsukura, Y.; Asashima, T.; Terasaki, T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J. Neurochem. 2008, 104, 147–154. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.M.; Baumholtz, A.I.; Ryan, A.K. Claudin-5 expression in the vasculature of the developing chick embryo. Gene Expr. Patterns 2012, 12, 123–129. [Google Scholar] [CrossRef]
- van Leeuwen, L.M.; Evans, R.J.; Jim, K.K.; Verboom, T.; Fang, X.; Bojarczuk, A.; Malicki, J.; Johnston, S.A.; van der Sar, A.M. A transgenic zebrafish model for the in vivo study of the blood and choroid plexus brain barriers using claudin 5. Biol. Open 2018, 7, bio030494. [Google Scholar] [CrossRef]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef]
- Matsuoka, R.L.; Buck, L.D.; Vajrala, K.P.; Quick, R.E.; Card, O.A. Historical and current perspectives on blood endothelial cell heterogeneity in the brain. Cell. Mol. Life Sci. 2022, 79, 372. [Google Scholar] [CrossRef] [PubMed]
- Parab, S.; Quick, R.E.; Matsuoka, R.L. Endothelial cell-type-specific molecular requirements for angiogenesis drive fenestrated vessel development in the brain. Elife 2021, 10, e64295. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Wichitnaowarat, V.; Lehmann, M.; Germano, R.F.; Mihova, D.; Macas, J.; Adams, R.H.; Taketo, M.M.; Plate, K.H.; Guerit, S.; et al. Low wnt/beta-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Elife 2019, 8, e43818. [Google Scholar] [CrossRef]
- Wang, Y.; Sabbagh, M.F.; Gu, X.; Rattner, A.; Williams, J.; Nathans, J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife 2019, 8, e43257. [Google Scholar] [CrossRef]
- Willis, C.L.; Garwood, C.J.; Ray, D.E. A size selective vascular barrier in the rat area postrema formed by perivascular macrophages and the extracellular matrix. Neuroscience 2007, 150, 498–509. [Google Scholar] [CrossRef]
- Bosma, E.K.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, H.; Hou, Y.; Wei, T.; Liu, J. Plasmalemma vesicle-associated protein: A crucial component of vascular homeostasis. Exp. Ther. Med. 2016, 12, 1639–1644. [Google Scholar] [CrossRef]
- Stan, R.V.; Tkachenko, E.; Niesman, I.R. PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol. Biol. Cell 2004, 15, 3615–3630. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Gordon, L.; Blechman, J.; Shimoni, E.; Gur, D.; Anand-Apte, B.; Levkowitz, G. The fenestrae-associated protein Plvap regulates the rate of blood-borne protein passage into the hypophysis. Development 2019, 146, dev177790. [Google Scholar] [CrossRef]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Xu, Y.; Zhu, X.; Jang, C.; Choi, W.; Bae, H.; Wang, W.; He, L.; Jin, S.W.; Arany, Z.; et al. Endothelium-derived lactate is required for pericyte function and blood-brain barrier maintenance. EMBO J. 2022, 41, e109890. [Google Scholar] [CrossRef]
- Stan, R.V.; Tse, D.; Deharvengt, S.J.; Smits, N.C.; Xu, Y.; Luciano, M.R.; McGarry, C.L.; Buitendijk, M.; Nemani, K.V.; Elgueta, R.; et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev. Cell 2012, 23, 1203–1218. [Google Scholar] [CrossRef]
- Herrnberger, L.; Seitz, R.; Kuespert, S.; Bosl, M.R.; Fuchshofer, R.; Tamm, E.R. Lack of endothelial diaphragms in fenestrae and caveolae of mutant Plvap-deficient mice. Histochem. Cell Biol. 2012, 138, 709–724. [Google Scholar] [CrossRef]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef]
- Sabbagh, M.F.; Nathans, J. A genome-wide view of the de-differentiation of central nervous system endothelial cells in culture. Elife 2020, 9, e51276. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef]
- Tran, K.A.; Zhang, X.; Predescu, D.; Huang, X.; Machado, R.F.; Gothert, J.R.; Malik, A.B.; Valyi-Nagy, T.; Zhao, Y.Y. Endothelial beta-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation 2016, 133, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bonney, S.; Dennison, B.J.C.; Wendlandt, M.; Siegenthaler, J.A. Retinoic Acid Regulates Endothelial beta-catenin Expression and Pericyte Numbers in the Developing Brain Vasculature. Front. Cell Neurosci. 2018, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Kamba, T.; Tam, B.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C.; et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef] [PubMed]
- Esser, S.; Wolburg, K.; Wolburg, H.; Breier, G.; Kurzchalia, T.; Risau, W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998, 140, 947–959. [Google Scholar] [CrossRef]
- Strickland, L.A.; Jubb, A.M.; Hongo, J.A.; Zhong, F.; Burwick, J.; Fu, L.; Frantz, G.D.; Koeppen, H. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF). J. Pathol. 2005, 206, 466–475. [Google Scholar] [CrossRef]
- Langlet, F.; Levin, B.E.; Luquet, S.; Mazzone, M.; Messina, A.; Dunn-Meynell, A.A.; Balland, E.; Lacombe, A.; Mazur, D.; Carmeliet, P.; et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013, 17, 607–617. [Google Scholar] [CrossRef]
- Jiang, H.; Gallet, S.; Klemm, P.; Scholl, P.; Folz-Donahue, K.; Altmuller, J.; Alber, J.; Heilinger, C.; Kukat, C.; Loyens, A.; et al. MCH Neurons Regulate Permeability of the Median Eminence Barrier. Neuron 2020, 107, 306–319.e9. [Google Scholar] [CrossRef]
- Morita, S.; Ukai, S.; Miyata, S. VEGF-dependent continuous angiogenesis in the median eminence of adult mice. Eur. J. Neurosci. 2013, 37, 508–518. [Google Scholar] [CrossRef]
- Furube, E.; Mannari, T.; Morita, S.; Nishikawa, K.; Yoshida, A.; Itoh, M.; Miyata, S. VEGF-dependent and PDGF-dependent dynamic neurovascular reconstruction in the neurohypophysis of adult mice. J. Endocrinol. 2014, 222, 161–179. [Google Scholar] [CrossRef]
- Anbalagan, S.; Gordon, L.; Blechman, J.; Matsuoka, R.L.; Rajamannar, P.; Wircer, E.; Biran, J.; Reuveny, A.; Leshkowitz, D.; Stainier, D.Y.R.; et al. Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Dev. Cell 2018, 47, 711–726.e5. [Google Scholar] [CrossRef] [PubMed]
- Gutnick, A.; Blechman, J.; Kaslin, J.; Herwig, L.; Belting, H.G.; Affolter, M.; Bonkowsky, J.L.; Levkowitz, G. The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev. Cell 2011, 21, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.S.; Walshe, T.E.; Saint-Geniez, M.; Venkatesha, S.; Maldonado, A.E.; Himes, N.C.; Matharu, K.S.; Karumanchi, S.A.; D’Amore, P.A. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 2008, 205, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Furube, E.; Mannari, T.; Okuda, H.; Tatsumi, K.; Wanaka, A.; Miyata, S. Vascular endothelial growth factor-dependent angiogenesis and dynamic vascular plasticity in the sensory circumventricular organs of adult mouse brain. Cell Tissue. Res. 2015, 359, 865–884. [Google Scholar] [CrossRef] [PubMed]
- Rahbarghazi, A.; Siahkouhian, M.; Rahbarghazi, R.; Ahmadi, M.; Bolboli, L.; Keyhanmanesh, R.; Mahdipour, M.; Rajabi, H. Role of melatonin in the angiogenesis potential; highlights on the cardiovascular disease. J. Inflamm. 2021, 18, 4. [Google Scholar] [CrossRef]
- This Figure Was Created in BioRender. Available online: https://biorender.com (accessed on 13 April 2025).
