Orally Administered Zinc Gluconate Induces Tight Junctional Remodeling and Reduces Passive Transmucosal Permeability Across Human Intestine in a Patient-Based Study
Abstract
1. Introduction
2. Results
2.1. Demographics and Zinc Administration
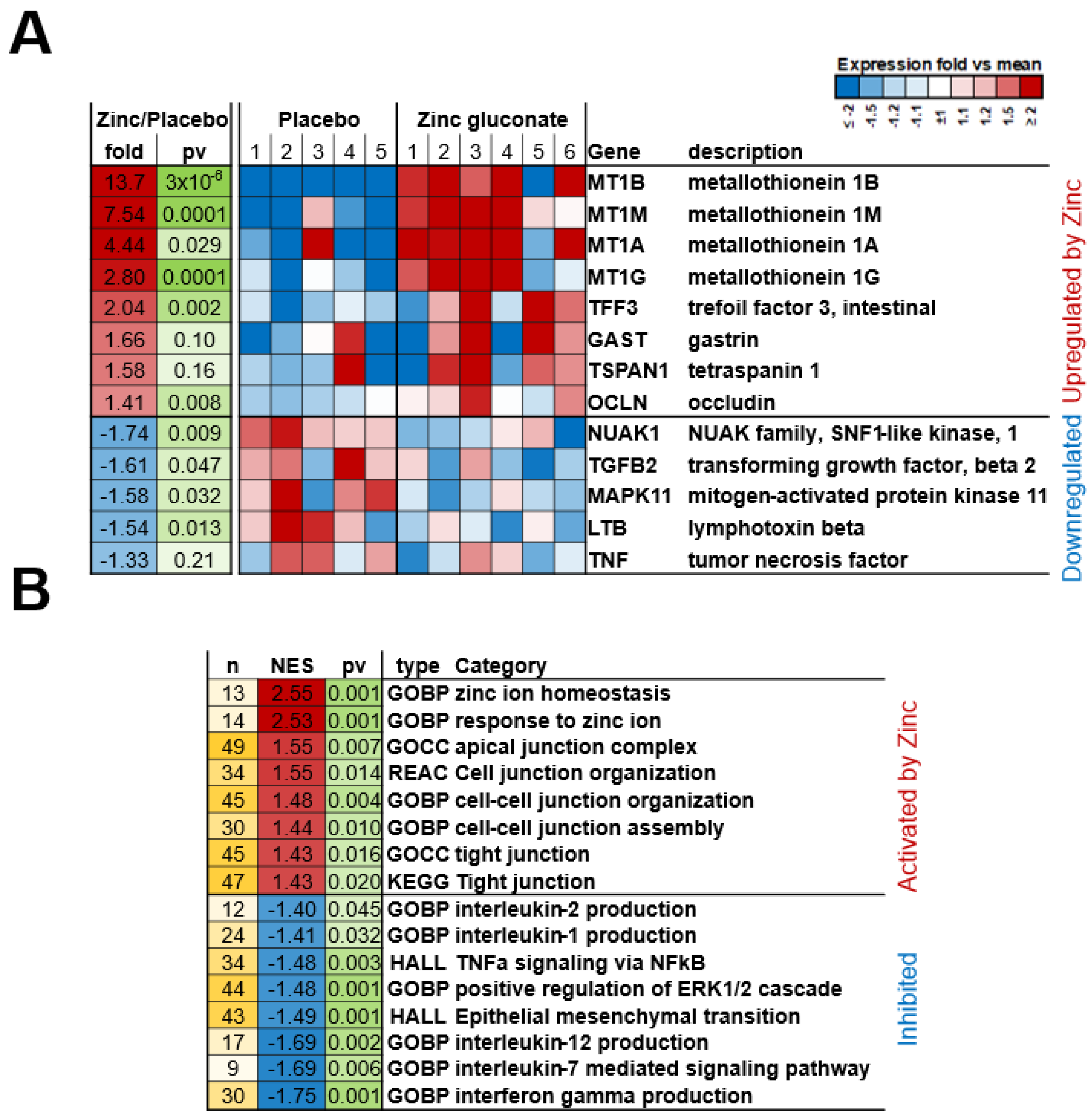
2.2. mRNA Expression
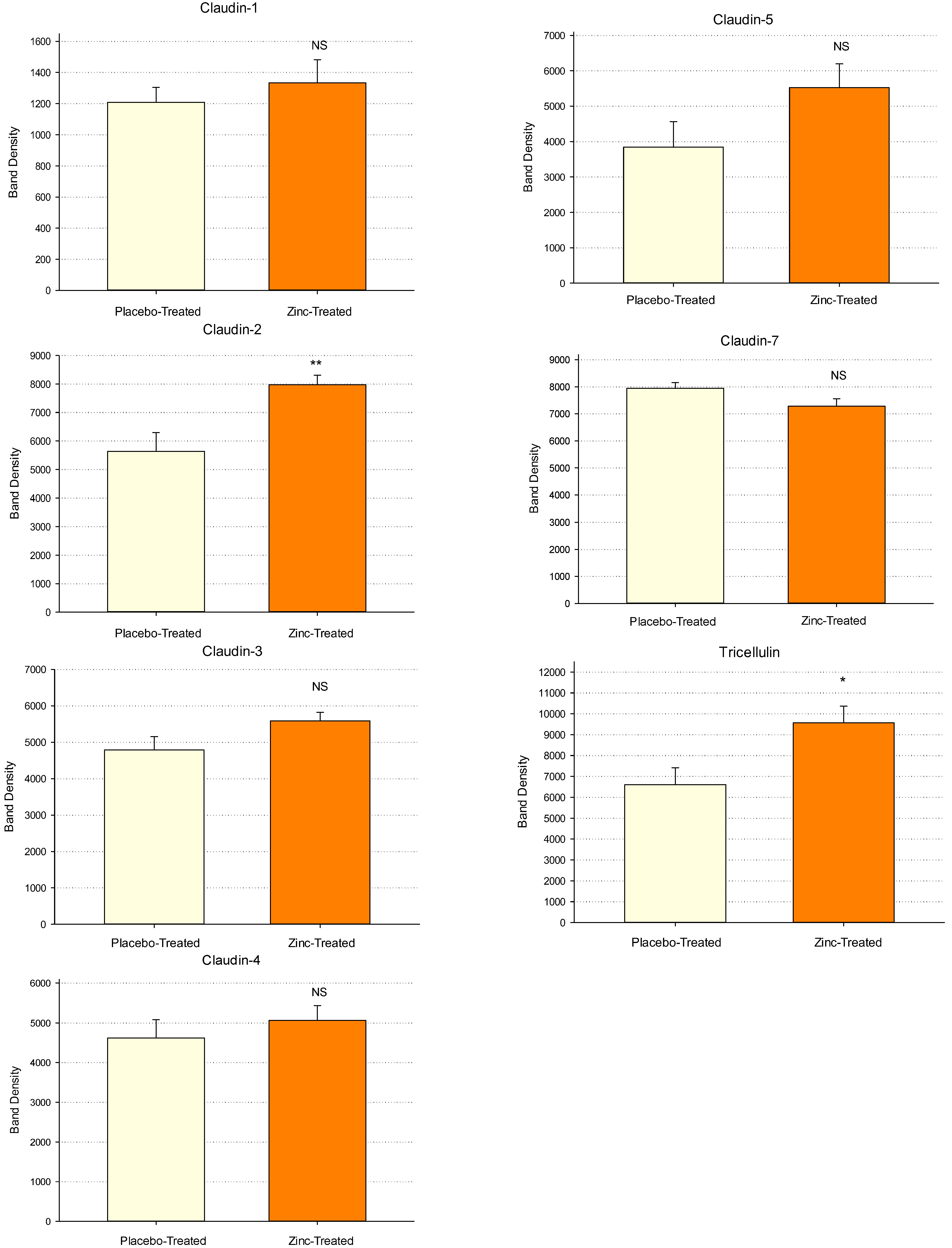
2.3. Western Immunoblot Analyses
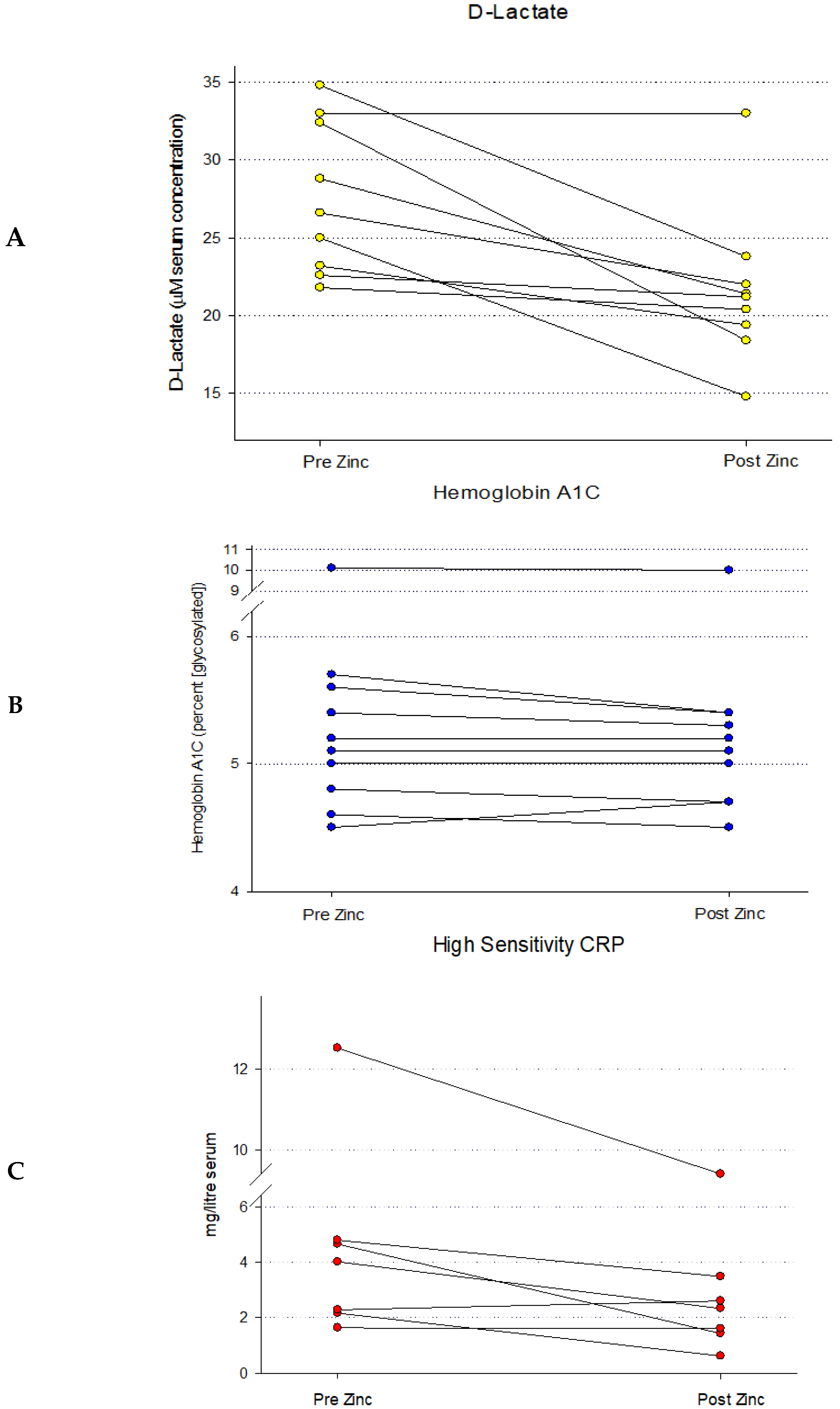
2.4. Serum D-Lactate Analyses
3. Discussion
4. Methods
4.1. Patient Demographics and Enrollment
- (1)
- Patient Recruitment for mRNA and Western Immunoblot Studies
- (2)
- Test Subject Recruitment for Zinc Leaky Gut Study
4.2. Zinc Administration
- (1)
- mRNA and Western Immunoblot Study
- (2)
- Zinc Leaky Gut Study
4.3. Duodenal Biopsy Collection and Processing
4.4. RNA-seq Data Analysis
4.5. Western Immunoblot Analyses
4.6. Blood Sampling
4.7. D-Lactate Analyses
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | C-Reactive Protein |
| GERD | Gastroesophageal Reflux Disease |
| GI | Gastrointestinal |
| GSEA | Gene Set Enrichment Analysis |
| HS CRP | High Sensitivity C-Reactive Protein |
| IBD | Inflammatory Bowel Disease |
| LG | Leaky Gut |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| RDA | Recommended Daily Allowance |
| TER | Transepithelial Electrical Resistance |
| TJ | Tight Junction |
| Zn | Zinc |
References
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef]
- Mullin, J.M.; Agostino, N.; Rendon-Huerta, E.; Thornton, J.J. Keynote review: Epithelial and endothelial barriers in human disease. Drug Discov. Today 2005, 10, 395–408. [Google Scholar] [CrossRef]
- Davidson, G.; Kritas, S.; Butler, R. Stressed mucosa. Nestle Nutr. Workshop Ser. Pediatr. Program 2007, 59, 133–142; discussion 143–146. [Google Scholar]
- Capaldo, C.T. Claudin Barriers on the Brink: How Conflicting Tissue and Cellular Priorities Drive IBD Pathogenesis. Int. J. Mol. Sci. 2023, 24, 8562. [Google Scholar] [CrossRef]
