QMR® and Patient Blood-Derived Secretome Modulate RPE microRNA Networks Under Oxidative Stress
Abstract
1. Introduction
- Define the time-resolved miRNA signature induced by QMR®, PDB secretome, and their combination.
- Integrate differential expression profiles with validated and in silico-predicted miRNA targetomes to reconstruct regulatory networks.
- Identify the signaling pathways and biological processes most susceptible to QMR®-mediated modulation, thereby elucidating the mechanistic bases of the observed cytoprotection.
2. Results
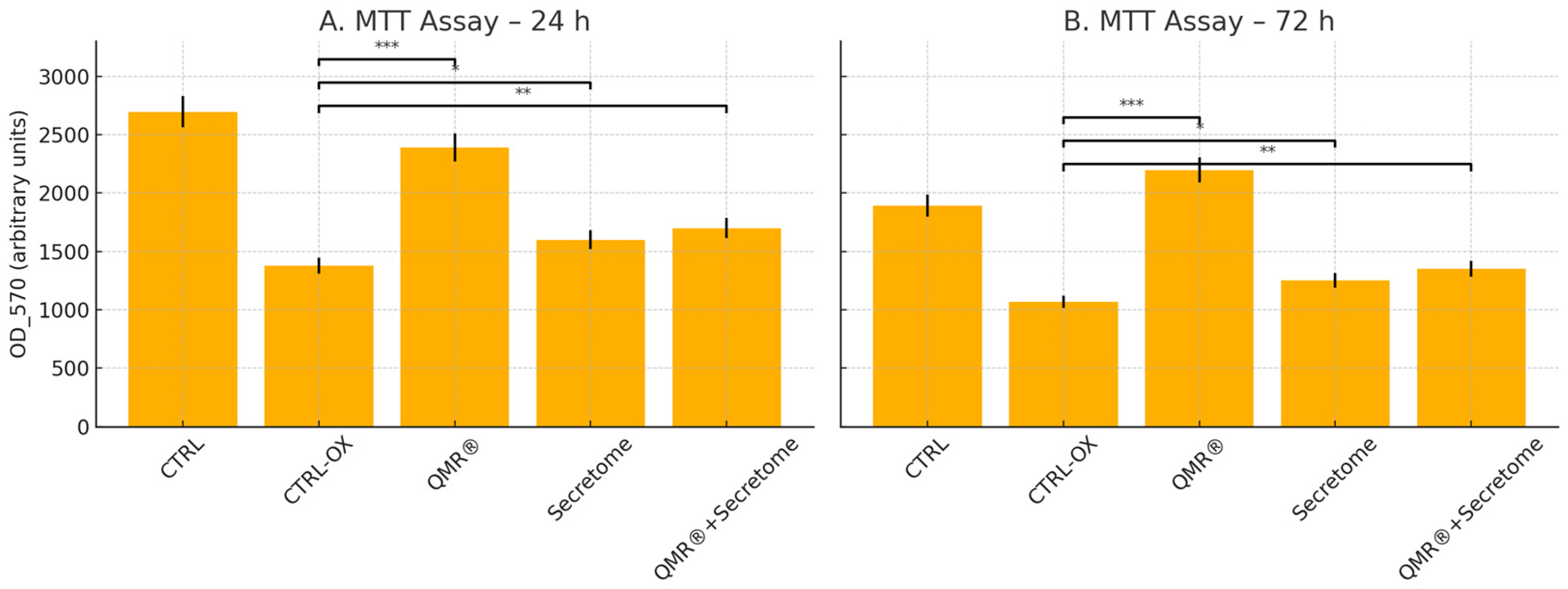
2.1. MTT Cell Viability Assay Reveals QMR®-Mediated Cytoprotection Under Oxidative Stress
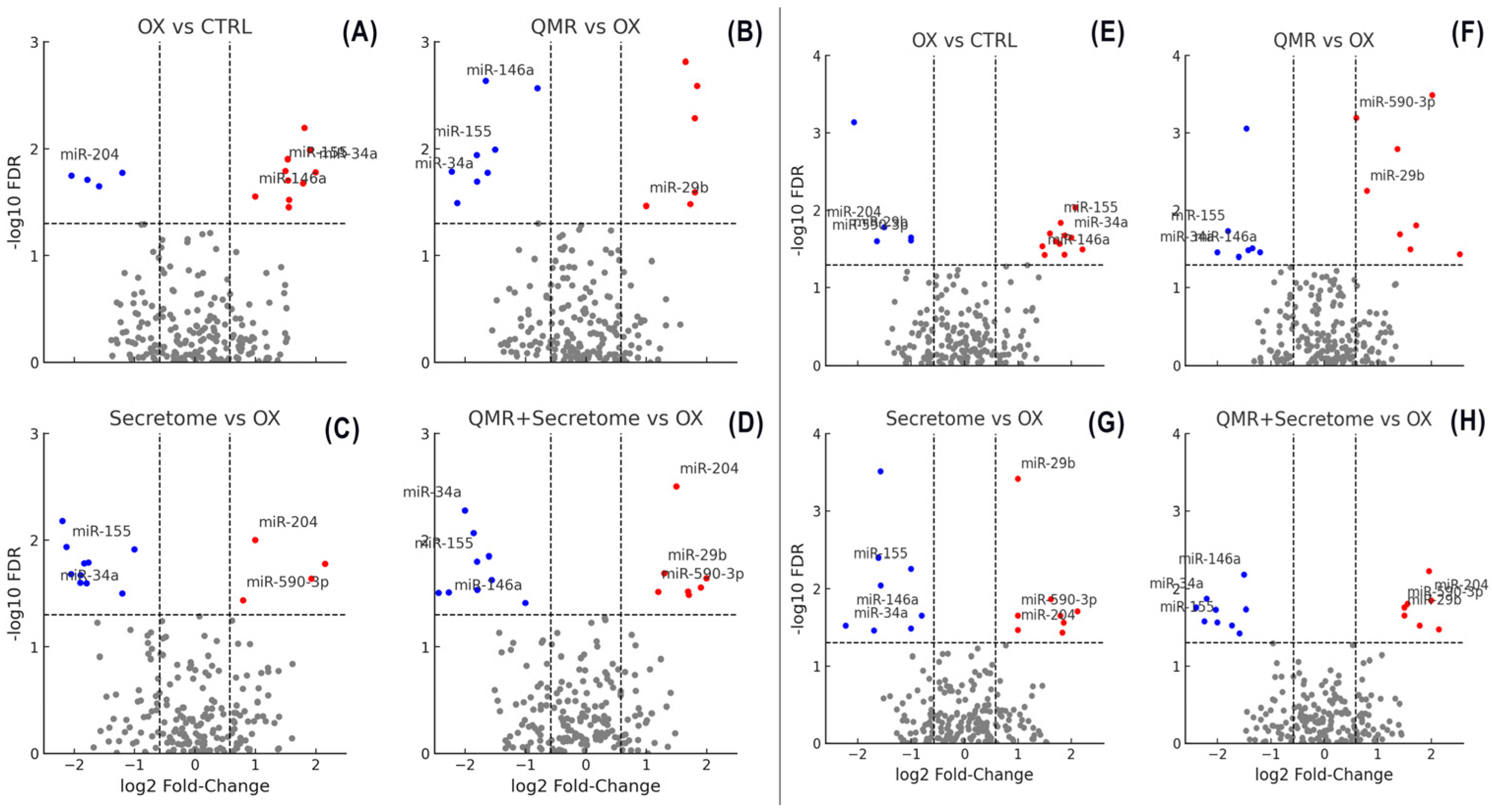
2.2. Overview of miRNA Responses to Oxidative Stress and Therapeutic Interventions
2.3. QMR® Treatment Modulates miRNAs in a Time-Dependent Manner
2.4. Secretome Treatment Elicits Overlapping and Distinct miRNA Changes
2.5. Combined QMR® + Secretome Treatment Amplifies the miRNA Response
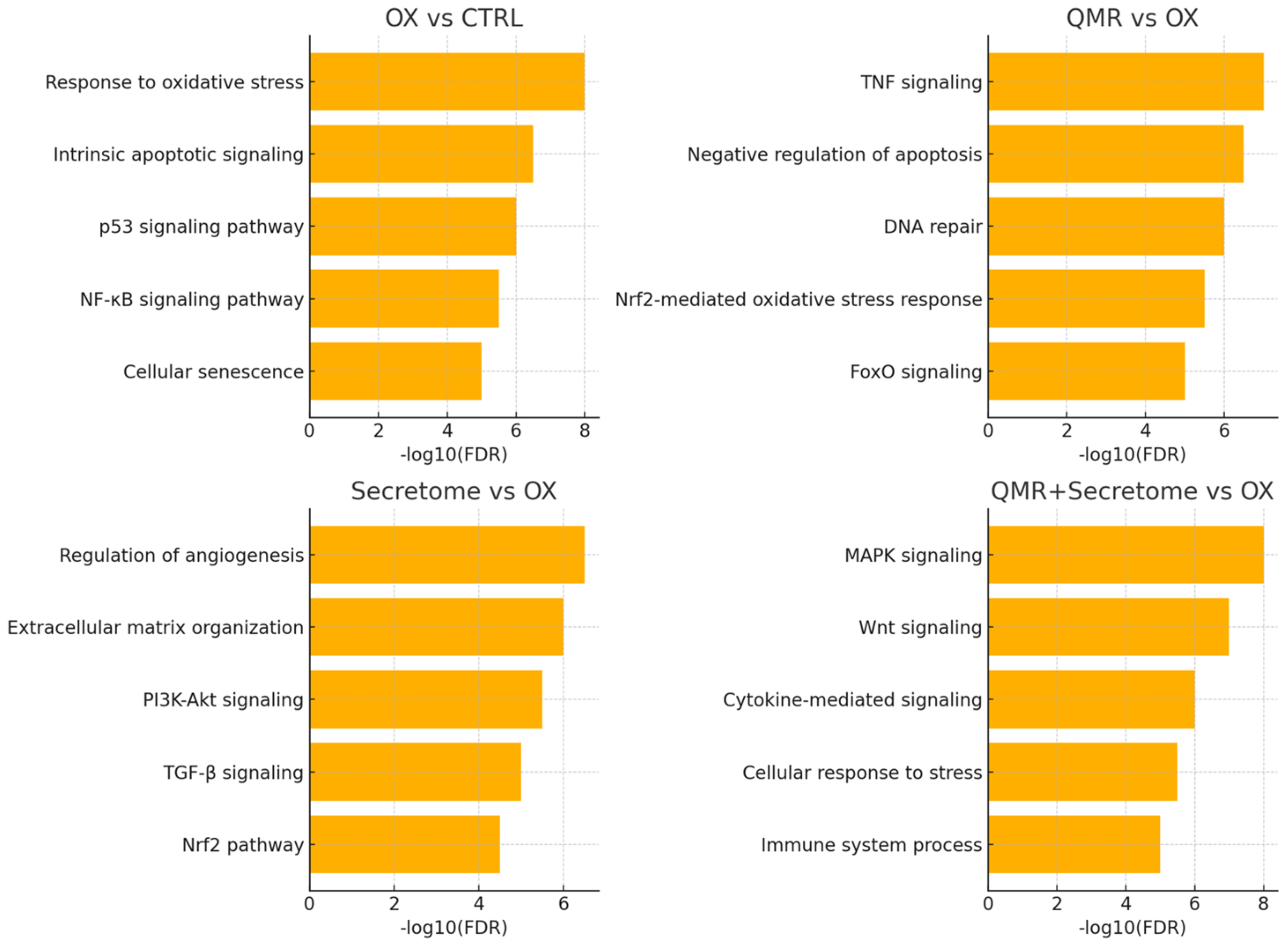
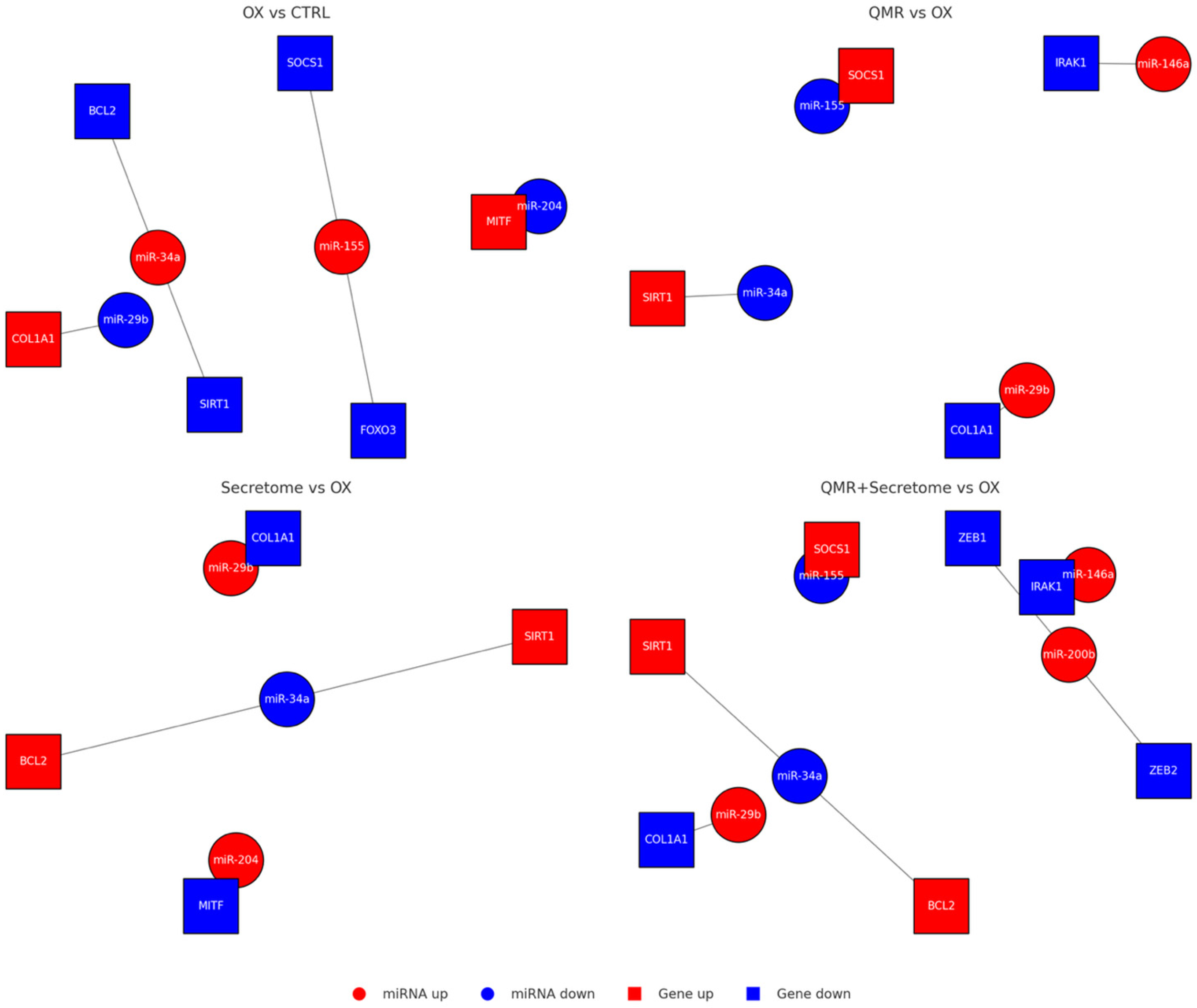
2.6. Predicted mRNA Targets and Affected Pathways
3. Discussion
3.1. QMR® Modulation of Redox-Regulatory miRNAs
3.2. Synergistic Effects of QMR® and PDB Secretome
3.3. Functional Impact on Oxidative Stress and Inflammation
3.4. Therapeutic Relevance and Translational Potential
3.5. Study Limitations and Future Directions
4. Materials and Methods
4.1. Cell Culture, Authentication, and Experimental Design
4.2. Patient Blood-Derived Secretome Preparation
4.3. QMR® Stimulation Setup
4.4. QMR® Treatment Protocol
4.5. Cell Viability Assay
4.6. RNA Extraction and Small RNA Library Preparation
4.7. Bioinformatic Analysis of Small RNA-Seq Data
4.8. Validation of miRNA Expression by RT-qPCR
4.9. miRNA Target Prediction and Functional Enrichment
4.10. miRNA–mRNA Network Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| miRNA | microRNA |
| QMR® | Quantum Molecular Resonance |
| PBD | Patient blood dependent |
| RPE | Retinal Pigment Epithelium |
| ROS | Reactive Oxygen Species |
| tBHP | tert-Butyl hydroperoxide |
| DEG | Differentially Expressed Gene |
| FDR | False Discovery Rate |
| FC/log2FC | Fold Change/log base 2-Fold Change |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| EMT | Epithelial-to-Mesenchymal Transition |
| ECM | Extracellular Matrix |
| IL | Interleukin |
| IL1B/IL6R | Interleukin-1 beta/Interleukin-6 receptor |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| TNF | Tumor Necrosis Factor |
| mRNA | Messenger RNA |
| RT-qPCR | Reverse Transcription quantitative Polymerase Chain Reaction |
| TS | TargetScan |
| DB | miRDB |
