Hepatic Histopathological Benefit, Microbial Cost: Oral Vancomycin Mitigates Non-Alcoholic Fatty Liver Disease While Disrupting the Cecal Microbiota
Abstract
1. Introduction
2. Results
2.1. Body Weight
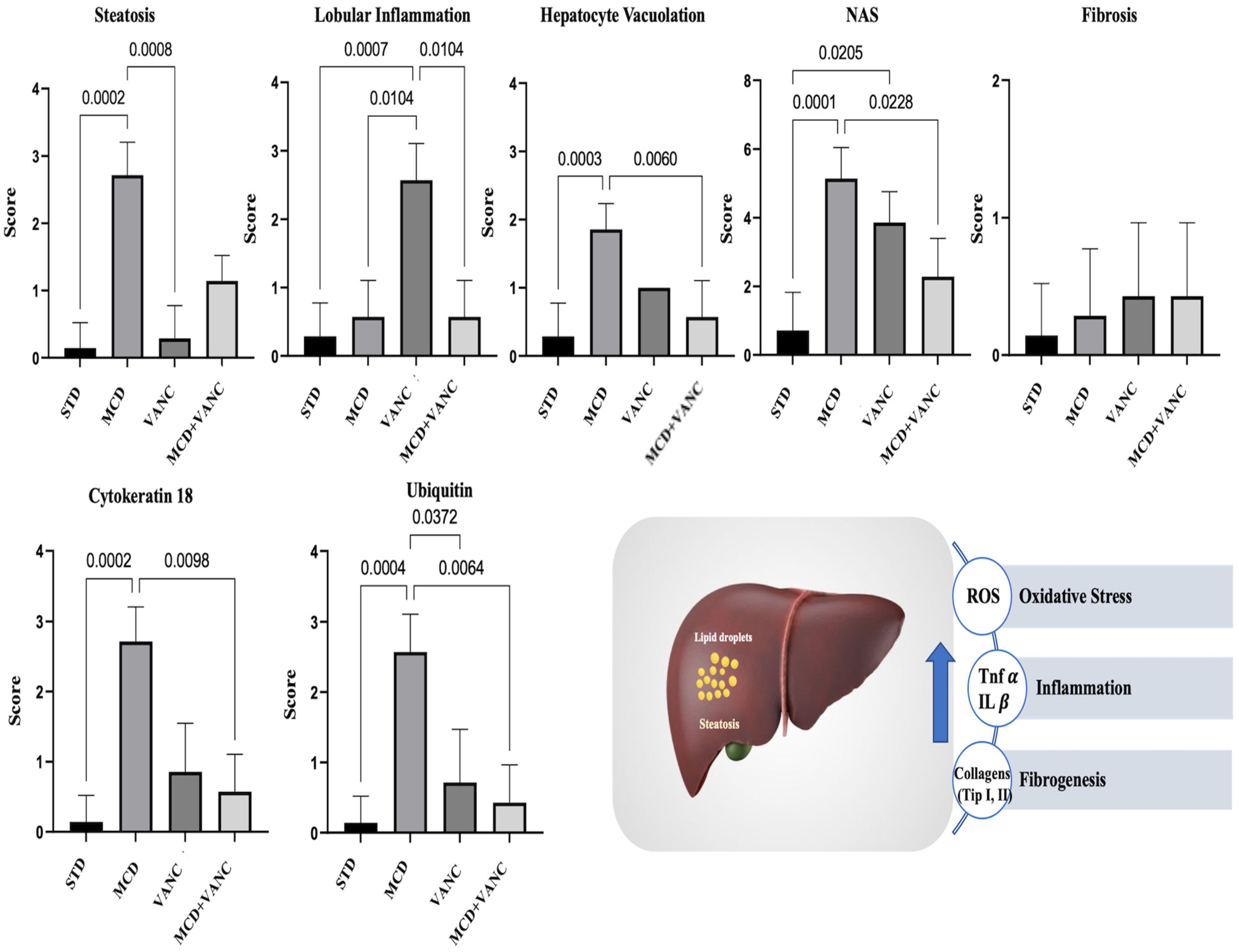
2.2. Liver Histological Findings
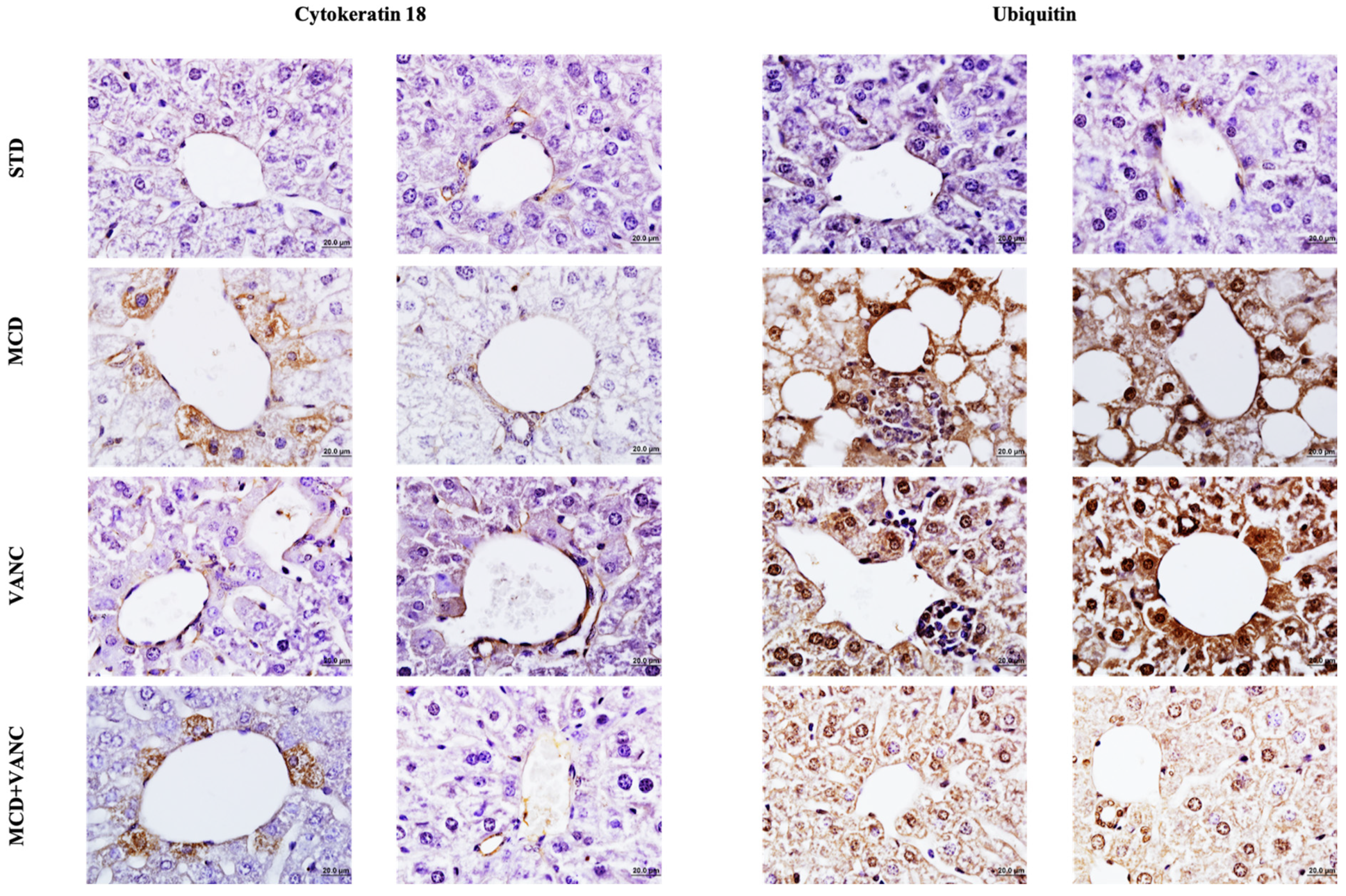
2.3. Immunohistochemistry
2.4. Hepatic Cytokine Findings
