Identification of a Novel Homozygous SLC34A1 Missense Mutation and a Heterozygous SLC34A3 Deletion in an Infant with Nephrocalcinosis, Failure to Thrive, and Hypercalcemia
Abstract
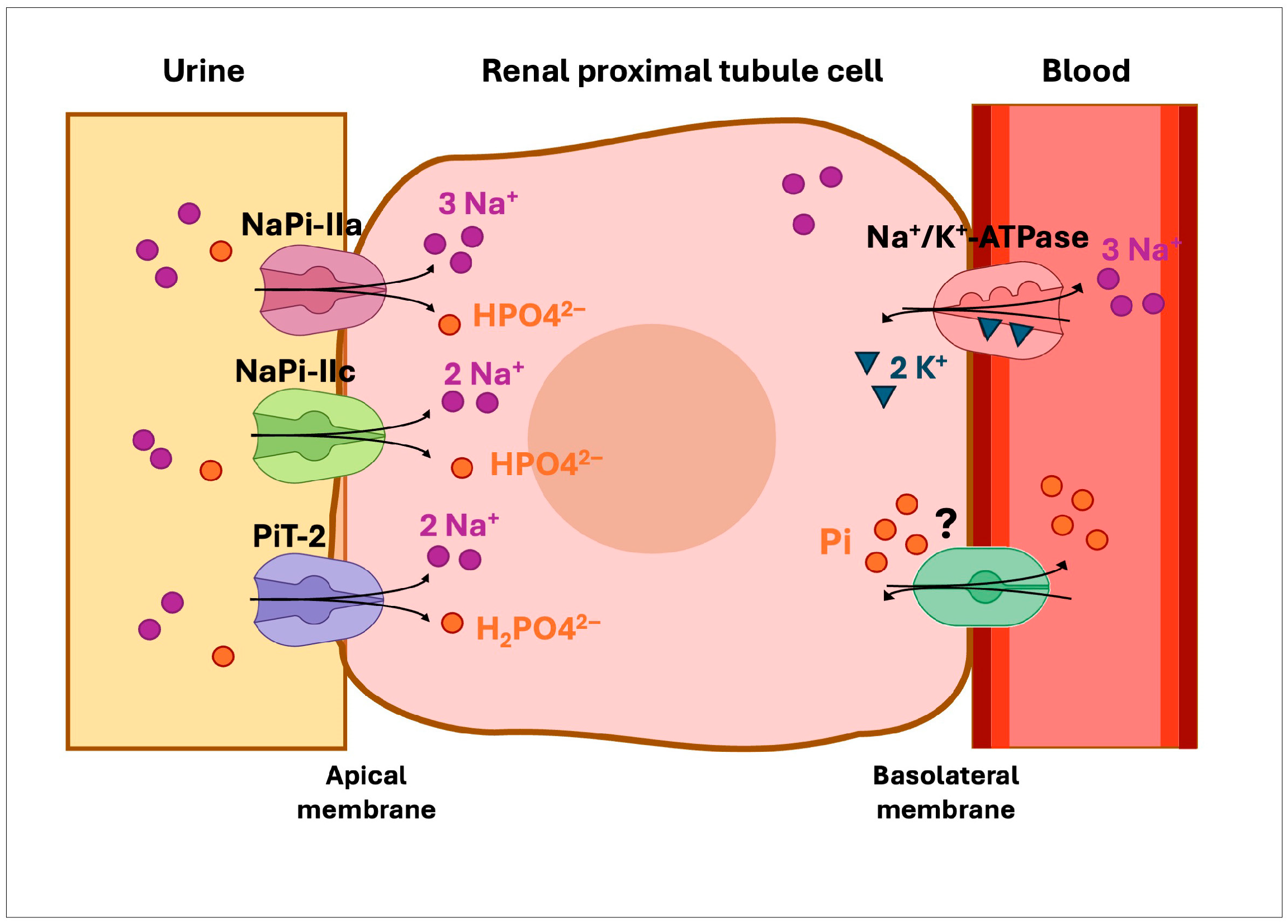
1. Introduction
2. Results
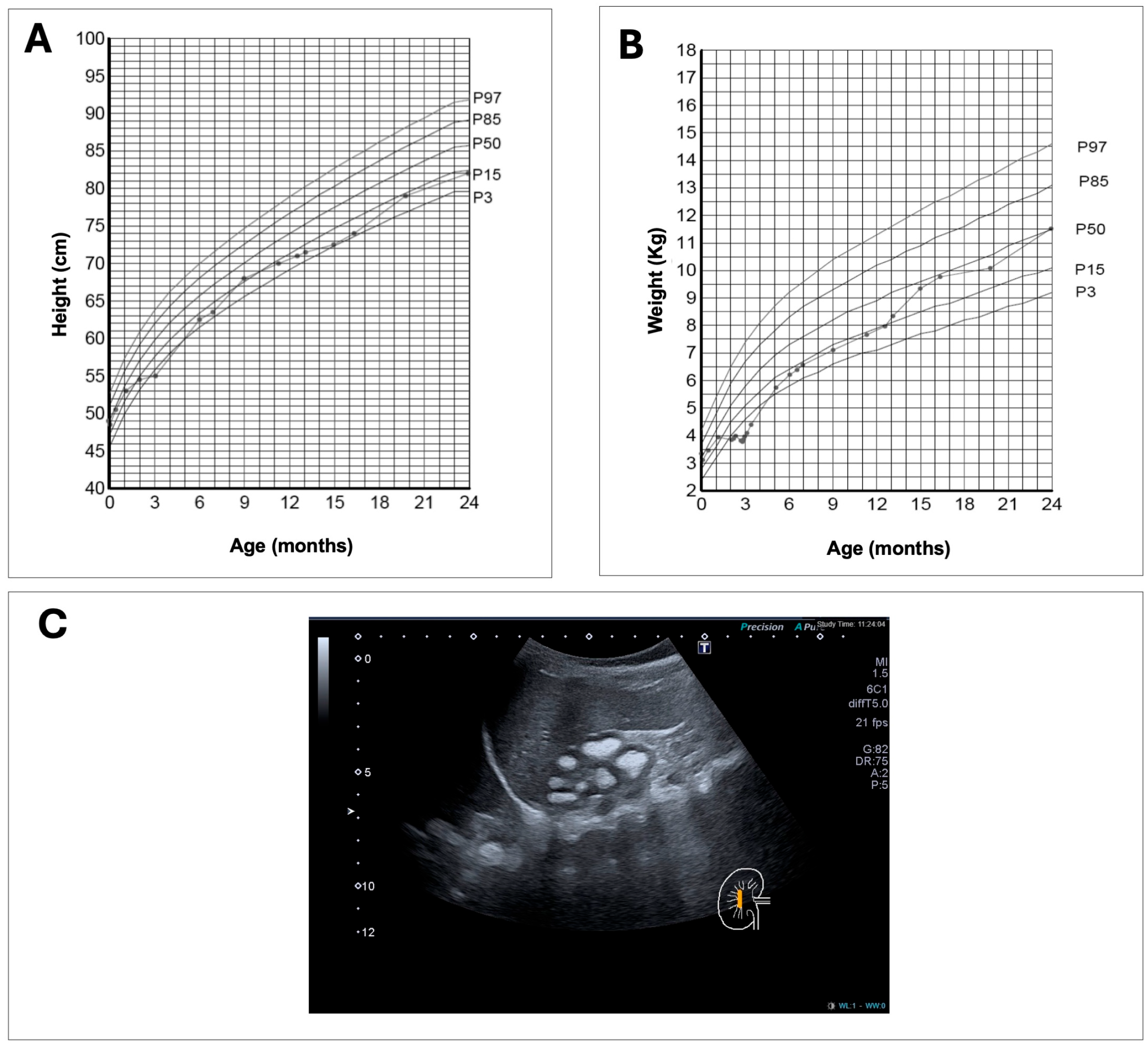
2.1. Clinical Case
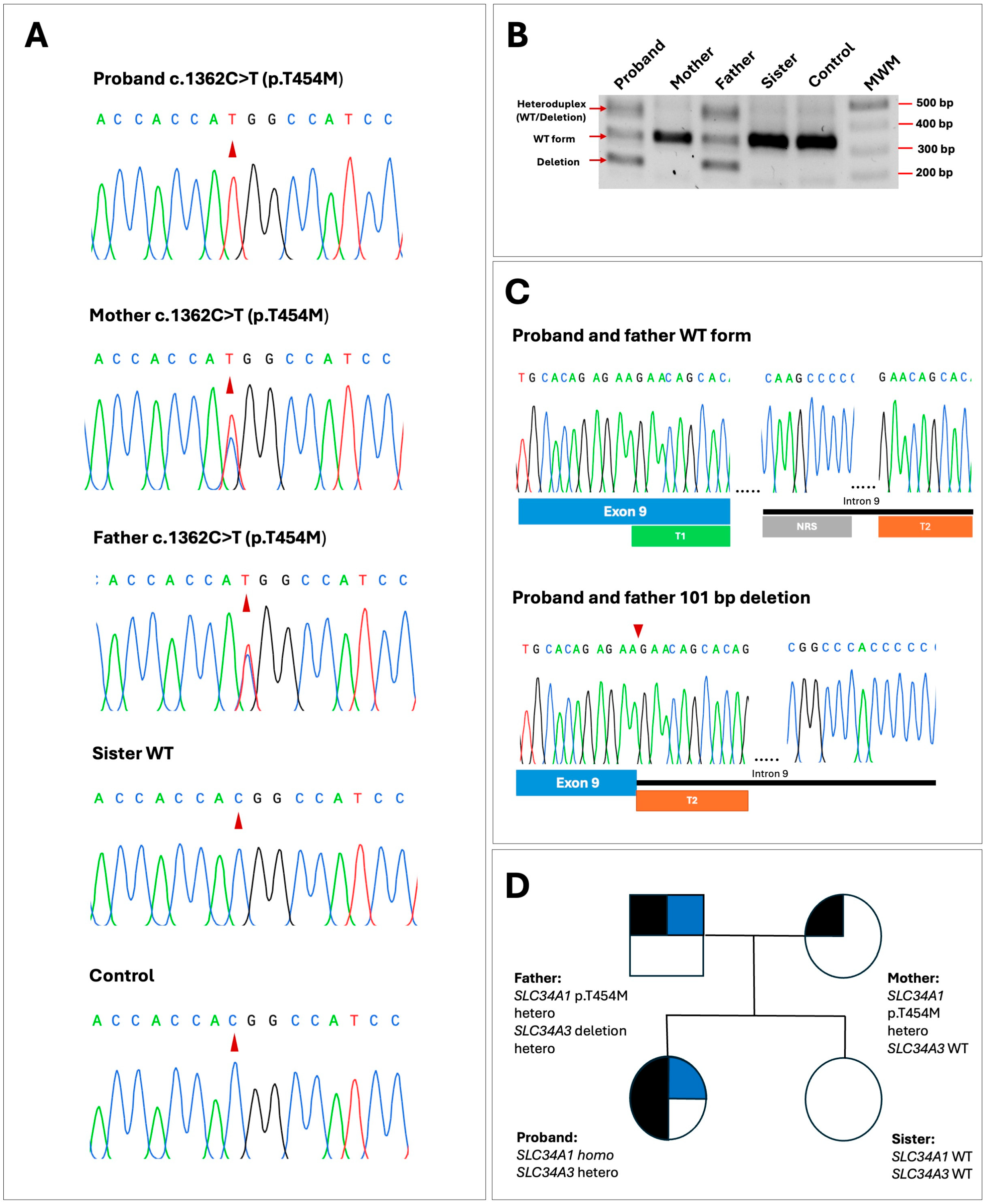
2.2. Genetic Findings
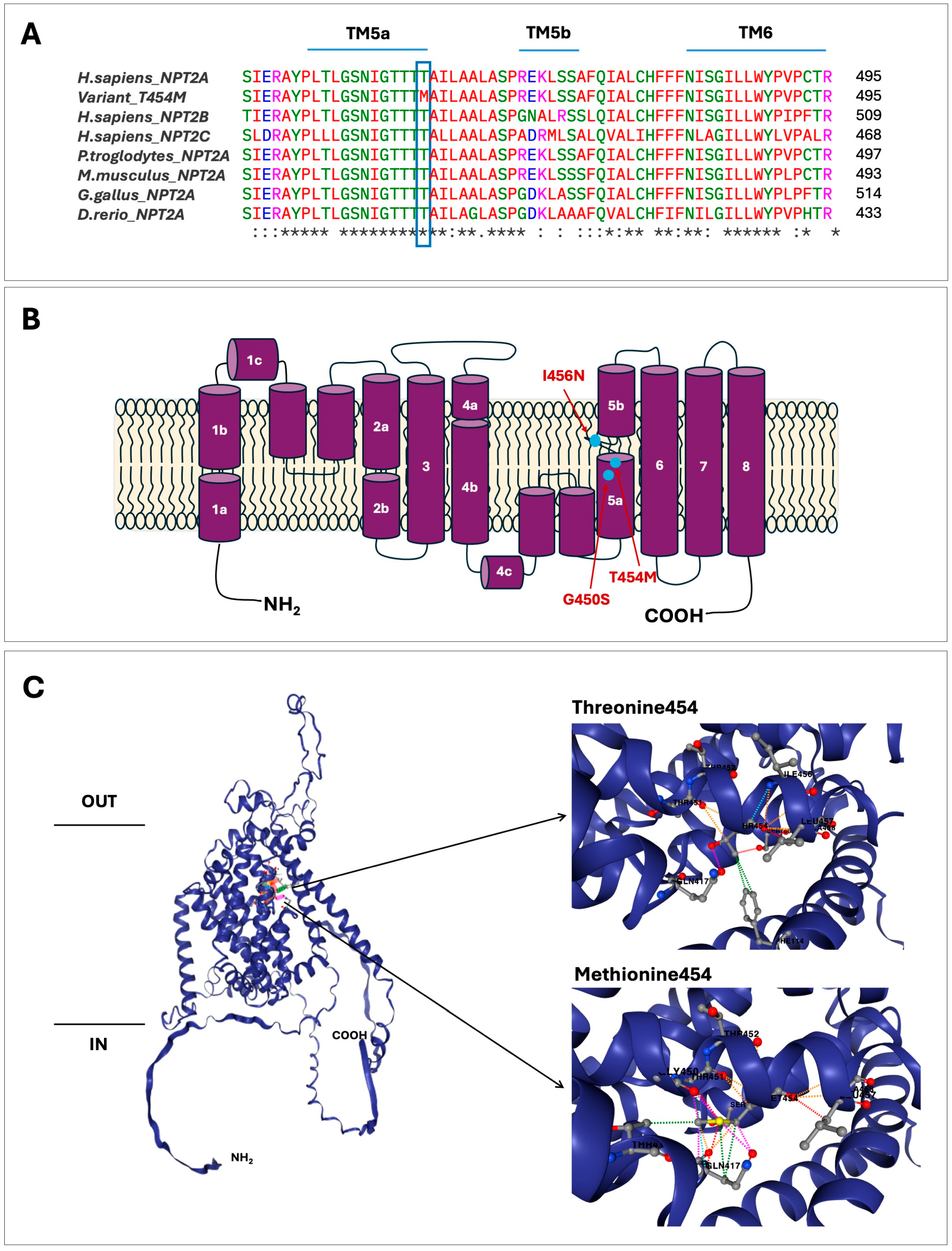
2.3. In Silico Assessment of Variant c.1361C>T; p.(T454M)
3. Discussion
Conclusions
4. Materials and Methods
4.1. Biochemical Determinations
4.2. Genetic Analysis
4.3. In Silico Prediction Analysis
4.4. Computational Protein Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a Signaling Molecule and Its Sensing Mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef]
- Hernando, N.; Gagnon, K.; Lederer, E. Phosphate Transport in Epithelial and Nonepithelial Tissue. Physiol. Rev. 2021, 101, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Kaneko, I.; Takahashi, A.; Kuwahata, M.; Ito, M.; Ohkido, I.; Tatsumi, S.; Miyamoto, K. Growth-related renal type II Na/Pi cotransporter. J. Biol. Chem. 2002, 277, 19665–19672. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Capuano, P.; Stange, G.; Mihailova, M.; Kaissling, B.; Murer, H.; Biber, J.; Wagner, C.A. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflug. Arch. 2010, 460, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C. The molecular mechanism of SLC34 proteins: Insights from two decades of transport assays and structure-function studies. Pflug. Arch. 2019, 471, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Gratton, E.; Forster, I.C.; Hernando, N.; Wagner, C.A.; Biber, J.; Sorribas, V.; Murer, H. Mechanisms of phosphate transport. Nat. Rev. Nephrol. 2019, 15, 482–500. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2008, 23, 22–44. [Google Scholar] [CrossRef]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 2013, 34, 386–395. [Google Scholar] [CrossRef]
- Beck, L.; Karaplis, A.C.; Amizuka, N.; Hewson, A.S.; Ozawa, H.; Tenenhouse, H.S. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 1998, 95, 5372–5377. [Google Scholar] [CrossRef]
- Ohkido, I.; Segawa, H.; Yanagida, R.; Nakamura, M.; Miyamoto, K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflug. Arch. 2003, 446, 106–115. [Google Scholar] [CrossRef]
- Segawa, H.; Onitsuka, A.; Furutani, J.; Kaneko, I.; Aranami, F.; Matsumoto, N.; Tomoe, Y.; Kuwahata, M.; Ito, M.; Matsumoto, M.; et al. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am. J. Physiol. Ren. Physiol. 2009, 297, F671–F678. [Google Scholar] [CrossRef]