- Soler, A.P.; Miller, R.D.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1431. [Google Scholar] [CrossRef]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe 2016, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D. Intestinal permeability, leaky gut, and intestinal disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Sgamato, C.; Rocco, A.; Coccoli, P.; Ambrosio, C.; Nardone, G. The Leaky Gut and Human Diseases: “Can’t Fill the Cup if You Don’t Plug the Holes First”. Dig. Dis. 2024, 46, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Balkovetz, D.F. Tight junction claudins and the kidney in sickness and in health. Biochim. Biophys. Acta 2009, 1788, 858–863. [Google Scholar] [CrossRef]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2000, 69, 46–67. [Google Scholar] [CrossRef]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Wang, F. Roles of Transepithelial Electrical Resistance in Mechanisms of Retinal Pigment Epithelial Barrier and Retinal Disorders. Discov. Med. 2022, 34, 19–24. [Google Scholar]
- John, L.J.; Fromm, M.; Schulzke, J.D. Epithelial barriers in intestinal inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut- Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.A.; Hou, Y.; Yi, D.; Qiu, Y.; Wu, G.; Kong, X.; Yin, Y. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim. Nutr. 2015, 1, 123–127. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef]
- Vargas-Robles, H.; Castro-Ochoa, K.F.; Citalán-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef]
- Hering, N.A.; Schulzke, J.D. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig. Dis. 2009, 27, 450–454. [Google Scholar] [CrossRef]
- Callaghan, P.J.; Rybakovsky, E.; Ferrick, B.; Thomas, S.; Mullin, J.M. Retinoic acid improves baseline barrier function and attenuates TNF-α-induced barrier leak in human bronchial epithelial cell culture model, 16HBE 14o. PLoS ONE 2020, 15, e0242536. [Google Scholar] [CrossRef]
- Rybakovsky, E.; DiGuilio, K.M.; Valenzano, M.C.; Geagan, S.; Pham, K.; Harty, R.N.; Mullin, J.M. Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lung-derived epithelial cell culture model, 16HBE 14o. Physiol. Rep. 2023, 11, e15592. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing Occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Incà, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef]
