Effects of L-Arginine on Bone Metabolism: Evidence from In Vitro and In Vivo Models
Abstract
1. Introduction
2. Results
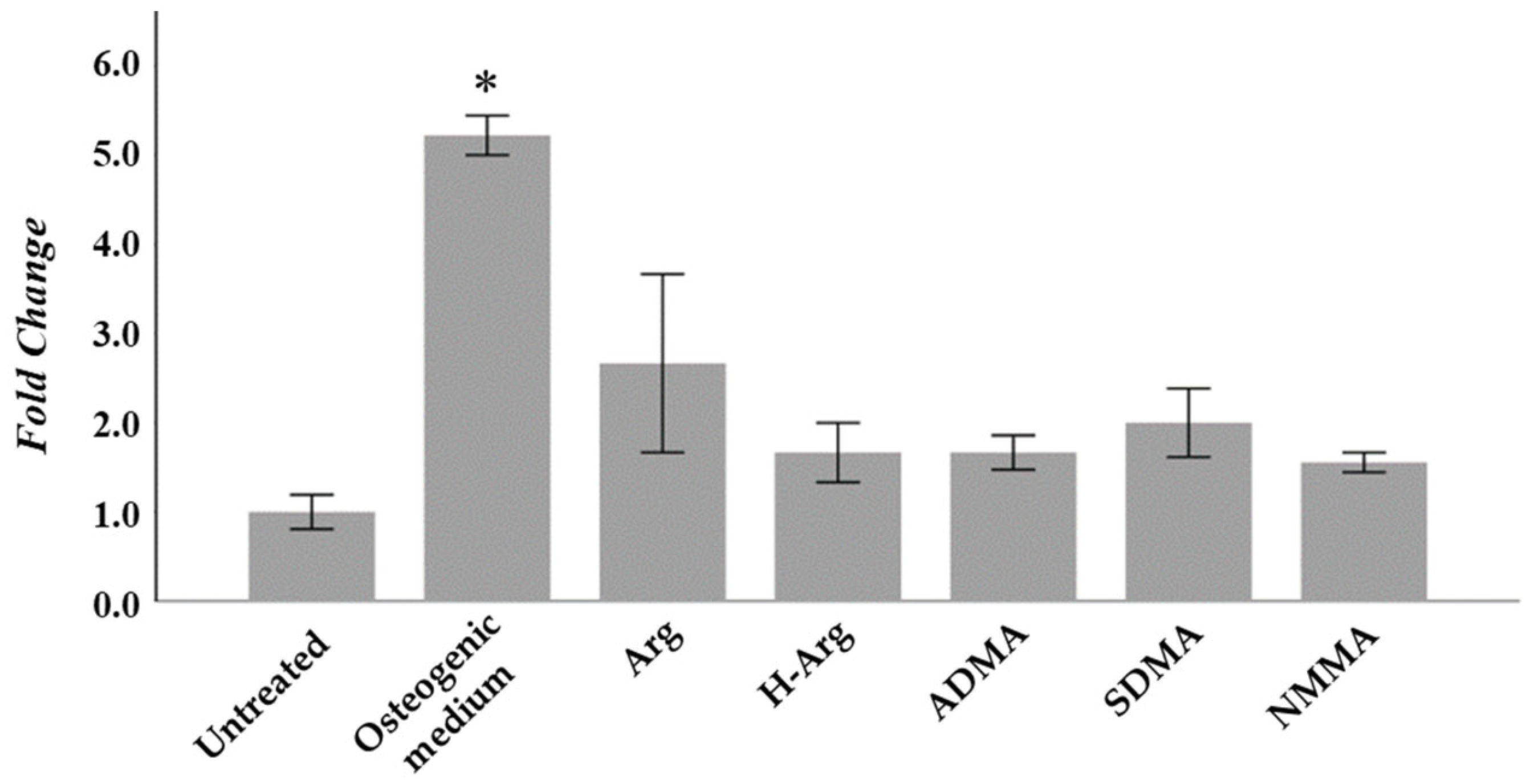
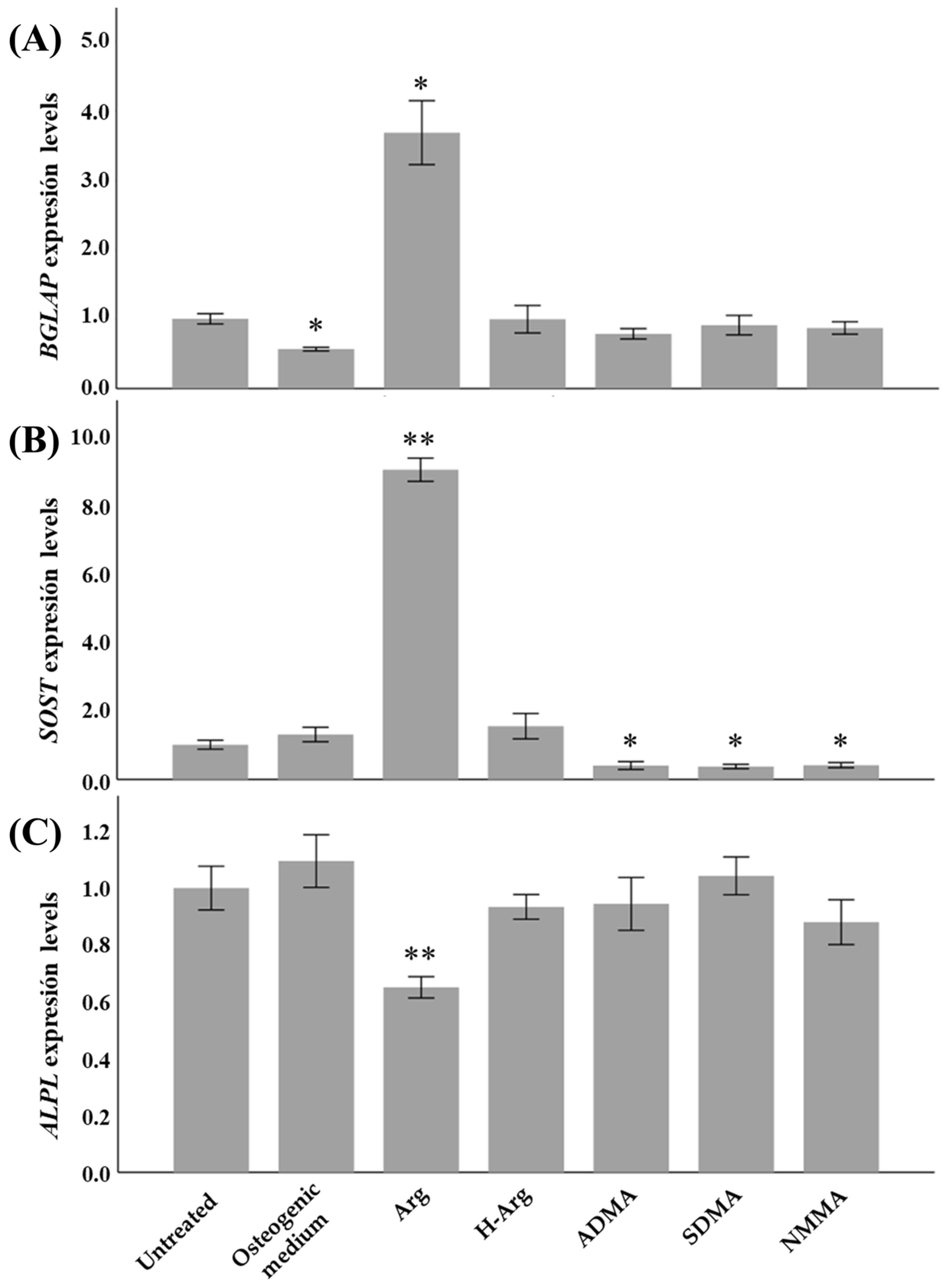
2.1. Osteogenic Function of Arginine In Vitro
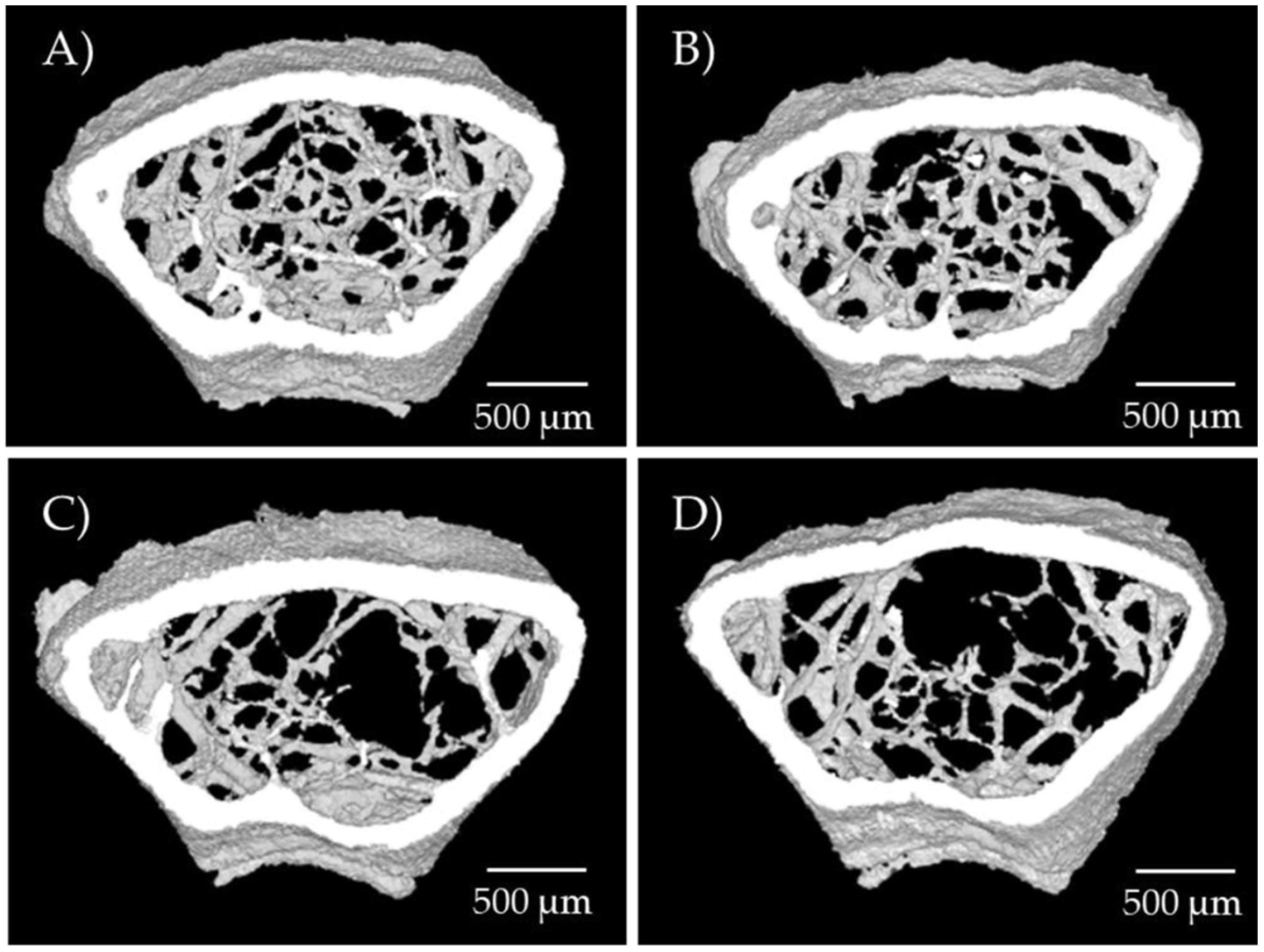
2.2. Osteogenic Function of Arginine In Vivo
3. Discussion
4. Materials and Methods
4.1. Study of the Osteogenic Function of Arginine In Vitro
4.2. Quantification of Calcium Mineral Deposition
4.3. Gene Expression
4.4. Study of Osteogenic Function of Arginine In Vivo
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorentzon, M.; Johansson, H.; Harvey, N.C.; Liu, E.; Vandenput, L.; McCloskey, E.V.; Kanis, J.A. Osteoporosis and Fractures in Women: The Burden of Disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef]
- Ward, R.J.; Roberts, C.C.; Bencardino, J.T.; Arnold, E.; Baccei, S.J.; Cassidy, R.C.; Chang, E.Y.; Fox, M.G.; Greenspan, B.S.; Gyftopoulos, S.; et al. ACR Appropriateness Criteria® Osteoporosis and Bone Mineral Density. J. Am. Coll. Radiol. 2017, 14, S189–S202. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Fuggle, N.R.; Cooper, C.; Harvey, N.C. Epigenetic Regulation of Bone Mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101612. [Google Scholar] [CrossRef] [PubMed]
- Panach, L.; Serna, E.; Tarín, J.J.; Cano, A.; García-Pérez, M.Á. A Translational Approach from an Animal Model Identifies CD80 as a Candidate Gene for the Study of Bone Phenotypes in Postmenopausal Women. Osteoporos. Int. 2017, 28, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Torricelli, P.; Giavaresi, G.; Carpi, A.; Nicolini, A.; Giardino, R. Effect of L-Lysine and L-Arginine on Primary Osteoblast Cultures from Normal and Osteopenic Rats. Biomed. Pharmacother. 2001, 55, 213–220. [Google Scholar] [CrossRef]
- Abdelkarem, H.M.; Fadda, L.H.; El-Sayed, E.M.; Radwan, O.K. Potential Role of L-Arginine and Vitamin E Against Bone Loss Induced by Nano-Zinc Oxide in Rats. J. Diet. Suppl. 2018, 15, 300–310. [Google Scholar] [CrossRef]