- Murray, R.D.; Holthouser, K.; Clark, B.J.; Salyer, S.A.; Barati, M.T.; Khundmiri, S.J.; Lederer, E.D. Parathyroid hormone (PTH) decreases sodium-phosphate cotransporter type IIa (NpT2a) mRNA stability. Am. J. Physiol. Ren. Physiol. 2013, 304, F1076–F1085. [Google Scholar] [CrossRef] [PubMed]
- Burris, D.; Webster, R.; Sheriff, S.; Faroqui, R.; Levi, M.; Hawse, J.R.; Amlal, H. Estrogen directly and specifically downregulates NaPi-IIa through the activation of both estrogen receptor isoforms (ERα and ERβ) in rat kidney proximal tubule. Am. J. Physiol. Ren. Physiol. 2015, 308, F522–F534. [Google Scholar] [CrossRef][Green Version]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflug. Arch. 2019, 471, 83–98. [Google Scholar] [CrossRef]
- Wagner, C.A.; Egli-Spichtig, D.; Rubio-Aliaga, I. Updates on renal phosphate transport. Curr. Opin. Nephrol. Hypertens. 2025, 34, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Ruminska, J.; Kaufmann, M.; Dursun, I.; Patti, M.; Kranz, B.; Pronicka, E.; Ciara, E.; Akcay, T.; Bulus, D.; et al. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J. Am. Soc. Nephrol. 2016, 27, 604–614. [Google Scholar] [CrossRef]
- Lederer, E.; Wagner, C.A. Clinical aspects of the phosphate transporters NaPi-IIa and NaPi-IIb: Mutations and disease associations. Pflug. Arch. 2019, 471, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Tieder, M.; Modai, D.; Samuel, R.; Arie, R.; Halabe, A.; Bab, I.; Gabizon, D.; Liberman, U.A. Hereditary