| CTRL | Untreated control condition |
| OX | Oxidative stress condition |
| Sec | Secretome from PDB |
| Q + S | Combined QMR® and Secretome treatment |
| MAPK | Mitogen-Activated Protein Kinase |
| HIF-1 | Hypoxia-Inducible Factor 1 |
| PI3K–Akt | Phosphoinositide 3-kinase–Protein kinase B pathway |
| TGF-β | Transforming Growth Factor beta |
| NFE2L2/NRF2 | Nuclear factor erythroid 2–related factor 2 |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| CFH | Complement Factor H |
| STAT1/3 | Signal Transducer and Activator of Transcription 1/3 |
| RELA | v-rel avian reticuloendotheliosis viral oncogene homolog A (p65 subunit of NF-κB) |
| IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 |
| TRAF6 | TNF Receptor Associated Factor 6 |
| CASP3 | Caspase-3 |
| BCL2 | B-cell lymphoma 2 |
| TP53 | Tumor Protein p53 |
| CDK6 | Cyclin-Dependent Kinase 6 |
| SIRT1 | Sirtuin 1 |
| ZEB1/2 | Zinc finger E-box-binding homeobox 1/2 |
| MET | Mesenchymal–epithelial transition factor (HGFR) |
| NOX4 | NADPH oxidase 4 |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| HMOX1 | Heme oxygenase 1 |
| FADD | Fas-Associated Death Domain protein |
| BCLAF1 | Bcl-2-associated transcription factor 1 |
| E2F1/2/3 | E2F Transcription Factor 1/2/3 |
| FOXO1/3 | Forkhead box protein O1/O3 |
| SOD1 | Superoxide Dismutase 1 |
| XBP1 | X-box binding protein 1 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
References
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Kapphahn, R.J.; Leary, M.M.; Atilano, S.R.; Terluk, M.R.; Karunadharma, P.; Chen, G.K.; Ratnapriya, R.; Swaroop, A.; Montezuma, S.R.; et al. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp. Eye Res. 2016, 145, 269–277. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xia, J.; Huang, L.L.; Li, Y.C. HIF-1alpha promotes NLRP3 inflammasome activation in bleomycin-induced acute lung injury. Mol. Med. Rep. 2019, 20, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; von der Emde, L.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Rubin, D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-related Macular Degeneration. JAMA Ophthalmol. 2020, 138, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Y.; Han, Z.; Wang, B.; Zheng, W.; Wei, L. Ferroptosis: A novel mechanism of cell death in ophthalmic conditions. Front. Immunol. 2024, 15, 1440309. [Google Scholar] [CrossRef]
- Yu, L.; Liu, P. cGAS/STING signalling pathway in senescence and oncogenesis. Semin. Cancer Biol. 2024, 106–107, 87–102. [Google Scholar] [CrossRef]
