Abstract
The orexin (hypocretin) system plays a central role in regulating the sleep–wake cycle through two neuropeptides, orexin-A and orexin-B, which act on OX1R and OX2R receptors. Emerging evidence links heightened orexin signaling with the pathophysiology of chronic insomnia. This review outlines the neurobiology of the orexinergic system, compares the pharmacological profile of dual orexin receptor antagonists (DORAs) to traditional GABAergic hypnotics, and evaluates the clinical efficacy and safety of Suvorexant, Lemborexant, and Daridorexant. DORAs function by selectively dampening orexin-driven arousal, thereby facilitating sleep onset and maintenance without disrupting natural sleep architecture. Clinical trials have shown that these agents significantly reduce sleep latency and enhance sleep continuity, with a favorable side effect profile. Overall, DORAs represent a distinct and clinically advantageous option for insomnia treatment, with growing interest in their potential utility across mood, anxiety, and neurodegenerative disorders.
1. Introduction
The orexin (also known as hypocretin) system is critically involved in the neurobiological regulation of the sleep-wake cycle [1]. Orexin-A and orexin-B, two neuropeptides produced predominantly by neurons in the lateral hypothalamus, mediate their physiological effects through two G-protein-coupled receptors including orexin receptor type 1 (OX1R) and type 2 (OX2R) [2]. These receptors are widely expressed across multiple brain regions implicated in arousal and vigilance, including but not limited to the locus coeruleus, dorsal raphe nuclei, thalamus, and cerebral cortex [3]. The distribution and function of the orexinergic network underscore its integral role in maintaining wakefulness and modulating transitions between behavioral states [4]. Dysregulation within this system, especially persistent overactivation of orexin signaling, has been increasingly recognized as a potential contributing factor in chronic insomnia [5]. Consequently, pharmacological agents that inhibit orexin receptors (notably dual orexin receptor antagonists, or DORAs) have emerged as promising alternatives to conventional hypnotics [6].
Sleep fulfills a range of critical physiological and cognitive functions, including cellular restoration, metabolic homeostasis, emotional regulation, and the consolidation of memory [7,8,9,10]. Factors like psychological stress, exposure to noise, artificial light, and disrupted daily routines are key contributors to the development of insomnia. These environmental challenges stimulate the central arousal network, especially the orexin system, thereby interfering with the ability to initiate and maintain sleep [5,11]. Persistent disruptions to sleep, as observed in insomnia disorder, can exert widespread negative effects on both psychological and somatic health [12]. Insomnia is commonly defined by difficulties in initiating or maintaining sleep, or by early morning awakenings, typically accompanied by significant daytime dysfunction [13]. While transient sleep disturbances may remit without intervention, chronic insomnia (defined as symptoms occurring at least three times per week for a minimum duration of three months) frequently necessitates clinical management [14,15]. Moreover, it is seldom an isolated condition, often co-occurring with other psychiatric or medical disorders [16,17].
Epidemiological data indicate that over 80% of individuals diagnosed with major depressive disorder (MDD) report significant disturbances in sleep [18,19]. Importantly, insomnia frequently precedes the onset of depressive episodes and has been identified as a predictive factor for both the initial development and subsequent recurrence of MDD [20]. Furthermore, even following remission of core depressive symptoms, persistent sleep disturbances are strongly correlated with an elevated risk of relapse and reduced therapeutic responsiveness [21]. As such, the effective identification and management of insomnia are essential components in the integrated treatment of mood disorders [22]. Furthermore, sleep disturbances are associated with anxiety disorders, post-traumatic stress disorder (PTSD), and bipolar disorder. In these conditions, hyperarousal, driven by dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, elevated cortisol levels, and increased autonomic activity, impairs sleep initiation and maintenance [23,24]. These physiological disruptions parallel those observed in chronic insomnia, indicating overlapping neurobiological mechanisms. In PTSD, sleep dysfunction is often compounded by nightmares and circadian misalignment, intensifying main symptoms [25,26]. Accordingly, targeting insomnia may not only relieve a primary complaint but also attenuate broader psychiatric symptomatology [26,27].
Conventional hypnotics, such as benzodiazepines and non-benzodiazepine “Z-drugs” (including Zolpidem), primarily potentiate GABAergic transmission to induce sedation [28]. Although effective acutely, their use is limited by risks of dependence, tolerance, cognitive impairment, and increased incidence of falls and fractures, particularly in the elderly [29,30]. In contrast, DORAs provide a more selective mechanism by attenuating the orexin-mediated arousal pathways implicated in the pathophysiology of insomnia [30,31,32]. By modulating this specific wake-promoting system, DORAs facilitate the initiation and maintenance of sleep without significantly disrupting overall neurophysiological balance [30,32]. This targeted action supports the preservation of normal sleep architecture and promotes sleep patterns that more closely resemble physiological sleep, while minimizing adverse cognitive and sedative effects.
Clinical trials involving agents such as Lemborexant and Daridorexant have demonstrated significant improvements in sleep latency, total sleep duration, and sleep efficiency [33,34]. These compounds also exhibit a favorable safety and tolerability profile, characterized by low abuse liability, minimal withdrawal symptoms, and limited impact on respiratory and cardiovascular function (as backed up by relative absence of such adverse effects in pharmacovigilance data) [35,36,37,38].
Such attributes render DORAs particularly suitable for use in older adults and populations with complex pharmacological regimens [39,40]. At the molecular level, orexin receptor antagonists influence a broad range of neurobiological processes beyond the regulation of arousal [41]. The orexin system plays a modulatory role across several major neurotransmitter circuits, most notably dopaminergic, serotonergic, noradrenergic, and glutamatergic pathways, all of which are commonly dysregulated in mood and anxiety disorders [41,42,43]. Orexinergic projections to the ventral tegmental area and dorsal raphe nucleus facilitate dopamine and serotonin release. Antagonism of orexin receptors may attenuate overactivation within these systems, contributing to mood stabilization and anxiolytic effects [44,45,46]. Moreover, suppression of orexin input to the locus coeruleus may reduce excessive noradrenergic tone, a key factor in the hyperarousal and stress reactivity observed in chronic insomnia [5,31,47]. In cortical regions, orexin signaling enhances glutamatergic excitation, promoting wakefulness. Suppressing this excitatory drive may aid in sleep initiation and consolidation [48,49]. Collectively, these neurochemical effects support the hypothesis that orexin receptor antagonists possess therapeutic potential that extends beyond insomnia, offering adjunctive benefits for comorbid psychiatric symptoms.
This efficacy has been observed in trials extending up to a year in duration [50]. Research revealed that close to 60% of both patients and clinicians reported improvements in their insomnia disorder, with no instances of discontinuation due to treatment-related adverse events. Its low potential for abuse, minimal risk of rebound effects, and lack of impact on cardiovascular or respiratory function make it a suitable option, especially for older adults and those with polypharmacy or a history of substance use [51,52]. For this reason, therapeutic strategies targeting the orexin system represent a promising and innovative approach for the treatment of insomnia.
Despite its widespread occurrence, insomnia remains largely underdiagnosed within current healthcare frameworks [53]. According to estimations, around 9% to 15% of the general population suffers from insomnia, accounting for elevated healthcare expenses and reduced work productivity. Moreover, research has consistently demonstrated a strong association between insomnia and neurodegenerative disorders or vascular diseases, highlighting its significant role in contemporary healthcare challenges [54]. The condition was found to be more prevalent in women, especially those experiencing hormonal changes including menopause, with 1.4-fold higher risk than men. Furthermore, it increases with age, affecting 40–70% of older adults, where frequent nighttime awakenings are the most common complaint. Early morning awakenings tend to be more frequent in those over 65 [55]. Recent studies suggest a significant association between insomnia and an increased risk of myocardial infarction (MI) in both men and women, further substantiating the growing body of evidence that underscores insomnia’s contribution to elevated cardiovascular risk [56]. Additionally, insomnia was found to be a significant factor in major depressive disorder (MDD), contributing to both clinical and economical challenges.
Despite decreasing depression severity, insomnia independently worsens health outcomes, making it a significant contributor in MDD progression [57,58]. Pathophysiology of insomnia involves an elevated state of arousal during non-REM sleep coupled with disturbed circadian rhythms and sleep maintenance. The condition might be categorized as either temporary or chronic based on duration and time intervals between which the symptoms occur. Although, in some cases, the diagnosis could be based purely on the patient’s history, it should be supported by sleep examination, including its duration, habits, and presence of any factors that could potentially affect the sleep quality, including alcohol [59]. The Insomnia Severity Index (ISI) is a seven-item scale used for assessment of both nocturnal and diurnal insomnia symptoms, measuring aspects like sleep onset, maintenance, and its impact on daily functioning, with higher scores reflecting more severe insomnia. In addition to its primary role in sleep medicine, the ISI is also utilized in other fields, including oncology, for examination of the relationship between sleep quality and patient health [60].
2. Mechanism of Action of Orexin System
2.1. Classical Neurochemistry of Wakefulness
The classical understanding of the neurochemistry of wakefulness primarily revolved around neurotransmitters such as norepinephrine, serotonin, GABA, and histamine. These neurotransmitters, central to early sleep research, were targeted by various pharmaceuticals, ranging from benzodiazepines and Z-drugs to tricyclic antidepressants, which exhibited sedative or stimulant effects. However, the discovery of a novel hypothalamic neuropeptide system added a fundamentally new dimension to our understanding: the orexin (also called hypocretin) system [3].
2.2. Orexin Peptides and Receptors
Orexinergic neurons in the lateral hypothalamus produce a precursor polypeptide that is cleaved into two isoforms; orexin A and orexin B. These peptides bind to two G-protein-coupled receptors: orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R) [61]. While both receptors interact with G-protein pathways, their downstream effects differ. OX1R predominantly modulates Na+/Ca2+ exchange, whereas OX2R enhances NMDA receptor transcription, suggesting a potential influence on synaptic plasticity and, consequently, neurological disease processes [49].
2.2.1. Differential Functions of OX1R and OX2R in Sleep and Arousal Regulation
The orexin receptor subtype OX2R is widely recognized as the principal mediator in the regulation of sleep–wake states [62]. Pharmacological antagonism of OX2R has been shown to reliably promote sleep, forming the basis for the clinical efficacy of DORAs such as Lemborexant and Daridorexant [63]. In contrast, OX1R appears to play a more modulatory role, particularly in relation to affective processes associated with arousal. Emerging evidence suggests that OX1R is implicated in the regulation of anxiety and emotional tension occurring during sleep initiation, rather than in the direct induction of sleep itself [64].
2.2.2. Orexin Receptor Specificity in Reward and Addiction
The orexin system contributes significantly to the regulation of reward-related behaviors, with OX1R playing a predominant role in the reinforcement mechanisms linked to substances such as alcohol, morphine, and cocaine [65]. Evidence indicates that prolonged ethanol exposure, particularly during the developmental window of adolescence, results in heightened orexinergic activity [66]. This hyperactivation is associated with alterations in normal sleep patterns, suggesting a complex interplay between orexin signaling and neurophysiological regulation [67]. Collectively, these findings support the therapeutic potential of orexin receptor modulation, especially targeting OX1R, as an avenue for mitigating addiction and its related behavioral disruptions.