- Montagne, A.; Nation, D.A.; Pa, J.; Sweeney, M.D.; Toga, A.W.; Zlokovic, B.V. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016, 131, 687–707. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Penalva, Y.C.M.; Paschkowsky, S.; Yang, J.; Recinto, S.J.; Cinkornpumin, J.K.; Ruelas, M.; Xiao, B.; Nitu, A.; Kwon, S.Y.; Wu, H.Y.; et al. Loss of the APP regulator RHBDL4 preserves memory in an Alzheimer’s disease mouse model. Cell Death. Dis. 2025, 16, 280. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Perez, J.M.; Evans, A.C.; Alzheimer’s Disease Neuroimaging, I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- Omar, O.M.F.; Kimble, A.L.; Cheemala, A.; Tyburski, J.D.; Pandey, S.; Wu, Q.; Reese, B.; Jellison, E.R.; Hao, B.; Li, Y.; et al. Endothelial TDP-43 depletion disrupts core blood-brain barrier pathways in neurodegeneration. Nat. Neurosci. 2025, 28, 973–984. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Libby, J.B.; Dumitrescu, L.; De Jager, P.L.; Menon, V.; Schneider, J.A.; Bennett, D.A.; Hohman, T.J. Association of ten VEGF family genes with Alzheimer’s disease endophenotypes at single cell resolution. Alzheimers Dement. 2025, 21, e14419. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.; Biswas, S.; Zhao, K.; Akcan, U.; Tuohy, M.C.; Glendinning, M.D.; Kurt, A.; Wayne, C.R.; Prochilo, G.; Price, M.Z.; et al. VEGF-A-mediated venous endothelial cell proliferation results in neoangiogenesis during neuroinflammation. Nat. Neurosci. 2024, 27, 1904–1917. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, S.; Romeo, E.; Albanesi, E.; Piccardi, F.; Catalano, F.; Debellis, D.; Bertozzi, F.; Reggiani, A. Combined in vivo effect of N-acylethanolamine-hydrolyzing acid amidase and glycogen synthase kinase-3beta inhibition to treat multiple sclerosis. Biomed. Pharmacother. 2024, 175, 116677. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Afridi, S.K.; Wang, T.; Zhu, F.; Xu, H.; Nazir, F.H.; Liu, C.; Wang, Y.; Long, Y.; et al. Astrocyte-derived CHI3L1 signaling impairs neurogenesis and cognition in the demyelinated hippocampus. Cell Rep. 2024, 43, 114226. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Zierfuss, B.; Larochelle, C.; Prat, A. Blood-brain barrier dysfunction in multiple sclerosis: Causes, consequences, and potential effects of therapies. Lancet Neurol. 2024, 23, 95–109. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Christensen, S.; Campbell, B.C.; Marks, M.P.; Albers, G.W.; Lansberg, M.G.; Investigators, D. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology 2016, 87, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Jen, S.S.; Hillis, A.E.; Krakauer, J.W.; Barker, P.B.; Stir; Investigators, V.I. Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke 2014, 45, 2030–2035. [Google Scholar] [CrossRef]
- Fetsko, A.R.; Sebo, D.J.; Taylor, M.R. Brain endothelial cells acquire blood-brain barrier properties in the absence of Vegf-dependent CNS angiogenesis. Dev. Biol. 2023, 494, 46–59. [Google Scholar] [CrossRef]
- Desilles, J.P.; Rouchaud, A.; Labreuche, J.; Meseguer, E.; Laissy, J.P.; Serfaty, J.M.; Lapergue, B.; Klein, I.F.; Guidoux, C.; Cabrejo, L.; et al. Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology 2013, 80, 844–851. [Google Scholar] [CrossRef]
- Villringer, K.; Sanz Cuesta, B.E.; Ostwaldt, A.C.; Grittner, U.; Brunecker, P.; Khalil, A.A.; Schindler, K.; Eisenblatter, O.; Audebert, H.; Fiebach, J.B. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology 2017, 88, 433–440. [Google Scholar] [CrossRef]
- Ji, B.; Zhou, F.; Han, L.; Yang, J.; Fan, H.; Li, S.; Li, J.; Zhang, X.; Wang, X.; Chen, X.; et al. Sodium Tanshinone IIA Sulfonate Enhances Effectiveness Rt-PA Treatment in Acute Ischemic Stroke Patients Associated with Ameliorating Blood-Brain Barrier Damage. Transl. Stroke Res. 2017, 8, 334–340. [Google Scholar] [CrossRef]
- Liu, M.; Wang, D.; Qi, C.; Zou, M.; Song, J.; Li, L.; Xie, H.; Ren, H.; Hao, H.; Yang, G.; et al. Brain ischemia causes systemic Notch1 activity in endothelial cells to drive atherosclerosis. Immunity 2024, 57, 2157–2172.e2157. [Google Scholar] [CrossRef]
- Kremer, R.; Williams, A.; Wardlaw, J. Endothelial cells as key players in cerebral small vessel disease. Nat. Rev. Neurosci. 2025, 26, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, R.; Wang, Y.; Liu, M.; Hu, D.; Wang, Y.; Yang, L. A blood-brain barrier- and blood-brain tumor barrier-penetrating siRNA delivery system targeting gliomas for brain tumor immunotherapy. J. Control. Release 2024, 369, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.G.; Vladacenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Hibbard, J.C.; Ma, E.; Natri, H.M.; Adhikarla, V.; Chimge, N.O.; Qiu, R.; Nguyen, C.; Melendez, E.; Aguilar, B.; et al. Targeting Wnt signaling for improved glioma immunotherapy. Front Immunol. 2024, 15, 1342625. [Google Scholar] [CrossRef]
- Rahmani, F.; Hashemian, P.; Tabrizi, A.T.; Ghorbani, Z.; Ziaeemehr, A.; Alijannejad, S.; Ferns, G.A.; Avan, A.; Shahidsales, S. Regulatory role of miRNAs on Wnt/beta-catenin signaling in tumorigenesis of glioblastoma. Indian J. Cancer 2023, 60, 295–302. [Google Scholar] [CrossRef]
- Li, H.; Ouyang, J.; Wang, X.; Qian, C. Platycodin D Enhances Glioma Sensitivity to Temozolomide by Inhibition of the Wnt/beta-Catenin Pathway. Drug Des. Devel. Ther. 2025, 19, 1811–1824. [Google Scholar] [CrossRef]
- Nowacka, A.; Sniegocki, M.; Smuczynski, W.; Bozilow, D.; Ziolkowska, E. Angiogenesis in Glioblastoma-Treatment Approaches. Cells 2025, 14, 407. [Google Scholar] [CrossRef]
- Rattner, A.; Wang, Y.; Nathans, J. Signaling Pathways in Neurovascular Development. Annu. Rev. Neurosci. 2022, 45, 87–108. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, F.; He, L.; Huang, H.; Chao, M.; Cao, H.; Hu, Y.; Fan, Z.; Zhai, Y.; Zhao, W.; et al. Single-cell dissection of the human blood-brain barrier and glioma blood-tumor barrier. Neuron 2024, 112, 3089–3105. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, D.; Wu, J.Y.; Xing, K.; Yeo, E.; Li, C.; Zhang, L.; Holland, E.; Yao, L.; Qin, L.; et al. Wnt-mediated endothelial transformation into mesenchymal stem cell-like cells induces chemoresistance in glioblastoma. Sci. Transl. Med. 2020, 12, eaay7522. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Beck, B.; Lapouge, G.; Rorive, S.; Drogat, B.; Desaedelaere, K.; Delafaille, S.; Dubois, C.; Salmon, I.; Willekens, K.; Marine, J.C.; et al. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell 2015, 16, 67–79. [Google Scholar] [CrossRef]