- Zhou, M.; Geathers, J.S.; Grillo, S.L.; Weber, S.R.; Wang, W.; Zhao, Y.; Sundstrom, J.M. Role of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium Dysfunction. Front. Cell Dev. Biol. 2020, 8, 501. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, S.B.; Yu, H.Y.; Kim, Y.H.; Kim, J. Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro. Molecules 2022, 27, 8970. [Google Scholar] [CrossRef]
- Rabin, D.M.; Rabin, R.L.; Blenkinsop, T.A.; Temple, S.; Stern, J.H. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: A novel culture model for dry AMD. Aging 2013, 5, 51–66. [Google Scholar] [CrossRef]
- Hanus, J.; Zhang, H.; Wang, Z.; Liu, Q.; Zhou, Q.; Wang, S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013, 4, e965. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Riascos, Z.V.; Ginesta, M.M.; Fabregat, J.; Serrano, T.; Busquets, J.; Buscail, L.; Cordelier, P.; Capella, G. Expression and Role of MicroRNAs from the miR-200 Family in the Tumor Formation and Metastatic Propensity of Pancreatic Cancer. Mol. Ther. Nucleic Acids 2019, 17, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhou, Q.; Ma, J.; Zhao, Y.; Wang, S. miR-146a is upregulated during retinal pigment epithelium (RPE)/choroid aging in mice and represses IL-6 and VEGF-A expression in RPE cells. J. Clin. Exp. Ophthalmol. 2016, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Stepien, P.W.; Nowakowska, K.N.; Stefaniak, M.; Osial, N.; Choragiewicz, T.; Toro, M.D.; Nowomiejska, K.; Rejdak, R. The Role of Dysregulated miRNAs in the Pathogenesis, Diagnosis and Treatment of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 7761. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Ouchi, Y.; Kiyokawa, M.; Sakuma, T.; Ito, R.; Ebihara, N. Upregulation of Mir-21 Levels in the Vitreous Humor Is Associated with Development of Proliferative Vitreoretinal Disease. PLoS ONE 2016, 11, e0158043. [Google Scholar] [CrossRef]
- Trivli, A.; Karmiris, E.; Dalianis, G.; Ruggeri, A.; Terzidou, C. Evaluating the efficacy of Quantum Molecular Resonance (QMR®) electrotherapy in mixed-type dry eye patients. J. Optom. 2023, 16, 128–134. [Google Scholar] [CrossRef]
- Ballesteros-Sanchez, A.; Sanchez-Gonzalez, J.M.; Tedesco, G.R.; Rocha-De-Lossada, C.; Russo, F.; Spinelli, A.; Ingrande, I.; Borroni, D. Efficacy and Safety of Quantum Molecular Resonance Electrotherapy in Patients with Aqueous-Deficient, Evaporative and Mixed-Type Dry Eye: A Randomized Interventional Study. Ophthalmol. Ther. 2024, 13, 495–507. [Google Scholar] [CrossRef]
- Trigo, C.M.; Rodrigues, J.S.; Camoes, S.P.; Sola, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124. [Google Scholar] [CrossRef]