2.5. Microbiota Analysis
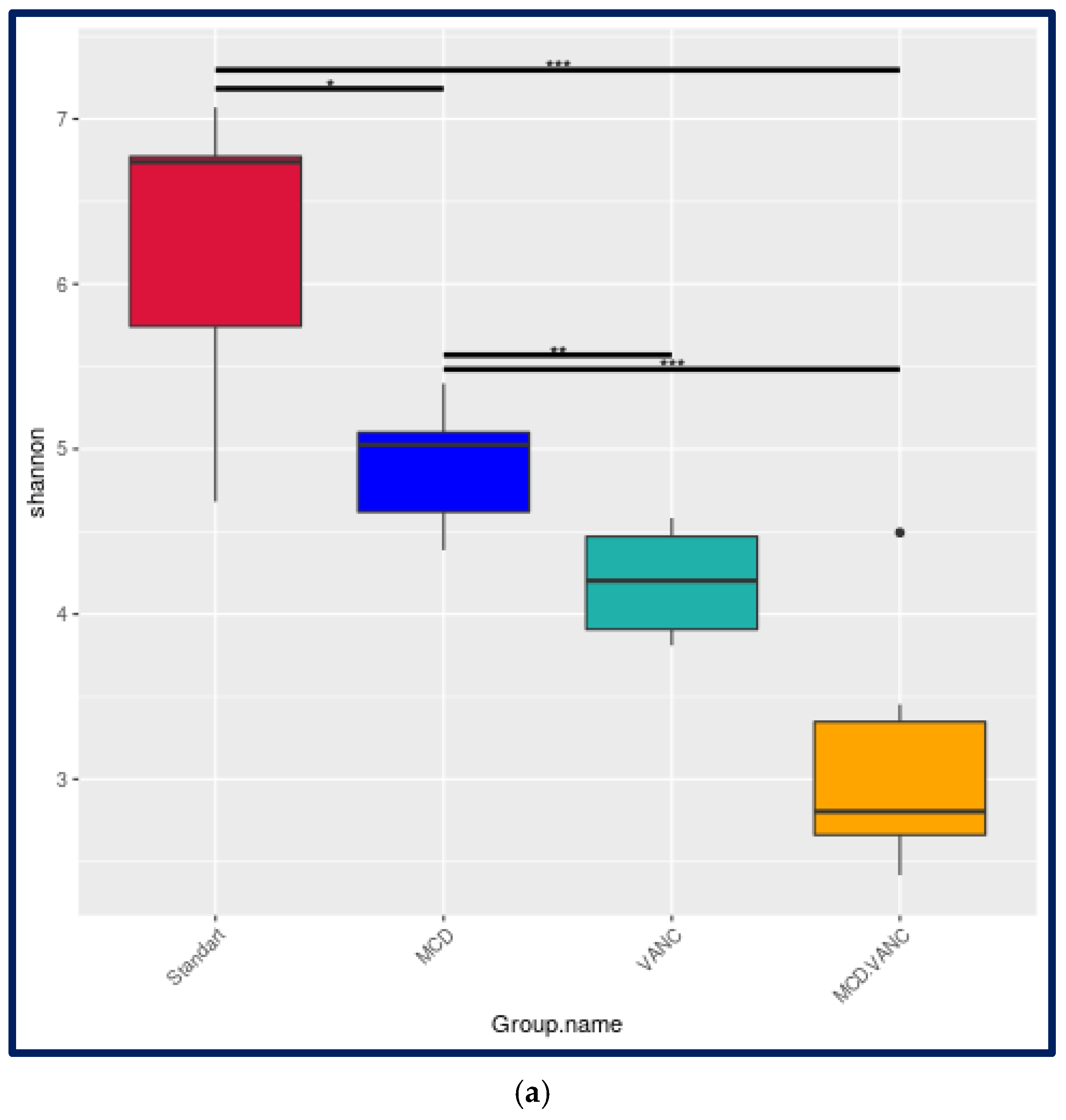
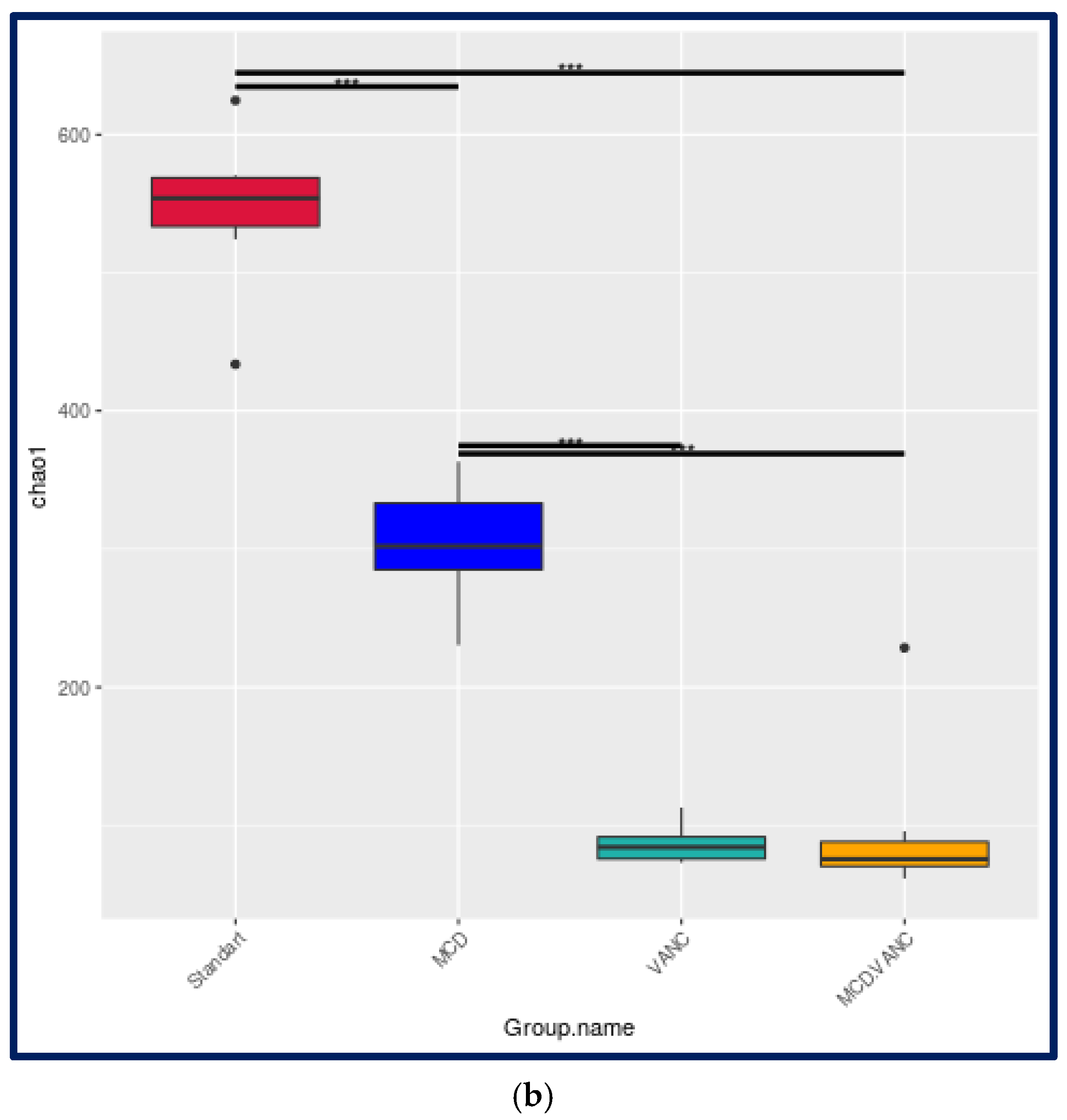
2.5.1. Alpha-Diversity
2.5.2. Beta-Diversity
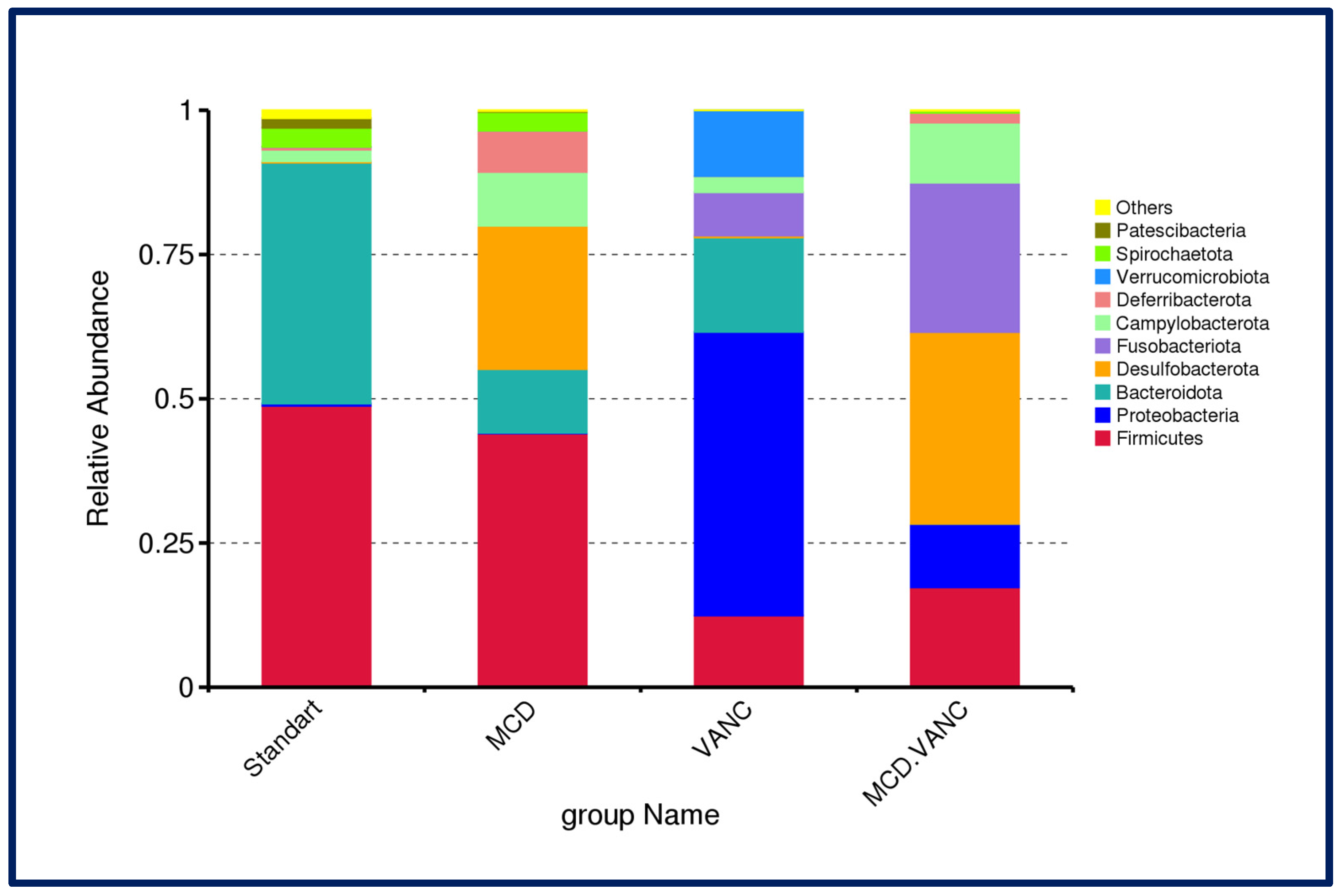