hypophosphatemic rickets with hypercalciuria. N. Engl. J. Med. 1985, 312, 611–617. [Google Scholar] [CrossRef]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Benet-Pages, A.; Eckstein, G.; Tenenbaum-Rakover, Y.; Wagenstaller, J.; Tiosano, D.; Gershoni-Baruch, R.; Albers, N.; Lichtner, P.; Schnabel, D.; et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 2006, 78, 193–201. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Bröking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, M.; Brunkhorst, L.; Martens, H.; Papizh, S.; Besouw, M.; Grasemann, C.; Turan, S.; Sikora, P.; Chromek, M.; Cornelissen, E.; et al. Presentation and outcome in carriers of pathogenic variants in SLC34A1 and SLC34A3 encoding sodium-phosphate transporter NPT2a and 2c. Kidney Int. 2025, 107, 116–129. [Google Scholar] [CrossRef]
- Dinour, D.; Beckerman, P.; Ganon, L.; Tordjman, K.; Eisenstein, Z.; Holtzman, E.J. Loss-of-function mutations of CYP24A1, the vitamin D 24-hydroxylase gene, cause long-standing hypercalciuric nephrolithiasis and nephrocalcinosis. J. Urol. 2013, 190, 552–557. [Google Scholar] [CrossRef]
- De Paolis, E.; Scaglione, G.L.; De Bonis, M.; Minucci, A.; Capoluongo, E. CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: From genotype to phenotype. Clin. Chem. Lab. Med. 2019, 57, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Lemoine, S.; Kaufmann, M.; Breton, P.; Nowoczyn, M.; Ballandonne, C.; Coudray, N.; Mittre, H.; Richard, N.; Ryckwaert, A.; et al. Overlapping phenotypes associated with CYP24A1, SLC34A1, and SLC34A3 mutations: A cohort study of patients with hypersensitivity to vitamin D. Front. Endocrinol. 2021, 12, 736240. [Google Scholar] [CrossRef]
- Nwachukwu, C.; Singh, G.; Moore, B.; Strande, N.T.; Bucaloiu, I.D.; Chang, A.R. Risk of nephrolithiasis in adults heterozygous for SLC34A3 Ser192Leu in an unselected health system cohort. J. Am. Soc. Nephrol. 2023, 34, 1819–1821. [Google Scholar] [CrossRef]
- Prié, D.; Huart, V.; Bakouh, N.; Planelles, G.; Dellis, O.; Gérard, B.; Hulin, P.; Benqué-Blanchet, F.; Silve, C.; Grandchamp, B.; et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N. Engl. J. Med. 2002, 347, 983–991. [Google Scholar] [CrossRef]
- Asociación Española de Nefrología Pediátrica (AENP). Nefrología Pediátrica. In Manual Práctico; Antón Gamero, M., Rodríguez Fernández, L.M., Eds.; AENP: Madrid, Spain, 2011; 400p, ISBN 9788498353020. [Google Scholar]
- Caliper. Reference Intervals for Creatinine (Jaffé Standardized, Abbott). Available online: https://caliper.research.sickkids.ca/#/search (accessed on 20 July 2025).