- Baecker, N.; Boese, A.; Schoenau, E.; Gerzer, R.; Heer, M. L-Arginine, the Natural Precursor of NO, Is Not Effective for Preventing Bone Loss in Postmenopausal Women. J. Bone Miner. Res. 2005, 20, 471–479. [Google Scholar] [CrossRef]
- Makama, M.; McDougall, A.R.A.; Cao, J.; Mills, K.; Nguyen, P.; Hastie, R.; Ammerdorffer, A.; Gülmezoglu, A.M.; Vogel, J.P. L-Arginine and L-Citrulline for Prevention and Treatment of Pre-Eclampsia: A Systematic Review and Meta-Analysis. BJOG 2025, 132, 698–708. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Q.; Li, W.; Liu, M.; Li, Q.; Chen, Q. Efficacy of L-Arginine and Pycnogenol® in the Treatment of Male Erectile Dysfunction: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1211720. [Google Scholar] [CrossRef]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef]
- Barros, C.D.S.; Coutinho, A.; Tengan, C.H. Arginine Supplementation in MELAS Syndrome: What Do We Know about the Mechanisms? Int. J. Mol. Sci. 2024, 25, 3629. [Google Scholar] [CrossRef]
- Shiraseb, F.; Asbaghi, O.; Bagheri, R.; Wong, A.; Figueroa, A.; Mirzaei, K. Effect of L-Arginine Supplementation on Blood Pressure in Adults: A Systematic Review and Dose–Response Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2022, 13, 1226–1242. [Google Scholar] [CrossRef]
- García-Pérez, M.A.; Del Val, R.; Noguera, I.; Hermenegildo, C.; Pineda, B.; Martinez-Romero, A.; Cano, A. Estrogen Receptor Agonists and Immune System in Ovariectomized Mice. Int. J. Immunopathol. Pharmacol. 2006, 19, 807–819. [Google Scholar] [CrossRef]
- Jung, G.H.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Extremely High White Blood Cell Counts on Postoperative Day 1 Do Not Predict Severe Complications Following Distal Pancreatectomy. Ann. Hepatobiliary Pancreat. Surg. 2019, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A. Introduction to Bone Biology. Bone 1992, 13, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Rowe, P.S.; Lim, H.P.; Welldon, K.J.; Ormsby, R.; Wijenayaka, A.R.; Zelenchuk, L.; Evdokiou, A.; Findlay, D.M. Sclerostin Is a Locally Acting Regulator of Late-Osteoblast/Preosteocyte Differentiation and Regulates Mineralization through a MEPE-ASARM-Dependent Mechanism. J. Bone Miner. Res. 2011, 26, 1425–1436. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Sophocleous, A.; Idris, A.I. Rodent Models of Osteoporosis. Bonekey Rep. 2014, 3, 614. [Google Scholar] [CrossRef]