- Gu, C.; Draga, D.; Zhou, C.; Su, T.; Zou, C.; Gu, Q.; Lahm, T.; Zheng, Z.; Qiu, Q. miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4215–4223. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Svegliati Baroni, S.; Pavani, M.; Moretti, M.; et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021, 193, 111413. [Google Scholar] [CrossRef]
- Paolucci, T.; Pino, V.; Elsallabi, O.; Gallorini, M.; Pozzato, G.; Pozzato, A.; Lanuti, P.; Reis, V.M.; Pesce, M.; Pantalone, A.; et al. Quantum Molecular Resonance Inhibits NLRP3 Inflammasome/Nitrosative Stress and Promotes M1 to M2 Macrophage Polarization: Potential Therapeutic Effect in Osteoarthritis Model In Vitro. Antioxidants 2023, 12, 1358. [Google Scholar] [CrossRef]
- Fraccalvieri, M.; Salomone, M.; Di Santo, C.; Ruka, E.; Morozzo, U.; Bruschi, S. Quantum molecular resonance technology in hard-to-heal extremity wounds: Histological and clinical results. Int. Wound J. 2017, 14, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and application strategies of miRNA-146a regulating inflammation and fibrosis at molecular and cellular levels (Review). Int. J. Mol. Med. 2023, 51, 7. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, M.; Leszczynska, A.; Llorenc, V.; Adan, A. Interleukin-6 blockade in ocular inflammatory diseases. Clin. Exp. Immunol. 2014, 176, 301–309. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forro, L.; Schlegel, W.; Krause, K.H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Ochoa Hernandez, M.E.; Lewis-Lujan, L.M.; Burboa Zazueta, M.G.; Del Castillo Castro, T.; De La Re Vega, E.; Galvez-Ruiz, J.C.; Trujillo-Lopez, S.; Lopez Torres, M.A.; Iloki-Assanga, S.B. Role of Oxidative Stress and Inflammation in Age Related Macular Degeneration: Insights into the Retinal Pigment Epithelium (RPE). Int. J. Mol. Sci. 2025, 26, 3463. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguilar, M.; Groman-Lupa, S.; Jimenez-Martinez, M.C. MicroRNAs as potential biomarkers and therapeutic targets in age-related macular degeneration. Front. Ophthalmol. 2023, 3, 1023782. [Google Scholar] [CrossRef] [PubMed]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Paquet-Durand, F.; Loscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- Bonilha, V.L. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp. Eye Res. 2014, 126, 38–45. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Di Pietro, C.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and Circulating miRNAs in Age-Related Macular Degeneration: An In vivo Animal and Human Study. Front. Pharmacol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Ahmado, A.; Carr, A.J.; Vugler, A.A.; Semo, M.; Gias, C.; Lawrence, J.M.; Chen, L.L.; Chen, F.K.; Turowski, P.; da Cruz, L.; et al. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7148–7159. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Harrison, T.E.; Bowler, J.; Cheng, C.I.; Reeves, K.D. Optimizing Platelet-Rich Plasma: Spin Time and Sample Source. Bioengineering 2023, 10, 1270. [Google Scholar] [CrossRef]