2.2.3. Selective Orexin Receptor Modulation and Therapeutic Progress
The development of selective ligands targeting orexin receptors has opened new avenues for clinical intervention. For instance, TAK-925, a highly selective OX2R agonist, demonstrates promising therapeutic efficacy with a reduced risk of side effects commonly associated with reward system activation [68]. This receptor-specific approach may also prove beneficial in conditions such as binge eating disorder, where OX1R appears to play a more prominent role [69]. Such targeted modulation underscores the potential for refined treatments tailored to distinct orexin receptor functions.
2.3. Regional Brain Activity and Orexin Stimulation
Orexin-producing neurons exhibit markedly reduced activity during sleep, with especially low firing rates observed during rapid eye movement (REM) sleep [70]. The functional consequences of orexinergic transmission are closely tied to the differential distribution of orexin receptor subtypes across key arousal-related brain regions. For instance, the locus coeruleus (LC), which is densely populated with OX1R, responds robustly to orexin A and plays a pivotal role in norepinephrine-mediated arousal [71]. In contrast, the tuberomammillary nucleus that is integral to histaminergic neurotransmission primarily expresses OX2R, implicating it in sustained wakefulness [72]. Moreover, several regions involved in dopaminergic and cholinergic signaling, including the ventral tegmental area, basal forebrain, and the pedunculopontine and laterodorsal tegmental nuclei, co-express both OX1R and OX2R. These structures collectively support arousal through diverse, receptor-specific mechanisms, underscoring the complexity of orexin-mediated modulation of wakefulness [73].
Inhibition of Sleep-Promoting Centers
Orexin suppresses sleep-promoting areas such as the medullary REM muscle atonia circuits, reinforcing its role in arousal. Serotonin released from the dorsal raphe nuclei acts on 5-HT1A receptors to hyperpolarize orexin-producing neurons, thereby reducing their excitability and contributing to the regulation of sleep onset through a feedback inhibitory mechanism [74,75].
2.4. Orexin, Narcolepsy, and Autoimmune Involvement
The orexin system is critically involved in narcolepsy type 1 (NT1), a disorder characterized by excessive daytime sleepiness, cataplexy, and sleep paralysis. A key diagnostic marker is the significant reduction in orexin A in cerebrospinal fluid, reflecting the loss of orexin-producing neurons in the hypothalamus [76,77]. Postmortem studies suggest this neuronal degeneration is driven by autoimmune T-cell activity [78]. NT1 shows a strong genetic association with the HLA-DQB1*06:02 allele, along with specific polymorphisms in T-cell receptor genes, supporting an autoimmune etiology [79]. Beyond sleep regulation, orexins also appear to participate in immune signaling, underscoring their broader physiological relevance [80].
A study of 77 participants explored how sleep deprivation influences inflammation, focusing on white blood cells (WBCs) and granulocytes (GRAs), and the role of physical activity. Sleep loss led to increased granulocyte counts in all individuals, while WBC elevation was mainly seen in those who were more physically active. Poor subjective sleep quality over several days was the strongest predictor of higher WBC levels. Additionally, granulocyte counts were linked to longer REM latency and lower sleep efficiency, highlighting a complex connection between sleep architecture and immune function [81]. Moreover, the research suggests that orexin peptides influence immune responses and neuroinflammation across a range of conditions, including sepsis, inflammatory bowel disease, and neurodegenerative disorders, by downregulating NF-κB activity and promoting anti-inflammatory signaling mechanisms, further highlighting their role in immune signaling [82].
2.5. Orexin and the Hypothalamic–Pituitary–Adrenal (HPA) Axis
Orexinergic pathways interact with the hypothalamic–pituitary–adrenal (HPA) axis, modulating levels of ACTH and corticosterone even in the absence of acute stress [83]. This regulatory link may help explain the sleep and mood-related effects of certain traditional Chinese herbal formulations. For example, modified Suanzaoren Decoction appears to stabilize orexin signaling and, in turn, modulate HPA activity, mechanisms thought to underlie its sedative and anxiolytic properties [83,84].
Herbal Modulation of Orexin Signaling Pathways
Herbal formulations like Zaoren Granules appear to reduce OX2R expression through serotonin-related mechanisms, affecting appetite, inflammation, and neuroendocrine function, probably via the cAMP/CREB pathway. Chaihu-Longgu-Muli Decoction has shown benefits in kidney disease models by suppressing NF-κB-driven inflammation through orexin-linked pathways. Additionally, orexin under conditions of sleep deprivation may involve signaling through CaMKK2/AMPK and PI3K/Akt/mTOR pathways, reflecting its role in diverse cellular processes.
2.6. Orexin Dysregulation Across Neuropsychiatric and Neurodegenerative Disorders
Studies measuring biomarkers have shown that orexin levels vary across different neuropsychiatric and neurodegenerative disorders. In some cases, elevated or decreased levels are associated with cognitive deterioration, but the direction of this relationship heavily depends on the specific condition.
This connection appears to be multifaceted, likely influenced by factors such as the phase of the illness, the body’s adaptive responses, and its interplay with sleep patterns and inflammatory processes. Reduced concentrations of orexin-A in the bloodstream have been linked to impairments in thinking and decision-making abilities among individuals with anorexia nervosa and epilepsy [85]. Lower blood levels of orexin-A in epilepsy were found to be tied to poorer thinking skills and increased inflammation, suggesting possible protective qualities of orexin-A against cognitive decline in this condition [86]. In Alzheimer’s disease, disturbances in the orexin system are closely linked to impaired sleep–wake regulation. Data from animal models and postmortem studies show that increased levels of orexin in the cerebrospinal fluid are associated with disrupted sleep patterns and worsening cognitive function in individuals with Alzheimer’s disease, especially in cases of mild cognitive impairment related to the condition [87,88,89]. Furthermore, a cross-sectional study involving 50 patients with AD demonstrated a significant association between elevated cerebrospinal orexin levels and reduced sleep duration, highlighting a potential role for orexin dysregulation in the progression of AD pathology, thereby confirming previous theories [90]. Evidence suggests that tau accumulation, particularly in regions such as the locus coeruleus, appears to interfere with orexin signaling, thereby exacerbating impairments in sleep regulation and arousal [91,92]. This dysfunction may hold promise as a biomarker or therapeutic target [4]. Likewise, in Niemann–Pick disease type C, degeneration of orexin-producing neurons has been observed, possibly secondary to toxic lipid accumulation, further highlighting the system’s vulnerability in neurodegenerative contexts [82,89,93].
2.7. The Influence of Orexins on the Development of Schizophrenia
Interestingly, schizophrenia is not devoid of orexin influence, as the MIR137 locus, which is responsible for silencing miRNA and miR-137, is a marker of genetic predisposition to this disease. Aforementioned miRNA modulates the hypocretin levels and it is conserved across species (Figure 1). The study showed a correlation of the MIR137 locus with sleep length in humans, but unfortunately not with typical narcolepsy cases. Speculation was made, however, that there might be an influence across a specific population of narcolepsy patients in whom HCRT-1 level is not as drastically decreased, but these cases are seldom [94].
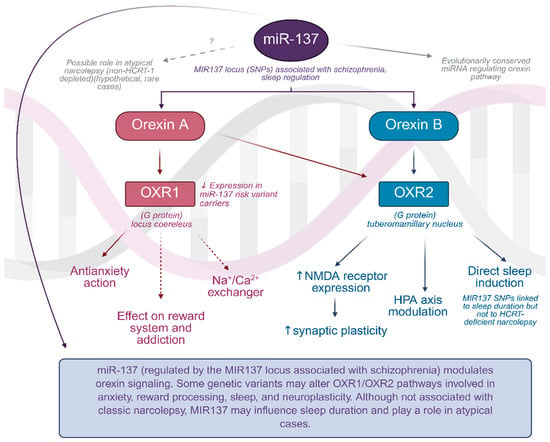
Figure 1.
The regulatory role of miR-137 in orexin signaling pathways. Graph was prepared using Biorender. Żełabowski, K.
2.8. Other Disorders Associated with the Orexin System
2.8.1. Anxiety Disorders, Post-Traumatic Stress Disorder, and Bipolar Disorder (BD)
In PTSD and BD, a heightened state of arousal is observed, associated with dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, elevated cortisol levels, and increased autonomic activity [23,24]. These mechanisms, similar to those described in chronic insomnia, contribute to difficulties in initiating and maintaining sleep. In PTSD, nightmares and circadian misalignment are additional problems that exacerbate core symptoms [25,26].The orexin system, through its impact on arousal, may play a significant role in these processes.
2.8.2. Addictions
The orexin system plays an important role in reward mechanisms, with OX1R being particularly involved in the reinforcement of alcohol, morphine, and cocaine use [65]. Prolonged ethanol exposure, especially during adolescence, has been shown to increase orexinergic activity [66], which is associated with disturbances in normal sleep patterns [67].
2.8.3. Eating Disorders—Binge Eating Disorder
In binge eating disorder, OX1R appears to play a predominant role in regulating feeding behavior. Targeted modulation of this receptor represents a potential therapeutic approach to alleviating symptoms [69].
2.8.4. Epilepsy
By modulating ascending cortical signals and promoting synchronization of cortical firing, the orexin system can reduce discharges typically associated with seizures. This suggests a potential therapeutic role for orexin system modulation in epilepsy management [95].
3. Description of Selected Representatives of the Dual Orexin Receptor Antagonists (DORAs) with Side Effects
The three main agents approved as DORAs by the FDA for insomnia treatment are Suvorexant (Belsomra), Lemborexant (Dayvigo), and Daridorexant (Quiviq) (Figure 2). They work by selective inhibition of orexinergic system signaling in the brain, resulting in quicker sleep onset times and prolonged sleep duration (Figure 2). In this chapter, their characteristics will be expanded upon. Additionally, some other orexinergic agents will be discussed at the end of the chapter, including Seltorexant, which has not been approved yet owing to its unique properties. At the end of the chapter, we will also present Table 1, which summarizes properties of Suvorexant, Lemborexant, and Daridorexant, and Table 2, which contains information about clinical efficacy studies of presented drugs.
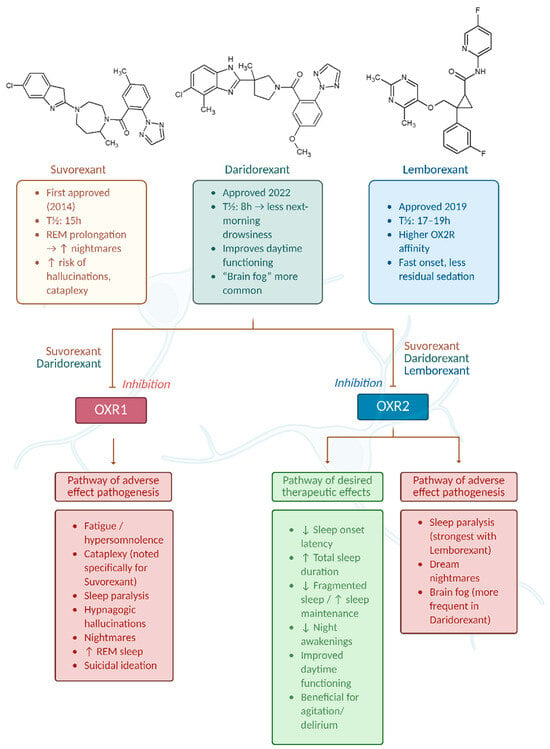
Figure 2.
