A Meta-Narrative Review of Channelopathies and Cannabis: Mechanistic, Epidemiologic, and Forensic Insights into Arrhythmia and Sudden Cardiac Death
Abstract
1. Introduction
1.1. Overview of Cannabinoids, Biphasic Effect, and Cardiac Ion Channel Modulation
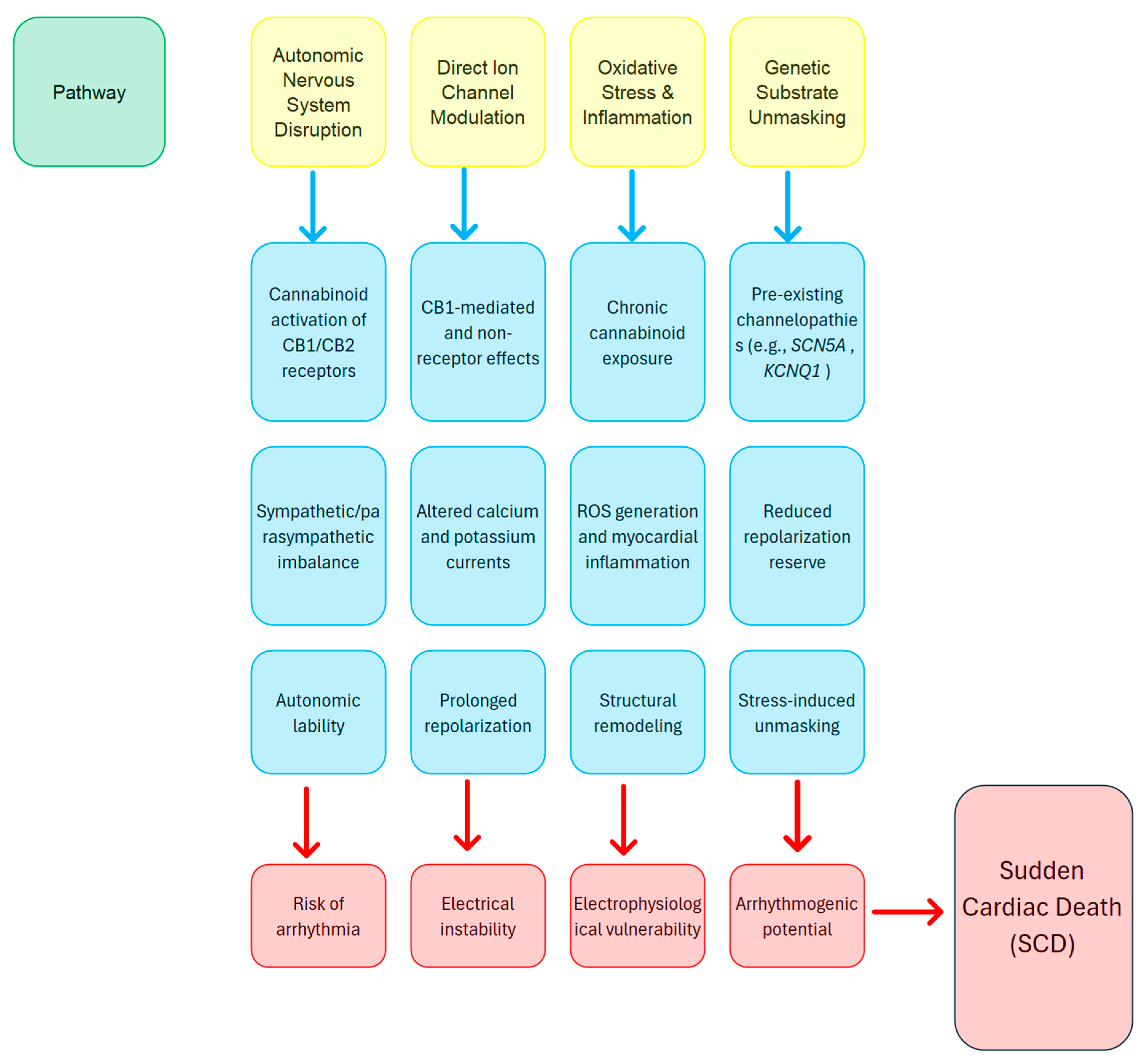
- Cannabinoids can affect both central and peripheral autonomic pathways, potentially causing changes that elevate the risk of arrhythmias [35].
- Cannabinoids may directly interact with ion channels, in addition to receptor-mediated effects, which could broaden their influence on current flow and extend repolarization phases [8].
- Extended exposure to cannabinoids can cause mild inflammation and oxidative stress, which may eventually result in changes to the structure and function of the myocardium [28].
- In individuals with pre-existing mutations, the extra stress caused by cannabinoid-related changes might reveal hidden arrhythmogenic substrates, increasing the risk of SCD [6].
1.2. Cannabinoids in Sudden Cardiac Death
- Synthetic cannabinoid predominance and potency—In 278 published human cases of synthetic cannabinoid exposure, 64 different Synthetic Cannabinoid Receptor Agonists (SCRAs) have been identified [44]. These compounds are often full agonists of CB1 receptors, with far higher potency than THC [45], leading to unpredictable cardiac effects (e.g., extremes of brady or tachycardia, autonomic lability). Fatalities cluster around the more potent SCRAs (e.g., MDMB-CHMICA, 5F-MDMB-PICA), reflecting their steep dose–response curves and narrow safety margins [20,44,46].
- A few years ago, in a Danish nationwide study of individuals aged 1–49 who died suddenly, it was found that 77% of medico-legal autopsies on SCD cases included toxicological analysis [43]. Of these, 57% tested positive for at least one substance, either licit or illicit. The most commonly detected substances were psychotropic drugs, identified in 62% of cases, mostly at therapeutic or subtherapeutic levels. Notably, cases with positive toxicology showed a higher incidence of sudden arrhythmic death syndrome (SADS) compared to those with negative toxicology—56% versus 42%—implying that these substances may contribute to arrhythmia risk even when not at lethal concentrations [47,48].
- To sharpen causal inferences in sudden death cases, a separate study combined toxicological, clinical, autopsy, and genetic data, particularly in young and middle-aged victims. This comprehensive approach aids in determining if substances played a role in the death or were incidental [49].
2. Aim and Hypotheses
3. Evidence and Clinical Observations
3.1. Aggregated Data on Cannabinoids and Sudden Cardiac Death
3.2. Aggregated Data on Poly-Substance Exposure and Sudden Cardiac Death
3.3. Limitations of the Evidence Base
3.4. Mechanisms by Which Poly-Drug Use Amplifies Risk
- When two or more agents prolong ventricular repolarization or depress myocardial excitability, their effects combine or even enhance each other, a phenomenon known as the pharmacodynamic combined effect. This effect leads to lowering the threshold for torsades de pointes and other malignant rhythms [78].