- Patel, H.; Pundkar, A.; Shrivastava, S.; Chandanwale, R.; Jaiswal, A.M. A Comprehensive Review on Platelet-Rich Plasma Activation: A Key Player in Accelerating Skin Wound Healing. Cureus 2023, 15, e48943. [Google Scholar] [CrossRef]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Res. Int. 2016, 2016, 6591717. [Google Scholar] [CrossRef] [PubMed]
- Prado-Yupanqui, J.W.; Ramirez-Orrego, L.; Cortez, D.; Vera-Ponce, V.J.; Chenet, S.M.; Tejedo, J.R.; Tapia-Limonchi, R. The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine-A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1926. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Afsar, E.; Kirimlioglu, E.; Ceker, T.; Yilmaz, C.; Demir, N.; Aslan, M. Effect of ER stress on sphingolipid levels and apoptotic pathways in retinal pigment epithelial cells. Redox Biol. 2020, 30, 101430. [Google Scholar] [CrossRef]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928–E931. [Google Scholar] [CrossRef]
- Sriram, H.; Khanka, T.; Kedia, S.; Tyagi, P.; Ghogale, S.; Deshpande, N.; Chatterjee, G.; Rajpal, S.; Patkar, N.V.; Subramanian, P.G.; et al. Improved protocol for plasma microRNA extraction and comparison of commercial kits. Biochem. Med. 2021, 31, 030705. [Google Scholar] [CrossRef]
- Shore, S.; Henderson, J.M.; Lebedev, A.; Salcedo, M.P.; Zon, G.; McCaffrey, A.P.; Paul, N.; Hogrefe, R.I. Small RNA Library Preparation Method for Next-Generation Sequencing Using Chemical Modifications to Prevent Adapter Dimer Formation. PLoS ONE 2016, 11, e0167009. [Google Scholar] [CrossRef]
- Potla, P.; Ali, S.A.; Kapoor, M. A bioinformatics approach to microRNA-sequencing analysis. Osteoarthr. Cartil. Open 2021, 3, 100131. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Love, M.I.; Anders, S.; Kim, V.; Huber, W. RNA-Seq workflow: Gene-level exploratory analysis and differential expression. F1000Research 2015, 4, 1070. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Ng, H.K.; Nadarajah, S.; Kaufman, H.L.; Yang, J.Y.; Deng, Y. Statistical methods on detecting differentially expressed genes for RNA-seq data. BMC Syst. Biol. 2011, 5 (Suppl. 3), S1. [Google Scholar] [CrossRef] [PubMed]