Mechanism of action and differential effects of dual orexin receptor antagonists (DORAs) on OX1R and OX2R pathways. Graph was prepared using Biorender. Żełabowski, K.

Table 1.
Properties of Suvorexant, Lemborexant, and Daridorexant.

Table 2.
Clinical efficacy studies of presented drugs.
We also illustrated the effects of DORA class drugs on OX1R and OX2R receptors in Figure 2.
3.1. Suvorexant
3.1.1. Drug Characteristics
As of now, three orexinergic agents have been FDA-approved for pharmacotherapy of insomnia [54], and out of these three, Suvorexant with the trade name Belsomra was the first one to obtain such approval [55], which officially happened in 2014 [58]. Because of its ability to block both OX1R and OX2R orexin receptors, the drug belongs to the dual orexin receptor antagonist class. This specific contribution to regulation of lateral hypothalamic efferent signaling differentiates it from classical hypnotics such as antihistamines or GABA modulators [56].
3.1.2. Pharmacokinetics
For the aspect of pharmacokinetics, the half-life of Suvorexant is approximated to be 15 h, which is the middle ground out of said three agents [56], Tmax is 2 h, and dosages tested vary between 5 and 20 mg [98]. Its enteral absorption is rapid [98]. Suvorexant’s main metabolic pathway involves CYP3A enzyme isoforms. In consequence, significant drug-to-drug interactions may take place during therapy with Suvorexant and clinically significant differences in Area Under the Curve (AUC) were observed when patients were administered rifampin—a CYP3A inducer—and its inhibitors (ketoconazole, diltiazem). A less significant contribution comes from the CYP2C19 enzyme. The metabolite that is produced by these liver enzymatic reactions is non-active, and excretion of both the metabolite and the parent compound was observed to occur in 66 percent by fecal route and 23 percent in urine. It should be noted that BMI and thus, indirectly, obesity decrease its elimination from the body [58].
3.1.3. Safety and Adverse Effects
Most of the adverse effects of Suvorexant noted in Barriera’s work [96] (based on phase 3 international and multicenter RCT by Michelson et al. lasting 1 year), which showed Suvorexant to be an overall safe agent to use for such an extended period of time, and its tolerance to be high for both elderly and non-elderly patients [96], were not significant, and were characteristic for a plethora of hypnotics, including fatigue, dry mouth, and of course, daytime hypersomnolence [54]. However, some serious reactions also occurred, including sleep paralysis, hypnagogic hallucinations, cataplexy, and suicidal ideation. These findings were attributed to 5% of the participants [54]. Incidence of increased sleep desire in the morning is not as profound though, and Suvorexant is still allowed to be administered to patients with morning arousal disturbances [55]. Asai et al. conducted a safety-oriented study of Belsomra with a large sample of 3428 and found the adverse effect subgroup to include 9.7% of the total sample, with as low as 0.2% constituting drug reactions that were severe, including delirium, agitation, fractures, and subdural hematoma [99,100]. Besides all of that, as Suvorexant does not interfere with benzodiazepine signaling, some traditional and problematic adverse effects typical for past sedatives do not apply to it as much, and findings such as amnesia, confusion, and gait disturbances are not associated with it [58]. For the aspect of the safety of the fetus, as of 2025, there is an absence of research in this area and possible risks have to be evaluated individually. A similar lack of knowledge was mentioned in the aspect of breastfeeding safety with concomitant Suvorexant therapy [58]. A particularly interesting retrospective study regarding the effect of Suvorexant on nightmare occurrence was carried out by Yasuda et al. [59], in which it was shown that Suvorexant prolongs the REM phase of the sleep cycle, which is characteristic for its class, but could also potentially increase the chance of such adverse effects. Said study concluded that the incidence of nightmares was indeed increased, but it was most pronounced in the younger age group, and the elderly seemed to be somewhat resistant to this effect [59].
For the aspect of pharmacovigilance, analysis of 1598 FAERS submissions between 2014 and 2015 made by Ali [101] pointed to a correlation with psychiatric risks and advised prescribers to watch out for changes in behaviors during therapy. Interesting work by Borchert et al. compared FAERS data (years 2015-Q1 through 2017-Q3) with 324 patient self-evaluated drug ratings and complaints found on Drugs.com. Said ratings were shown to be highly similar to FAERS reports in terms of nightmare occurrence, abnormal dreams, sleep paralysis, and other adverse effects [102]. To our knowledge, the most recent pharmacovigilance analysis [38] collected data from Q3rd 2014 to Q4th 2023 and supported earlier findings of nightmares, abnormal dreams, and sleep paralysis being the most common adverse effects.
3.1.4. Clinical Efficacy
Suvorexant’s primary use is for the onset as well as maintenance of sleep [55]. In a study addressing the drug’s therapeutic effect [96] that is mentioned by León-Barriera et al. [54], higher-end doses were used, namely 30 mg for patients above 65 year old and 40 mg for the patients younger than 65 years old, with a notable increase in subjective total sleep length and a decrease in time to fall asleep over the control, placebo group [54]. As insomnia is a chronic condition, it is exceptionally important to address the persistence of the therapeutic effect during such pharmacotherapy. Roughly 62% of patients continued the trial (phase 3 RCT) for 1 year, with therapeutic actions (subjective sleep onset and maintenance improvements) not fading off in such a period of time [96]. Unfortunately, many insomnia comorbidities are contraindications to Suvorexant treatment, including depression, narcolepsy, and obstructive sleep apnea [58]. On top of that, Suvorexant is not considered a go-to alternative for insomnia, as its cost remains relatively high and similarly to Zolpidem, it is associated with notable abuse potential [57]. Aside from its effect on insomnia, Suvorexant was shown to have a positive effect on the extent of delirium associated with Alzheimer’s disease. The underlying mechanism was postulated to involve increased orexin levels in patients with the disease [103].
3.2. Lemborexant
3.2.1. Drug Characteristics
The next agent of the DORA class to be brought to the public was Lemborexant. The official FDA approval date was December 2019, and the drug was released the next year by Merck [55]. It is worth noting that Lemborexant has higher in vitro activity for the OX2R receptor isoform compared to Suvorexant [104], and this might contribute to its somewhat more beneficial profile for insomnia treatment, in comparison with Suvorexant [105].
3.2.2. Pharmacokinetics
For 5 mg and 10 mg doses, Landry et al. [106] found the mean half-life to be 17 h and 19 h, respectively [106]. Dosage adjustments are not needed in hepatic dysfunction unless it is moderate; for such cases, 5 mg a night should not be exceeded to avoid excessive adverse effects, particularly daytime sedation [107]. Lemborexant is metabolized by the CYP3A enzyme family in the liver; nevertheless, the influence of kidney injury and insufficiency on the plasma level of the agent was assessed by Landry et al., and was shown to be not worthy of dose adjustment [107]. Concomitant Lemborexant therapy and alcohol exposure is not advised, as alcohol has additive effects on Lemborexant’s plasma level with a subsequent increase in occurrence of negative cognitive symptoms [108]. Shorter time to achieve maximum concentration puts Lemborexant over Suvorexant when quick therapy initiation is needed [104].
3.2.3. Safety and Adverse Effects
In contrast to Suvorexant, Lemborexant was evaluated not to be harmful to patients with obstructive sleep apnea, as a study by Cheng et al. concluded [109]. The abuse potential for the drug is of importance, and it appeared to be comparable to both Zolpidem and Suvorexant [57]. Next morning sleepiness was not associated with the treatment, as evaluated by tests like the Karolinska Scale. Individual features like sex, age, and race had no effect on pharmacokinetics (PK), pharmacodynamics (PD), or overall safety of the therapy [106]. Evaluation of possible emergent adverse effects during long-standing (12 month) therapy was performed by Yardley et al., in an analysis of the SUNRISE-2 study in which no new safety signals were found and Lemborexant was shown to be a safe option for long-term therapy [110].
Considering the relative novelty of Lemborexant and subsequent lack of longitudinal trials, a particularly significant fraction of new safety information can be extracted from pharmacovigilance studies, based on databases such as FAERS. A 2024 study by Jiang et al. [38], also included in Section 3.1, in fact covered both Suvorexant, Lemborexant, and Daridorexant (pan-DORA study). In the same fashion as for Suvorexant, Lemborexant results were in line with previous trials, although it may be worth noting that the sleep paralysis signal was strongest in this agent [38].
3.2.4. Clinical Efficacy
An international 1-month Phase 3 SUNRISE 1 study on the effectiveness of Lemborexant completed by Rosenberg et al., with Zolpidem and placebo as reference points, made it evident that this orexinergic agent was overall the most plausible therapeutic option of the three. Doses tested were 5 mg and 10 mg [60]. SUNRISE 1 successor SUNRISE 2 (Study E2006-G000-303) possesses significant value as it assesses effects for longer, and thus, is more practical in the context of chronic insomnia than 12-month Lemborexant therapy. Therapeutic benefits were persistent in both the first 6 months and total 12 months, as reported by Yardley et al., and a lack of rebound insomnia and withdrawal should also be noted [110]. A small pilot study addressed the effect of Lemborexant on cancer patients with delirium, emphasizing its particular OX2R affinity. The same receptor isoform was shown to have the most influence in agitation states [111]. It should be noted that research of DORAs’ usefulness is highly concentrated on insomnia only. The Kishi group’s network meta-analysis that ultimately covered four studies—SUNRISE-1, SUNRISE-2, and work by Herring et al. [112]—proposed that Lemborexant might be somewhat more beneficial than Suvorexant, but the evidence was not that clear, and data quality was lacking [111]. Later findings by Xue et al. [113] were more promising and showed some superiority of Lemborexant in terms of subjective sleep onset time reduction, subjective total sleep time, as well as subjective wake time after sleep onset [113]; said findings were based on nine RCTs, three of which covered Lemborexant efficacy (SUNRISE 1, 2 and a multicenter study by Murphy et al.) [114]. This study grounded the result of the previous article by Xue’s group.
3.3. Daridorexant
3.3.1. Drug Characteristics
Daridorexant is the newest DORA in terms of FDA approval (2022) [54]. In contrast to Lemborexant, Daridorexant exhibits equipotent antagonizing properties to both orexin receptors [115]. It is special in that its short half-life of 8 h could potentially make it a better alternative for patients in whom morning residual somnolence is especially undesired [54]. This feature was the basis for its selection from a plethora of potential pharmaceutical candidates [116].
3.3.2. Pharmacokinetics
Besides its half-life of 8 h, its onset of action is fast, owing to quick absorption [117]. Doses of 25 and the more efficacious [118] 50 mg are officially used [54] in adults at least 18 years old [117]. The hepatic metabolism of the drug is significant with 89% being mediated by CYP3A4, opening doors for many problematic interactions. Additive negative effects of simultaneous Daridorexant and ethanol consumption were noted, same as for Lemborexant [116]. Compromised kidney function, based on Cmax increase and AUC, did not affect Daridorexant exposure [118].