- Co-ingested drugs can inhibit or compete for the same cytochrome P450 enzymes, causing one or more substances (e.g., SCRAs) to accumulate to toxic levels and extend their cardiac effects [79].
- Increased oxidative stress and inflammation refer to the presence of multiple xenobiotics, which elevate reactive oxygen species and induce low-grade inflammation in the myocardium, further impairing structural and cellular damage and disrupting ion channel function and conduction homogeneity [82,83].
- Additive hemodynamic effects may be noticed when several central nervous system depressants or stimulants are used together, causing alternating episodes of hypotension, hypertension, tachycardia, or bradycardia, with each one recognized as an arrhythmic trigger when the heart’s compensatory reserve is overwhelmed [84].
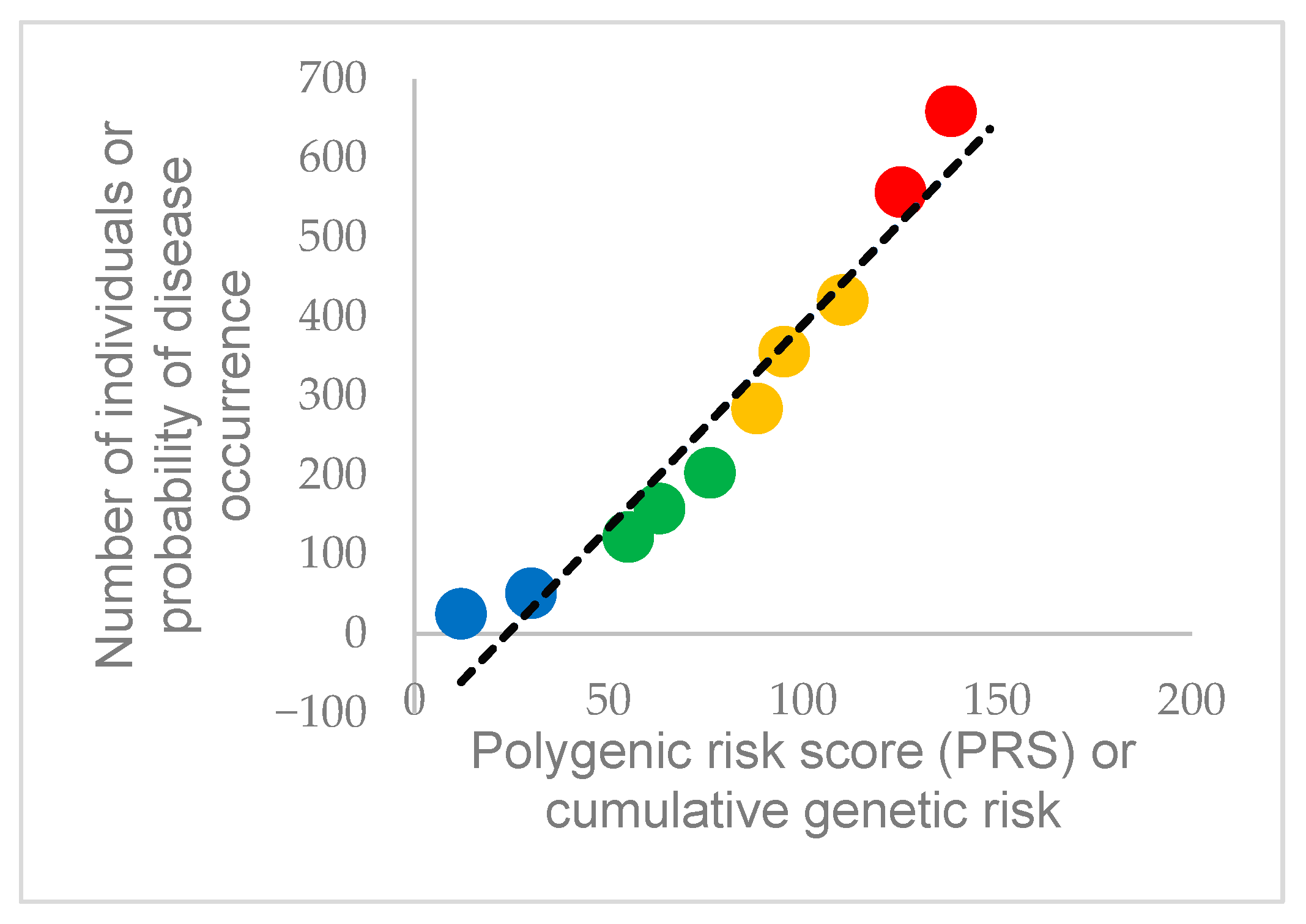
4. Interplay Between Genetics and Cannabinoid Exposure
4.1. Cardiac Electrophysiology and Sudden Cardiac Death
4.2. Genetic Variants Implicated in Cardiac Arrhythmias
4.3. Channelopathies, Their Genetic Background, and Relation to Cannabinoids
5. Illustrative Model
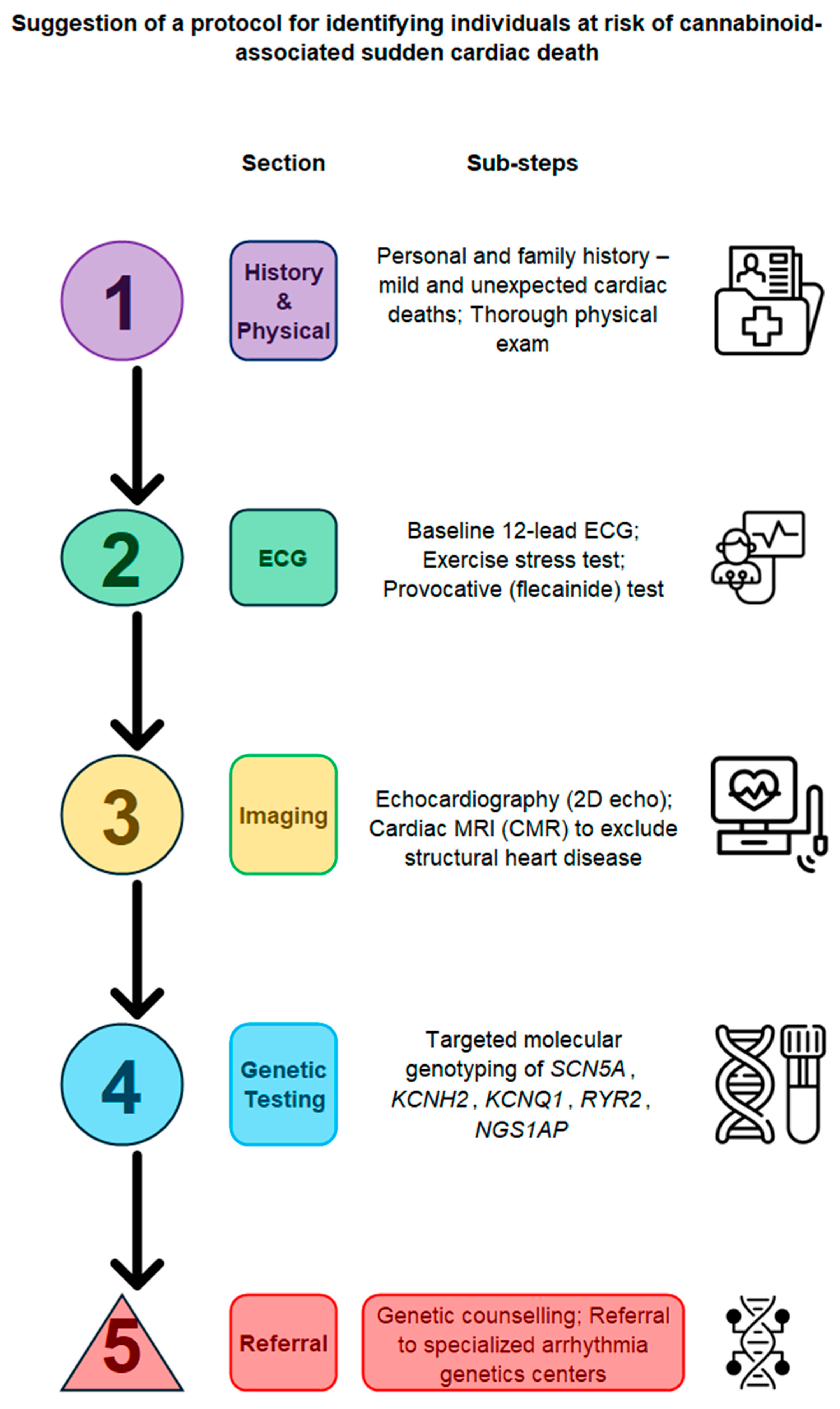
6. Clinical Implications and Future Directions
Future Directions and Research Challenges
- Additional animal and cell-based research is required to clearly understand the molecular interactions between cannabinoids and different ion channels.
- In addition, observational studies with genetic screening can help measure the rate of SCD among cannabinoid users and identify groups at higher risk.
- Likewise, trials in progress should be carefully designed to evaluate interventions or guidelines that can mitigate arrhythmogenic risk in individuals who are genetically predisposed.
- Personal risk assessment tools must be developed and activated, and their implementation clearly defined. Tools may include genetic testing, targeted ECG monitoring, and accurate dosing strategies to minimize risks while maintaining therapeutic effects
- Progress in fields related to genomics (e.g., bioinformatics) could eventually lead to personalized cannabinoid use for treatment, optimizing the benefits and reducing the adverse cardiac effects of such treatment.
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full term |
| 2-AG | 2-arachidonoylglycerol (endocannabinoid) |
| ACC | American College of Cardiology |
| ACS | Acute coronary syndrome |
| ANS | Autonomic nervous system |
| CALM2 | Calmodulin 2 |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CBD | Cannabidiol |
| CI | Confidence interval |
| CNS | Central nervous system |
| CYP | Cytochrome P450 |
| DNA | Deoxyribonucleic acid |
| ECG | Electrocardiogram |
| GPCR | Gi/o-protein-coupled receptor |
| KCNH2 | Potassium voltage-gated channel subfamily H member 2 |
| KCNQ1 | Potassium voltage-gated channel subfamily Q member 1 |
| LC-MS/MS | Liquid chromatography–mass spectrometry |
| MI | Myocardial infarction |
| MYBPC3 | Myosin binding protein C |
| MYH7 | Myosin heavy chain 7 |
| NCBI | National Center for Biotechnology Information |
| NOS1AP | Nitric oxide synthase 1 adaptor protein |
| OR | Odds ratio |
| PK/PD | Pharmacokinetic/pharmacodynamic |
| PKP2 | Plakophilin-2 |
| PRS | Polygenic risk score |
| QT | Interval between Q and T waves on ECG |
| RR | Relative risk |
| RYR2 | Ryanodine receptor 2 |
| SADS | Sudden arrhythmic death syndrome |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 |
| SCD | Sudden cardiac death |
| SCRA | Synthetic cannabinoid receptor agonist |
| SNP | Single-nucleotide polymorphism |
| THC | Δ9-Tetrahydrocannabinol |
| TNNI3 | Troponin I type 3 |
References
- Hoch, E.; Volkow, N.D.; Friemel, C.M.; Lorenzetti, V.; Freeman, T.P.; Hall, W. Cannabis, cannabinoids and health: A review of evidence on risks and medical benefits. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 281–292. [Google Scholar] [CrossRef]
- Duczmal, D.; Bazan-Wozniak, A.; Niedzielska, K.; Pietrzak, R. Cannabinoids—Multifunctional Compounds, Applications and Challenges—Mini Review. Molecules 2024, 29, 4923. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L.; Allen, L.A.; Kloner, R.A.; Carriker, C.R.; Martel, C.; Morris, A.A.; Piano, M.R.; Rana, J.S.; Saucedo, J.F. Medical marijuana, recreational cannabis, and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2020, 142, e131–e152. [Google Scholar] [CrossRef]