- Sharbati-Tehrani, S.; Kutz-Lohroff, B.; Bergbauer, R.; Scholven, J.; Einspanier, R. miR-Q: A novel quantitative RT-PCR approach for the expression profiling of small RNA molecules such as miRNAs in a complex sample. BMC Mol. Biol. 2008, 9, 34. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Dias, B.D.S.; Diniz, L.F.A.; Correa, L.D.; Souza, R.P.; Ferreira, L.T.; Pasqualin, D.D.C.; Cicco, R.; Silva, E.; Severino, P. Comparative analysis of miRNA-mRNA interaction prediction tools based on experimental head and neck cancer data. Einstein 2025, 23, eAO1372. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Leon, L.E.; Calligaris, S.D. Visualization and Analysis of MiRNA-Targets Interactions Networks. Methods Mol. Biol. 2017, 1509, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef] [PubMed]


| Comparison | Time | miRNA | log2FC | FDR |
|---|---|---|---|---|
| QMR® vs. Control | 24 h | hsa-miR-21-5p | 1.46 | 0.003 |
| 24 h | hsa-miR-126-3p | 0.85 | 0.02 | |
| 24 h | hsa-miR-146a-5p | 1.02 | 0.015 | |
| 24 h | hsa-miR-34a-5p | −1.23 | 0.009 | |
| 24 h | hsa-miR-155-5p | −0.98 | 0.018 | |
| 72 h | hsa-miR-21-5p | 2.2 | 0.0005 | |
| 72 h | hsa-miR-146a-5p | 1.77 | 0.001 | |
| 72 h | hsa-miR-126-3p | 1.05 | 0.01 | |
| 72 h | hsa-miR-29b-3p | 1.19 | 0.03 | |
| 72 h | hsa-miR-34a-5p | −1.68 | 0.0008 | |
| 72 h | hsa-miR-155-5p | −1.31 | 0.004 | |
| Secretome vs. Control | 24 h | hsa-miR-146a-5p | 1.1 | 0.012 |
| 24 h | hsa-miR-21-5p | 0.78 | 0.027 | |
| 24 h | hsa-miR-9-5p | 0.95 | 0.021 | |
| 24 h | hsa-miR-126-3p | 1.34 | 0.005 | |
| 24 h | hsa-let-7f-5p | −0.81 | 0.034 | |
| 24 h | hsa-miR-34a-5p | −1.1 | 0.011 | |
| 24 h | hsa-miR-155-5p | −1.05 | 0.016 | |
| 72 h | hsa-miR-146a-5p | 1.98 | 0.0008 | |
| 72 h | hsa-miR-21-5p | 1.53 | 0.003 | |
| 72 h | hsa-miR-204-5p | 1.21 | 0.009 | |
| 72 h | hsa-miR-126-3p | 0.88 | 0.025 | |
| 72 h | hsa-let-7f-5p | −1.12 | 0.006 | |
| 72 h | hsa-miR-34a-5p | −1.79 | 0.0004 | |
| 72 h | hsa-miR-155-5p | −1.43 | 0.002 | |
| QMR® + Secretome vs. Control | 24 h | hsa-miR-146a-5p | 2.45 | 0.0003 |
| 24 h | hsa-miR-21-5p | 2.02 | 0.001 | |
| 24 h | hsa-miR-126-3p | 1.2 | 0.008 | |
| 24 h | hsa-miR-200a-3p | 1.05 | 0.017 | |
| 24 h | hsa-miR-34a-5p | −2.05 | 0.0001 | |
| 24 h | hsa-miR-155-5p | −1.82 | 0.0009 | |
| 24 h | hsa-let-7f-5p | −0.95 | 0.028 | |
| 72 h | hsa-miR-146a-5p | 2.1 | 0.0002 | |
| 72 h | hsa-miR-21-5p | 1.67 | 0.0007 | |
| 72 h | hsa-miR-126-3p | 1.3 | 0.004 | |
| 72 h | hsa-miR-204-5p | 1.45 | 0.002 | |
| 72 h | hsa-miR-34a-5p | −2.2 | 0.0001 | |
| 72 h | hsa-miR-155-5p | −1.55 | 0.001 | |
| 72 h | hsa-let-7f-5p | −1.3 | 0.0005 | |
| 72 h | hsa-miR-210-3p | −0.78 | 0.032 | |
| OX vs. Control | 24 h | hsa-miR-21-5p | 1.5 | 0.004 |
| 72 h | hsa-miR-34a-5p | 2.1 | 0.001 | |
| 72 h | hsa-miR-146a-5p | 1.8 | 0.005 | |
| QMR® vs. Control | 24 h | hsa-miR-223-3p | 1.2 | 0.02 |
| 72 h | hsa-miR-21-5p | 1.3 | 0.015 | |
| 72 h | hsa-miR-146a-5p | 1.0 | 0.03 | |