3.3.3. Safety and Adverse Effects
Residual effects of the therapy, mentioned earlier, were evaluated by Muehlan and Brooks, particularly in the context of their influence on driving performance. A custom-built Green Dino driving simulator was used for this purpose, and its role was to quantify Standard deviation of lateral position (SDLP) as a measure of driving impairment. Zolpidem was used to assess the simulator’s accuracy and it indeed showed a significant decrease in driving performance. On the contrary, Daridorexant was found to have an effect comparable with placebo. However, subjective driving performance was compromised on the initial 2 days of therapy. Thus, individual assessment of potential negative response is recommended [119]. Adverse effects of the Daridorexant regime were similar to other orexinergic agents and were not severe with their occurrence being not dose-dependent [120]. Comorbid psychiatric disorders were not found to change therapy tolerance or safety of Japanese subjects [121]. Daridorexant partook in a particularly interesting study regarding the dampening of responsiveness to external stimuli during sleep [122]. Such an effect is uncompromisingly a significant issue whenever a CNS depressant drug is being used in therapy. Magliocca et al. showed that Daridorexant does not induce such a response, and subsequently, awakening in emergency situations was not disrupted [122]. Even though Daridorexant was observed to favor REM sleep presence, which is known to be involved in dream recalling, it did not induce significant abnormalities in dreaming or the presence of nightmares [56]. A pharmacovigilance study by Jian et al. highlighted ‘’brain fog’’ being more common in patients taking Daridorexant than in those taking the other two agents [38].
3.3.4. Clinical Efficacy
Daytime functioning impairment was not reported during Daridorexant therapy and in fact, for a 50 mg dose, daytime functioning was improved [97]. Similarly to the other two agents, Daridorexant was shown in phase 3 clinical trials to improve sleep quality of patients with insomnia [97,123]. In a recent paper by Cardiroli et al., Daridorexant is mentioned in the context of a possible novel way of managing eating disorders because of their strong association with the orexinergic system [124]. As with previously discussed agents, it is important to assess long-term efficacy and tolerability in light of disease chronicity. Kunz et al. conducted a 12-month extension study with a total of 804 adult and elderly participants. Similarly to earlier orexants, Daridorexant showed to be a safe agent for persistent maintenance therapy [117].
3.4. Other Agents
Of note, yet another orexinergic agent currently in a phase III clinical trial, Seltorexant (Figure 3), evoked unexpected antidepressant activity while it was being evaluated for other actions. Such action was observed in monotherapy in MDD patients, but an even more profound outcome was obtained in adjunction with typical antidepressants. Nevertheless, later investigations with broader samples showed no benefit in these conditions and as of now, evidence for it is absent. The particularly short half-life of Seltorexant of 2–3 h is also worth mentioning [125]. TS-142, another experimental agent, was assessed in the context of efficacy in insomnia by Uchiyama et al. [126]. TS-142′s favorable pharmacokinetics (fast absorption and rapid elimination) as well as absence of significant residual side effects and overall positive results of clinical efficacy and consistency in Uchiyama’s study, despite the relatively small sample size, put this agent into the spotlight for the future of orexinergic system-oriented therapy [126]. Yet another agent, Fazamorexant, showed to have promising kinetics as well as safety, as pointed out in Ni’s randomized controlled trial [127]. Fazamorexant’s most common adverse effects came to be drowsiness and dizziness, although the small sample size is also a feature of this study and should be taken into account. Possible saturation of Fazamorexant’s (Figure 3) absorption process should also be considered [127]. Going further, a compound known as TAK-925 (Figure 3) or Danavorexton needs a mention, as it is an agonist of orexinergic receptor 2, contrary to all of the other drugs mentioned in this paper, which are antagonists. Because of this opposite molecular mechanism, it is not being researched in the area of insomnia, but rather in the acceleration of emergence from anesthesia produced by opioids like fentanyl [128].
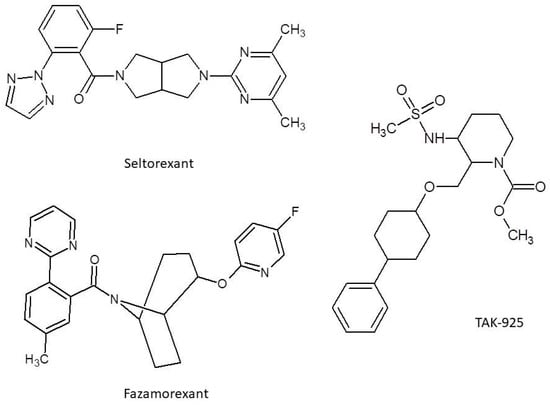
Figure 3.
Structure for Seltorexant, Fazamorexant, and TAK-925.
4. DORAs in Contrast to GABAergic Hypnotics: Comparison of Clinical, Economic, and Environmental Perspectives
It is essential to contextualize DORAs alongside GABAergic hypnotics. Benzodiazepines and Z-drugs are known for risks in tolerance, dependence, and falls in the elderly, increasing both health and environmental burdens [129]. Conversely, DORAs present lower potential for overdosing and fewer cognitive or motor side effects, thus positioning them as potentially safer therapeutic options [113].
4.1. Impact of DORAs and GABAergic Hypnotics on Sleep Architecture and Neural Pathways
In contrast to conventional hypnotic agents—such as benzodiazepines and non-benzodiazepine “Z-drugs”—which exert their sedative effects by potentiating GABAergic inhibition, orexin receptor antagonists facilitate sleep through a distinct mechanism that preserves physiological sleep architecture [28]. Traditional GABAergic sedatives often alter the natural distribution of sleep stages, potentially diminishing restorative sleep and contributing to residual daytime drowsiness. By selectively targeting the arousal-promoting orexin system, agents such as DORAs maintain the proportional integrity of REM and non-REM sleep, thereby enhancing overall sleep quality with a lower risk of next-day impairment [31,130]. Notably, the ventrolateral preoptic nucleus (VLPO), a key GABAergic node involved in sleep initiation and maintenance, exerts inhibitory control over orexin-producing neurons [131]. This interaction represents a critical point of convergence between GABAergic and orexinergic systems, highlighting the complementary roles these pathways play in sleep–wake regulation.
4.2. Question of Healthcare and Economic Impact of DORAs Compared to GABAergic Hypnotics
From the perspective of the healthcare system, although DORAs stand out to have higher acquisition costs than GABAergic agents, their superior safety profile—particularly the reduced risk of falls, dependence, and cognitive impairment [132]—may result in downstream savings and contribute to a more efficient healthcare model.
Moreover, recent cost-effectiveness analyses suggest that Lemborexant may provide favorable value compared to Suvorexant or Zolpidem, supporting its rational integration into healthcare systems [133]. Behavioral interventions such as cognitive behavioral therapy for insomnia (CBT-I) is to remain the most environmentally sustainable approach, lacking pharmaceutical residues and demonstrating cost-effectiveness compared with medication or no treatment [134].
Improved sleep quality itself yields broader health and sustainability advantages, including reduction in comorbidity-related medication use and hospital admissions [135]. Decline in both the number of prescriptions issued and the number of hospital admissions has an effect of reducing both pharmaceutical residues and healthcare-related emissions. This outcome serves to underscore the potential environmental co-benefits of such actions.
4.3. Environmental Footprint of GABAergic Hypnotics and Orexin Antagonists
Traditional GABAergic hypnotics such as benzodiazepines and “Z-drugs” are detected in wastewater and surface waters due to their environmental persistence and resistance to standard treatment processes (e.g., diazepam, oxazepam, bromazepam; [136]. In contrast, data on the environmental footprint of dual orexin receptor antagonists (DORAs) are limited, but their generally shorter elimination half-lives and lower risk of bioaccumulation may imply reduced ecological impact, though systematic ecotoxicological assessments are needed.
5. Discussion
The introduction of dual orexin receptor antagonists (DORAs) has profoundly altered the pharmacological approach to insomnia treatment, providing a targeted and physiologically coherent solution to the disorder. In contrast to traditional sedative-hypnotic agents, such as benzodiazepines and non-benzodiazepine “Z-drugs,” which broadly potentiate GABAergic inhibition, DORAs act by selectively modulating the orexinergic arousal system, thereby facilitating the onset and maintenance of sleep without disrupting natural sleep architecture [54,55]. This distinction offers a number of clinical benefits, particularly in terms of safety, tolerability, and long-term efficacy.
Suvorexant, the first FDA-approved DORA, has demonstrated efficacy in reducing sleep latency and improving its maintenance [55]. Its dual mechanism, targeting both OX1R and OX2R, makes it effective across a broad spectrum of insomnia presentations. However, its relatively long half-life (~15 h) raises concerns about next-day sedation, especially in vulnerable populations such as the elderly [58]. Although studies report generally mild adverse effects, including fatigue and dry mouth, rare but serious events such as sleep paralysis and hallucinations have also been documented [54]. Notably, Suvorexant has been linked with increased incidence of nightmares, particularly in younger users [59]. Lemborexant, approved in 2019, offers an improved pharmacokinetic profile, with a more favorable receptor selectivity for OX2R, which is predominantly involved in sleep–wake transitions [104]. The receptor specificity of the aforementioned pharmaceutical agent has been demonstrated to result in a number of clinical benefits, including a reduction in residual sedation and a decreased risk of respiratory depression. This characteristic renders the pharmaceutical agent a safer alternative for patients suffering from comorbid conditions, such as obstructive sleep apnea [109]. Comparative studies indicate that Lemborexant may offer superior efficacy in reducing wake time after sleep onset and increasing total sleep duration compared to Suvorexant [113].
Practical prescribing considerations are increasingly important in the clinical use of DORAs. It is noteworthy that in contrast to conventional hypnotics such as Zolpidem (≈20 USD/month), DORAs are substantially more expensive, with average monthly costs of approximately USD 290 for Lemborexant and USD 380 for Suvorexant in the United States [137]. Although manufacturer-sponsored savings programs reduce out-of-pocket costs for many commercially insured patients to ≤USD 35 per month.
Outside of the US, pricing varies considerably, with the UK National Institute for Health and Care Excellence (NICE) reporting a price for Daridorexant of GBP 2.12 per day (≈83.8 USD/month) [138].
A cost-effectiveness model from Japan suggests that when considering both benefits and cost offsets such as reductions in falls and collision, the use of Lemborexant may offer a more favorable balance of “quality-adjusted life-years” (QALYs) compared to Suvorexant or Zolpidem [133].
In the case of Daridorexant, the most recent addition to the DORA class, it stands out due to its shorter half-life (~8 h), which significantly minimizes the risk of next-day sedation [117]. Its balanced affinity for OX1R and OX2R receptors enables it to address both sleep onset and maintenance difficulties. The efficacy of the treatment has been confirmed through a series of clinical trials. These trials have demonstrated that the treatment is effective in improving objective and subjective sleep parameters, without compromising daytime functioning [97]. Importantly, Daridorexant has shown no significant effect on psychomotor performance, as demonstrated in driving simulation studies [119]. This profile renders it an appealing option for individuals who require high levels of daytime alertness.
The orexin system’s broader neurobiological functions, in addition to its role in insomnia, present significant therapeutic prospects. Orexin signaling intersects with key neurotransmitter pathways implicated in mood, stress, and arousal regulation, including the serotonergic, noradrenergic, and dopaminergic systems [42,139]. Suppression of orexinergic tone may attenuate hyperarousal and overactivation of the hypothalamic–pituitary–adrenal (HPA) axis, which are central to both chronic insomnia and comorbid psychiatric disorders such as anxiety, PTSD, and major depressive disorder [20,23].