- Ghosh, A.; Majie, A.; Karmakar, V.; Chatterjee, K.; Chakraborty, S.; Pandey, M.; Jain, N.; Roy Sarkar, S.; Nair, A.B.; Gorain, B. In-depth Mechanism, Challenges, and Opportunities of Delivering Therapeutics in Brain Using Intranasal Route. AAPS PharmSciTech. 2024, 25, 96. [Google Scholar] [CrossRef]
- Reiss, Y.; Bauer, S.; David, B.; Devraj, K.; Fidan, E.; Hattingen, E.; Liebner, S.; Melzer, N.; Meuth, S.G.; Rosenow, F.; et al. The neurovasculature as a target in temporal lobe epilepsy. Brain Pathol. 2023, 33, e13147. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Tong, Y.; Mu, R.; Han, L. Wnt-Regulated Therapeutics for Blood-Brain Barrier Modulation and Cancer Therapy. Bioconjug. Chem. 2025, 36, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lee, S.J.; Vlahos, L.; Yuki, K.; Rada, C.C.; van Unen, V.; Vuppalapaty, M.; Chen, H.; Sura, A.; McCormick, A.K.; et al. Therapeutic blood-brain barrier modulation and stroke treatment by a bioengineered FZD(4)-selective WNT surrogate in mice. Nat. Commun. 2023, 14, 2947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abedin, M.; Jo, H.N.; Levey, J.; Dinh, Q.C.; Chen, Z.; Angers, S.; Junge, H.J. A Frizzled4-LRP5 agonist promotes blood-retina barrier function by inducing a Norrin-like transcriptional response. iScience 2023, 26, 107415. [Google Scholar] [CrossRef]
- Martin, M.; Vermeiren, S.; Bostaille, N.; Eubelen, M.; Spitzer, D.; Vermeersch, M.; Profaci, C.P.; Pozuelo, E.; Toussay, X.; Raman-Nair, J.; et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science 2022, 375, eabm4459. [Google Scholar] [CrossRef]
- Yu, L.; Huang, L.; Zhao, Y.; Liu, S.; Zhou, R.; Yue, Y.; Sun, H.; Su, X.; Liu, Q.; Li, S.; et al. Atorvastatin Promotes Pro/anti-inflammatory Phenotypic Transformation of Microglia via Wnt/beta-catenin Pathway in Hypoxic-Ischemic Neonatal Rats. Mol. Neurobiol. 2024, 61, 3559–3577. [Google Scholar] [CrossRef]
- Sebo, D.J.; Ali, I.; Fetsko, A.R.; Trimbach, A.A.; Taylor, M.R. Activation of Wnt/beta-catenin in neural progenitor cells regulates blood-brain barrier development and promotes neuroinflammation. Sci. Rep. 2025, 15, 3496. [Google Scholar] [CrossRef]
- Xi, M.; Zhao, P.; Li, F.; Bao, H.; Ding, S.; Ji, L.; Yan, J. MicroRNA-16 inhibits the TLR4/NF-kappaB pathway and maintains tight junction integrity in irritable bowel syndrome with diarrhea. J. Biol. Chem. 2022, 298, 102461. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cui, K.; Mao, W.; Du, Y.; Yao, N.; Li, Z.; Zhao, H.; Ma, W. Weissella cibaria Attenuated LPS-Induced Dysfunction of Intestinal Epithelial Barrier in a Caco-2 Cell Monolayer Model. Front. Microbiol. 2020, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Tianrun, W.; Jiaqi, Y.; Xin, L.; Ruxue, D.; Peng, Z. Bta-miR-149-3p suppresses inflammatory response in bovine Sertoli cells exposed to microcystin-leucine arginine (MC-LR) through TLR4/NF-kB signaling pathway. Ecotoxicol. Environ. Saf. 2024, 281, 116636. [Google Scholar] [CrossRef]
- Ismail Hassan, F.; Didari, T.; Baeeri, M.; Gholami, M.; Haghi-Aminjan, H.; Khalid, M.; Navaei-Nigjeh, M.; Rahimifard, M.; Solgi, S.; Abdollahi, M.; et al. Metformin Attenuates Brain Injury by Inhibiting Inflammation and Regulating Tight Junction Proteins in Septic Rats. Cell J. 2020, 22, 29–37. [Google Scholar] [CrossRef]
- Cai, C.; Gu, C.; Meng, C.; He, S.; Thashi, L.; Deji, D.; Zheng, Z.; Qiu, Q. Therapeutic Effects of Metformin on Central Nervous System Diseases: A Focus on Protection of Neurovascular Unit. Pharm. Res. 2024, 41, 1907–1920. [Google Scholar] [CrossRef]
- Jin, L.; Jin, F.; Guo, S.; Liu, W.; Wei, B.; Fan, H.; Li, G.; Zhang, X.; Su, S.; Li, R.; et al. Metformin Inhibits NLR Family Pyrin Domain Containing 3 (NLRP)-Relevant Neuroinflammation via an Adenosine-5’-Monophosphate-Activated Protein Kinase (AMPK)-Dependent Pathway to Alleviate Early Brain Injury After Subarachnoid Hemorrhage in Mice. Front. Pharmacol. 2022, 13, 796616. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Kazem Nezhad, S.; Farmoudeh, A.; Babaei, A.; Ebrahimnejad, P.; Akbari, E.; Siahposht-Khachaki, A. Design and optimization of metformin-loaded solid lipid nanoparticles for neuroprotective effects in a rat model of diffuse traumatic brain injury: A biochemical, behavioral, and histological study. Eur. J. Pharm. Biopharm. 2022, 181, 122–135. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Reddy, N.M.; Faridi, H.M.; Shahid, M.; Shanley, T.P. Metformin alleviates lung-endothelial hyperpermeability by regulating cofilin-1/PP2AC pathway. Front. Pharmacol. 2023, 14, 1211460. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Z.; Wang, X.; He, H.; Xu, M.; Tan, C.; Wu, X.; Zhang, J.; Dong, W. Metformin attenuates colitis via blocking STAT3 acetylation by reducing acetyl-CoA production. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Tian, B.; Ye, P.; Zhou, X.; Hu, J.; Wang, P.; Cai, M.; Yang, K.; Sun, P.; Zou, X. Gallic Acid Ameliorated Chronic DSS-Induced Colitis Through Gut Microbiota Modulation, Intestinal Barrier Improvement, and Inflammation. Mol. Nutr. Food Res. 2025, e70024. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Li, G.; Gao, W.; Tang, H.; Fan, S.; Tang, X.; Zhao, L.; Wang, H.; Peng, A.; et al. Metformin Alleviates Delayed Hydrocephalus after Intraventricular Hemorrhage by Inhibiting Inflammation and Fibrosis. Transl. Stroke Res. 2023, 14, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Li, X.; Tian, Y.; Yu, Y.; Tang, L.; Sun, Q.; Zhang, T.; Fan, M.; Zhang, L.; et al. Semaglutide Alleviates Ovary Inflammation via the AMPK/SIRT1/NF-kappaB Signaling Pathway in Polycystic Ovary Syndrome Mice. Drug Des. Devel. Ther. 2024, 18, 3925–3938. [Google Scholar] [CrossRef]