- García-Nieto, V.M.; González-Rodríguez, J.D.; Cabrera-Sevilla, J.E.; Martín-Fernández de Basoa, M.C.; Luis-Yanes, M.I. Reflections on TRP and TP/GFR in the definition of renal phosphate loss: Conceptual review. Pediatr. Nephrol. 2023, 38, 3845–3848. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sorenson, A.H.; Imel, E.A.; Friedman, N.E.; Gertner, J.M.; Econs, M.J. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J. Clin. Endocrinol. Metab. 2006, 91, 4022–4027. [Google Scholar] [CrossRef]
- Hasani-Ranjbar, S.; Ejtahed, H.S.; Amoli, M.M.; Bitarafan, F.; Qorbani, M.; Soltani, A.; Yarjoo, B. SLC34A3 intronic deletion in an Iranian kindred with hereditary hypophosphatemic rickets with hypercalciuria. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Fearn, A.; Allison, B.; Rice, S.J.; Edwards, N.; Halbritter, J.; Bourgeois, S.; Pastor-Arroyo, E.M.; Hildebrandt, F.; Tasic, V.; Wagner, C.A.; et al. Clinical, biochemical, and pathophysiological analysis of SLC34A1 mutations. Physiol. Rep. 2018, 6, e13715. [Google Scholar] [CrossRef]
- Braun, D.A.; Lawson, J.A.; Gee, H.Y.; Halbritter, J.; Shril, S.; Tan, W.; Stein, D.; Wassner, A.J.; Ferguson, M.A.; Gucev, Z.; et al. Prevalence of monogenic causes in pediatric patients with nephrolithiasis or nephrocalcinosis. Clin. J. Am. Soc. Nephrol. 2016, 11, 664–672. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.J.; Wei, L.Y.; Ding, Y.; Liu, M.; Li, W.J.; Su, C.; Gong, C.X. Biallelic and monoallelic pathogenic variants in CYP24A1 and SLC34A1 genes cause idiopathic infantile hypercalcemia. Orphanet J. Rare Dis. 2024, 19, 126. [Google Scholar] [CrossRef]
- Gordon, R.J.; Li, D.; Doyle, D.; Zaritsky, J.; Levine, M.A. Digenic heterozygous mutations in SLC34A3 and SLC34A1 cause dominant hypophosphatemic rickets with hypercalciuria. J. Clin. Endocrinol. Metab. 2020, 105, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Marini, F.; Al-Alwani, H.; Marasco, E.; Garagnani, P.; Khan, A.A.; Brandi, M.L. A novel heterozygous mutation c.1627G>T (p.Gly543Cys) in the SLC34A1 gene in a male patient with recurrent nephrolithiasis and early onset osteopenia: A case report. Int. J. Mol. Sci. 2023, 24, 17289. [Google Scholar] [CrossRef] [PubMed]
- Lenherr-Taube, N.; Young, E.J.; Furman, M.; Elia, Y.; Assor, E.; Chitayat, D.; Uster, T.; Kirwin, S.; Robbins, K.; Vinette, K.M.B.; et al. Mild idiopathic infantile hypercalcemia—Part 1: Biochemical and genetic findings. J. Clin. Endocrinol. Metab. 2021, 106, 2915–2937. [Google Scholar] [CrossRef]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Chapman, M.; Evans, K.; Azevedo, L.; Hayden, M.; Heywood, S.; Millar, D.S.; Phillips, A.D.; et al. The Human Gene Mutation Database (HGMD®): Optimizing its use in a clinical diagnostic or research setting. Hum. Genet. 2020, 139, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Perwad, F.; Azam, N.; Zhang, M.Y.; Yamashita, T.; Tenenhouse, H.S.; Portale, A.A. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 2005, 146, 5358–5364. [Google Scholar] [CrossRef]
- Fenollar-Ferrer, C.; Forrest, L.R. Structural models of the NaPi-II sodium-phosphate cotransporters. Pflug. Arch. 2019, 471, 43–52. [Google Scholar] [CrossRef]