Clinical and preclinical studies have begun exploring the potential of DORAs in reducing psychiatric symptoms. By dampening orexin input to the locus coeruleus and dorsal raphe nucleus, these agents may help modulate stress reactivity and emotional dysregulation [45]. These findings underscore the potential of orexin antagonists as adjunctive therapies in mood and anxiety disorders, especially when sleep disturbances are prominent.
Another area of growing interest is the role of the orexin system in neurodegenerative diseases. Alterations in orexin signaling have been linked to sleep disturbances in Alzheimer’s disease, with increased orexin levels correlating with disrupted REM sleep and accelerated cognitive decline [87]. Furthermore, tau pathology in brainstem nuclei may interfere with orexinergic regulation, presenting a possible target for future intervention [92].
Despite the considerable promise inherent in DORAs, it is imperative to address the limitations that are associated with this technology. Long-term safety data remain limited, and questions persist regarding their use in special populations such as pregnant or lactating women, patients with hepatic impairment, and individuals with histories of substance abuse [58]. Moreover, while DORAs generally exhibit lower abuse potential than benzodiazepines, they are not devoid of risks, and pharmacovigilance remains essential [38,57].
Future research endeavors should prioritize the delineation of the comparative effectiveness of individual DORAs. Additionally, there is a need to explore their adjunctive use in psychiatric and neurodegenerative disorders. Furthermore, there is a need to refine biomarkers to identify patients that are most likely to benefit from orexin-targeted therapies.
6. Conclusions
The usage of dual orexin receptor antagonists (DORAs) signifies a substantial progression in the management of insomnia, as they provide a more precise approach in contrast to conventional sedative-hypnotics, offering targeted efficacy with a favorable safety profile. DORAs have influence over sleep, mood regulation, neuroprotection, and overall quality of life. By selectively modulating the orexin system, DORAs facilitate natural sleep without impairing sleep architecture. The clinical evidence supports their efficacy, safety, and favorable tolerability profiles, particularly with agents like Lemborexant and Daridorexant. Beyond the indication for insomnia, DORAs have managed to demonstrate efficacy in the management of psychiatric and neurodegenerative disorders, attributable to their comprehensive impact on mood and arousal regulation. Nevertheless, the long-term safety data and usage in special populations necessitate further investigation. With ongoing research and refinement, these agents may become cornerstone therapies in both sleep medicine and integrative neuropsychiatric care.
Author Contributions
Conceptualization, K.Ż.; software, K.Ż.; validation, K.Ż., K.B., M.W. and A.C.-K., formal analysis, K.Ż. and A.C.-K., investigation, K.Ż.; resources, K.Ż. and A.C.-K.; data curation, K.Ż., K.B., M.W. and A.C.-K.; writing—original draft preparation, W.P., K.W., Z.R., K.F., D.Ś., M.R., M.S. and I.R.; writing—review and editing, K.Ż., W.P., K.W., Z.R. and K.B.; visualization, K.Ż.; supervision, K.Ż.; project administration, K.Ż.; funding acquisition, A.C.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OX1R | Orexin Receptor Type 1 |
| DORA | Dual Orexin Receptor Antagonist |
| PTSD | Post-traumatic Stress Disorder |
| HPA | Hypothalamic–Pituitary–Adrenal |
| MI | Myocardial infarction |
| MDD | Major Depressive Disorder |
| ISI | Insomnia Severity Index |
| LC | Locus Coeruleus |
| VLPO | Ventrolateral Preoptic Nucleus |
| NT-1 | Narcolepsy Type 1 |
| BD | Bipolar Disorder |
References
- Toor, B.; Ray, L.B.; Pozzobon, A.; Fogel, S.M. Sleep, Orexin and Cognition. Front. Neurol. Neurosci. 2021, 45, 38–51. [Google Scholar] [CrossRef]
- Pizza, F.; Barateau, L.; Dauvilliers, Y.; Plazzi, G. The orexin story, sleep and sleep disturbances. J. Sleep Res. 2022, 31, e13665. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Jacobson, L.H.; Hoyer, D.; Rinne, M.K.; Borgland, S.L. International Union of Basic and Clinical Pharmacology CXIV: Orexin Receptor Function, Nomenclature and Pharmacology. Pharmacol. Rev. 2024, 76, 625–688. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, S. Effect of a dual orexin receptor antagonist on Alzheimer’s disease: Sleep disorders and cognition. Front. Med. 2022, 9, 984227. [Google Scholar] [CrossRef]
- Palagini, L.; Geoffroy, P.A.; Balestrieri, M.; Miniati, M.; Biggio, G.; Liguori, C.; Menicucci, D.; Ferini-Strambi, L.; Nobili, L.; Riemann, D.; et al. Current models of insomnia disorder: A theoretical review on the potential role of the orexinergic pathway with implications for insomnia treatment. J. Sleep Res. 2023, 32, e13825. [Google Scholar] [CrossRef]
- Muehlan, C.; Vaillant, C.; Zenklusen, I.; Kraehenbuehl, S.; Dingemanse, J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1063–1078. [Google Scholar] [CrossRef]
- Gilley, R.R. The Role of Sleep in Cognitive Function: The Value of a Good Night’s Rest. Clin. EEG Neurosci. 2023, 54, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kong, J.; Li, X.; Ren, Q. Sex differences in the effects of sleep disorders on cognitive dysfunction. Neurosci. Biobehav. Rev. 2023, 146, 105067. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.M.; Lokhandwala, S.; Riggins, T.; Spencer, R.M.C. Sleep and human cognitive development. Sleep Med. Rev. 2021, 57, 101472. [Google Scholar] [CrossRef] [PubMed]
- Fjell, A.M.; Walhovd, K.B. Individual sleep need is flexible and dynamically related to cognitive function. Nat. Hum. Behav. 2024, 8, 422–430. [Google Scholar] [CrossRef]
- Palagini, L.; Geoffroy, P.A.; Gehrman, P.R.; Miniati, M.; Gemignani, A.; Riemann, D. Potential genetic and epigenetic mechanisms in insomnia: A systematic review. J. Sleep Res. 2023, 32, e13868. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Benz, F.; Dressle, R.J.; Espie, C.A.; Johann, A.F.; Blanken, T.F.; Leerssen, J.; Wassing, R.; Henry, A.L.; Kyle, S.D.; et al. Insomnia disorder: State of the science and challenges for the future. J. Sleep Res. 2022, 31, e13604. [Google Scholar] [CrossRef] [PubMed]
- McNamara, S.; Spurling, B.C.; Bollu, P.C. Chronic Insomnia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK526136/ (accessed on 28 August 2025).
- Sutton, E.L. Insomnia. Ann. Intern. Med. 2021, 174, ITC33–ITC48. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gordon, C.J.; Chapman, J.L.; Hoyos, C.M.; Marshall, N.S.; Miller, C.B.; Grunstein, R.R. The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 59, 101456. [Google Scholar] [CrossRef]
- Li, X.; Sotres-Alvarez, D.; Gallo, L.C.; Ramos, A.R.; Aviles-Santa, L.; Perreira, K.M.; Isasi, C.R.; Zee, P.C.; Savin, K.L.; Schneiderman, N.; et al. Associations of Sleep-disordered Breathing and Insomnia with Incident Hypertension and Diabetes. The Hispanic Community Health Study/Study of Latinos. Am. J. Respir. Crit. Care Med. 2021, 203, 356–365. [Google Scholar] [CrossRef]
- Shan, W.; Peng, X.; Tan, W.; Zhou, Z.; Xie, H.; Wang, S. Prevalence of insomnia and associations with depression, anxiety among adults in guangdong, China: A large-scale cross-sectional study. Sleep Med. 2024, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bard, H.A.; O’Driscoll, C.; Miller, C.B.; Henry, A.L.; Cape, J.; Espie, C.A. Insomnia, depression, and anxiety symptoms interact and individually impact functioning: A network and relative importance analysis in the context of insomnia. Sleep Med. 2023, 101, 505–514. [Google Scholar] [CrossRef]
- Riemann, D.; Dressle, R.J.; Benz, F.; Spiegelhalder, K.; Johann, A.F.; Nissen, C.; Hertenstein, E.; Baglioni, C.; Palagini, L.; Krone, L.; et al. Chronic insomnia, REM sleep instability and emotional dysregulation: A pathway to anxiety and depression? J. Sleep Res. 2025, 34, e14252. [Google Scholar] [CrossRef]
- Morin, C.M.; Bertisch, S.M.; Pelayo, R.; Watson, N.F.; Winkelman, J.W.; Zee, P.C.; Krystal, A.D. What Should Be the Focus of Treatment When Insomnia Disorder Is Comorbid with Depression or Anxiety Disorder? J. Clin. Med. 2023, 12, 1975. [Google Scholar] [CrossRef]
- Meaklim, H.; Saunders, W.J.; Byrne, M.L.; Junge, M.F.; Varma, P.; Finck, W.A.; Jackson, M.L. Insomnia is a key risk factor for persistent anxiety and depressive symptoms: A 12-month longitudinal cohort study during the COVID-19 pandemic. J. Affect. Disord. 2023, 322, 52–62. [Google Scholar] [CrossRef]
- Cox, R.C.; Olatunji, B.O. Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med. Rev. 2020, 51, 101282. [Google Scholar] [CrossRef]
- Richards, A.; Kanady, J.C.; Neylan, T.C. Sleep disturbance in PTSD and other anxiety-related disorders: An updated review of clinical features, physiological charac-teristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology 2020, 45, 55–73, Erratum in: Neuropsychopharmacology 2020, 45, 240–241. https://doi.org/10.1038/s41386-019-0529-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weber, F.C.; Wetter, T.C. The Many Faces of Sleep Disorders in Post-Traumatic Stress Disorder: An Update on Clinical Features and Treatment. Neuropsychobiology 2022, 81, 85–97. [Google Scholar] [CrossRef]
- Lancel, M.; van Marle, H.J.F.; Van Veen, M.M.; van Schagen, A.M. Disturbed Sleep in PTSD: Thinking Beyond Nightmares. Front. Psychiatry 2021, 12, 767760. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Liu, S.; Wu, D.J.H.; Wang, J.; Qian, Y.; Ye, D.; Mao, Y. Sleep disturbance and psychiatric disorders: A bidirectional Mendelian randomisation study. Epidemiol. Psychiatr. Sci. 2022, 31, e26. [Google Scholar] [CrossRef]
- Álamo, C.; Ruiz, J.S.; Arnáez, C.Z. Orexinergic Receptor Antagonists as a New Therapeutic Target to Overcome Limitations of Current Pharmacological Treatment of Insomnia Disorder. Actas Esp. Psiquiatr. 2024, 52, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.-L.; Chang, X.-W.; Zheng, J.-W.; Shi, L.; Xiang, Y.-J.; Que, J.-Y.; Yuan, K.; Deng, J.-H.; Teng, T.; Li, Y.-Y.; et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: A systematic review and network meta-analysis. Sleep Med. Rev. 2023, 68, 101746. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, Y.; Ye, C.; Guo, L.; Luo, S.; Dai, S.; Chen, N.; Wang, E. A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults. Neurosci. Biobehav. Rev. 2021, 131, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Muehlan, C.; Roch, C.; Vaillant, C.; Dingemanse, J. The orexin story and orexin receptor antagonists for the treatment of insomnia. J. Sleep Res. 2023, 32, e13902. [Google Scholar] [CrossRef]
- Waters, K. Review of the Efficacy and Safety of Lemborexant, a Dual Receptor Orexin Antagonist (DORA), in the Treatment of Adults with Insomnia Disorder. Ann. Pharmacother. 2022, 56, 213–221. [Google Scholar] [CrossRef]
- Di Marco, T.; Scammell, T.E.; Meinel, M.; Seboek Kinter, D.; Datta, A.N.; Zammit, G.; Dauvilliers, Y. Number, Duration, and Distribution of Wake Bouts in Patients with Insomnia Disorder: Effect of Daridorexant and Zolpidem. CNS Drugs 2023, 37, 639–653. [Google Scholar] [CrossRef]
- Arnold, V.; Ancoli-Israel, S.; Dang-Vu, T.T.; Mishima, K.; Pinner, K.; Malhotra, M.; Moline, M. Efficacy of Lemborexant in Adults ≥ 65 Years of Age with Insomnia Disorder. Neurol. Ther. 2024, 13, 1081–1098. [Google Scholar] [CrossRef]
- Fietze, I.; Bassetti, C.L.A.; Mayleben, D.W.; Pain, S.; Seboek Kinter, D.; McCall, W.V. Efficacy and Safety of Daridorexant in Older and Younger Adults with Insomnia Disorder: A Secondary Analysis of a Randomised Placebo-Controlled Trial. Drugs Aging 2022, 39, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Zammit, G.K.; Cheng, J.Y.; Kumar, D.; Moline, M. Effect of lemborexant on sleep architecture in participants with insomnia disorder and mild obstructive sleep apnea. Sleep Med. 2025, 127, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Boof, M.-L.; Dingemanse, J.; Lederer, K.; Fietze, I.; Ufer, M. Effect of the New Dual Orexin Receptor Antagonist Daridorexant on Nighttime Respiratory Function and Sleep in Patients with Mild and Moderate Obstructive Sleep Apnea|SLEEP|Oxford Academic. Available online: https://academic.oup.com/sleep/article/44/6/zsaa275/6030922 (accessed on 2 August 2025).