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Singla, N.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Blood-brain barrier: Emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol. Brain 2022, 15, 49. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, J.; Zhang, H.; Lu, Q.; Zhang, Y.; Liu, C.; Liang, Y.; Tian, S.; Zhao, Y.; Fan, H. Traumatic brain injury: Bridging pathophysiological insights and precision treatment strategies. Neural Regen. Res. 2025, 21, 887–907. [Google Scholar] [CrossRef]
- Xue, K.; Qi, M.; She, T.; Jiang, Z.; Zhang, Y.; Wang, X.; Wang, G.; Xu, L.; Peng, B.; Liu, J.; et al. Argon mitigates post-stroke neuroinflammation by regulating M1/M2 polarization and inhibiting NF-kappaB/NLRP3 inflammasome signaling. J. Mol. Cell Biol. 2023, 14, mjac077. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dong, X. Unlocking Neuroinflammation: A Balanced Art for Therapeutics of Prion Disease. ACS Chem. Neurosci. 2025, 16, 281–283. [Google Scholar] [CrossRef]
- Du, X.; Amin, N.; Xu, L.; Botchway, B.O.A.; Zhang, B.; Fang, M. Pharmacological intervention of curcumin via the NLRP3 inflammasome in ischemic stroke. Front. Pharmacol. 2023, 14, 1249644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, W.; Chen, H.; Cai, Z.; Xu, G. Mitochondrial dynamics and mitochondrial autophagy: Molecular structure, orchestrating mechanism and related disorders. Mitochondrion 2024, 75, 101847. [Google Scholar] [CrossRef]
- Gureev, A.P.; Alimova, A.A.; Silachev, D.N.; Plotnikov, E.Y. Noncoupled Mitochondrial Respiration as Therapeutic Approach for the Treatment of Metabolic Diseases: Focus on Transgenic Animal Models. Int. J. Mol. Sci. 2023, 24, 16491. [Google Scholar] [CrossRef]
- Li, H.; Cai, Z. SIRT3 regulates mitochondrial biogenesis in aging-related diseases. J. Biomed. Res. 2022, 37, 77–88. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Reddy, A.P.; Reddy, P.H. Beneficial effects of mitophagy enhancers on amyloid beta-induced mitochondrial and synaptic toxicities in Alzheimer’s disease. Mitochondrion 2025, 83, 102038. [Google Scholar] [CrossRef] [PubMed]
- Reutzel, M.; Grewal, R.; Joppe, A.; Eckert, G.P. Age-Dependent Alterations of Cognition, Mitochondrial Function, and Beta-Amyloid Deposition in a Murine Model of Alzheimer’s Disease-A Longitudinal Study. Front. Aging Neurosci. 2022, 14, 875989. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Q.; Hicks, J.L.; Trabzonlu, L.; Ozbek, B.; Jones, T.; Vaghasia, A.M.; Larman, T.C.; Wang, R.; Markowski, M.C.; et al. MYC-driven increases in mitochondrial DNA copy number occur early and persist throughout prostatic cancer progression. JCI Insight 2023, 8, e169868. [Google Scholar] [CrossRef]
- Pegoraro, C.; Domingo-Orti, I.; Conejos-Sanchez, I.; Vicent, M.J. Unlocking the Mitochondria for Nanomedicine-based Treatments: Overcoming Biological Barriers, Improving Designs, and Selecting Verification Techniques. Adv. Drug Deliv. Rev. 2024, 207, 115195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.M.; Wei, L.S.; Ye, J.F. Advancements in mitochondrial-targeted nanotherapeutics: Overcoming biological obstacles and optimizing drug delivery. Front. Immunol. 2024, 15, 1451989. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.C.; Maarman, G.J.; Dube, A.; Bardien, S. Mitochondria targeted nanoparticles for the treatment of mitochondrial dysfunction-associated brain disorders. Front. Bioeng. Biotechnol. 2025, 13, 1563701. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Sun, J.; Han, Y.; Gong, W.; Li, Y.; Feng, Y.; Wang, H.; Yang, M.; Li, Z.; et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020, 108, 285–299. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.R.; Lee, M.J.; Park, Y.J.; Mun, S.; Yune, C.J.; Chung, T.N.; Bae, J.; Kim, M.J.; Choi, Y.S.; et al. The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated. Int. J. Mol. Sci. 2023, 24, 10113. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Starinets, V.S.; Mikheeva, I.B.; Belosludtsev, M.N.; Dubinin, M.V.; Mironova, G.D.; Belosludtsev, K.N. Effect of Chronic Treatment with Uridine on Cardiac Mitochondrial Dysfunction in the C57BL/6 Mouse Model of High-Fat Diet-Streptozotocin-Induced Diabetes. Int. J. Mol. Sci. 2022, 23, 10633. [Google Scholar] [CrossRef]
- Pan, X.; Hao, E.; Zhang, F.; Wei, W.; Du, Z.; Yan, G.; Wang, X.; Deng, J.; Hou, X. Diabetes cardiomyopathy: Targeted regulation of mitochondrial dysfunction and therapeutic potential of plant secondary metabolites. Front. Pharmacol. 2024, 15, 1401961. [Google Scholar] [CrossRef]
- Yao, L.; Hai, Q.; Zhang, T. The Application of Nucleic Acid Nanomaterials in the Treatment of Mitochondrial Dysfunction. Curr. Drug Metab. 2023, 24, 393–403. [Google Scholar] [CrossRef]
- Dave, K.M.; Venna, V.R.; Rao, K.S.; Stolz, D.B.; Brady, B.; Quaicoe, V.A.; Maniskas, M.E.; Hildebrand, E.E.; Green, D.; Chen, M.; et al. Mitochondria-containing extracellular vesicles from mouse vs. human brain endothelial cells for ischemic stroke therapy. J. Control. Release 2024, 373, 803–822. [Google Scholar] [CrossRef]
- Szirmai, Z.; Marton, A.; Kemeny, J.; Szever, Z.; Fodor, M.; Gal, E. Neonatal pneumonia caused by Chlamydia trachomatis. Orv. Hetil. 1985, 126, 1027–1030. [Google Scholar]
- Zhang, M.; Xiang, C.; Niu, R.; He, X.; Luo, W.; Liu, W.; Gu, R. Liposomes as versatile agents for the management of traumatic and nontraumatic central nervous system disorders: Drug stability, targeting efficiency, and safety. Neural. Regen. Res. 2025, 20, 1883–1899. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, J.; Chen, L.; Xu, Q.; Yao, S.; Chen, H. Targeting Neuroinflammation in Central Nervous System Diseases by Oral Delivery of Lipid Nanoparticles. Pharmaceutics 2025, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, H.; Du, B.; He, Y. Nanoscale drug formulations for the treatment of Alzheimer’s disease progression. RSC Adv. 2025, 15, 4031–4078. [Google Scholar] [CrossRef]