- García-Pérez, M.A. Physiological Regulation of Bone Metabolism and Estrogen Agonism. In Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs; Cano, A., Calaf i Alsina, J., Dueñas-Díez, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 161–186. [Google Scholar]
- Cano, A.; Dapía, S.; Noguera, I.; Pineda, B.; Hermenegildo, C.; Del Val, R.; Caeiro, J.R.; García-Pérez, M.A. Comparative Effects of 17β-Estradiol, Raloxifene and Genistein on Bone 3D Microarchitecture and Volumetric Bone Mineral Density in the Ovariectomized Mice. Osteoporos. Int. 2008, 19, 793–800. [Google Scholar] [CrossRef]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast Differentiation and Bone Formation Gene Expression in Strontium-Inducing Bone Marrow Mesenchymal Stem Cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar]
- Lorentzon, M.; Branco, J.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Cooper, C.; Cortet, B.; Diez-Perez, A.; Ferrari, S.; Gasparik, A.; et al. Algorithm for the Use of Biochemical Markers of Bone Turnover in the Diagnosis, Assessment and Follow-Up of Treatment for Osteoporosis. Adv. Ther. 2019, 36, 2811–2824. [Google Scholar] [CrossRef]
- Hale, L.V.; Galvin, R.J.S.; Risteli, J.; Ma, Y.L.; Harvey, A.K.; Yang, X.; Cain, R.L.; Zeng, Q.; Frolik, C.A.; Sato, M.; et al. PINP: A Serum Biomarker of Bone Formation in the Rat. Bone 2007, 40, 1103–1109. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Yao, Q.; Qin, J. Early Effects of Ovariectomy on Bone Microstructure, Bone Turnover Markers and Mechanical Properties in Rats. BMC Musculoskelet. Disord. 2022, 23, 316. [Google Scholar] [CrossRef]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Morko, J.; Rasi, S.; Risteli, J.; Halleen, J.M. Short-Term Changes in Serum PINP Predict Long-Term Changes in Trabecular Bone in the Rat Ovariectomy Model. Calcif. Tissue Int. 2008, 82, 155–161. [Google Scholar] [CrossRef]