- Valenzano, M.C.; Rybakovsky, E.; Chen, V.; Leroy, K.; Lander, J.; Richardson, E.; Yalamanchili, S.; McShane, S.; Mathew, A.; Mayilvaganan, B.; et al. Zinc Gluconate Induces Potentially Cancer Chemopreventive Activity in Barrett’s Esophagus: A Phase 1 Pilot Study. Dig. Dis. Sci. 2021, 66, 1195–1211. [Google Scholar] [CrossRef]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-Lactate: Implications for Gastrointestinal Diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef]
- Murray, M.J.; Barbose, J.J.; Cobb, C.F. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J. Surg. Res. 1993, 54, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, A.B.; Oehme, F.W. The effect of various dietary zinc concentrations on the biological interactions of zinc, copper, and iron in rats. Biol. Trace Elem. Res. 1991, 29, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Shibamoto, M.; Ogawa, M.; Miyake, S.; Mizumoto, K.; Ohishi, A.; Nishida, K.; Nagasawa, K. The effect of divalent metal cations on zinc uptake by mouse Zrt/Irt-like protein 1 (ZIP1). Life Sci. 2014, 113, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig. Dis. Sci. 2013, 58, 77–87. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Guo, J.; Geng, B.; Ji, W.; Zhao, Q.; Li, J.; Liu, X.; Liu, J.; Guo, Z.; et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 2018, 6, 66. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Z.H.; Cui, W.; Fu, J.L.; Wang, Y.R.; Liu, P. Tumor necrosis factor alpha increases intestinal permeability in mice with fulminant hepatic failure. World J. Gastroenterol. 2012, 18, 5042–5050. [Google Scholar] [CrossRef]
- Tossou, M.C.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L.; et al. Effect of High Dietary Tryptophan on Intestinal Morphology and Tight Junction Protein of Weaned Pig. BioMed Res. Int. 2016, 2016, 2912418, Erratum in BioMed Res. Int. 2017, 2017, 8309364. [Google Scholar] [CrossRef]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Robles-Osorio, M.L.; Sabath, E. Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J. Diabetes 2023, 14, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Chegini, Z.; Noei, M.; Hemmati, J.; Arabestani, M.R.; Shariati, A. The destruction of mucosal barriers, epithelial remodeling, and impaired mucociliary clearance: Possible pathogenic mechanisms of Pseudomonas aeruginosa and Staphylococcus aureus in chronic rhinosinusitis. Cell Commun. Signal. 2023, 21, 306. [Google Scholar] [CrossRef]