- Jiang, M.; Li, H.; Kong, L. Data mining and safety analysis of dual orexin receptor antagonists (DORAs): A real-world pharmacovigilance study based on the FAERS database. Front. Pharmacol. 2024, 15, 1436405. [Google Scholar] [CrossRef]
- Kärppä, M.; Yardley, J.; Pinner, K.; Filippov, G.; Zammit, G.; Moline, M.; Perdomo, C.; Inoue, Y.; Ishikawa, K.; Kubota, N. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: Results from the phase 3 randomized clinical trial SUNRISE 2. Sleep 2020, 43, zsaa123. [Google Scholar] [CrossRef]
- Gotfried, M.H.; Auerbach, S.H.; Dang-Vu, T.T.; Mishima, K.; Kumar, D.; Moline, M.; Malhotra, M. Efficacy and safety of insomnia treatment with lemborexant in older adults: Analyses from three clinical trials. Drugs Aging 2024, 41, 741–752. [Google Scholar] [CrossRef]
- Bonifazi, A.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Saab, E.; Botticelli, L.; Cifani, C.; Micioni Di Bonaventura, E.; Micioni Di Bonaventura, M.V.; Quaglia, W. Targeting orexin receptors: Recent advances in the development of subtype selective or dual ligands for the treatment of neuropsychiatric disorders. Med. Res. Rev. 2023, 43, 1607–1667. [Google Scholar] [CrossRef]
- Carpi, M.; Palagini, L.; Fernandes, M.; Calvello, C.; Geoffroy, P.A.; Miniati, M.; Pini, S.; Gemignani, A.; Mercuri, N.B.; Liguori, C. Clinical usefulness of dual orexin receptor antagonism beyond insomnia: Neurological and psychiatric comorbidities. Neuropharmacology 2024, 245, 109815. [Google Scholar] [CrossRef]
- Grandjean, C.M.; Kiry, M.; Vaillant, C.; Nayler, O.; Gatfield, J. 059 Daridorexant: A dual, equipotent, and insurmountable antagonist of both orexin-1 and orexin-2 receptors. Sleep 2021, 44 (Suppl. 2), A25. [Google Scholar] [CrossRef]
- Summers, C.H.; Yaeger, J.D.W.; Staton, C.D.; Arendt, D.H.; Summers, T.R. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy. Brain Res. 2020, 1731, 146085. [Google Scholar] [CrossRef] [PubMed]
- Shariq, A.S.; Rosenblat, J.D.; Alageel, A.; Mansur, R.B.; Rong, C.; Ho, R.C.; Ragguett, R.-M.; Pan, Z.; Brietzke, E.; McIntyre, R.S. Evaluating the role of orexins in the pathophysiology and treatment of depression: A comprehensive review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 1–7. [Google Scholar] [CrossRef]
- Salvadore, G.; Bonaventure, P.; Shekhar, A.; Johnson, P.L.; Lord, B.; Shireman, B.T.; Lebold, T.P.; Nepomuceno, D.; Dugovic, C.; Brooks, S.; et al. Translational evaluation of novel selective orexin-1 receptor antagonist JNJ-61393215 in an experimental model for panic in rodents and humans. Transl. Psychiatry 2020, 10, 308. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Lakis, G.A.; Zhoba, H. Sleep-wake and arousal dysfunctions in post-traumatic stress disorder: Role of orexin systems. Brain Res. Bull. 2022, 186, 106–122. [Google Scholar] [CrossRef]
- Schöne, C.; Burdakov, D. Orexin/Hypocretin and Organizing Principles for a Diversity of Wake-Promoting Neurons in the Brain. In Behavioral Neuroscience of Orexin/Hypocretin; Lawrence, A.J., de Lecea, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 51–74. ISBN 978-3-319-57535-3. [Google Scholar]
- Chatterjee, O.; Gopalakrishnan, L.; Pullimamidi, D.; Raj, C.; Yelamanchi, S.; Gangadharappa, B.S.; Nair, B.; Mahadevan, A.; Raju, R.; Keshava Prasad, T.S. A molecular network map of orexin-orexin receptor signaling system. J. Cell Commun. Signal. 2023, 17, 217–227. [Google Scholar] [CrossRef]
- Yin, J.; Kang, Y.; McGrath, A.P.; Chapman, K.; Sjodt, M.; Kimura, E.; Okabe, A.; Koike, T.; Miyanohana, Y.; Shimizu, Y.; et al. Molecular mechanism of the wake-promoting agent TAK-925. Nat. Commun. 2022, 13, 2902. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Chen, M.; Yu, X.; Chen, C.; Chen, Y.; Zhang, L.; Shu, Y. A comprehensive study on the regulation of Compound Zaoren Granules on cAMP/CREB signaling pathway and metabolic disorder in CUMS-PCPA induced insomnia rats. J. Ethnopharmacol. 2024, 332, 118401. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-L.; Peng, X.-M.; Li, G.-B.; Ding, W.-S.; Wang, K.-Z.; Wang, X.-L.; Xiong, Y.-Y.; Xiong, W.-J.; Li, F.; Song, M. Chaihu-Longgu-Muli decoction improves sleep disorders by restoring orexin-A function in CKD mice. Front. Endocrinol. 2023, 14, 1206353. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Y.; Xiong, H.; Wang, L.; Shi, Y.; Wang, D.; Wang, Z.; Ren, J.; Wang, S. Mechanism of action of Panax ginseng alcohol extract based on orexin-mediated autophagy in the treatment of sleep and cognition in aged sleep-deprived rats. J. Ethnopharmacol. 2025, 337 Pt 2, 118907. [Google Scholar] [CrossRef]
- León-Barriera, R.; Chaplin, M.M.; Kaur, J.; Modesto-Lowe, V. Insomnia in older adults: A review of treatment options. Cleve. Clin. J. Med. 2025, 92, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Porwal, A.; Yadav, Y.C.; Pathak, K.; Yadav, R. An Update on Assessment, Therapeutic Management, and Patents on Insomnia. BioMed Res. Int. 2021, 2021, 6068952. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mishima, K.; Yagi, T.; Yoshihara, T.; Eto, T.; Muehlan, C.; Togo, O.; Inoue, Y. Pharmacokinetics, safety, and efficacy of daridorexant in Japanese subjects: Results from phase 1 and 2 studies. J. Sleep Res. 2025, 34, e14302. [Google Scholar] [CrossRef]
- Landry, I.; Hall, N.; Aluri, J.; Filippov, G.; Reyderman, L.; Setnik, B.; Henningfield, J.; Moline, M. Abuse Potential of Lemborexant, a Dual Orexin Receptor Antagonist, Compared with Zolpidem and Suvorexant in Recreational Sedative Users. J. Clin. Psychopharmacol. 2022, 42, 365–373. [Google Scholar] [CrossRef]
- Han, A.H.; Burroughs, C.R.; Falgoust, E.P.; Hasoon, J.; Hunt, G.; Kakazu, J.; Lee, T.; Kaye, A.M.; Kaye, A.D.; Ganti, L. Suvorexant, a Novel Dual Orexin Receptor Antagonist, for the Management of Insomnia. Health Psychol. Res. 2022, 10, 67898. [Google Scholar] [CrossRef]
- Yasuda, K.; Hirano, Y.; Takeda, R.; Ikeda, R.; Ishida, Y. Characteristics of psychiatric patients with nightmares after suvorexant administration: A retrospective study. Neuropsychopharmacol. Rep. 2025, 45, e12506. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Murphy, P.; Zammit, G.; Mayleben, D.; Kumar, D.; Dhadda, S.; Filippov, G.; LoPresti, A.; Moline, M. Comparison of Lemborexant with Placebo and Zolpidem Tartrate Extended Release for the Treatment of Older Adults with Insomnia Disorder: A Phase 3 Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1918254, Erratum in: JAMA Netw. Open 2020, 3, e206497. https://doi.org/10.1001/jamanetworkopen.2020.6497. Erratum in: JAMA Netw. Open 2021, 4, e2127643. https://doi.org/10.1001/jamanetworkopen.2021.27643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katzman, M.A.; Katzman, M.P. Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sci. 2022, 12, 150. [Google Scholar] [CrossRef]
- Hopf, F.W. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology 2020, 168, 108013. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nagumo, Y.; Ishikawa, Y.; Irukayama-Tomobe, Y.; Namekawa, Y.; Nemoto, T.; Tanaka, H.; Takahashi, G.; Tokuda, A.; Saitoh, T.; et al. OX2R-selective orexin agonism is sufficient to ameliorate cataplexy and sleep/wake fragmentation without inducing drug-seeking behavior in mouse model of narcolepsy. PLoS ONE 2022, 17, e0271901. [Google Scholar] [CrossRef]
- Sun, Y.; Tisdale, R.K.; Kilduff, T.S. Hypocretin/Orexin Receptor Pharmacology and Sleep Phases. Available online: https://karger.com/books/book/380/chapter/5563796/Hypocretin-Orexin-Receptor-Pharmacology-and-Sleep (accessed on 2 August 2025).