- Pennisi, P.; Clementi, G.; Prato, A.; Luca, T.; Martinez, G.; Mangiafico, R.A.; Pulvirenti, I.; Muratore, F.; Fiore, C.E. L-Arginine Supplementation Normalizes Bone Turnover and Preserves Bone Mass in Streptozotocin-Induced Diabetic Rats. J. Endocrinol. Investig. 2009, 32, 546–551. [Google Scholar] [CrossRef]
- Choi, M.-J.; Chang, K.J. Effect of Dietary Taurine and Arginine Supplementation on Bone Mineral Density in Growing Female Rats. Adv. Exp. Med. Biol. 2013, 776, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine Supplementation and Wound Healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Preli, R.B.; Klein, K.P.; Herrington, D.M. Vascular Effects of Dietary L-Arginine Supplementation. Atherosclerosis 2002, 162, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McConell, G.K. Effects of L-Arginine Supplementation on Exercise Metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 46–51. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Nitric Oxide and Bone. Ann. N. Y. Acad. Sci. 2010, 1192, 391–403. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; De Marco, G.; Gangula, P.; Yallampalli, C. Nitric Oxide Donor Alleviates Ovariectomy-Induced Bone Loss. Bone 1996, 18, 301–304. [Google Scholar] [CrossRef]
- Wallner, S.; Hermetter, A.; Mayer, B.; Wascher, T.C. The Alpha-amino Group of L-arginine Mediates Its Antioxidant Effect. Eur. J. Clin. Investig. 2001, 31, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Suessenbacher, A.; Wölkart, G.; Mayer, B.; Brunner, F. Functional and Analytical Evidence for Scavenging of Oxygen Radicals by L-Arginine. Mol. Pharmacol. 2002, 61, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; Tell, G.; Robbie-Ryan, M.; Gao, Y.; Terauchi, M.; Yang, X.; Romanello, M.; Jones, D.P.; Weitzmann, M.N.; Pacifici, R. Oxidative Stress Causes Bone Loss in Estrogen-Deficient Mice through Enhanced Bone Marrow Dendritic Cell Activation. Proc. Natl. Acad. Sci. USA 2007, 104, 15087–15092. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Zhong, Z.-M.; Hou, G.; Jiang, H.; Chen, J.-T. Involvement of Oxidative Stress in Age-Related Bone Loss. J. Surg. Res. 2011, 169, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Browner, W.S.; Bauer, D.C.; Cummings, S.R. Intermittent Use of Nitrates Increases Bone Mineral Density: The Study of Osteoporotic Fractures. J. Bone Miner. Res. 1998, 13, 1755–1759. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Mosekilde, L. Decreased Fracture Risk in Users of Organic Nitrates: A Nationwide Case-Control Study. J. Bone Miner. Res. 2006, 21, 1811–1817. [Google Scholar] [CrossRef]
- Weaver, C.; Bishop, A.E.; Polak, J.M. Pancreatic Changes Elicited by Chronic Administration of Excess L-Arginine. Exp. Mol. Pathol. 1994, 60, 71–87. [Google Scholar] [CrossRef]
- Mizunuma, T.; Kawamura, S.; Kishino, Y. Effects of Injecting Excess Arginine on Rat Pancreas. J. Nutr. 1984, 114, 467–471. [Google Scholar] [CrossRef]
- Matull, W.R. Biochemical Markers of Acute Pancreatitis. J. Clin. Pathol. 2006, 59, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J.A.; Barkin, J.S. Chronic Pancreatitis and Bone Disease. J. Clin. Densitom. 2020, 23, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Jobgen, W.S.; Kim, S.W.; Lassala, A.; Li, P.; Matis, J.H.; Meininger, C.J.; Spencer, T.E. Pharmacokinetics and Safety of Arginine Supplementation in Animals. J. Nutr. 2007, 137, 1673S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Santaolaria, M.L.; Meneu, V.; Alonso, E. Dietary Arginine Slightly and Variably Affects Tissue Polyamine Levels in Male Swiss Albino Mice. J. Nutr. 2002, 132, 3715–3720. [Google Scholar] [CrossRef]
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and Prospects: Public Health Regulation of Dietary Supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef]
- Bode-Böger, S.M. Effect of L-Arginine Supplementation on NO Production in Man. Eur. J. Clin. Pharmacol. 2006, 62, 91–99. [Google Scholar] [CrossRef]