- Yang, Y.F.; Shen, Y.D. Choroid plexus and its relations with age-related diseases. Yi Chuan 2024, 46, 109–125. [Google Scholar]
- Berni Canani, R.; Caminati, M.; Carucci, L.; Eguiluz-Gracia, I. Skin, gut, and lung barrier: Physiological interface and target of intervention for preventing and treating allergic diseases. Allergy 2024, 79, 1485–1500. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rybakovsky, E.; Abdavies, R.; Chamoun, R.; Flounders, C.A.; Shepley-McTaggart, A.; Harty, R.N.; Mullin, J.M. Micronutrient Improvement of Epithelial Barrier Function in Various Disease States: A Case for Adjuvant Therapy. Int. J. Mol. Sci. 2022, 23, 2995. [Google Scholar] [CrossRef]
- Valenzano, M.C.; DiGuilio, K.; Mercado, J.; Teter, M.; To, J.; Ferraro, B.; Mixson, B.; Manley, I.; Baker, V.; Moore, B.A.; et al. Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients. PLoS ONE 2015, 10, e0133926. [Google Scholar] [CrossRef]
- Hu, X.; Wang, R.; Kille, P.; Maret, W.; Hogstrand, C. Zinc amino acid chelate and the Aryl Hydrocarbon Receptor (AHR) cooperate in improving the barrier function of a Caco-2 cell intestinal epithelium. J. Nutr. Biochem. 2025, 141, 109909. [Google Scholar] [CrossRef]
- Brenner, H.; Holleczek, B.; Schöttker, B. Vitamin D Insufficiency and Deficiency and Mortality from Respiratory Diseases in a Cohort of Older Adults: Potential for Limiting the Death Toll during and beyond the COVID-19 Pandemic? Nutrients 2020, 12, 2488. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Wasiak, J.; Gawlik-Kotelnicka, O. Intestinal permeability and its significance in psychiatric disorders—A narrative review and future perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef]