- Vargas, R.; Lizano-Barrantes, C.; Romero, M.; Valencia-Clua, K.; Narvaez-Narvaez, D.A.; Sune-Negre, J.M.; Perez-Lozano, P.; Garcia-Montoya, E.; Martinez-Martinez, N.; Hernandez-Munain, C.; et al. The piper at the gates of brain: A systematic review of surface modification strategies on lipid nanoparticles to overcome the Blood-Brain-Barrier. Int. J. Pharm. 2024, 665, 124686. [Google Scholar] [CrossRef] [PubMed]
- Picciolini, S.; Roda, F.; Gualerzi, A.; Mangolini, V.; Forleo, L.; Mangolini, A.; Sesana, S.; Antoniou, A.; Re, F.; Seneci, P.; et al. SPRi analysis of molecular interactions of mApoE-functionalized liposomes as drug delivery systems for brain diseases. Analyst 2023, 148, 6070–6077. [Google Scholar] [CrossRef]
- Constantinou, C.; Meliou, K.; Skouras, A.; Siafaka, P.; Christodoulou, P. Liposomes against Alzheimer’s Disease: Current Research and Future Prospects. Biomedicines 2024, 12, 1519. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer’s disease therapy. Int. J. Pharm. 2022, 626, 122167. [Google Scholar] [CrossRef]
- Qi, N.; Duan, W.; Gao, D.; Ma, N.; Zhang, J.; Feng, J.; Li, A. “Guide” of muscone modification enhanced brain-targeting efficacy and anti-glioma effect of lactoferrin modified DTX liposomes. Bioeng. Transl. Med. 2023, 8, e10393. [Google Scholar] [CrossRef] [PubMed]
- Giofre, S.; Renda, A.; Sesana, S.; Formicola, B.; Vergani, B.; Leone, B.E.; Denti, V.; Paglia, G.; Groppuso, S.; Romeo, V.; et al. Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics 2022, 14, 2402. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tao, Y.; Jiang, Y.; Qin, F. Recent progress of nanomedicine in the treatment of Alzheimer’s disease. Front. Cell Dev. Biol. 2023, 11, 1228679. [Google Scholar] [CrossRef]
- Wu, Y.; Angelova, A. Recent Uses of Lipid Nanoparticles, Cell-Penetrating and Bioactive Peptides for the Development of Brain-Targeted Nanomedicines against Neurodegenerative Disorders. Nanomaterials 2023, 13, 3004. [Google Scholar] [CrossRef]
- Razavi, Z.S.; Razavi, F.S.; Alizadeh, S.S. Inorganic nanoparticles and blood-brain barrier modulation: Advancing targeted neurological therapies. Eur. J. Med. Chem. 2025, 287, 117357. [Google Scholar] [CrossRef]
- Tang, S.; Han, E.L.; Mitchell, M.J. Peptide-functionalized nanoparticles for brain-targeted therapeutics. Drug Deliv. Transl. Res. 2025. [Google Scholar] [CrossRef]
- Wu, Y.; Moonshi, S.S.; Ta, H.T. Advancements in Using Polymeric Nanoparticles for Blood-Brain Barrier Penetration in Neurological Disorders. ACS Appl. Bio Mater. 2025, 8, 4416–4431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Q.; Chen, J.; Wei, Y.; Chen, J. Novel Development of Nanoparticles-A Promising Direction for Precise Tumor Management. Pharmaceutics 2022, 15, 24. [Google Scholar] [CrossRef]
- Cestarollo, L.; Utomo, N.; Htet, H.W.; Chen, Y.; Archer, L.A.; El-Ghazaly, A. Amplifying Magneto-Mechanical Performance of Magnetorheological Elastomers through Surface Functionalization of Iron Nanoparticles. ACS Appl. Mater. Interfaces 2025, 17, 15849–15858. [Google Scholar] [CrossRef]
- Rehman, F.U.; Liu, Y.; Zheng, M.; Shi, B. Exosomes based strategies for brain drug delivery. Biomaterials 2023, 293, 121949. [Google Scholar] [CrossRef]
- Rana, R.; Devi, S.N.; Bhardwaj, A.K.; Yashavarddhan, M.H.; Bohra, D.; Ganguly, N.K. Exosomes as nature’s nano carriers: Promising drug delivery tools and targeted therapy for glioma. Biomed. Pharmacother. 2025, 182, 117754. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, H.; Duan, H.; Sheng, G.; Tian, N.; Liu, D.; Sun, Z. Isolation and usage of exosomes in central nervous system diseases. CNS Neurosci. Ther. 2024, 30, e14677. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Geng, J.X.; Lu, Y.F.; Zhou, J.N.; Huang, B.; Qin, Y. Exosome technology: A novel and effective drug delivery system in the field of cancer therapy. World J. Gastrointest. Oncol. 2025, 17, 101857. [Google Scholar] [CrossRef]
- Chung, S.; Sugimoto, Y.; Huang, J.; Zhang, M. Iron Oxide Nanoparticles Decorated with Functional Peptides for a Targeted siRNA Delivery to Glioma Cells. ACS Appl. Mater. Interfaces 2023, 15, 106–119. [Google Scholar] [CrossRef]
- Pourmasoumi, P.; Abdouss, M.; Farhadi, M.; Jameie, S.B.; Khonakdar, H.A. Co-delivery of temozolomide and quercetin with folic acid-conjugated exosomes in glioblastoma treatment. Nanomedicine 2024, 19, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, Q.; Zhang, X.; Di, Z.; Wang, X.; Di, L. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids Surf. B. Biointerfaces 2022, 215, 112505. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Das, S.; Nandi, S.; Dhara, D.; Mandal, M. Magnolol and Temozolomide exhibit a synergistic anti-glioma activity through MGMT inhibition. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166782. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Zhou, J.; Wang, Q.; Kang, C. Expert opinion on translational research for advanced glioblastoma treatment. Cancer Biol. Med. 2023, 20, 344–352. [Google Scholar] [CrossRef]
- Bai, L.; Yu, L.; Ran, M.; Zhong, X.; Sun, M.; Xu, M.; Wang, Y.; Yan, X.; Lee, R.J.; Tang, Y.; et al. Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders. Int. J. Mol. Sci. 2025, 26, 2491. [Google Scholar] [CrossRef]
- Zhang, X.; Artz, N.; Steindler, D.A.; Hingtgen, S.; Satterlee, A.B. Exosomes: Traversing the blood-brain barrier and their therapeutic potential in brain cancer. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189300. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Gharalari, N.; Ghahremani-Nasab, M.; Naderi, R.; Chodari, L.; Nezhadshahmohammad, F. The potential of exosomal biomarkers: Revolutionizing Parkinson’s disease: How do they influence pathogenesis, diagnosis, and therapeutic strategies? AIMS Neurosci. 2024, 11, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, Y.; Zhao, C.; Liu, H.; Shao, Y.; Zhu, C.; An, L.; Chen, X.; Chen, Z. Engineered exosomes with enhanced stability and delivery efficiency for glioblastoma therapy. J. Control. Release 2024, 368, 170–183. [Google Scholar] [CrossRef]