- Gregory, C.A.; Grady Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin Red-Based Assay of Mineralization by Adherent Cells in Culture: Comparison with Cetylpyridinium Chloride Extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- García-Pérez, M.A.; Noguera, I.; Hermenegildo, C.; Martínez-Romero, A.; Tarín, J.J.; Cano, A. Alterations in the Phenotype and Function of Immune Cells in Ovariectomy-Induced Osteopenic Mice. Human Reprod. 2006, 21, 880–887. [Google Scholar] [CrossRef]
- Pertusa, C.; Tarín, J.J.; Cano, A.; García-Pérez, M.Á.; Mifsut, D. Serum MicroRNAs in Osteoporotic Fracture and Osteoarthritis: A Genetic and Functional Study. Sci. Rep. 2021, 11, 19372. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. J. Opt. Soc. Am. A 1984, 1, 612. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A New Method for the Model-independent Assessment of Thickness in Three-dimensional Images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Ulrich, D.; van Rietbergen, B.; Laib, A.; Rüegsegger, P. The Ability of Three-Dimensional Structural Indices to Reflect Mechanical Aspects of Trabecular Bone. Bone 1999, 25, 55–60. [Google Scholar] [CrossRef]
| SHAM (N) | SHAM + Arg (N) | OVX (N) | OVX + Arg (N) | p-Value | |
|---|---|---|---|---|---|
| Biochemical Parameters | |||||
| Urea (mg/dL) | 41.0 ± 8.2 (5) | 40.3 ± 4.4 (4) | 41.8 ± 5.7 (8) | 45.0 ± 10.8 (7) | 0.430 |
| Albumin (g/dL) | 4.0 ± 0.3 (5) | 3.8 ± 0.6 (4) | 3.9 ± 0.2 (8) | 3.9 ± 0.1 (7) | 0.279 |
| Alkaline phosphatase (U/L) | 115.2 ± 6.9 (5) | 110.8 ± 30.6 (4) | 110.6 ± 7.5 (8) | 107.4 ± 5.8 (7) | 0.150 |
| Alanine transaminase (U/L) | 55.8 ± 52.3 (5) | 33.0 ± 5.2 (4) | 60.4 ± 38.8 (8) | 53.7 ± 50.6 (7) | 0.052 |
| Amylase (U/dL) | 361.8 ± 133.9 (5) | 256.5 ± 47.9 (4) | 370.4 ± 283.1 (8) | 272.9 ± 32.4 (7) | 0.441 |
| Aspartate transaminase (U/dL) | 118.4 ± 100.0 (5) | 81.5 ± 14.4 (4) | 119.0 ± 55.3 (8) | 97.3 ± 50.9 (7) | 0.309 |
| Blood urea nitrogen(mg/dL) | 19.1 ± 3.8 (5) | 18.9 ± 2.1 (4) | 19.5 ± 2.7 (8) | 21.0 ± 5.0 (7) | 0.430 |
| Cholesterol (mg/dL) | 91.8 ± 10.8 (5) | 91.5 ± 3.1 (4) | 99.0 ± 6.3 (8) | 93.1 ± 17.2 (7) | 0.428 |