- Brown, G.C.; Heneka, M.T. The endotoxin hypothesis of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Walker, C.L.; Chan, K.Y.; Jian, W.Y.; Black, R.E. Oral zinc supplementation for the treatment of acute diarrhea in children: A systematic review and meta-analysis. Nutrients 2013, 5, 4715–4740. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wolf, P.G.; Guo, S.; Guo, Y.; Gaskins, H.R.; Zhang, B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Wong, T.; Howard, S.C. Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics. Med. Sci. 2021, 9, 9. [Google Scholar] [CrossRef]
- Hu, C.; Song, J.; Li, Y.; Luan, Z.; Zhu, K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 2013, 110, 681–688. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef]
- Fan, P.; Tan, Y.; Jin, K.; Lin, C.; Xia, S.; Han, B.; Zhang, F.; Wu, L.; Ma, X. Supplemental lipoic acid relieves post-weaning diarrhoea by decreasing intestinal permeability in rats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 136–146. [Google Scholar] [CrossRef]
- Ranaldi, G.; Caprini, V.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc stores protect the intestinal epithelium from Ochratoxin A toxicity. Toxicol. In Vitro 2009, 23, 1516–1521. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Choudhry, N.; Scott, F.; Edgar, M.; Sanger, G.J.; Kelly, P. Reversal of Pathogen-Induced Barrier Defects in Intestinal Epithelial Cells by Contra-pathogenicity Agents. Dig. Dis. Sci. 2021, 66, 88–104. [Google Scholar] [CrossRef]
- Sarkar, P.; Saha, T.; Sheikh, I.A.; Chakraborty, S.; Aoun, J.; Chakrabarti, M.K.; Rajendran, V.M.; Ameen, N.A.; Dutta, S.; Hoque, K.M. Zinc ameliorates intestinal barrier dysfunctions in shigellosis by reinstating claudin-2 and -4 on the membranes. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G229–G246. [Google Scholar] [CrossRef]
- Song, Z.H.; Ke, Y.L.; Xiao, K.; Jiao, L.F.; Hong, Q.H.; Hu, C.H. Diosmectite-zinc oxide composite improves intestinal barrier restoration and modulates TGF-β1, ERK1/2, and Akt in piglets after acetic acid challenge. J. Anim. Sci. 2015, 93, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.; Marchbank, T.; March, D.S.; Thatcher, R.; Playford, R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016, 104, 526–536. [Google Scholar] [CrossRef]
- Roy, S.K.; Behrens, R.H.; Haider, R.; Akramuzzaman, S.M.; Mahalanabis, D.; Wahed, M.A.; Tomkins, A.M. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 289–296. [Google Scholar]