- Rajagopal, A.; Braslavsky, D.; Lu, J.T.; Kleppe, S.; Clément, F.; Cassinelli, H.; Liu, D.S.; Liern, J.M.; Vallejo, G.; Bergadá, I.; et al. Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J. Clin. Endocrinol. Metab. 2014, 99, E2451–E2456. [Google Scholar] [CrossRef] [PubMed]
- Dinour, D.; Davidovits, M.; Ganon, L.; Ruminska, J.; Forster, I.C.; Hernando, N.; Eyal, E.; Holtzman, E.J.; Wagner, C.A. Loss of function of NaPiIIa causes nephrocalcinosis and possibly kidney insufficiency. Pediatr. Nephrol. 2016, 31, 2289–2297. [Google Scholar] [CrossRef]
- Brazier, F.; Courbebaisse, M.; David, A.; Bergerat, D.; Leroy, C.; Lindner, M.; Maruani, G.; Saint Jacques, C.; Letavernier, E.; Hureaux, M.; et al. Relationship between clinical phenotype and in vitro analysis of 13 NPT2c/SCL34A3 mutants. Sci. Rep. 2023, 13, 85. [Google Scholar] [CrossRef]
- Zhu, Z.; Bo-Ran Ho, B.; Chen, A.; Amrhein, J.; Apetrei, A.; Carpenter, T.O.; Lazaretti-Castro, M.; Colazo, J.M.; McCrystal Dahir, K.; Geßner, M.; et al. An update on clinical presentation and responses to therapy of patients with hereditary hypophosphatemic rickets with hypercalciuria (HHRH). Kidney Int. 2024, 105, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, G.; Carpenter, T.O.; Forman, S.; Jüppner, H.; Bergwitz, C. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am. J. Physiol. Ren. Physiol. 2008, 295, F371–F379. [Google Scholar] [CrossRef]
- Dasgupta, D.; Wee, M.J.; Reyes, M.; Li, Y.; Simm, P.J.; Sharma, A.; Schlingmann, K.P.; Janner, M.; Biggin, A.; Lazier, J.; et al. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J. Am. Soc. Nephrol. 2014, 25, 2366–2375. [Google Scholar] [CrossRef]
- Sadeghi-Alavijeh, O.; Chan, M.M.Y.; Moochhala, S.H.; Genomics England Research Consortium; Howles, S.; Gale, D.P.; Böckenhauer, D. Rare variants in the sodium-dependent phosphate transporter gene SLC34A3 explain missing heritability of urinary stone disease. Kidney Int. 2023, 104, 975–984. [Google Scholar] [CrossRef]
- Tabibzadeh, N.; Cheddani, L.; Daudon, M.; Haymann, J.P.; Toussaint, A.; Silve, C.; Letavernier, E. The case: Epistasis and urolithiasis. Kidney Int. 2017, 92, 523–524. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Basic Local Alignment Search Tool (BLAST). Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 October 2024).
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Steinhaus, R.; Proft, S.; Schuelke, M.; Cooper, D.N.; Schwarz, J.M.; Seelow, D. MutationTaster2021. Nucleic Acids Res. 2021, 49, W446–W451. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Schubach, M.; Maass, T.; Nazaretyan, L.; Röner, S.; Kircher, M. CADD v1.7: Using protein language models, regulatory CNNs and other nucleotide-level scores to improve genome-wide variant predictions. Nucleic Acids Res. 2024, 52, D1143–D1154. [Google Scholar] [CrossRef]
- Harrison, S.M.; Biesecker, L.G.; Rehm, H.L. Overview of specifications to the ACMG/AMP variant interpretation guidelines. Curr. Protoc. Hum. Genet. 2019, 103, e93. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.J.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 2020, 11, 5918. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Venselaar, H.; Te Beek, T.A.; Kuipers, R.K.; Hekkelman, M.L.; Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform. 2010, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Ittisoponpisan, S.; Islam, S.A.; Khanna, T.; Alhuzimi, E.; David, A.; Sternberg, M.J.E. Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J. Mol. Biol. 2019, 431, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. DynaMut2: Assessing changes in stability and flexibility upon single and multiple point missense mutations. Protein Sci. 2021, 30, 60–69. [Google Scholar] [CrossRef] [PubMed]