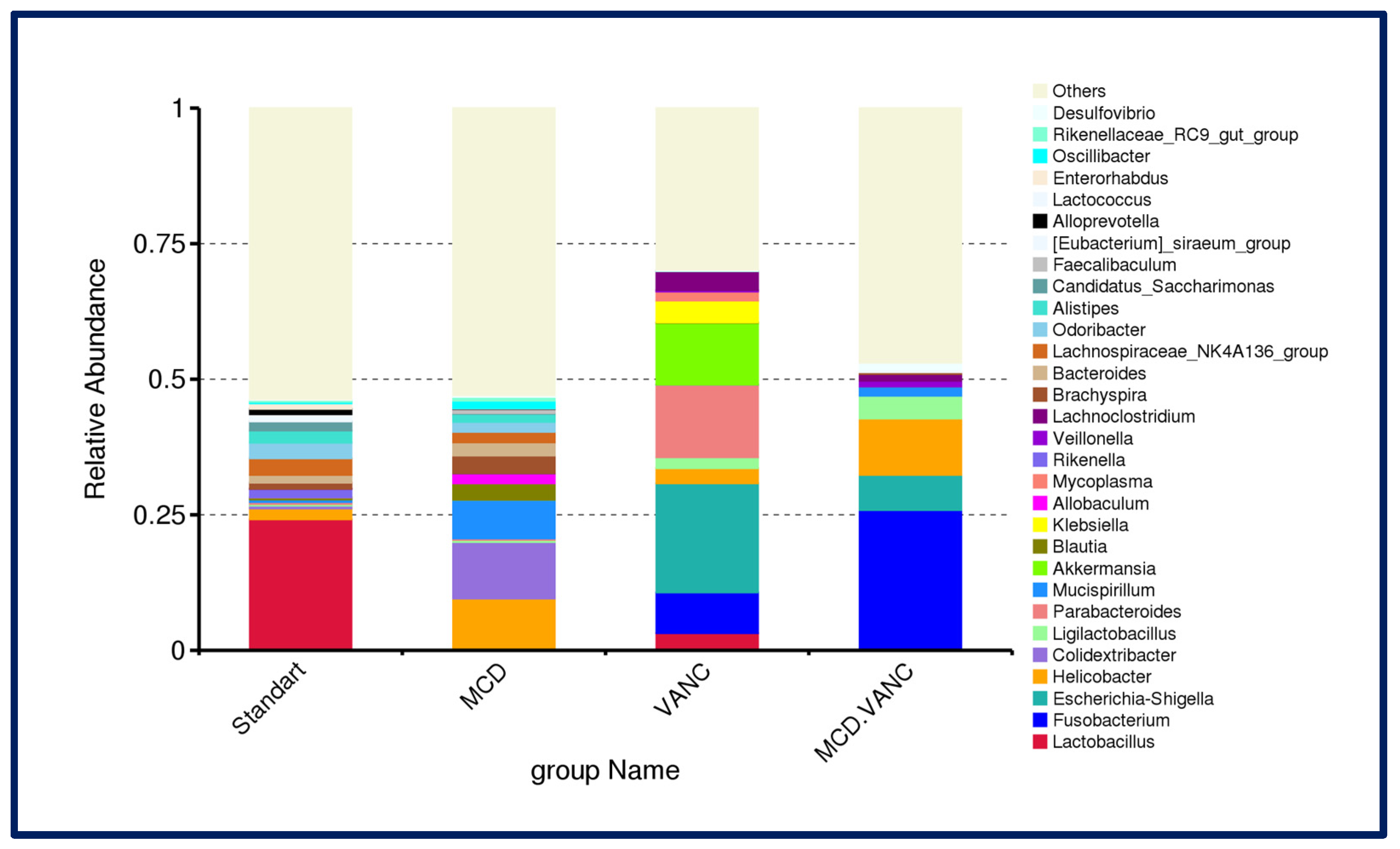
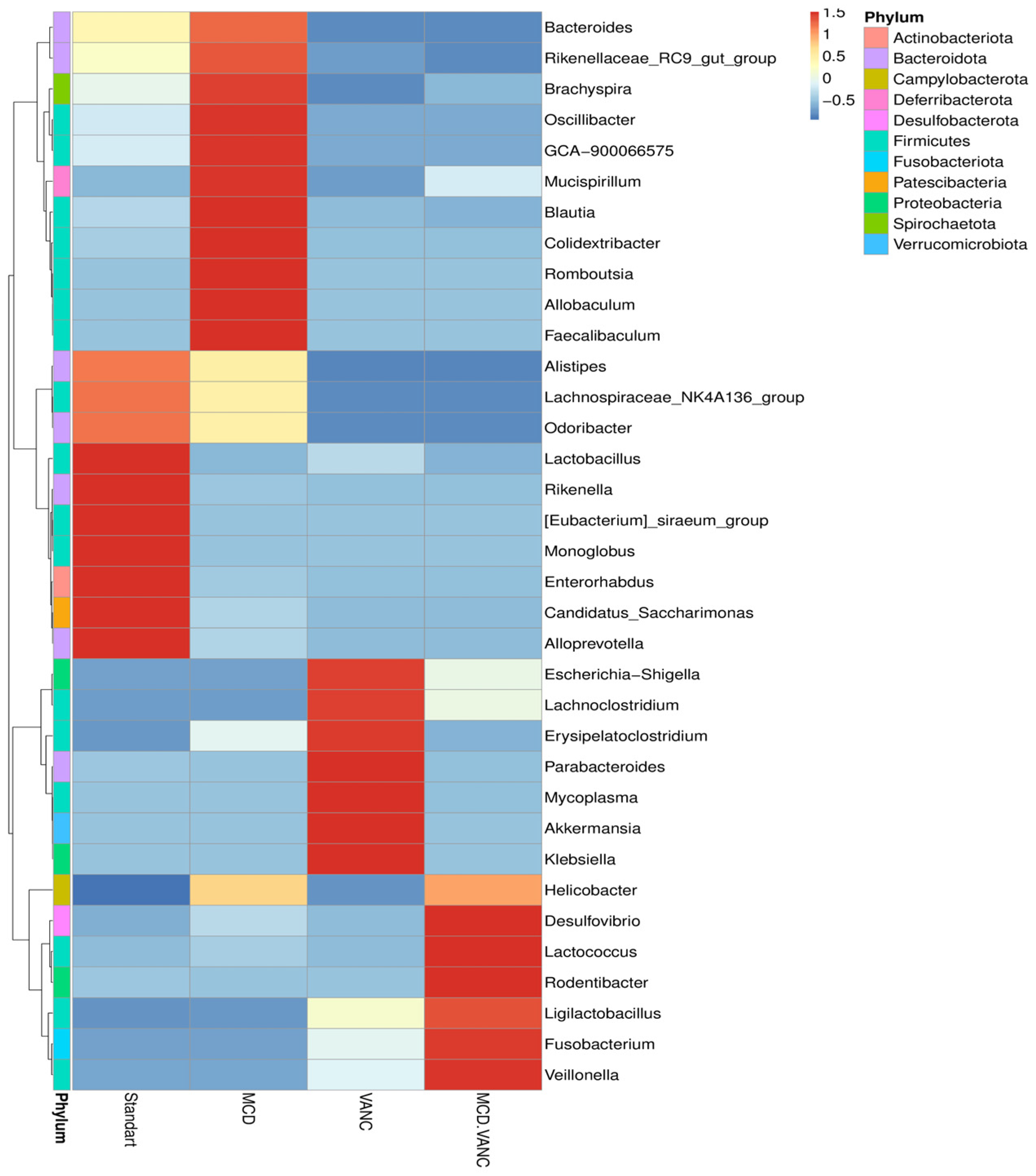
2.5.3. Cecal Microbiota Composition
2.5.4. Differential Abundance Snapshot
- STD vs. MCD diet