- Rincon, N.; Gerke, S.; Wagner, J.K. Implications of An Evolving Regulatory Landscape on the Development of AI and ML in Medicine. Pac. Symp. Biocomput. 2025, 30, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Chye, D.M.; Sampaio Rodrigues, T.; Quarto, L.J.G.; Young, N.; Hamilton, G.W.; Burrell, L.M.; Teh, A.W.; Lim, H.S.; Koshy, A.N. Cannabis use and atrial arrhythmias: A systematic review and meta-analysis of large populational studies. Heart Rhythm 2025. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, S.; Upreti, P.; Tamirisa, K.; Batnyam, U. Arrhythmias and cannabis use: A comprehensive overview. Heart Rhythm O2 2025, 6, 78–85. [Google Scholar] [CrossRef]
- Drummer, O.H.; Gerostamoulos, D.; Woodford, N.W. Cannabis as a cause of death: A review. Forensic Sci. Int. 2019, 298, 298–306. [Google Scholar] [CrossRef]
- Malheiro, R.F.; Carmo, H.; Carvalho, F.; Silva, J.P. Cannabinoid-mediated targeting of mitochondria on the modulation of mitochondrial function and dynamics. Pharmacol. Res. 2023, 187, 106603. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef]
- Chandy, M.; Jimenez-Tellez, N.; Wu, J.C. The relationship between cannabis and cardiovascular disease: Clearing the haze. Nat. Rev. Cardiol. 2025, 22, 467–481. [Google Scholar] [CrossRef]
- Mensah, E.; Tabrizchi, R.; Daneshtalab, N. Pharmacognosy and Effects of Cannabinoids in the Vascular System. ACS Pharmacol. Transl. Sci. 2022, 5, 1034–1049. [Google Scholar] [CrossRef] [PubMed]
- Raïch, I.; Lillo, J.; Rivas-Santisteban, R.; Rebassa, J.B.; Capó, T.; Santandreu, M.; Cubeles-Juberias, E.; Reyes-Resina, I.; Navarro, G. Potential of CBD Acting on Cannabinoid Receptors CB1 and CB2 in Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 6708. [Google Scholar] [CrossRef]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef]
- Fonseca, D.J.; Vaz da Silva, M.J. Cardiac channelopathies: The role of sodium channel mutations. Rev. Port. Cardiol. 2018, 37, 179–199. [Google Scholar] [CrossRef]
- Ponce-Balbuena, D.; Deschenes, I. Long QT syndrome—Bench to bedside. Heart Rhythm O2 2021, 2, 89–106. [Google Scholar] [CrossRef]
- Topal, L.; Naveed, M.; Orvos, P.; Paszti, B.; Prorok, J.; Bajtel, A.; Kiss, T.; Csupor-Loffler, B.; Csupor, D.; Baczko, I.; et al. The electrophysiological effects of cannabidiol on action potentials and transmembrane potassium currents in rabbit and dog cardiac ventricular preparations. Arch. Toxicol. 2021, 95, 2497–2505. [Google Scholar] [CrossRef]
- Lemos, N.P.; Ingle, E.A. Cannabinoids in Postmortem Toxicology. J. Anal. Toxicol. 2011, 35, 394–401. [Google Scholar] [CrossRef]
- Ali, S.M.; Abdelaal, G.M.M.; Moawed, D.M.N.A.; Shehata, S.A. Association of designer drug use and sudden cardiac death: A systematic review. Egypt. J. Forensic Sci. 2025, 15, 46. [Google Scholar] [CrossRef]
- Giorgetti, A.; Busardo, F.P.; Tittarelli, R.; Auwarter, V.; Giorgetti, R. Post-Mortem Toxicology: A Systematic Review of Death Cases Involving Synthetic Cannabinoid Receptor Agonists. Front. Psychiatry 2020, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, G.; Stair, J.L.; Kirton, S.B.; Baker, D.; Guirguis, A. Screening and Quantification of the Synthetic Cannabinoid Receptor Agonist 5F-MDMB-PINACA From Seized Prison Paper Using Ultraperformance Liquid Chromatography-Mass Spectrometry Approaches. Drug Test. Anal. 2025, 17, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Alzu’bi, A.; Almahasneh, F.; Khasawneh, R.; Abu-El-Rub, E.; Baker, W.B.; Al-Zoubi, R.M. The synthetic cannabinoids menace: A review of health risks and toxicity. Eur. J. Med. Res. 2024, 29, 49. [Google Scholar] [CrossRef] [PubMed]
- Shustorovich, A.; Corroon, J.; Wallace, M.S.; Sexton, M. Biphasic effects of cannabis and cannabinoid therapy on pain severity, anxiety, and sleep disturbance: A scoping review. Pain Med. 2024, 25, 387–399. [Google Scholar] [CrossRef]