| Creatinine (mg/dL) | 0.38 ± 0.05 (5) | 0.35 ± 0.06 (4) | 0.34 ± 0.08 (8) | 0.38 ± 0.04 (7) | 0.202 |
| Calcium (mg/dL) | 9.9 ± 0.6 (5) | 9.8 ± 0.4 (4) | 9.9 ± 0.4 (8) | 9.8 ± 0.2 (7) | 0.521 |
| Glucose (mg/dL) | 224.6 ± 38.2 (5) | 203.0 ± 38.7 (4) | 206.8 ± 23.9 (8) | 201.1 ± 46.0 (7) | 0.205 |
| Phosphate (mg/dL) | 6.6 ± 1.0 (5) | 6.8 ± 1.9 (4) | 6.9 ± 2.1 (8) | 5.7 ± 1.0 (7) | 0.326 |
| Total bilirubin (mg/dL) | 0.32 ± 0.11 (5) | 0.35 ± 0.27 (4) | 0.21 ± 0.18 (8) | 0.44 ± 0.19 (7) | 0.045 (a) |
| Total protein (mg/dL) | 5.1 ± 0.4 (5) | 5.4 ± 0.4 (4) | 5.3 ± 0.3 (8) | 5.1 ± 0.2 (7) | 0.619 |
| Hematopoietic parameters | |||||
| Hemoglobin (g/dL) | 14.8 ± 0.4 (5) | 14.2 ± 1.2 (4) | 14.4 ± 0.5 (8) | 14.3 ± 0.9 (8) | 0.420 |
| Haematocrit (%) | 44.1 ± 1.0 (5) | 42.5 ± 3.4 (4) | 42.9 ± 1.2 (8) | 43.0 ± 2.1 (8) | 0.488 |
| Erythrocytes (1012/L) | 9.5 ± 0.1 (5) | 9.3 ± 0.6 (4) | 9.3 ± 0.3 (8) | 9.2 ± 0.6 (8) | 0.439 |
| Leukocytes (109/L) | 4.8 ± 2.0 (5) | 3.9 ± 1.0 (4) | 7.1 ± 4.7 (8) | 6.8 ± 1.8 (8) | 0.018 (b) |
| Platelets (109/L) | 584.2 ± 330.1 (5) | 487.5 ± 377.2 (4) | 750.4 ± 401.4 (8) | 797.0 ± 404.6 (8) | 0.054 |
| SHAM (N) | SHAM + Arg (N) | OVX (N) | OVX + Arg (N) | p-Value | |
|---|---|---|---|---|---|
| tbBMD (g/cm3) | 0.132 ± 0.021 (4) | 0.120 ± 0.010 (3) | 0.117 ± 0.011 (7) | 0.095 ± 0.018 (8) | 0.002 (a, b) |
| BV/TV (%) | 6.3 ± 0.5 (4) | 5.6 ± 0.4 (3) | 5.3 ± 0.8 (7) | 4.5 ± 0.5 (8) | <0.001 (c, d) |
| Trabecular thickness (mm) | 0.045 ± 0.004 (4) | 0.042 ± 0.001 (3) | 0.042 ± 0.002 (7) | 0.040 ± 0.001 (8) | 0.005 (c) |
| Number of trabeculae (1/mm) | 1.36 ± 0.10 (4) | 1.33 ± 0.07 (3) | 1.28 ± 0.17 (7) | 1.12 ± 0.11 (8) | 0.011 (a, b) |
| Trabecular separation (mm) | 0.278 ± 0.007 (4) | 0.278 ± 0.009 (3) | 0.310 ± 0.020 (7) | 0.315 ± 0.025 (8) | <0.001 (e) |
| Trabecular bone pattern factor (1/mm) | 35.2 ± 1.7 (4) | 38.7 ± 0.6 (3) | 36.4 ± 1.9 (7) | 38.8 ± 2.0 (8) | 0.002 (d) |
| coBMD (g/cm3) | 1.27 ± 0.02 (5) | 1.27 ± 0.01 (4) | 1.26 ± 0.02 (8) | 1.27 ± 0.02 (8) | 0.285 |
| Cortical bone volume (mm3) | 0.663 ± 0.040 (5) | 0.693 ± 0.057 (4) | 0.625 ± 0.048 (8) | 0.610 ± 0.023 (8) | 0.015 (a) |