- STD vs. VANC
- STD vs. MCD + VANC
- MCD vs. VANC
- MCD vs. MCD + VANC
- VANC vs. MCD + VANC
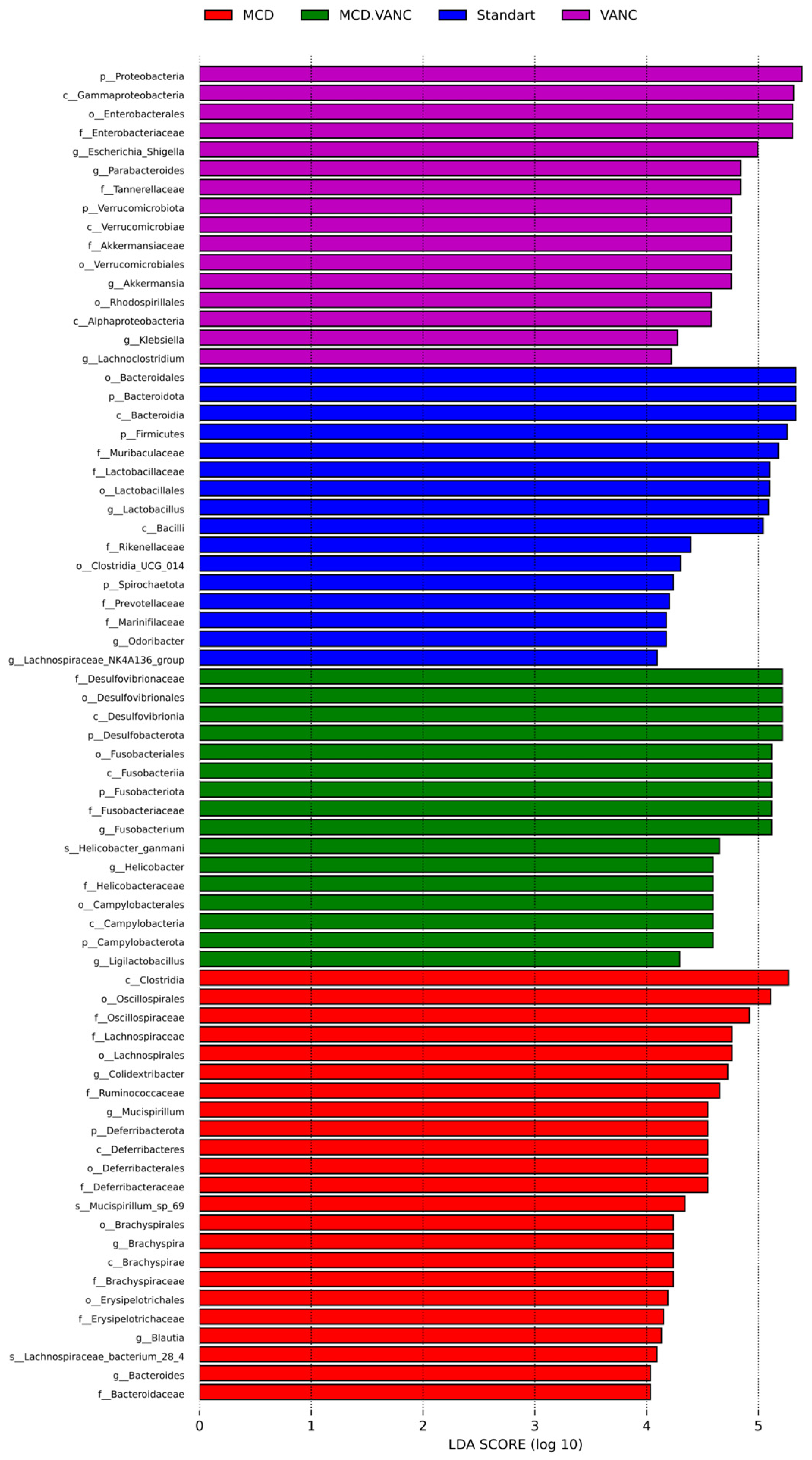
2.5.5. LEfSe Multiclass Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups and Numbers
- STD group (n = 7): Mice were fed a standard diet (STD) and water and administered 1 mL of serum physiological (SP) via oral gavage.