- Arnold, J.C. A primer on medicinal cannabis safety and potential adverse effects. Aust. J. Gen. Pract. 2021, 50, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Kamel, I.; Mahmoud, A.K.; Twayana, A.R.; Younes, A.M.; Horn, B.; Dietzius, H. Myocardial Infarction and Cardiovascular Risks Associated With Cannabis Use: A Multicenter Retrospective Study. JACC Adv. 2025, 4, 101698. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Müller, D.; Kithinji, J.; Angerer, V.; Franz, F.; Eyer, F.; Neurath, H.; Liebetrau, G.; Auwärter, V. Acute side effects after consumption of the new synthetic cannabinoids AB-CHMINACA and MDMB-CHMICA. Clin. Toxicol. 2018, 56, 404–411. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Ventura, A.L.M.; Silva, T.M.; Franca, G.R. Cannabinoids Activate Endoplasmic Reticulum Stress Response and Promote the Death of Avian Retinal Muller Cells in Culture. Brain Sci. 2025, 15, 291. [Google Scholar] [CrossRef]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.M.; Liang, Y.; Muratori, C.; Sabia, J.J. The effects of recreational marijuana legalization on employment and earnings. J. Popul. Econ. 2025, 38, 47. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB(1) and CB(2) Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Boczek, T.; Zylinska, L. Receptor-Dependent and Independent Regulation of Voltage-Gated Ca(2+) Channels and Ca(2+)-Permeable Channels by Endocannabinoids in the Brain. Int. J. Mol. Sci. 2021, 22, 8168. [Google Scholar] [CrossRef]
- Richards, J.R. Mechanisms for the Risk of Acute Coronary Syndrome and Arrhythmia Associated With Phytogenic and Synthetic Cannabinoid Use. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 508–522. [Google Scholar] [CrossRef]
- Holt, A.; Nouhravesh, N.; Strange, J.E.; Kinnberg Nielsen, S.; Schjerning, A.M.; Vibe Rasmussen, P.; Torp-Pedersen, C.; Gislason, G.H.; Schou, M.; McGettigan, P.; et al. Cannabis for chronic pain: Cardiovascular safety in a nationwide Danish study. Eur. Heart J. 2024, 45, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, G.M.M.; Shehata, S.A.; Ali, S.M.; Moawed, D.M.N.A. Genetic variants associated with sudden cardiac death in the young: A systematic review. Egypt. J. Forensic Sci. 2025, 15, 34. [Google Scholar] [CrossRef]
- Mohammadi, L.; Navabzadeh, M.; Jimenez-Tellez, N.; Han, D.D.; Reagan, E.; Naughton, J.; Zhou, L.Y.; Almeida, R.; Castaneda, L.M.; Abdelaal, S.A.; et al. Association of Endothelial Dysfunction With Chronic Marijuana Smoking and THC-Edible Use. JAMA Cardiol. 2025, 10, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Villalobos, M.; Guevara, Y.; Mitchell, J.; Ryskamp, D.; Conner, J.; Bush, M.; Periel, L.; Uribe, A.; Weaver, T.E. Potential perioperative cardiovascular outcomes in cannabis/cannabinoid users. A call for caution. Front. Cardiovasc. Med. 2024, 11, 1343549. [Google Scholar] [CrossRef]
- Storck, W.; Elbaz, M.; Vindis, C.; Deguilhem, A.; Lapeyre-Mestre, M.; Jouanjus, E. Cardiovascular risk associated with the use of cannabis and cannabinoids: A systematic review and meta-analysis. Heart 2025. [Google Scholar] [CrossRef]
- Glantz, S.; Silver, L. It is time to treat cannabis as an important risk factor for cardiovascular disease. Heart 2025. [Google Scholar] [CrossRef]
- Page Ii, R.L. Cannabis by any name does not smell as sweet: Potential cardiovascular events with medical cannabis. Eur. Heart J. 2024, 45, 485–487. [Google Scholar] [CrossRef]
- Culic, V. Cannabis use and cardiovascular diseases. Eur. Heart J. 2024, 45, 2573. [Google Scholar] [CrossRef]
- Bjune, T.; Risgaard, B.; Kruckow, L.; Glinge, C.; Ingemann-Hansen, O.; Leth, P.M.; Linnet, K.; Banner, J.; GregersWinkel, B.; Tfelt-Hansen, J. Post-mortem toxicology in young sudden cardiac death victims: A nationwide cohort study. Europace 2018, 20, 614–621. [Google Scholar] [CrossRef]
- Thomsen, L.R.; Rosengren, R.J.; Glass, M. Potential Implications of Multi-Drug Exposure with Synthetic Cannabinoids: A Scoping Review of Human Case Studies. Psychoactives 2024, 3, 365–383. [Google Scholar] [CrossRef]
- Marusich, J.A.; Gamage, T.F.; Zhang, Y.; Akinfiresoye, L.R.; Wiley, J.L. In Vitro and In Vivo pharmacology of nine novel synthetic cannabinoid receptor agonists. Pharmacol. Biochem. Behav. 2022, 220, 173467. [Google Scholar] [CrossRef] [PubMed]