- Perrey, D.A.; Zhang, Y. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. 2020, 1731, 145922. [Google Scholar] [CrossRef]
- Fragale, J.E.; James, M.H.; Avila, J.A.; Spaeth, A.M.; Aurora, R.N.; Langleben, D.; Aston-Jones, G. The Insomnia-Addiction Positive Feedback Loop: Role of the Orexin System. Front. Neurosci. 2021, 45, 117–127. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, K.; Zheng, Y.; Lu, L. Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders. Neurosci. Bull. 2020, 36, 432–448. [Google Scholar] [CrossRef]
- Yukitake, H.; Fujimoto, T.; Ishikawa, T.; Suzuki, A.; Shimizu, Y.; Rikimaru, K.; Ito, M.; Suzuki, M.; Kimura, H. TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol. Biochem. Behav. 2019, 187, 172794. [Google Scholar] [CrossRef]
- Steiner, M.A.; Botticelli, L.; Bergamini, G.; Micioni Di Bonaventura, E.; Gatfield, J.; Williams, J.T.; Treiber, A.; Vaillant, C.; Cifani, C.; Micioni Di Bonaventura, M.V. Evaluating the efficacy of the selective orexin 1 receptor antagonist nivasorexant in an animal model of binge-eating disorder. Int. J. Eat. Disord. 2024, 57, 1418–1432. [Google Scholar] [CrossRef]
- Hung, C.; Yamanaka, A. The role of orexin neuron activity in sleep/wakefulness regulation. Peptides 2023, 165, 171007. [Google Scholar] [CrossRef]
- Li, N.; Huang, L.; Zhang, B.; Zhu, W.; Dai, W.; Li, S.; Xu, H. The mechanism of different orexin/hypocretin neuronal projections in wakefulness and sleep. Brain Res. 2025, 1850, 149408. [Google Scholar] [CrossRef]
- Li, S.-B.; de Lecea, L. The hypocretin (orexin) system: From a neural circuitry perspective. Neuropharmacology 2020, 167, 107993. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Saito, Y.C.; Yanagisawa, M. Interaction between Orexin Neurons and Monoaminergic Systems. Available online: https://karger.com/books/book/380/chapter/5563735/Interaction-between-Orexin-Neurons-and (accessed on 2 August 2025).
- Saito, Y.C.; Tsujino, N.; Abe, M.; Yamazaki, M.; Sakimura, K.; Sakurai, T. Serotonergic Input to Orexin Neurons Plays a Role in Maintaining Wakefulness and REM Sleep Architecture. Front. Neurosci. 2018, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Barateau, L.; Pizza, F.; Plazzi, G.; Dauvilliers, Y. Narcolepsy. J. Sleep Res. 2022, 31, e13631. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, A.C.; Maski, K.; Scammell, T.E.; Tucker, D.; Orban, Z.S.; Koralnik, I.J. Children with Narcolepsy type 1 have increased T-cell responses to orexins. Ann. Clin. Transl. Neurol. 2019, 6, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Kornum, B.R. Narcolepsy type 1: What have we learned from immunology? Sleep 2020, 43, zsaa055. [Google Scholar] [CrossRef]
- Mahoney, C.E.; Cogswell, A.; Koralnik, I.J.; Scammell, T.E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 2019, 20, 83–93. [Google Scholar] [CrossRef]
- Sochal, M.; Ditmer, M.; Turkiewicz, S.; Karuga, F.F.; Białasiewicz, P.; Gabryelska, A. The effect of sleep and its restriction on selected inflammatory parameters. Sci. Rep. 2024, 14, 17379. [Google Scholar] [CrossRef]
- Couvineau, A.; Voisin, T.; Nicole, P.; Gratio, V.; Abad, C.; Tan, Y.-V. Orexins as Novel Therapeutic Targets in Inflammatory and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 709. [Google Scholar] [CrossRef]
- Spinazzi, R.; Andreis, P.G.; Rossi, G.P.; Nussdorfer, G.G. Orexins in the Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Pharmacol. Rev. 2006, 58, 46–57. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Jiang, N.-H.; Zhan, L.-H.; Teng, X.; Fang, X.; Lin, M.-Q.; Xie, Z.-Y.; Luo, R.; Li, L.-Z.; Li, B.; et al. Soporific effect of modified Suanzaoren Decoction on mice models of insomnia by regulating Orexin-A and HPA axis homeostasis. Biomed. Pharmacother. 2021, 143, 112141. [Google Scholar] [CrossRef]
- Steward, T.; Mestre-Bach, G.; Granero, R.; Sánchez, I.; Riesco, N.; Vintró-Alcaraz, C.; Sauchelli, S.; Jiménez-Murcia, S.; Agüera, Z.; Fernández-García, J.C.; et al. Reduced Plasma Orexin-A Concentrations are Associated with Cognitive Deficits in Anorexia Nervosa. Sci. Rep. 2019, 9, 7910. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Qian, J.; Chen, X.; Li, D.; Song, C. Correlation of serum Orexin-A level with cognitive function and serum inflammatory cytokines in epileptic patients. Am. J. Transl. Res. 2023, 15, 4110–4117. [Google Scholar] [PubMed]
- Liguori, C.; Nuccetelli, M.; Izzi, F.; Sancesario, G.; Romigi, A.; Martorana, A.; Amoroso, C.; Bernardini, S.; Marciani, M.G.; Mercuri, N.B.; et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol. Aging 2016, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Yu, K.-Y.; Cui, Y.-J.; Wang, Z.-J.; Cai, H.-Y.; Cao, J.-M.; Wu, M.-N. Orexin-A aggravates cognitive deficits in 3xTg-AD mice by exacerbating synaptic plasticity impairment and affecting amyloid β metabolism. Neurobiol. Aging 2023, 124, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, F.; Wu, Y. Orexinergic System in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 713201. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, W.-J.; Lian, T.-H.; Zhang, W.-J.; He, M.-Y.; Zhang, Y.-N.; Huang, Y.; Ding, D.-Y.; Guan, H.-Y.; Li, J.-H.; et al. Alzheimer’s disease with sleep insufficiency: A cross-sectional study on correlations among clinical characteristics, orexin, its receptors, and the blood-brain barrier. Neural Regen. Res. 2022, 18, 1757–1762. [Google Scholar] [CrossRef]
- Treu, S.P.; Plante, D.T. Cerebrospinal fluid orexin in Alzheimer’s disease: A systematic review and meta-analysis. Sleep Med. 2021, 85, 230–238. [Google Scholar] [CrossRef]
- Lucey, B.P.; Liu, H.; Toedebusch, C.D.; Freund, D.; Redrick, T.; Chahin, S.L.; Mawuenyega, K.G.; Bollinger, J.G.; Ovod, V.; Barthélemy, N.R.; et al. Suvorexant Acutely Decreases Tau Phosphorylation and Aβ in the Human CNS. Ann. Neurol. 2023, 94, 27–40. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Possovre, M.-L.; Bandarabadi, M.; Moseholm, K.F.; Justinussen, J.L.; Bozic, I.; Lemcke, R.; Arribat, Y.; Amati, F.; Silahtaroglu, A.; et al. The evolutionarily conserved miRNA-137 targets the neuropeptide hypocretin/orexin and modulates the wake to sleep ratio. Proc. Natl. Acad. Sci. USA 2022, 119, e2112225119. [Google Scholar] [CrossRef]
- Mogavero, M.P.; Silvani, A.; Lanza, G.; DelRosso, L.M.; Ferini-Strambi, L.; Ferri, R. Targeting Orexin Receptors for the Treatment of Insomnia: From Physiological Mechanisms to Current Clinical Evidence and Recommendations. Nat. Sci. Sleep 2023, 15, 17–38. [Google Scholar] [CrossRef]
- Michelson, D.; Snyder, E.; Paradis, E.; Chengan-Liu, M.; Snavely, D.B.; Hutzelmann, J.; Walsh, J.K.; Krystal, A.D.; Benca, R.M.; Cohn, M.; et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: A phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014, 13, 461–471. [Google Scholar] [CrossRef]
- Mignot, E.; Mayleben, D.; Fietze, I.; Leger, D.; Zammit, G.; Bassetti, C.L.A.; Pain, S.; Kinter, D.S.; Roth, T. Safety and efficacy of daridorexant in patients with insomnia disorder: Results from two multicentre, randomised, dou-ble-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022, 21, 125–139, Erratum in: Lancet Neurol. 2022, 21, e3. https://doi.org/10.1016/S1474-4422(22)00029-1. Erratum in: Lancet Neurol. 2022, 21, e6. https://doi.org/10.1016/S1474-4422(22)00144-2. [Google Scholar] [CrossRef] [PubMed]
- Wrishko, R.E.; McCrea, J.B.; Yee, K.L.; Liu, W.; Panebianco, D.; Mangin, E.; Chakravarthy, M.; Martinez-Cantarin, M.P.; Kraft, W.K. Effect of CYP3A Inhibition and Induction on the Pharmacokinetics of Suvorexant: Two Phase I, Open-Label, Fixed-Sequence Trials in Healthy Subjects. Clin. Drug Investig. 2019, 39, 441–451, Erratum in: Clin. Drug Investig. 2019, 39, 453–454. https://doi.org/10.1007/s40261-019-00777-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asai, Y.; Miyazaki, M.; Iwakura, M.; Hara, M. The interim report about the drug use—Results survey of orexinergic receptor antagonist, Belsomra, in patients with insomnia. Jpn. J. Sleep Med. 2017, 11, 249–263. [Google Scholar]
- Asai, Y.; Sano, H.; Miyazaki, M.; Iwakura, M.; Maeda, Y.; Hara, M. Suvorexant (Belsomra® Tablets 10, 15, and 20 mg): Japanese Drug-Use Results Survey. Drugs RD 2019, 19, 27–46. [Google Scholar] [CrossRef]
- Ali, A.K. Psychiatric Safety Profile Of Suvorexant, A First-In-Class Orexin Receptor Antagonist. Value Health 2016, 19, A59. [Google Scholar] [CrossRef]
- Borchert, J.S.; Wang, B.; Ramzanali, M.; Stein, A.B.; Malaiyandi, L.M.; Dineley, K.E. Adverse Events Due to Insomnia Drugs Reported in a Regulatory Database and Online Patient Reviews: Comparative Study. J. Med. Internet Res. 2019, 21, e13371. [Google Scholar] [CrossRef] [PubMed]
- Hatta, K.; Kishi, Y.; Wada, K.; Takeuchi, T.; Ito, S.; Kurata, A.; Murakami, K.; Sugita, M.; Usui, C.; Nakamura, H.; et al. Preventive Effects of Suvorexant on Delirium: A Randomized Placebo-Controlled Trial. J. Clin. Psychiatry 2017, 78, e970–e979. [Google Scholar] [CrossRef]
- Kishi, T.; Nishida, M.; Koebis, M.; Taninaga, T.; Muramoto, K.; Kubota, N.; Moline, M.; Sakuma, K.; Okuya, M.; Nomura, I.; et al. Evidence-based insomnia treatment strategy using novel orexin antagonists: A review. Neuropsychopharmacol. Rep. 2021, 41, 450–458. [Google Scholar] [CrossRef]
- Kishi, T.; Nomura, I.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Ikuta, T.; Iwata, N. Lemborexant vs suvorexant for insomnia: A systematic review and network meta-analysis. J. Psychiatr. Res. 2020, 128, 68–74. [Google Scholar] [CrossRef]
- Landry, I.; Nakai, K.; Ferry, J.; Aluri, J.; Hall, N.; Lalovic, B.; Moline, M.L. Pharmacokinetics, Pharmacodynamics, and Safety of the Dual Orexin Receptor Antagonist Lemborexant: Findings From Single-Dose and Multiple-Ascending-Dose Phase 1 Studies in Healthy Adults. Clin. Pharmacol. Drug Dev. 2021, 10, 153–165. [Google Scholar] [CrossRef]
- Dayal, S.; Aluri, J.; Hall, N.; Filippov, G.; Moline, M.; Reyderman, L.; Landry, I. Effect of hepatic impairment on pharmacokinetics, safety, and tolerability of lemborexant. Pharmacol. Res. Perspect. 2021, 9, e00758. [Google Scholar] [CrossRef]
- Landry, I.; Hall, N.; Aluri, J.; Filippov, G.; Setnik, B.; Dayal, S.; Reyderman, L.; Moline, M. Effect of alcohol coadministration on the pharmacodynamics, pharmacokinetics, and safety of lemborexant: A randomized, placebo-controlled crossover study. J. Psychopharmacol. Oxf. Engl. 2022, 36, 745–755. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Lorch, D.; Lowe, A.D.; Uchimura, N.; Hall, N.; Shah, D.; Moline, M. A randomized, double-blind, placebo-controlled, crossover study of respiratory safety of lemborexant in moderate to severe obstructive sleep apnea. J. Clin. Sleep Med. 2024, 20, 57–65. [Google Scholar] [CrossRef]
- Yardley, J.; Kärppä, M.; Inoue, Y.; Pinner, K.; Perdomo, C.; Ishikawa, K.; Filippov, G.; Kubota, N.; Moline, M. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: Results from a phase 3 randomized clinical trial. Sleep Med. 2021, 80, 333–342. [Google Scholar] [CrossRef]
- Terada, T.; Hirayama, T.; Sadahiro, R.; Wada, S.; Nakahara, R.; Matsuoka, H. Pilot Study of Lemborexant for Insomnia in Cancer Patients with Delirium|Journal of Palliative Medicine. Available online: https://www.liebertpub.com/doi/10.1089/jpm.2021.0509 (accessed on 2 August 2025).