| Age (Months) | 7 | 10 | 16 | 22 | 28 | Reference Values | |
|---|---|---|---|---|---|---|---|
| Blood | Creatinine (mg/dL) * | 0.47 (+0.91) | 0.42 (0.00) | 0.42 (–1.25) | 0.44 (–0.75) | 0.41 (–1.50) | 15 days-<1 year: 0.31–0.53. 1–2 years: 0.39–0.55. |
| Calcium (mg/dL) | 11.2 (+3.5) | 11.5 (+4.25) | 10.7 (+2.25) | 10.4 (+1.50) | 10.0 (+0.50) | 10 days-2 years: 9–10.6 | |
| Phosphate (mg/dL) | 4.6 (–2.31) | 4.5 (–2.46) | 4.2 (–2.60) | 3.9 (–3.20) | 3.2 (–4.60) | 0–1 year: 4.8–7.4 1–5 years: 4.5–6.5 | |
| PTH a (pg/mL) | 13.8 (–2.42) | 16 (–2.30) | - | 12.2 (–2.50) | - | 22.0–101.0 | |
| 25-(OH) vitamin D (ng/mL) | 58.6 (+0.54) | 54.0 (+0.13) | 36.1 (–1.46) | - | 31.1 (–1.90) | 30–75 | |
| 1,25-(OH)2D3 (pg/mL) | 78 (+4.82) | 43 (+0.71) | 74 (+4.35) | - | 71 (+4.00) | 20–54 | |
| Urine | TRP b (%) ** | 75.29 (–0.41) | 69.68 (–1.07) | 54.69 (–2.86) | 61.32 (–2.07) | 64.22 (–6.61) | 0–2 years: 78.7 ± 8.4 >2 years: 92 ± 4.2 |
| Calcium/Creatinine (mg/mg) | 0.33 (+0.10) | 0.47 (+1.08) | 0.51 (+2.45) | 0.15 (–0.88) | 0.31 (+4.44) | 0.1–0.5 years: 0.03–0.80 0.6–1 year: 0.03–0.60 1–2 years: 0.03–0.46 2–3 years: 0.02–0.20 | |
| Protein/Creatinine (mg/mg) | 0.56 | 0.44 | - | 0.00 | 0.41 | <2 years: <0.5 >2 years<0.2 | |
| V/GFR c (%) | 2.70 | 2.57 | 2.94 (+10.68) | 3.21 (+11.91) | 2.05 (+6.64) | >1 year: 0.59 ± 0.22 |
| VarSome a | Franklin b | CADD c | Polyphen2 d | SIFT e | MutPred f |
|---|---|---|---|---|---|
| PP3 | PM2, PP3 | 32 | 1.00 | 0.00 | 0.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mura-Escorche, G.; García-Suarez, L.C.; Lebredo-Álvarez, I.; Ramos-Trujillo, E.; Claverie-Martin, F. Identification of a Novel Homozygous SLC34A1 Missense Mutation and a Heterozygous SLC34A3 Deletion in an Infant with Nephrocalcinosis, Failure to Thrive, and Hypercalcemia. Int. J. Mol. Sci. 2025, 26, 8541. https://doi.org/10.3390/ijms26178541
Mura-Escorche G, García-Suarez LC, Lebredo-Álvarez I, Ramos-Trujillo E, Claverie-Martin F. Identification of a Novel Homozygous SLC34A1 Missense Mutation and a Heterozygous SLC34A3 Deletion in an Infant with Nephrocalcinosis, Failure to Thrive, and Hypercalcemia. International Journal of Molecular Sciences. 2025; 26(17):8541. https://doi.org/10.3390/ijms26178541
Chicago/Turabian StyleMura-Escorche, Glorián, Leire C. García-Suarez, Isis Lebredo-Álvarez, Elena Ramos-Trujillo, and Felix Claverie-Martin. 2025. "Identification of a Novel Homozygous SLC34A1 Missense Mutation and a Heterozygous SLC34A3 Deletion in an Infant with Nephrocalcinosis, Failure to Thrive, and Hypercalcemia" International Journal of Molecular Sciences 26, no. 17: 8541. https://doi.org/10.3390/ijms26178541
APA StyleMura-Escorche, G., García-Suarez, L. C., Lebredo-Álvarez, I., Ramos-Trujillo, E., & Claverie-Martin, F. (2025). Identification of a Novel Homozygous SLC34A1 Missense Mutation and a Heterozygous SLC34A3 Deletion in an Infant with Nephrocalcinosis, Failure to Thrive, and Hypercalcemia. International Journal of Molecular Sciences, 26(17), 8541. https://doi.org/10.3390/ijms26178541