- Tokarczyk, B.; Jurczyk, A.; Krupinska, J.; Adamowicz, P. Fatal intoxication with new synthetic cannabinoids 5F-MDMB-PICA and 4F-MDMB-BINACA-parent compounds and metabolite identification in blood, urine and cerebrospinal fluid. Forensic Sci. Med. Pathol. 2022, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Nielsen, J.L.; Risgaard, B.; van der Werf, C.; Winkel, B.G.; Tfelt-Hansen, J. Causes of sudden cardiac death according to age and sex in persons aged 1–49 years. Heart Rhythm 2023, 20, 61–68. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Y.; Shen, Z.; Jiang, L.; Zhang, X.; Wei, X.; Han, Z.; Liu, H.; Yang, T. Mapping the Knowledge of Antipsychotics-Induced Sudden Cardiac Death: A Scientometric Analysis in CiteSpace and VOSviewer. Front. Psychiatry 2022, 13, 925583. [Google Scholar] [CrossRef]
- Ripoll, T.; Garcia, A.B.; Gomila, I.; Heine, D.; Poncela, J.L.; Sanchez, N.; Perez, C.; Garcia, E.; Hernandez, E.; Barcelo, A.; et al. Post-mortem toxicology in the diagnosis of sudden death in young and middle-aged victims. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9135–9149. [Google Scholar] [CrossRef]
- Russo, E.B.; Whiteley, V.L. Cannabinoid hyperemesis syndrome: Genetic susceptibility to toxic exposure. Front. Toxicol. 2024, 6, 1465728. [Google Scholar] [CrossRef]
- Crowley, R.; Cline, K.; Hilden, D.; Beachy, M.; Health and Public Policy Committee of the American College of Physicians. Regulatory Framework for Cannabis: A Position Paper From the American College of Physicians. Ann. Intern. Med. 2024, 177, 1104–1105. [Google Scholar] [CrossRef]
- Paratz, E.D.; Rowsell, L.; Zentner, D.; Parsons, S.; Morgan, N.; Thompson, T.; James, P.; Pflaumer, A.; Semsarian, C.; Smith, K.; et al. Cardiac arrest and sudden cardiac death registries: A systematic review of global coverage. Open Heart 2020, 7, e001195. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.; Farcas, A.O.; Nyulas, V.; Radu, C.C.; Brinzaniuc, K. Sudden Cardiac Death-Etiology, Risk Factors and Demographic Characteristics: An Extensive Study of 1618 Forensic Autopsies. Diseases 2024, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Risgaard, B.; Banner, J.; Nielsen, J.L.; Jespersen, T.; Stampe, N.K.; Albert, C.M.; Winkel, B.G.; Tfelt-Hansen, J. Nationwide burden of sudden cardiac death: A study of 54,028 deaths in Denmark. Heart Rhythm 2021, 18, 1657–1665. [Google Scholar] [CrossRef]
- Arzamendi, D.; Benito, B.; Tizon-Marcos, H.; Flores, J.; Tanguay, J.F.; Ly, H.; Doucet, S.; Leduc, L.; Leung, T.K.; Campuzano, O.; et al. Increase in sudden death from coronary artery disease in young adults. Am. Heart J. 2011, 161, 574–580. [Google Scholar] [CrossRef]
- Lin, R.; Huang, Z.; Liu, Y.; Zhou, Y. Analysis of Personalized Cardiovascular Drug Therapy: From Monitoring Technologies to Data Integration and Future Perspectives. Biosensors 2025, 15, 191. [Google Scholar] [CrossRef]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing Precision Medicine: A Review of Innovative In Silico Approaches for Drug Development, Clinical Pharmacology and Personalized Healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, A.M.; Glantz, S.; Byers, A.L.; Keyhani, S. Association of Cannabis Use With Cardiovascular Outcomes Among US Adults. J. Am. Heart Assoc. 2024, 13, e030178. [Google Scholar] [CrossRef]
- Rock, K.L.; Englund, A.; Morley, S.; Rice, K.; Copeland, C.S. Can cannabis kill? Characteristics of deaths following cannabis use in England (1998–2020). J. Psychopharmacol. 2022, 36, 1362–1370. [Google Scholar] [CrossRef]
- Malta, G.; Albano, G.D.; Lavanco, G.; Brancato, A.; Cannizzaro, C.; Argo, A.; Contorno, S.; Plescia, F.; Zerbo, S. Acute cannabis intoxication among the paediatric population. Front. Toxicol. 2025, 7, 1558721. [Google Scholar] [CrossRef]
- Sampat, P.J.; Riaz, S.; Bisen, M.; Carhart, R. An Unusual Case of Ventricular Tachycardia in a Young Patient Associated with Cannabis Use. Case Rep. Cardiol. 2020, 2020, 8813930. [Google Scholar] [CrossRef] [PubMed]
- Xpress, M. Pooled Real-World Analysis of Cannabis Use and Cardiovascular Death; Medical Xpress: Fort Myers, FL, USA, 2025. [Google Scholar]
- Smith, A.B.; Lopez, C.; Nguyen, T. Meta-analysis of cannabis use and risk of myocardial infarction [Abstract LBA-XX]. In Proceedings of the American College of Cardiology: 74th Annual Scientific Session and Expo, Chicago, IL, USA, 29–31 March 2025. [Google Scholar]
- National Center for Biotechnology Information. Injury & Death. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 10 June 2025).
