Temporal Molecular Signatures of Early Human Clavicle Fracture Healing: Characterization of Hematological, Cytokine, and miRNA Profiles
Abstract
1. Introduction
2. Results
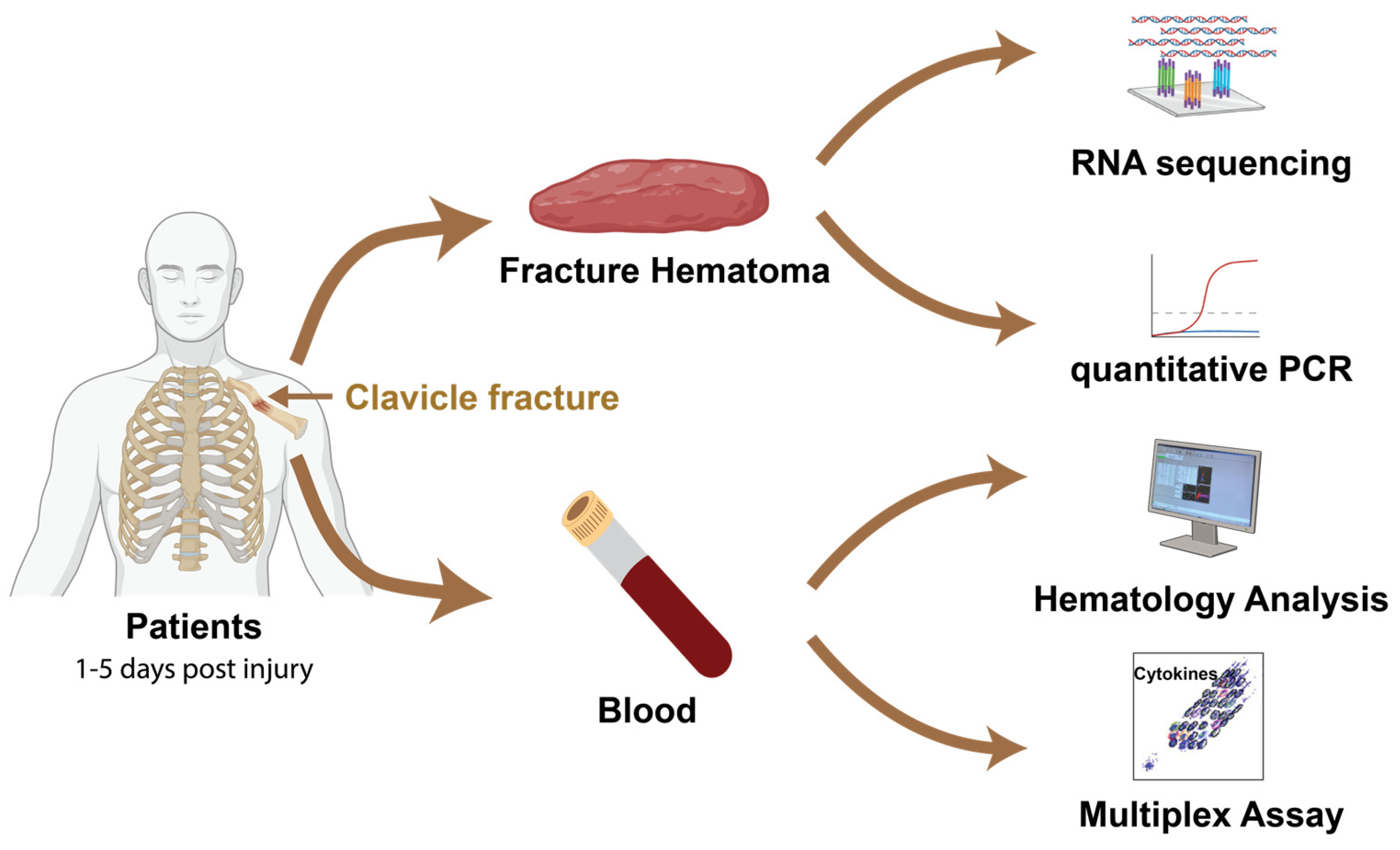
2.1. Study Population and Design
2.1.1. Study Design and Patient Characteristics