- Khatami, S.H.; Karami, N.; Taheri-Anganeh, M.; Taghvimi, S.; Tondro, G.; Khorsand, M.; Soltani Fard, E.; Sedighimehr, N.; Kazemi, M.; Rahimi Jaberi, K.; et al. Exosomes: Promising Delivery Tools for Overcoming Blood-Brain Barrier and Glioblastoma Therapy. Mol. Neurobiol. 2023, 60, 4659–4678. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.I.; Khan, M.U.; Khan, N.M.; Bungau, S.; Hassan, S.S.U. Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering 2022, 10, 51. [Google Scholar] [CrossRef]
- Ramezani, A.; Rahnama, M.; Mahmoudian, F.; Shirazi, F.; Ganji, M.; Bakhshi, S.; Khalesi, B.; Hashemi, Z.S.; Khalili, S. Current Understanding of the Exosomes and Their Associated Biomolecules in the Glioblastoma Biology, Clinical Treatment, and Diagnosis. J. Neuroimmune Pharmacol. 2025, 20, 48. [Google Scholar] [CrossRef]
- Mehta, R.I.; Carpenter, J.S.; Mehta, R.I.; Haut, M.W.; Wang, P.; Ranjan, M.; Najib, U.; D’Haese, P.F.; Rezai, A.R. Ultrasound-mediated blood-brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer’s disease. Fluids Barriers CNS 2023, 20, 46. [Google Scholar] [CrossRef]
- Antoniou, A.; Stavrou, M.; Evripidou, N.; Georgiou, E.; Kousiappa, I.; Koupparis, A.; Papacostas, S.S.; Kleopa, K.A.; Damianou, C. FUS-mediated blood-brain barrier disruption for delivering anti-Abeta antibodies in 5XFAD Alzheimer’s disease mice. J. Ultrasound 2024, 27, 251–262. [Google Scholar] [CrossRef]
- Bathini, P.; Sun, T.; Schenk, M.; Schilling, S.; McDannold, N.J.; Lemere, C.A. Acute Effects of Focused Ultrasound-Induced Blood-Brain Barrier Opening on Anti-Pyroglu3 Abeta Antibody Delivery and Immune Responses. Biomolecules 2022, 12, 951. [Google Scholar] [CrossRef]
- Martinez, P.J.; Song, J.J.; Castillo, J.I.; DeSisto, J.; Song, K.H.; Green, A.L.; Borden, M. Effect of Microbubble Size, Composition, and Multiple Sonication Points on Sterile Inflammatory Response in Focused Ultrasound-Mediated Blood-Brain Barrier Opening. ACS Biomater. Sci. Eng. 2024, 10, 7451–7465. [Google Scholar] [CrossRef]
- Noel, R.L.; Batts, A.J.; Ji, R.; Pouliopoulos, A.N.; Bae, S.; Kline-Schoder, A.R.; Konofagou, E.E. Natural aging and Alzheimer’s disease pathology increase susceptibility to focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2023, 13, 6757. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kusunose, J.; Phipps, M.A.; Wang, F.; Chen, L.M.; Caskey, C.F. Guiding and monitoring focused ultrasound mediated blood-brain barrier opening in rats using power Doppler imaging and passive acoustic mapping. Sci. Rep. 2022, 12, 14758. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Hu, A.; Dong, L.; Lin, X.; Li, M.; Wang, Y.; Li, W.; Chang, L.; Chang, Y.; et al. Ultrasound combined with glial cell line-derived neurotrophic factor-loaded microbubbles for the targeted treatment of drug addiction. Front. Bioeng. Biotechnol. 2022, 10, 961728. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, Y.; Chen, G.; Cai, M.; Zhong, H.; Xiao, Z.; Lin, M.; Li, T.; Cai, Y.; Shuai, X.; et al. Anchoring Microbubbles on Cerebrovascular Endothelium as a New Strategy Enabling Low-Energy Ultrasound-Assisted Delivery of Varisized Agents Across Blood-Brain Barrier. Adv. Sci. 2023, 10, e2302134. [Google Scholar] [CrossRef]
- Lin, Y.; McMahon, D.; Jones, R.M.; Hynynen, K. A Transmit-Receive Phased Array for Microbubble-Mediated Focused Ultrasound Brain Therapy in Small Animals. IEEE Trans. Biomed. Eng. 2025, 72, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Klaus, S.P. Focused ultrasound therapy for Alzheimer’s disease: Exploring the potential for targeted amyloid disaggregation. Front. Neurol. 2024, 15, 1426075. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, Y.; Pople, C.B.; Bethune, A.; Jones, R.M.; Abrahao, A.; Hamani, C.; Kalia, S.K.; Kalia, L.V.; Lipsman, N.; et al. Cavitation Feedback Control of Focused Ultrasound Blood-Brain Barrier Opening for Drug Delivery in Patients with Parkinson’s Disease. Pharmaceutics 2022, 14, 2607. [Google Scholar] [CrossRef]
- Song, G.; Plumlee, P.; Ahn, J.Y.; Wong, S.T.; Zhao, H. Translational strategies and systems biology insights for blood-brain barrier opening and delivery in brain tumors and Alzheimer’s disease. Biomed. Pharmacother. 2023, 167, 115450. [Google Scholar] [CrossRef]
- Angolano, C.; Hansen, E.; Ajjawi, H.; Nowlin, P.; Zhang, Y.; Thunemann, N.; Ferran, C.; Todd, N. Characterization of focused ultrasound blood-brain barrier disruption effect on inflammation as a function of treatment parameters. Biomed. Pharmacother. 2025, 182, 117762. [Google Scholar] [CrossRef]
- Ador, T.; Fournie, M.; Rigollet, S.; Counil, C.; Stupar, V.; Barbier, E.L.; Pichon, C.; Delalande, A. Ultrasound-Assisted Blood-Brain Barrier Opening Monitoring by Photoacoustic and Fluorescence Imaging Using Indocyanine Green. Ultrasound Med. Biol. 2025, 51, 1059–1069. [Google Scholar] [CrossRef]
- Kazeem, H. Outdated ECT machines. Br. J. Psychiatry 1994, 165, 555. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chu, C.; Zhang, J.; Bie, C.; Chen, L.; Aafreen, S.; Xu, J.; Kamson, D.O.; van Zijl, P.C.M.; Walczak, P.; et al. Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood-Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging. Pharmaceutics 2022, 14, 2529. [Google Scholar] [CrossRef] [PubMed]
- Conq, J.; Joudiou, N.; Ucakar, B.; Vanvarenberg, K.; Preat, V.; Gallez, B. Assessment of Hyperosmolar Blood-Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI. Biomedicines 2023, 11, 1957. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Chen, J.; Long, T.; Zhong, C.; Li, Y. Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells. Open Life Sci. 2022, 17, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Romanova, N.; Schmitz, J.; Strakeljahn, M.; Grunberger, A.; Bahnemann, J.; Noll, T. Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response. Cells 2022, 11, 1763. [Google Scholar] [CrossRef]
- Cai, Q.; Fan, H.; Li, X.; Giannotta, M.; Bachoo, R.; Qin, Z. Optical Modulation of the Blood-Brain Barrier for Glioblastoma Treatment. Bio-Protoc. 2024, 14, e4920. [Google Scholar] [CrossRef]