- Daldegan-Bueno, D.; Maia, L.O.; Glass, M.; Jutras-Aswad, D.; Fischer, B. Co-exposure of cannabinoids with amphetamines and biological, behavioural and health outcomes: A scoping review of animal and human studies. Psychopharmacology 2022, 239, 1211–1230. [Google Scholar] [CrossRef]
- Ceelen, M.; Dorn, T.; Buster, M.; Stomp, J.; Zweipfenning, P.; Das, K. Post-mortem toxicological urine screening in cause of death determination. Hum. Exp. Toxicol. 2011, 30, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Paloczi, J.; Gunduz-Cinar, O.; Yokus, B.; Paes-Leme, B.; Hasko, G.; Kunos, G.; Holmes, A.; Pacher, P. Exacerbated cardiac dysfunction from combined alcohol binge and synthetic cannabinoid use. Biomed. Pharmacother. 2025, 187, 118053. [Google Scholar] [CrossRef]
- Westin, A.A.; Frost, J.; Brede, W.R.; Gundersen, P.O.M.; Einvik, S.; Aarset, H.; Slordal, L. Sudden Cardiac Death Following Use of the Synthetic Cannabinoid MDMB-CHMICA. J. Anal. Toxicol. 2016, 40, 86–87. [Google Scholar] [CrossRef]
- Colombo, G.; Selimi, A.; Cesari, A.; Pedrotti, P.; Sacco, A. When coronary spasm strikes: A case report on the dreadful association between cannabinoids and caffeine. Eur. Heart J. Case Rep. 2025, 9, ytaf252. [Google Scholar] [CrossRef]
- Coll, M.; Fernandez-Falgueras, A.; Tiron, C.; Iglesias, A.; Buxo, M.; Simon, A.; Nogue-Navarro, L.; Moral, S.; Perez-Serra, A.; Puigmule, M.; et al. Post-mortem toxicology analysis in a young sudden cardiac death cohort. Forensic Sci. Int. Genet. 2022, 59, 102723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, X.; Sheng, C.; Cai, W.; Geng, X.; Liu, H.; Song, S. Effect of long-term use of antipsychotics on the ventricular repolarization index. BMC Psychiatry 2024, 24, 505. [Google Scholar] [CrossRef]
- Manolis, T.A.; Apostolopoulos, E.J.; Manolis, A.A.; Melita, H.; Manolis, A.S. The proarrhythmic conundrum of alcohol intake. Trends Cardiovasc. Med. 2022, 32, 237–245. [Google Scholar] [CrossRef]
- Piano, M.R.; Marcus, G.M.; Aycock, D.M.; Buckman, J.; Hwang, C.L.; Larsson, S.C.; Mukamal, K.J.; Roerecke, M.; on behalf the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; et al. Alcohol Use and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2025, 152, e7–e21. [Google Scholar] [CrossRef]
- Menshov, V.A.; Trofimov, A.V.; Zagurskaya, A.V.; Berdnikova, N.G.; Yablonskaya, O.I.; Platonova, A.G. Influence of Nicotine from Diverse Delivery Tools on the Autonomic Nervous and Hormonal Systems. Biomedicines 2022, 10, 121. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef]
- Caicedo, D.A.; Perez-Mana, C.; Farre, M.; Papaseit, E. An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans. Pharmaceutics 2025, 17, 319. [Google Scholar] [CrossRef]
- Radaelli, D.; Manfredi, A.; Zanon, M.; Fattorini, P.; Scopetti, M.; Neri, M.; Frisoni, P.; D’Errico, S. Synthetic Cannabinoids and Cathinones Cardiotoxicity: Facts and Perspectives. Curr. Neuropharmacol. 2021, 19, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Nánási, P.P.; Pueyo, E.; Virág, L. Perspectives of Antiarrhythmic Drug Therapy: Disappointing Past, Current Efforts, and Faint Hopes. Front. Pharmacol. 2020, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450; StatPearls Publishing: Tampa, FL, USA; St. Petersburg, FL, USA, 2025. [Google Scholar]
- Brunner, S.; Winter, R.; Werzer, C.; von Stulpnagel, L.; Clasen, I.; Hameder, A.; Stover, A.; Graw, M.; Bauer, A.; Sinner, M.F. Impact of acute ethanol intake on cardiac autonomic regulation. Sci. Rep. 2021, 11, 13255. [Google Scholar] [CrossRef]
- Göçen, H.B.; Özden, A.V. Autonomic dysfunction in psychiatric disorders. Psikiyatr. Güncel Yaklaşımlar 2024, 16, 401–409. [Google Scholar] [CrossRef]
- Taghdiri, A. Inflammation and arrhythmogenesis: A narrative review of the complex relationship. Int. J. Arrhythmia 2024, 25, 4. [Google Scholar] [CrossRef]
- Balan, A.I.; Halatiu, V.B.; Scridon, A. Oxidative Stress, Inflammation, and Mitochondrial Dysfunction: A Link between Obesity and Atrial Fibrillation. Antioxidants 2024, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Leslie, S.W. Autonomic dysreflexia. Urologiia 2025, 3, 75–80. [Google Scholar]