| CS/BV (%) | 15.2 ± 0.3 (5) | 15.1 ± 0.1 (4) | 16.8 ± 0.5 (8) | 16.7 ± 0.3 (8) | <0.001 (e) |
| Cortical bone thickness (mm) | 0.183 ± 0.008 (5) | 0.185 ± 0.002 (4) | 0.170 ± 0.005 (8) | 0.167 ± 0.004 (8) | <0.001 (e) |
| Polar moment of inertia (mm4) | 0.339 ± 0.044 (5) | 0.362 ± 0.063 (4) | 0.319 ± 0.017 (8) | 0.314 ± 0.024 (8) | 0.098 (a) |
| Biochemical Markers | SHAM (N) | SHAM + Arg (N) | OVX (N) | OVX + Arg (N) | p-Value |
|---|---|---|---|---|---|
| CTx (ng/mL) | 28.4 ± 23.3 (5) | 9.6 ± 0.9 (4) | 26.6 ± 15.0 (8) | 21.5 ± 8.5 (8) | 0.005 (a, b) |
| PINP (ng/mL) | 67.6 ± 23.8 (5) | 51.1 ± 24.8 (4) | 79.4 ± 18.7 (8) | 72.9 ± 12.1 (8) | 0.058 (c) |
| ALP (units/mL) | 131.3 ± 15.1 (5) | 128.5 ± 61.7 (4) | 128.2 ± 17.1 (8) | 122.6 ± 15.1 (8) | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertusa, C.; Carrasco-García, Á.; Aliaga, R.; Suay, L.; Alonso-Iglesias, E.; Cano, A.; Tarín, J.J.; García-Pérez, M.Á. Effects of L-Arginine on Bone Metabolism: Evidence from In Vitro and In Vivo Models. Int. J. Mol. Sci. 2025, 26, 8484. https://doi.org/10.3390/ijms26178484
Pertusa C, Carrasco-García Á, Aliaga R, Suay L, Alonso-Iglesias E, Cano A, Tarín JJ, García-Pérez MÁ. Effects of L-Arginine on Bone Metabolism: Evidence from In Vitro and In Vivo Models. International Journal of Molecular Sciences. 2025; 26(17):8484. https://doi.org/10.3390/ijms26178484
Chicago/Turabian StylePertusa, Clara, Álvaro Carrasco-García, Rosa Aliaga, Loreto Suay, Eulalia Alonso-Iglesias, Antonio Cano, Juan J. Tarín, and Miguel Ángel García-Pérez. 2025. "Effects of L-Arginine on Bone Metabolism: Evidence from In Vitro and In Vivo Models" International Journal of Molecular Sciences 26, no. 17: 8484. https://doi.org/10.3390/ijms26178484
APA StylePertusa, C., Carrasco-García, Á., Aliaga, R., Suay, L., Alonso-Iglesias, E., Cano, A., Tarín, J. J., & García-Pérez, M. Á. (2025). Effects of L-Arginine on Bone Metabolism: Evidence from In Vitro and In Vivo Models. International Journal of Molecular Sciences, 26(17), 8484. https://doi.org/10.3390/ijms26178484