- Menegaz de Almeida, A.; Viana, P.; Marinheiro, G.; Hoffmann Relvas, J.; Lopes, L.; Lima Guilherme, G.; Zanette Giusti, J.A.; Oliveira, P.; Azevedo Silva Kaiser Cabral, M.A.; Carvalho Santos, R.; et al. Hypertonic Saline Solution Versus Mannitol for Brain Relaxation During Craniotomies: A Systematic Review and Updated Meta-Analysis. Neurosurgery 2024, 95, 517–526. [Google Scholar] [CrossRef]
- Huang, Q.; Chan, K.Y.; Wu, J.; Botticello-Romero, N.R.; Zheng, Q.; Lou, S.; Keyes, C.; Svanbergsson, A.; Johnston, J.; Mills, A.; et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science 2024, 384, 1220–1227. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Jiang, L.; Wang, L.; Li, Y.; Liu, Y.; Wang, Y.; Yang, G.Y.; Ding, J.; Zhang, Z. Endothelial progenitor cell transplantation attenuates synaptic loss associated with enhancing complement receptor 3-dependent microglial/macrophage phagocytosis in ischemic mice. J. Cereb. Blood Flow Metab. 2023, 43, 379–392. [Google Scholar] [CrossRef]
- Rudnicka-Drozak, E.; Drozak, P.; Mizerski, G.; Drozak, M. Endothelial Progenitor Cells in Neurovascular Disorders-A Comprehensive Overview of the Current State of Knowledge. Biomedicines 2022, 10, 2616. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, X.; He, Y.; Guo, H.; Zhang, Y. Stem cell-derived exosomes for ischemic stroke: A conventional and network meta-analysis based on animal models. Front. Pharmacol. 2024, 15, 1481617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Q.; Cheng, H.; Zhang, Y.; Xie, Y.; Zhang, Q. Extracellular vesicles derived from endothelial progenitor cells modified by Houshiheisan promote angiogenesis and attenuate cerebral ischemic injury via miR-126/PIK3R2. Sci. Rep. 2024, 14, 28166. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cheng, T.; Lai, X. Mechanism of ischemic brain injury repair by endothelial progenitor cell-derived exosomes. Mol. Med. Rep. 2022, 26, 269. [Google Scholar] [CrossRef]
- Bansal, A.; Singh, A.; Nag, T.C.; Sharma, D.; Garg, B.; Bhatla, N.; Choudhury, S.D.; Ramakrishnan, L. Augmenting the Angiogenic Profile and Functionality of Cord Blood Endothelial Colony-Forming Cells by Indirect Priming with Bone-Marrow-Derived Mesenchymal Stromal Cells. Biomedicines 2023, 11, 1372. [Google Scholar] [CrossRef]
- Canjuga, D.; Steinle, H.; Mayer, J.; Uhde, A.K.; Klein, G.; Wendel, H.P.; Schlensak, C.; Avci-Adali, M. Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium. Pharmaceutics 2022, 14, 1194. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of Endothelial Progenitor Cell: Past, Present, and Future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef]
- Du, Y.T.; Pan, Z.G.; Chen, B.C.; Sun, F.Y. Carotid artery transplantation of brain endothelial cells enhances neuroprotection and neurorepair in ischaemic stroke rats. Acta Pharmacol. Sin. 2024, 45, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Thakore, A.; Tada, Y.; Pedroza, A.J.; Ikeda, G.; Chen, I.Y.; Chan, D.; Jaatinen, K.J.; Yajima, S.; Pfrender, E.M.; et al. Angiogenic stem cell delivery platform to augment post-infarction neovasculature and reverse ventricular remodeling. Sci. Rep. 2022, 12, 17605. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Yang, L.Y.; Chen, Y.T.; Chou, S.C.; Chen, K.W.; Chen, Y.H.; Deng, C.R.; Chen, I.C.; Chou, W.J.; Chang, C.C.; et al. Endothelial progenitor cell-derived conditioned medium mitigates chronic cerebral ischemic injury through macrophage migration inhibitory factor-activated AKT pathway. Stem Cell Res. Ther. 2024, 15, 428. [Google Scholar] [CrossRef]
- Heng, Y.Y.; Shang, H.J.; Zhang, X.Z.; Wei, W. Sodium tanshinone IIA sulfonate ameliorates neointima by protecting endothelial progenitor cells in diabetic mice. BMC Cardiovasc. Disord. 2023, 23, 446. [Google Scholar] [CrossRef]
- Radtke, L.; Majchrzak-Celinska, A.; Awortwe, C.; Vater, I.; Nagel, I.; Sebens, S.; Cascorbi, I.; Kaehler, M. CRISPR/Cas9-induced knockout reveals the role of ABCB1 in the response to temozolomide, carmustine and lomustine in glioblastoma multiforme. Pharmacol. Res. 2022, 185, 106510. [Google Scholar] [CrossRef]
- Roy, L.O.; Lemelin, M.; Blanchette, M.; Poirier, M.B.; Aldakhil, S.; Fortin, D. Expression of ABCB1, ABCC1 and 3 and ABCG2 in glioblastoma and their relevance in relation to clinical survival surrogates. J. Neurooncol. 2022, 160, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.A.; Hartz, A.M.S.; Bauer, B. ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery. Pharmacol. Rev. 2023, 75, 815–853. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Hu, C.; Chen, Y.; Chen, Z.; Chen, Z.S.; Zhang, J.Y.; Fang, S. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol. Cancer 2022, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, C.; Gregori, A.; Hagens, T.M.S.; van der Noord, V.E.; van de Water, B.; Zweemer, A.J.M.; Coban, B.; Capula, M.; Mantini, G.; Botto, A.; et al. ABCB1 overexpression through locus amplification represents an actionable target to combat paclitaxel resistance in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2024, 43, 4. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shang, P.; Mohanraju, P.; Geijsen, N. Prime editing: Advances and therapeutic applications. Trends Biotechnol. 2023, 41, 1000–1012. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, M.S.; Singh, B. Deciphering the functional role of clinical mutations in ABCB1, ABCC1, and ABCG2 ABC transporters in endometrial cancer. Front. Pharmacol. 2024, 15, 1380371. [Google Scholar] [CrossRef]
- Lin, B.H.; Li, Y.C.; Murakami, M.; Wu, Y.S.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V.; Wu, C.P. Epertinib counteracts multidrug resistance in cancer cells by antagonizing the drug efflux function of ABCB1 and ABCG2. Biomed. Pharmacother. 2024, 180, 117542. [Google Scholar] [CrossRef]