- Alam, J.; Nuzhat, S.; Billal, S.M.; Ahmed, T.; Khan, A.I.; Hossain, M.I. Nutritional Profiles and Zinc Supplementation among Children with Diarrhea in Bangladesh. Am. J. Trop. Med. Hyg. 2023, 108, 837–843. [Google Scholar] [CrossRef]
- Samman, S.; Roberts, D.C. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Med. J. Aust. 1987, 146, 246–249. [Google Scholar] [CrossRef]
- Field, H.P.; Whitley, A.J.; Srinivasan, T.R.; Walker, B.E.; Kelleher, J. Plasma and leucocyte zinc concentrations and their response to zinc supplementation in an elderly population. Int. J. Vitam. Nutr. Res. 1987, 57, 311–317. [Google Scholar]
- Farrell, C.P.; Morgan, M.; Rudolph, D.S.; Hwang, A.; Albert, N.E.; Valenzano, M.C.; Wang, X.; Mercogliano, G.; Mullin, J.M. Proton Pump Inhibitors Interfere with Zinc Absorption and Zinc Body Stores. Gastroenterol. Res. 2011, 4, 243–251. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Bogden, J.D.; Oleske, J.M.; Lavenhar, M.A.; Munves, E.M.; Kemp, F.W.; Bruening, K.S.; Holding, K.J.; Denny, T.N.; Guarino, M.A.; Krieger, L.M.; et al. Zinc and immunocompetence in elderly people: Effects of zinc supplementation for 3 months. Am. J. Clin. Nutr. 1988, 48, 655–663. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Keen, C.L. Trace elements and human health. Mol. Asp. Med. 2005, 26, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 1999, 4, D286–D298. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA- seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge- based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The use of total protein stains as loading controls: An alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef]
| RNA Microarray | |||
|---|---|---|---|
| Mean Age (range 64–79) | Gender Distribution | Racial Composition | |
| Placebo Group (n = 5) | 71.6 (range 64–79) | 2 males 3 females | Caucasian |
| Zn-Treated Group (n = 6) | 71.0 (range 61–76) | 1 male 5 females | Caucasian (5) African American (1) |
| Western Immunoblot Study | |||
| Mean Age (range 64–79) | Gender Distribution | Racial Composition | |
| Placebo Group (n = 12) | 68.0 (range 55–78) | 7 males 5 females | Caucasian |