- MCD group (n = 7): Mice were fed a methionine–choline-deficient (MCD) diet and water and administered 1 mL of SP via oral gavage.
- VANC group (n = 7): Mice were fed a standard diet and water and administered 1 mL of vancomycin (VANC) via oral gavage. Vancomycin was administered at a dose of 2 mg/mouse every three days.
- MCD + VANC group (n = 7): Mice were fed an MCD diet and water and administered 1 mL of vancomycin via oral gavage. Vancomycin was administered at a dose of 2 mg/mouse every three days.
4.3. Diet
4.4. Vancomycin Dosing Rationale and Use
4.5. Sample Collection
4.6. Histochemical Evaluation
4.7. Cytokine Analyses
4.8. Microbiota Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 85–89. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Chen, L.; Zhao, Z.; Du, S.; Dong, Q.; Xin, Y.; Xuan, S. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Exp. Ther. Med. 2019, 18, 1935–1944. [Google Scholar] [CrossRef]
- Mouzaki, M.; Bandsma, R. Targeting the Gut Microbiota for the Treatment of Non-Alcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1324–1331. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Halabitska, I.; Petakh, P.; Kunduzova, O.; Oksenych, V.; Kamyshnyi, O. Unlocking the gut-liver axis: Microbial contributions to the pathogenesis of metabolic-associated fatty liver disease. Front. Microbiol. 2025, 16, 1577724. [Google Scholar] [CrossRef]
- Lee, H.S. Nonalcoholic fatty liver disease in children and adolescents. Clin. Exp. Pediatr. 2024, 67, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zou, J.; Ran, W.; Qi, X.; Chen, Y.; Cui, H.; Guo, J. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 2023, 13, 1087260. [Google Scholar] [CrossRef]
- Abarbanel, D.N.; Seki, S.M.; Davies, Y.; Marlen, N.; Benavides, J.A.; Cox, K.; Nadeau, K.C.; Cox, K.L. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J. Clin. Immunol. 2013, 33, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Tulone, A.; Nicastro, E.; Norsa, L.; Sonzogni, A.; D’Antiga, L. Use of oral vancomycin in children with autoimmune liver disease: A single centre experience. World J. Hepatol. 2021, 13, 2113–2127. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.; Xu, H.; Wenping; Fang, M.; Xu, Y. Hepatocyte-specific deletion of Brg1 alleviates methionine-and-choline-deficient diet (MCD) induced non-alcoholic steatohepatitis in mice. Biochem. Biophys. Res. Commun. 2018, 503, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Li, H.; Zhou, J.; Li, Q.; Glaser, S.; Francis, H.; Alpini, G.; Wu, C. Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis Is Associated with Increased Intestinal Inflammation. Am. J. Pathol. 2021, 191, 1743–1753. [Google Scholar] [CrossRef]
- Korver, S.; Bowen, J.; Pearson, K.; Gonzalez, R.J.; French, N.; Park, K.; Jenkins, R.; Goldring, C. The application of cytokeratin-18 as a biomarker for drug-induced liver injury. Arch. Toxicol. 2021, 95, 3435–3448. [Google Scholar] [CrossRef]
- Banner, B.F.; Savas, L.; Zivny, J.; Tortorelli, K.; Bonkovsky, H.L. Ubiquitin as a marker of cell injury in nonalcoholic steatohepatitis. Am. J. Clin. Pathol. 2000, 114, 860–866. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Zhang, J.; Li, H. Cytokeratin 18 in nonalcoholic fatty liver disease: Value and application. Expert Rev. Mol. Diagn. 2024, 24, 1009–1022. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, M.S.; Jung, H.R.; Hwang, M.S.; Lee, C.H.; Han, D.H.; Lee, Y.H.; Yi, E.C.; Im, S.S.; Hwang, I.; et al. Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Exp. Mol. Med. 2023, 55, 1520–1530. [Google Scholar] [CrossRef]