2.1.2. Patient Demographics and Clinical Characteristics
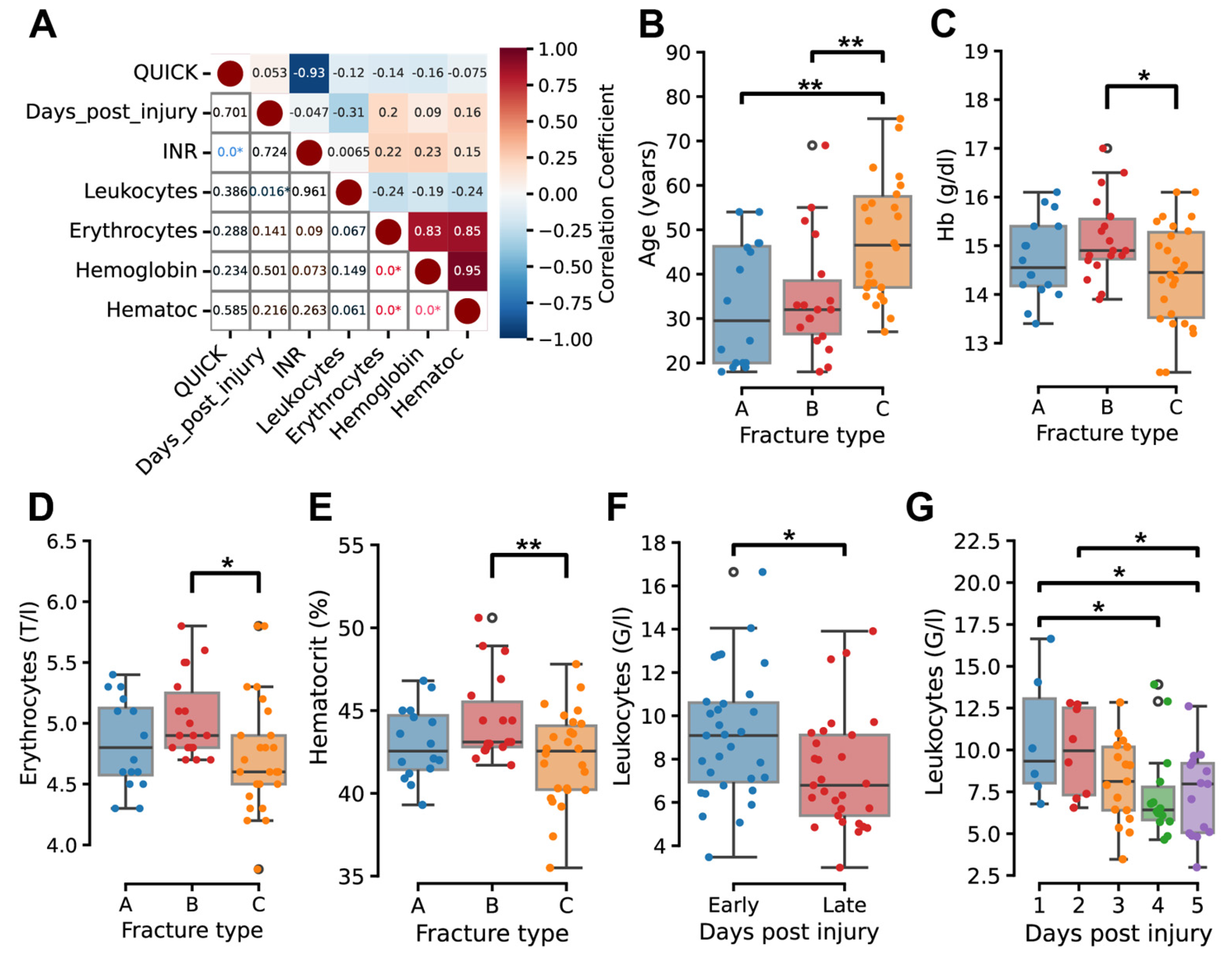
2.2. Temporal and Severity-Dependent Hematological Changes