| Zn-Treated Group (n = 11) | 68.0 (range 54–75) | 5 males 6 females | Caucasian (10) African American (1) |
| Serum D-Lactate Study | |||
| Mean Age (range 64–79) | Gender Distribution | Racial Composition | |
| (n = 19) | 44.0 (range 21–71) | 11 males 8 females | Caucasian (16) African American (2) Asian (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Rio, E.A.; Valenzano, M.C.; DiGuilio, K.M.; Rybakovsky, E.; Kjelstrom, S.; Montone, G.; Mercogliano, G.; Newman, G.; Wong, P.; Albert, N.; et al. Orally Administered Zinc Gluconate Induces Tight Junctional Remodeling and Reduces Passive Transmucosal Permeability Across Human Intestine in a Patient-Based Study. Int. J. Mol. Sci. 2025, 26, 8540. https://doi.org/10.3390/ijms26178540
Del Rio EA, Valenzano MC, DiGuilio KM, Rybakovsky E, Kjelstrom S, Montone G, Mercogliano G, Newman G, Wong P, Albert N, et al. Orally Administered Zinc Gluconate Induces Tight Junctional Remodeling and Reduces Passive Transmucosal Permeability Across Human Intestine in a Patient-Based Study. International Journal of Molecular Sciences. 2025; 26(17):8540. https://doi.org/10.3390/ijms26178540
Chicago/Turabian StyleDel Rio, Elizabeth A., Mary Carmen Valenzano, Katherine M. DiGuilio, Elizabeth Rybakovsky, Stephanie Kjelstrom, Georgia Montone, Giancarlo Mercogliano, Gary Newman, Patricia Wong, Nicole Albert, and et al. 2025. "Orally Administered Zinc Gluconate Induces Tight Junctional Remodeling and Reduces Passive Transmucosal Permeability Across Human Intestine in a Patient-Based Study" International Journal of Molecular Sciences 26, no. 17: 8540. https://doi.org/10.3390/ijms26178540
APA StyleDel Rio, E. A., Valenzano, M. C., DiGuilio, K. M., Rybakovsky, E., Kjelstrom, S., Montone, G., Mercogliano, G., Newman, G., Wong, P., Albert, N., Burris, V., Szymanski, K., Rodriguez, A., Hollis, E., Kossenkov, A., & Mullin, J. M. (2025). Orally Administered Zinc Gluconate Induces Tight Junctional Remodeling and Reduces Passive Transmucosal Permeability Across Human Intestine in a Patient-Based Study. International Journal of Molecular Sciences, 26(17), 8540. https://doi.org/10.3390/ijms26178540