- Rafaqat, S.; Gluscevic, S.; Mercantepe, F.; Rafaqat, S.; Klisic, A. Interleukins: Pathogenesis in Non-Alcoholic Fatty Liver Disease. Metabolites 2024, 14, 153. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Li, H.; Niu, Q.; Tao, Y.; Zhao, X.; Zeng, Z.; Dong, H. The role of the interleukin family in liver fibrosis. Front Immunol. 2025, 16, 1497095. [Google Scholar] [CrossRef]
- Kasai, K.; Igarashi, N.; Tada, Y.; Kani, K.; Takano, S.; Yanagibashi, T.; Usui-Kawanishi, F.; Fujisaka, S.; Watanabe, S.; Ichimura-Shimizu, M.; et al. Impact of Vancomycin Treatment and Gut Microbiota on Bile Acid Metabolism and the Development of Non-Alcoholic Steatohepatitis in Mice. Int. J. Mol. Sci. 2023, 24, 4050. [Google Scholar] [CrossRef]
- Takano, S.; Kani, K.; Kasai, K.; Igarashi, N.; Kato, M.; Goto, K.; Matsuura, Y.; Ichimura-Shimizu, M.; Watanabe, S.; Tsuneyama, K.; et al. Antibiotic effects on gut microbiota modulate diet-induced metabolic dysfunction-associated steatohepatitis development in C57BL/6 mice. Genes Cells 2024, 29, 635–649. [Google Scholar] [CrossRef]
- Schneider, K.M.; Mohs, A.; Kilic, K.; Candels, L.S.; Elfers, C.; Bennek, E.; Schneider, L.B.; Heymann, F.; Gassler, N.; Penders, J.; et al. Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis. Int. J. Mol. Sci. 2019, 20, 308. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Briner Crawley, A.; Theriot, C.M.; Barrangou, R. The Lactobacillus Bile Salt Hydrolase Repertoire Reveals Niche-Specific Adaptation. mSphere 2018, 3, e00140-18. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Chen, X.; Katchar, K.; Goldsmith, J.D.; Nanthakumar, N.; Cheknis, A.; Gerding, D.N.; Kelly, C.P. A mouse model of Clostridium difficile-associated disease. Gastroenterology 2008, 135, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.K.; Gayer, C.P.; Roberts, A.; Golden, J.M.; Aladegbami, B.G.; Guo, J.; Erwin, C.R.; Warner, B.W. Liver steatosis induced by small bowel resection is prevented by oral vancomycin. Surgery 2016, 160, 1485–1495. [Google Scholar] [CrossRef][Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]




| Group | IL-6 (pg/mg) | IL-8 (pg/mg) | TGF-β1 (ng/mg) |
|---|---|---|---|
| STD | 20.6 ± 1.3 | 2833 ± 544 | 2973 ± 632 |
| MCD | 15.8 ± 0.9 | 1660 ± 404 | 2089 ± 523 |
| VANC | 17.8 ± 0.76 | 1953 ± 384 | 2114 ± 334 |
| MCD + VANC | 17.9 ± 2.6 | 999 ± 124 * | 1045 ± 95.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çirkin, G.; Aydemir, S.; Açıkgöz, B.; Çelik, A.; Güler, Y.; Kiray, M.; Baykara, B.; Dinleyici, E.Ç.; Öztürk, Y. Hepatic Histopathological Benefit, Microbial Cost: Oral Vancomycin Mitigates Non-Alcoholic Fatty Liver Disease While Disrupting the Cecal Microbiota. Int. J. Mol. Sci. 2025, 26, 8616. https://doi.org/10.3390/ijms26178616
Çirkin G, Aydemir S, Açıkgöz B, Çelik A, Güler Y, Kiray M, Baykara B, Dinleyici EÇ, Öztürk Y. Hepatic Histopathological Benefit, Microbial Cost: Oral Vancomycin Mitigates Non-Alcoholic Fatty Liver Disease While Disrupting the Cecal Microbiota. International Journal of Molecular Sciences. 2025; 26(17):8616. https://doi.org/10.3390/ijms26178616
Chicago/Turabian StyleÇirkin, Gül, Selma Aydemir, Burcu Açıkgöz, Aslı Çelik, Yunus Güler, Müge Kiray, Başak Baykara, Ener Çağrı Dinleyici, and Yeşim Öztürk. 2025. "Hepatic Histopathological Benefit, Microbial Cost: Oral Vancomycin Mitigates Non-Alcoholic Fatty Liver Disease While Disrupting the Cecal Microbiota" International Journal of Molecular Sciences 26, no. 17: 8616. https://doi.org/10.3390/ijms26178616
APA StyleÇirkin, G., Aydemir, S., Açıkgöz, B., Çelik, A., Güler, Y., Kiray, M., Baykara, B., Dinleyici, E. Ç., & Öztürk, Y. (2025). Hepatic Histopathological Benefit, Microbial Cost: Oral Vancomycin Mitigates Non-Alcoholic Fatty Liver Disease While Disrupting the Cecal Microbiota. International Journal of Molecular Sciences, 26(17), 8616. https://doi.org/10.3390/ijms26178616