- de Oliveira Souza, L.B.; Sicoli, J.P.G.; Olalla Saad, S.T.; Benites, B.D. Modulation of the endocannabinoid system in chronic conditions: A potential therapeutic intervention yet to be explored in sickle cell disease. Expert Rev. Hematol. 2025, 18, 215–224. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Xiao, P. Associations between common ion channel single nucleotide polymorphisms and sudden cardiac death in adults: A MOOSE-compliant meta-analysis. Medicine 2018, 97, e12428. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Crotti, L.; George, A.L., Jr. Modifier genes for sudden cardiac death. Eur. Heart J. 2018, 39, 3925–3931. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Li, S.; Li, Q.; Meng, L.; Yang, J.; Liu, L.; Lv, C.; Zhang, P. The Role of RyR2 Mutations in Congenital Heart Diseases: Insights Into Cardiac Electrophysiological Mechanisms. J. Cardiovasc. Electrophysiol. 2025, 36, 683–692. [Google Scholar] [CrossRef]
- Ronchi, C.; Bernardi, J.; Mura, M.; Stefanello, M.; Badone, B.; Rocchetti, M.; Crotti, L.; Brink, P.; Schwartz, P.J.; Gnecchi, M.; et al. NOS1AP polymorphisms reduce NOS1 activity and interact with prolonged repolarization in arrhythmogenesis. Cardiovasc. Res. 2021, 117, 472–483. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Liao, H.; Xue, Y.; Zhan, X.; Fang, X.; Liang, Y.; Wei, W.; Rao, F.; Zhang, Q.; et al. Genetic Variants on SCN5A, KCNQ1, and KCNH2 in Patients with Ventricular Arrhythmias during Acute Myocardial Infarction in a Chinese Population. Cardiology 2020, 145, 38–45. [Google Scholar] [CrossRef]
- Sharma, V.; Bhardwaj, A.; Sahai, A.; Singh, S.; Shamim, S. Myocardial Infarction Triggered by Marijuana Use. JACC Case Rep. 2025, 30, 103202. [Google Scholar] [CrossRef]
- Abdullah, N.M.; Ali, A. RYR2 receptor gene mutation associated with catecholaminergic polymorphic ventricular tachycardia in children: A case report & literature review. Transl. Pediatr. 2024, 13, 359–369. [Google Scholar] [CrossRef]
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular Complications of Marijuana and Related Substances: A Review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.A.; de Castro, L.R.; Bortolin, R.H.; Bastos, G.M.; de Oliveira, V.F.; Ferreira, G.M.; Hirata, T.D.C.; Fajardo, C.M.; Sampaio, M.F.; Moreira, D.A.R.; et al. Association of variants in and with sudden cardiac death-related risk factors in Brazilian patients with hypertrophic cardiomyopathy. Forensic Sci. Int. Genet. 2021, 52, 102478. [Google Scholar] [CrossRef] [PubMed]
- Hylind, R.J.; Pereira, A.C.; Quiat, D.; Chandler, S.F.; Roston, T.M.; Pu, W.T.; Bezzerides, V.J.; Seidman, J.G.; Seidman, C.E.; Abrams, D.J. Population Prevalence of Premature Truncating Variants in Plakophilin-2 and Association with Arrhythmogenic Right Ventricular Cardiomyopathy: A UK Biobank Analysis. Circ. Genom. Precis. Med. 2022, 15, e003507. [Google Scholar] [CrossRef] [PubMed]
- Association, A.H. Marijuana Use Linked with Increased Risk of Heart Attack, Heart Failure. Available online: https://newsroom.heart.org/news/marijuana-use-linked-with-increased-risk-of-heart-attack-heart-failure (accessed on 21 August 2025).
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.; Alencar, A.K.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazaleh, A.K.; Zhou, X.; Bhuyan, D.J.; Münch, G.W.; Al-Dalabeeh, E.A.; Jaye, K.; Chang, D. The Neurotherapeutic Arsenal in Cannabis sativa: Insights into Anti-Neuroinflammatory and Neuroprotective Activity and Potential Entourage Effects. Molecules 2024, 29, 410. [Google Scholar] [CrossRef]
- Zamarripa, C.A.; Spindle, T.R.; Surujunarain, R.; Weerts, E.M.; Bansal, S.; Unadkat, J.D.; Paine, M.F.; Vandrey, R. Assessment of Orally Administered Delta9-Tetrahydrocannabinol When Coadministered With Cannabidiol on Delta9-Tetrahydrocannabinol Pharmacokinetics and Pharmacodynamics in Healthy Adults: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2254752. [Google Scholar] [CrossRef]
- Fu, M.; Valiente-Banuet, L.; Wadhwa, S.S.; Pasaniuc, B.; Vossel, K.; Chang, T.S. Improving genetic risk modeling of dementia from real-world data in underrepresented populations. Commun. Biol. 2024, 7, 1049. [Google Scholar] [CrossRef]
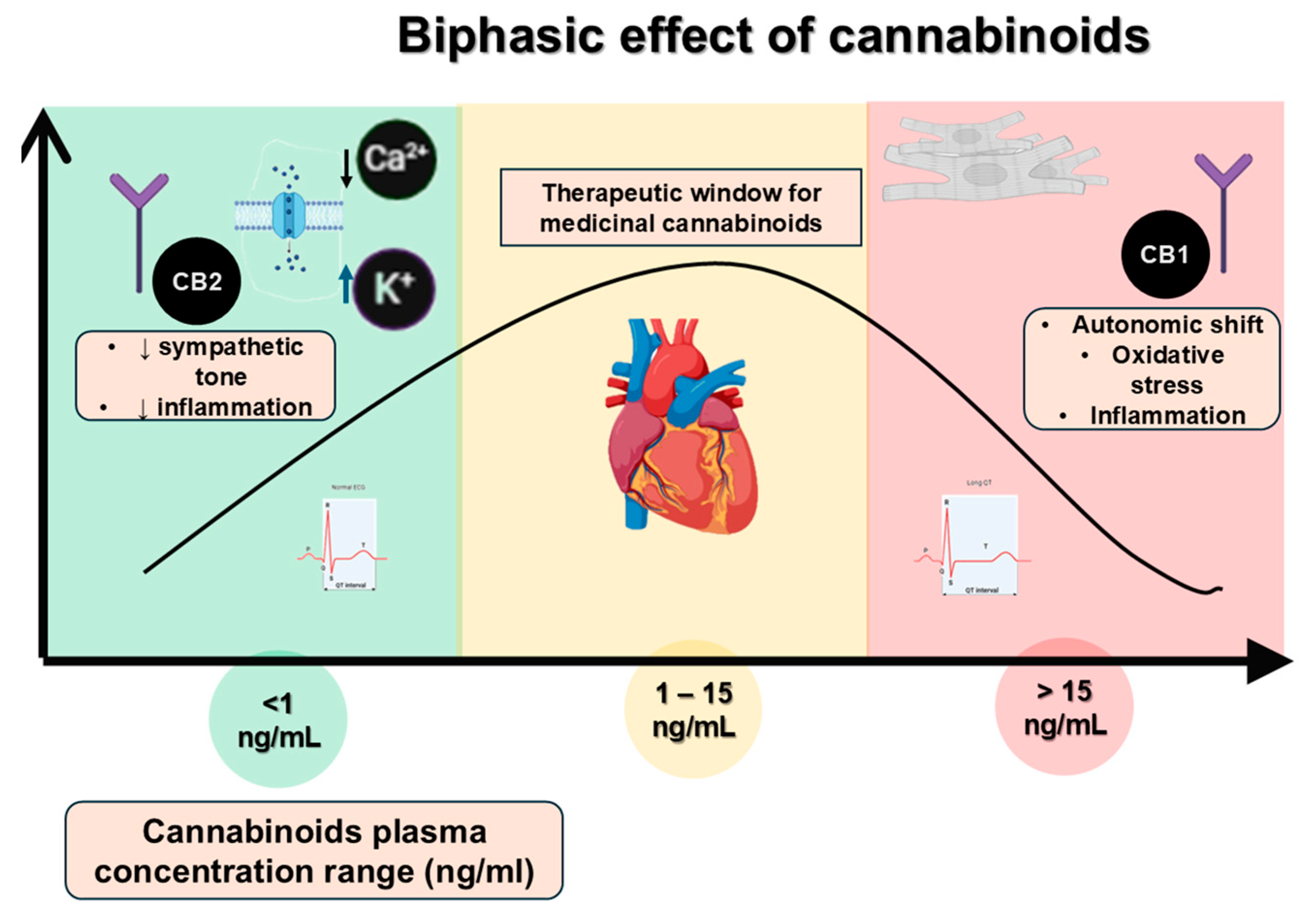