| Secretome vs. Control | 24 h | hsa-miR-126-3p | 1.1 | 0.04 |
| 72 h | hsa-miR-21-5p | 1.4 | 0.01 | |
| QMR® + Secretome vs. Control | 24 h | hsa-miR-21-5p | 0.9 | 0.048 |
| OX vs. Control | 24 h | hsa-miR-200a-3p | −0.8 | 0.018 |
| 72 h | hsa-miR-204-5p | −1.1 | 0.007 | |
| 72 h | hsa-miR-211-5p | −1.3 | 0.003 | |
| QMR® vs. Control | 72 h | hsa-miR-204-5p | −0.6 | 0.04 |
| Secretome vs. Control | 72 h | hsa-miR-211-5p | −0.7 | 0.033 |
| QMR® vs. OX | 24 h | hsa-miR-34a-5p | −0.9 | 0.012 |
| 72 h | hsa-miR-146a-5p | −0.7 | 0.02 | |
| Secretome vs. OX | 24 h | hsa-miR-21-5p | −1.0 | 0.005 |
| 72 h | hsa-miR-34a-5p | −0.8 | 0.015 | |
| QMR® + Secretome vs. OX | 24 h | hsa-miR-21-5p | −1.4 | 0.001 |
| 24 h | hsa-miR-34a-5p | −1.1 | 0.004 | |
| 72 h | hsa-miR-146a-5p | −1.2 | 0.008 |
| Treatment and Time | Pathway/Term (Category) | Adjusted p-Value | Genes (n°) |
|---|---|---|---|
| QMR® 24 h | Inflammatory response (GO BP) | 8.0 × 10−4 | 18 |
| Regulation of apoptotic process (GO BP) | 1.2 × 10−3 | 15 | |
| NF-κB signaling pathway (KEGG) | 3.0 × 10−3 | 8 | |
| Cytokine signaling in immune system (Reactome) | 2.0 × 10−3 | 12 | |
| QMR® 72 h | Cellular response to oxidative stress (GO BP) | 5.0 × 10−5 | 20 |
| Regulation of cell cycle (GO BP) | 8.0 × 10−4 | 18 | |
| p53 signaling pathway (KEGG) | 1.0 × 10−4 | 10 | |
| Cellular senescence and autophagy (Reactome) | 1.0 × 10−3 | 9 | |
| Secretome 24 h | Angiogenesis (GO BP) | 5.0 × 10−4 | 14 |
| Regulation of cell proliferation (GO BP) | 1.0 × 10−3 | 16 | |
| PI3K–Akt signaling pathway (KEGG) | 2.0 × 10−3 | 10 | |
| TGF-β receptor signaling (Reactome) | 8.0 × 10−4 | 9 | |
| Secretome 72 h | Regulation of inflammatory response (GO BP) | 4.0 × 10−4 | 17 |
| Positive regulation of autophagy (GO BP) | 5.0 × 10−3 | 8 | |
| FoxO signaling pathway (KEGG) | 1.0 × 10−3 | 12 | |
| Extracellular matrix organization (Reactome) | 3.0 × 10−4 | 10 | |
| QMR® + Secretome 24 h | Regulation of ROS metabolic process (GO BP) | 1.0 × 10−5 | 22 |
| Inflammatory response (GO BP) | 2.0 × 10−4 | 20 | |
| TNF signaling pathway (KEGG) | 7.0 × 10−4 | 9 | |
| Cellular senescence (Reactome) | 4.0 × 10−4 | 10 | |
| QMR® + Secretome 72 h | Response to oxidative stress (GO BP) | 5.0 × 10−6 | 25 |
| Regulation of cell proliferation (GO BP) | 1.0 × 10−4 | 18 | |
| HIF-1 signaling pathway (KEGG) | 3.0 × 10−4 | 11 | |
| Apoptosis (Reactome) | 2.0 × 10−4 | 12 |
| Condition | Key Deregulated miRNAs | Expected Effect (Functional Outcome) | Major Affected Pathways |
|---|---|---|---|
| QMR® | miR-21 ↑, miR-146a ↑, miR-126 ↑; miR-34a ↓, miR-155 ↓ | Anti-inflammatory and anti-apoptotic shift, promoting cell survival | NF-κB/TNF inflammatory signaling; p53-mediated apoptosis |