- Herring, W.J.; Connor, K.M.; Ivgy-May, N.; Snyder, E.; Liu, K.; Snavely, D.B.; Krystal, A.D.; Walsh, J.K.; Benca, R.M.; Rosenberg, R.; et al. Suvorexant in Patients with Insomnia: Results From Two 3-Month Randomized Controlled Clinical Trials. Biol. Psychiatry 2016, 79, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Wu, X.; Li, J.; Chen, S.; Wang, Z.; Tan, X.; Wang, Z.; Zhang, J. Different doses of dual orexin receptor antagonists in primary insomnia: A Bayesian network analysis. Front. Pharmacol. 2023, 14, 1175372. [Google Scholar] [CrossRef]
- Murphy, P.; Moline, M.; Mayleben, D.; Rosenberg, R.; Zammit, G.; Pinner, K.; Dhadda, S.; Hong, Q.; Giorgi, L.; Satlin, A. Lemborexant, A Dual Orexin Receptor Antagonist (DORA) for the Treatment of Insomnia Disorder: Results From a Bayesian, Adaptive, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Sleep Med. 2017, 13, 1289–1299. [Google Scholar] [CrossRef]
- Roch, C.; Bergamini, G.; Steiner, M.A.; Clozel, M. Nonclinical pharmacology of daridorexant: A new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology 2021, 238, 2693–2708. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Daridorexant: First Approval. Drugs 2022, 82, 601–607, Erratum in: Drugs 2022, 82, 841. https://doi.org/10.1007/s40265-022-01719-x. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunz, D.; Dauvilliers, Y.; Benes, H.; García-Borreguero, D.; Plazzi, G.; Seboek Kinter, D.; Coloma, P.; Rausch, M.; Sassi-Sayadi, M.; Thein, S. Long-Term Safety and Tolerability of Daridorexant in Patients with Insomnia Disorder. CNS Drugs 2023, 37, 93–106. [Google Scholar] [CrossRef]
- Berger, B.; Muehlan, C.; Klein, G.; Dingemanse, J. Pharmacokinetics of daridorexant, a dual orexin receptor antagonist, are not affected by renal impairment. Clin. Transl. Sci. 2021, 14, 2132–2138. [Google Scholar] [CrossRef]
- Muehlan, C.; Brooks, S.; Vaillant, C.; Meinel, M.; Jacobs, G.E.; Zuiker, R.G.; Dingemanse, J. Driving Performance after Bedtime Administration of Daridorexant, Assessed in a Sensitive Simulator. Clin. Pharmacol. Ther. 2022, 111, 1334–1342. [Google Scholar] [CrossRef]
- Nie, T.; Blair, H.A. Daridorexant in Insomnia Disorder: A Profile of Its Use. CNS Drugs 2023, 37, 267–274, Erratum in: CNS Drugs 2023, 37, 291. https://doi.org/10.1007/s40263-023-00994-w. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchimura, N.; Ozone, M.; Suzuki, M.; Taniguchi, M.; Kuriyama, K.; Togo, O.; Uchiyama, M. Long-term safety and efficacy of daridorexant in Japanese patients with insomnia disorder. Sleep Med. 2024, 122, 64–70. [Google Scholar] [CrossRef]
- Magliocca, M.; Koopmans, I.; Vaillant, C.; Lemoine, V.; Zuiker, R.; Dingemanse, J.; Muehlan, C. Nighttime safety of daridorexant: Evaluation of responsiveness to an external noise stimulus, postural stability, walking, and cognitive function. J. Psychopharmacol. 2025, 39, 223–232. [Google Scholar] [CrossRef]
- Boss, C.; Gatfield, J.; Brotschi, C.; Heidmann, B.; Sifferlen, T.; von Raumer, M.; Schmidt, G.; Williams, J.T.; Treiber, A.; Roch, C. The Quest for the Best Dual Orexin Receptor Antagonist (Daridorexant) for the Treatment of Insomnia Disorders. ChemMedChem 2020, 15, 2286–2305. [Google Scholar] [CrossRef]
- Caldiroli, A.; Affaticati, L.M.; Capuzzi, E.; La Tegola, D.; Colmegna, F.; Clerici, M.; Dakanalis, A.; Buoli, M. The potential use of daridorexant in eating disorders: Beyond the treatment of insomnia? Int. Clin. Psychopharmacol. 2025, 40, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.; Fanelli, G.; De Ronchi, D.; Serretti, A.; Fabbri, C. Clinical specificity profile for novel rapid acting antidepressant drugs. Int. Clin. Psychopharmacol. 2023, 38, 297–328. [Google Scholar] [CrossRef]
- Uchiyama, M.; Kambe, D.; Imadera, Y.; Kajiyama, Y.; Ogo, H.; Uchimura, N. Effects of TS-142, a novel dual orexin receptor antagonist, on sleep in patients with insomnia: A randomized, double-blind, placebo-controlled phase 2 study. Psychopharmacology 2022, 239, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Jin, L.; Zhao, D.; Zhang, W.; Li, B.; Huang, X.; Hao, X. Pharmacokinetics, Pharmacodynamic, Safety and Tolerability of Fazamorexant, a Novel Dual Orexin Receptor Antagonist: Report of the First-in-Human Study. Drug Des. Devel. Ther. 2025, 19, 5271–5282. [Google Scholar] [CrossRef] [PubMed]
- van Lemmen, M.; Dahan, A.; Hang, Y.; Jansen, S.C.; Lu, H.; Naylor, M.; Olsson, T.; Sheikh, S.; Sullivan, D.; Tolkoff, M.; et al. TAK-925 (Danavorexton), an Orexin Receptor 2 Agonist, Reduces Opioid-induced Respiratory Depression and Sedation Without Affecting Analgesia in Healthy Men. Anesthesiology 2025, 142, 628. [Google Scholar] [CrossRef]
- Billioti de Gage, S.; Bégaud, B.; Bazin, F.; Verdoux, H.; Dartigues, J.-F.; Pérès, K.; Kurth, T.; Pariente, A. Benzodiazepine use and risk of dementia: Prospective population based study. BMJ 2012, 345, e6231. [Google Scholar] [CrossRef]
- Hoyer, D.; Allen, A.; Jacobson, L.H. Hypnotics with novel modes of action. Br. J. Clin. Pharmacol. 2020, 86, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W.; Daykin, H.; Metha, J.A.; Allocca, G.; Hoyer, D.; Drummond, S.P.A.; Jacobson, L.H. Manipulation of rapid eye movement sleep via orexin and GABAA receptor modulators differentially affects fear extinction in mice: Effect of stable versus disrupted circadian rhythm. Sleep 2021, 44, zsab068. [Google Scholar] [CrossRef]
- Matsumoto, S.; Tamiya, H.; Yamana, H.; Hosoi, T.; Matsui, H.; Fushimi, K.; Akishita, M.; Yasunaga, H.; Ogawa, S. Association between the type of hypnotic drug and in-hospital fractures in older patients with neurocognitive disorders: A case-control study using a nationwide database. Geriatr. Gerontol. Int. 2023, 23, 500–505. [Google Scholar] [CrossRef]
- Ikeda, S.; Azuma, M.K.; Fujimoto, K.; Shibahara, H.; Inoue, S.; Moline, M.; Ishii, M.; Mishima, K. Cost-effectiveness analysis of lemborexant for treating insomnia in Japan: A model-based projection, incorporating the risk of falls, motor vehicle collisions, and workplace accidents. Psychol. Med. 2022, 52, 2847, Erratum for: https://doi.org/10.1017/S0033291722000356. Published online by Cambridge University Press, 4 May 2022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natsky, A.N.; Vakulin, A.; Chai-Coetzer, C.L.; Lack, L.; McEvoy, R.D.; Lovato, N.; Sweetman, A.; Gordon, C.J.; Adams, R.J.; Kaambwa, B. Economic evaluation of cognitive behavioural therapy for insomnia (CBT-I) for improving health outcomes in adult populations: A systematic review. Sleep Med. Rev. 2020, 54, 101351. [Google Scholar] [CrossRef]
- Rao, H. ASL Imaging of Brain Function Changes During Sleep Restriction. Sleep 2012, 35, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Perko, S.; Zupanc, M.; Zanoški Hren, M.; Landeka Dragičević, T.; Zigon, D.; Kompare, B.; Heath, E. Environmental occurrence, fate and transformation of benzodiazepines in water treatment. Water Res. 2012, 46, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.A. Lemborexant (Dayvigo) for the Treatment of Insomnia. Am. Fam. Physician 2021, 103, 241–242. [Google Scholar]
- Briggs, A.H.; Chalet, F.-X.; Cooper, J.; Graham, P.; Palmer, S.; Miller, P.; Walker, A.; Greenwood, B.; Morin, C.M. Cost-Effectiveness Analysis of Daridorexant for the Pharmacological Treatment of Chronic Insomnia Disorder in Adults. PharmacoEconomics Open 2025, 9, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Sattari, N.; Upadhya, A.; Vinces, K.; Mednick, S. 058 The interactions of Sleep, Heart Rate Variability and Aging on an Emotional Directed Forgetting Memory Task. Sleep 2021, 44 (Suppl. 2), A24–A25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).