| Cannabinoid | Plasma Concentration Range (ng/mL) | Observed Effects | References |
|---|---|---|---|
| Δ9-THC (low dose) | 1–5 | Sedation, anti-inflammatory benefits | [24] |
| Δ9-THC (moderate dose) | 5–15 | Mild tachycardia, autonomic fluctuation | [25] |
| Δ9-THC (high dose) | >15 | Marked tachycardia, hypertension, autonomic instability | [26] |
| Synthetic cannabinoids (low dose) | <1 | Anxiety, mild autonomic symptoms | [27] |
| Synthetic cannabinoids (high dose) | ≥1 | Severe autonomic dysregulation, malignant arrhythmias | [27] |
| Endocannabinoids (low dose) | <2 | Homeostatic regulation, mild anti-inflammatory effects | [9] |
| Endocannabinoids (high dose) | ≥2 | Dysregulation of autonomic tone, pro-arrhythmic potential | [8] |
| Study/Source | Design | n (Approx.) | Outcome | Effect Size | Citation |
|---|---|---|---|---|---|
| Injury and Death—NCBI case report | Case report | 1 | SCD | Anecdotal | [64] |
| Storck et al. (Heart, 2025) | Systematic review + meta-analysis | 200 million | Cardiovascular death | RR 2.10 (1.29–3.42) | [39] |
| Medical Xpress pooled analysis (June 2025) | Pooled epidemiology | 200 million | Cardiovascular death | 2× risk | [62] |
| TriNetX retrospective (ACC.25) | Cohort, follow-up 3 y | 4.6 million | Composite CV death/MI/stroke | 3× risk | [26] |
| ACC meta-analysis (12 studies) | Meta-analysis | 75 million | Myocardial infarction (heart attack) | OR 1.50 | [63] |
| Gene | SNP ID | Effect Size (Odds Ratio, OR) | Variant Type | High-Frequency Regions | Notes |
|---|---|---|---|---|---|
| SCN5A | rs11720524 | 0.76 | Common and rare SNPs | East Asia, Europe, South Asia | Associated with reduced SCD risk in Europeans [18,86] |
| KCNQ1 | rs2283222 | 0.73 | SNPs and deletions | Japan, Korea, Northern Europe | Linked to QT interval modulation and lower SCD risk [18,86] |
| KCNQ1 | rs12296050 | 0.85 | SNPs and missense | Europe, North America | Protective effect observed in Koreans and Americans [18,86] |
| RYR2 | rs790896 | 0.66 | Missense mutations | Japan, Italy, USA | Associated with reduced risk of catecholaminergic polymorphic VT [18,86] |
| NOS1AP | rs16847548 | 1.28 | Common SNPs | Europe, North America | Increases QT interval and risk of cardiac events, including SCD [18,87] |
| Component | Description | References |
|---|---|---|
| Genetic Markers | Single-nucleotide polymorphisms linked to cardiac arrhythmias (e.g., SCN5A, RYR2, KCNQ1, KCNH2, and NOS1AP) | [18,36,88,89,90] |
| Epigenetic Modifiers | DNA methylation changes triggered by cannabis or alcohol exposure | [28,34,67,76] |
| Cannabis Use Profile | Frequency, duration, type (THC/CBD ratio), age of initiation | [26,30,39,58] |
| Family History | Sudden death, inherited cardiac diseases, substance use disorders | [6,7,36,70] |
| Lifestyle Factors | Alcohol use, physical activity, stress, sleep quality | [71,72,74,82,83] |
| Heart Health Parameters | ECG abnormalities, QT interval, previous arrhythmia episodes | [5,14,35,91] |
| Interaction | Synergistic risk from substance interactions (e.g., alcohol + cannabinoids) | [19,44,66,67,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I. A Meta-Narrative Review of Channelopathies and Cannabis: Mechanistic, Epidemiologic, and Forensic Insights into Arrhythmia and Sudden Cardiac Death. Int. J. Mol. Sci. 2025, 26, 8635. https://doi.org/10.3390/ijms26178635
Šoša I. A Meta-Narrative Review of Channelopathies and Cannabis: Mechanistic, Epidemiologic, and Forensic Insights into Arrhythmia and Sudden Cardiac Death. International Journal of Molecular Sciences. 2025; 26(17):8635. https://doi.org/10.3390/ijms26178635
Chicago/Turabian StyleŠoša, Ivan. 2025. "A Meta-Narrative Review of Channelopathies and Cannabis: Mechanistic, Epidemiologic, and Forensic Insights into Arrhythmia and Sudden Cardiac Death" International Journal of Molecular Sciences 26, no. 17: 8635. https://doi.org/10.3390/ijms26178635
APA StyleŠoša, I. (2025). A Meta-Narrative Review of Channelopathies and Cannabis: Mechanistic, Epidemiologic, and Forensic Insights into Arrhythmia and Sudden Cardiac Death. International Journal of Molecular Sciences, 26(17), 8635. https://doi.org/10.3390/ijms26178635