| Secretome | miR-146a ↑, miR-21 ↑, miR-204 ↑; miR-155 ↓, let-7f ↓, miR-34a ↓ | Pro-survival and pro-regenerative response (reduced senescence, enhanced cell viability and proliferation) | PI3K–Akt survival pathway; TGF-β/EMT signaling suppression; inflammatory cytokine signaling |
| QMR® + Secretome | miR-146a ↑, miR-21 ↑, miR-126 ↑, miR-204 ↑; miR-34a ↓, miR-155 ↓, let-7f ↓ | Strongly anti-inflammatory, anti-apoptotic, and anti-senescent effect, restoring a protective homeostatic state | Oxidative stress response pathways (FoxO, HIF-1); cell cycle/senescence (p53, telomere) pathways; NF-κB inflammatory pathway |
| miRNA (Direction) | Validated/Predicted Targets | Associated Pathway | Expected Functional Effect |
|---|---|---|---|
| miR-590-3p ↑ | NLRP1, NOX4 | Inflammasome, ROS generation | ↓ Pyroptosis, ↓ ROS |
| miR-146a-5p ↑ | IRAK1, TRAF6, IL-6, VEGFA | NF-κB, Inflammation | ↓ Cytokines, ↓ Angiogenesis |
| miR-34a-5p ↓ | SIRT1, BCL2 | Apoptosis, oxidative defense | ↑ Survival, ↑ Anti-oxidant |
| miR-29b-3p ↑ | COL1A1, ZEB1/2 | Fibrosis, EMT | ↓ ECM deposition |
| miR-204-5p ↑ | MITF, TGF-β pathway components | Regeneration, anti-fibrosis | ↑ Differentiation, ↓ EMT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibrandi, S.; Mordà, D.; Scimone, C.; D’ascola, A.; Aliquò, F.; Pozzato, A.; Scalinci, S.Z.; D’Angelo, R.; Sidoti, A.; Donato, L. QMR® and Patient Blood-Derived Secretome Modulate RPE microRNA Networks Under Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 8614. https://doi.org/10.3390/ijms26178614
Alibrandi S, Mordà D, Scimone C, D’ascola A, Aliquò F, Pozzato A, Scalinci SZ, D’Angelo R, Sidoti A, Donato L. QMR® and Patient Blood-Derived Secretome Modulate RPE microRNA Networks Under Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(17):8614. https://doi.org/10.3390/ijms26178614
Chicago/Turabian StyleAlibrandi, Simona, Domenico Mordà, Concetta Scimone, Angela D’ascola, Federica Aliquò, Alessandro Pozzato, Sergio Zaccaria Scalinci, Rosalia D’Angelo, Antonina Sidoti, and Luigi Donato. 2025. "QMR® and Patient Blood-Derived Secretome Modulate RPE microRNA Networks Under Oxidative Stress" International Journal of Molecular Sciences 26, no. 17: 8614. https://doi.org/10.3390/ijms26178614
APA StyleAlibrandi, S., Mordà, D., Scimone, C., D’ascola, A., Aliquò, F., Pozzato, A., Scalinci, S. Z., D’Angelo, R., Sidoti, A., & Donato, L. (2025). QMR® and Patient Blood-Derived Secretome Modulate RPE microRNA Networks Under Oxidative Stress. International Journal of Molecular Sciences, 26(17), 8614. https://doi.org/10.3390/ijms26178614