- Chuapoco, M.R.; Flytzanis, N.C.; Goeden, N.; Christopher Octeau, J.; Roxas, K.M.; Chan, K.Y.; Scherrer, J.; Winchester, J.; Blackburn, R.J.; Campos, L.J.; et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nat. Nanotechnol. 2023, 18, 1241–1251. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Liu, Y.; Qu, Y.; Wang, K.; Zhang, Y.; Chang, Y.; Yang, Z.; Wan, J.; Liu, J.; et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat. Biomed. Eng. 2022, 6, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Hudak, A.; Roach, M.; Pusztai, D.; Pettko-Szandtner, A.; Letoha, A.; Szilak, L.; Azzouz, M.; Letoha, T. Syndecan-4 Mediates the Cellular Entry of Adeno-Associated Virus 9. Int J. Mol. Sci. 2023, 24, 3141. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.C.; Hoffman, B.A.; Chen, W.; Shah, I.; Ren, X.Q.; Knox, T.; Liu, J.; Wang, W.; Li, J.; Khalid, H.; et al. Highly conserved brain vascular receptor ALPL mediates transport of engineered AAV vectors across the blood-brain barrier. Mol. Ther. 2025. [Google Scholar] [CrossRef]
- Ellison, S.; Liao, A.; Gleitz, H.F.E.; Parker, H.; Booth, L.; Robinson, J.; Wood, S.; Taylor, J.; Holley, R.; Bigger, B.W. Sustained long-term disease correction in a murine model of MPSII following stem cell gene therapy. Mol. Ther. Methods Clin. Dev. 2023, 31, 101127. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Malviya, R. Vector-Mediated Delivery of Transgenes and RNA Interference-Based Gene Silencing Sequences to Astrocytes for Disease Management: Advances and Prospectives. Curr. Gene Ther. 2024, 24, 110–121. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Ji, W.; Xu, L.; Xie, Y.; He, S.; Lai, C.; Hou, K.; Li, Z.; Chen, G.; et al. High-titer AAV disrupts cerebrovascular integrity and induces lymphocyte infiltration in adult mouse brain. Mol. Ther. Methods Clin. Dev. 2023, 31, 101102. [Google Scholar] [CrossRef]
- Song, R.; Pekrun, K.; Khan, T.A.; Zhang, F.; Pasca, S.P.; Kay, M.A. Selection of rAAV vectors that cross the human blood-brain barrier and target the central nervous system using a transwell model. Mol. Ther. Methods Clin. Dev. 2022, 27, 73–88. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Lee, S.; Yoon, S.; Cho, Y.; Min, D.; Ann, D.; Shin, J.; Paik, Y.K.; Jo, D. AAV-aMTD-Parkin, a therapeutic gene delivery cargo, enhances motor and cognitive functions in Parkinson’s and Alzheimer’s diseases. Pharmacol. Res. 2024, 208, 107326. [Google Scholar] [CrossRef]
- Hong, S.; Piao, J.; Hu, J.; Liu, X.; Xu, J.; Mao, H.; Piao, J.; Piao, M.G. Advances in cell-penetrating peptide-based nose-to-brain drug delivery systems. Int. J. Pharm. 2025, 678, 125598. [Google Scholar] [CrossRef]
- Munji, R.N.; Soung, A.L.; Weiner, G.A.; Sohet, F.; Semple, B.D.; Trivedi, A.; Gimlin, K.; Kotoda, M.; Korai, M.; Aydin, S.; et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 2019, 22, 1892–1902. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef]
- Chiaretti, A.; Genovese, O.; Aloe, L.; Antonelli, A.; Piastra, M.; Polidori, G.; Di Rocco, C. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv. Syst. 2005, 21, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pare, A.; Mailhot, B.; Levesque, S.A.; Juzwik, C.; Ignatius Arokia Doss, P.M.; Lecuyer, M.A.; Prat, A.; Rangachari, M.; Fournier, A.; Lacroix, S. IL-1beta enables CNS access to CCR2(hi) monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1194–E1203. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef]
- Maier, C.M.; Hsieh, L.; Crandall, T.; Narasimhan, P.; Chan, P.H. Evaluating therapeutic targets for reperfusion-related brain hemorrhage. Ann. Neurol. 2006, 59, 929–938. [Google Scholar] [CrossRef]
- Pun, P.B.; Lu, J.; Moochhala, S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef]
- Relton, J.K.; Beckey, V.E.; Hanson, W.L.; Whalley, E.T. CP-0597, a selective bradykinin B2 receptor antagonist, inhibits brain injury in a rat model of reversible middle cerebral artery occlusion. Stroke 1997, 28, 1430–1436. [Google Scholar] [CrossRef]
- Gidday, J.M.; Gasche, Y.G.; Copin, J.C.; Shah, A.R.; Perez, R.S.; Shapiro, S.D.; Chan, P.H.; Park, T.S. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H558–H568. [Google Scholar] [CrossRef]
- Ugarte-Berzal, E.; Berghmans, N.; Boon, L.; Martens, E.; Vandooren, J.; Cauwe, B.; Thijs, G.; Proost, P.; Van Damme, J.; Opdenakker, G. Gelatinase B/matrix metalloproteinase-9 is a phase-specific effector molecule, independent from Fas, in experimental autoimmune encephalomyelitis. PLoS ONE 2018, 13, e0197944. [Google Scholar] [CrossRef]
- Wang, Y.; Rattner, A.; Zhou, Y.; Williams, J.; Smallwood, P.M.; Nathans, J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 2012, 151, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Mancuso, M.R.; Maier, C.; Liang, X.; Yuki, K.; Yang, L.; Kwong, J.W.; Wang, J.; Rao, V.; Vallon, M.; et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Seidner, G.; Alvarez, M.G.; Yeh, J.I.; O’Driscoll, K.R.; Klepper, J.; Stump, T.S.; Wang, D.; Spinner, N.B.; Birnbaum, M.J.; De Vivo, D.C. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. 1998, 18, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Tarlungeanu, D.C.; Deliu, E.; Dotter, C.P.; Kara, M.; Janiesch, P.C.; Scalise, M.; Galluccio, M.; Tesulov, M.; Morelli, E.; Sonmez, F.M.; et al. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 2016, 167, 1481–1494. [Google Scholar] [CrossRef]
- Vatine, G.D.; Al-Ahmad, A.; Barriga, B.K.; Svendsen, S.; Salim, A.; Garcia, L.; Garcia, V.J.; Ho, R.; Yucer, N.; Qian, T.; et al. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell 2017, 20, 831–843. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Gu, Q.; Liu, D. Brain Endothelial Cells in Blood–Brain Barrier Regulation and Neurological Therapy. Int. J. Mol. Sci. 2025, 26, 5843. https://doi.org/10.3390/ijms26125843
Xiang Y, Gu Q, Liu D. Brain Endothelial Cells in Blood–Brain Barrier Regulation and Neurological Therapy. International Journal of Molecular Sciences. 2025; 26(12):5843. https://doi.org/10.3390/ijms26125843
Chicago/Turabian StyleXiang, Yuqing, Qiuxiang Gu, and Dong Liu. 2025. "Brain Endothelial Cells in Blood–Brain Barrier Regulation and Neurological Therapy" International Journal of Molecular Sciences 26, no. 12: 5843. https://doi.org/10.3390/ijms26125843
APA StyleXiang, Y., Gu, Q., & Liu, D. (2025). Brain Endothelial Cells in Blood–Brain Barrier Regulation and Neurological Therapy. International Journal of Molecular Sciences, 26(12), 5843. https://doi.org/10.3390/ijms26125843