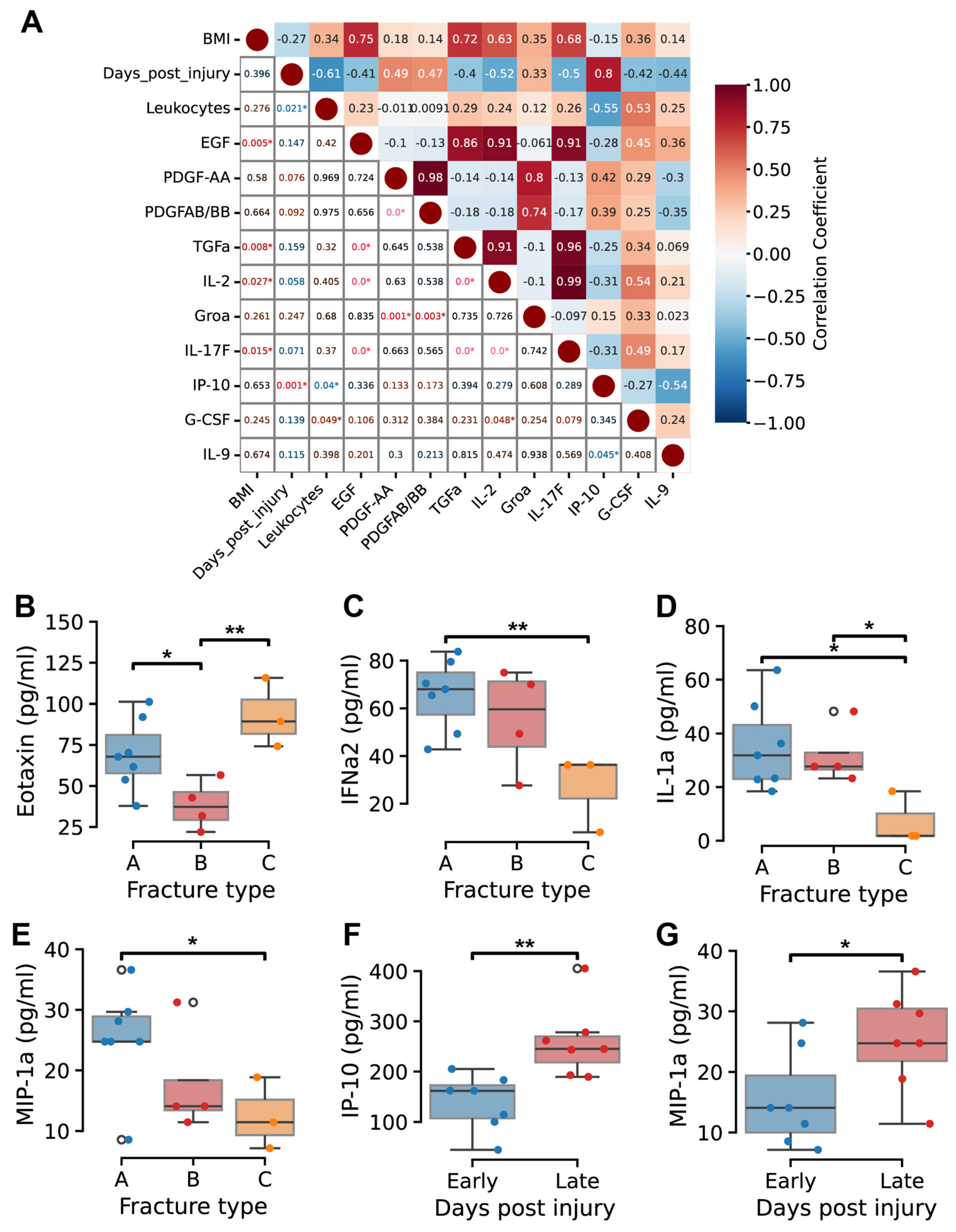
2.3. Temporal Dynamics of Cytokine Profiles
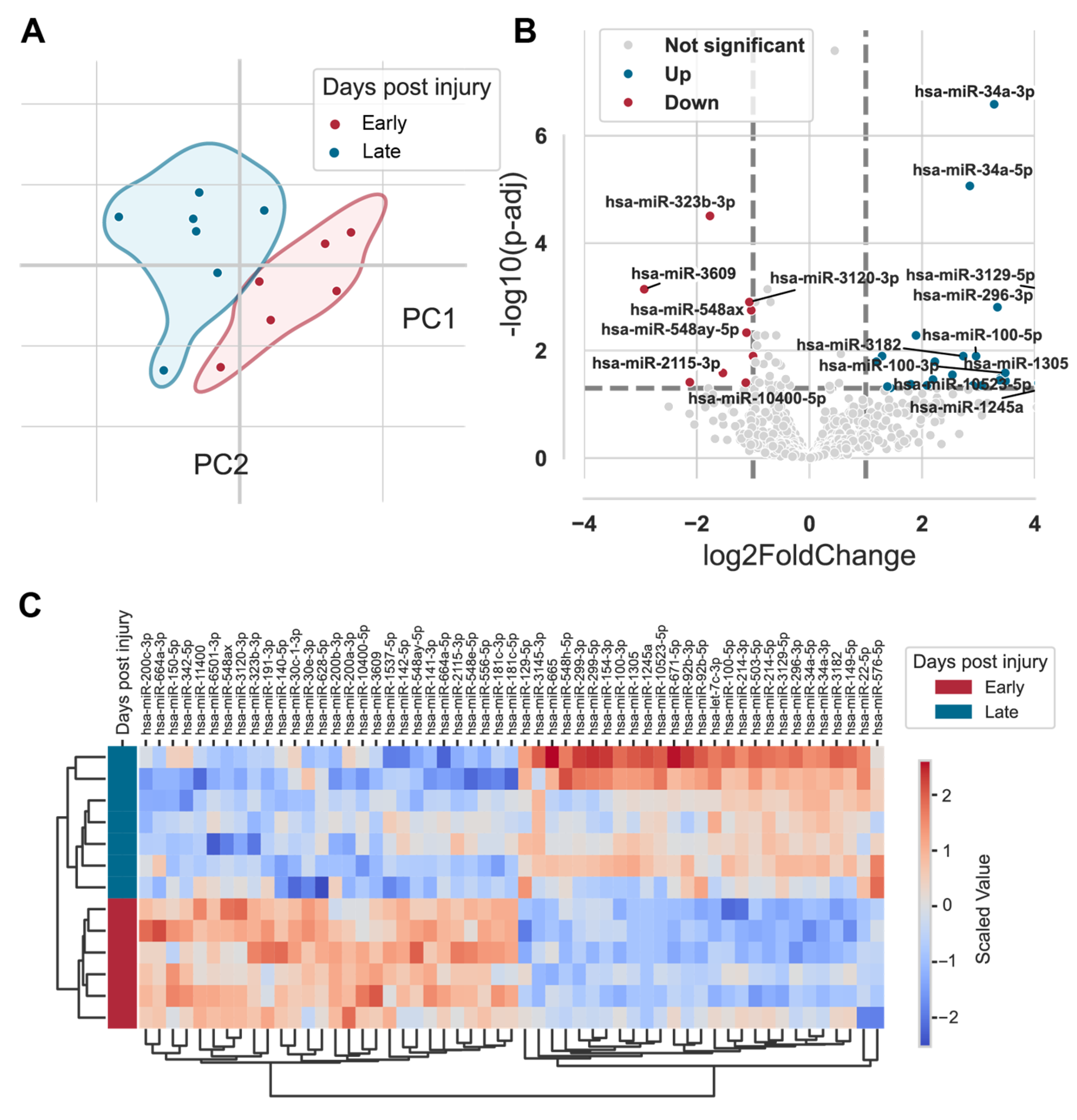
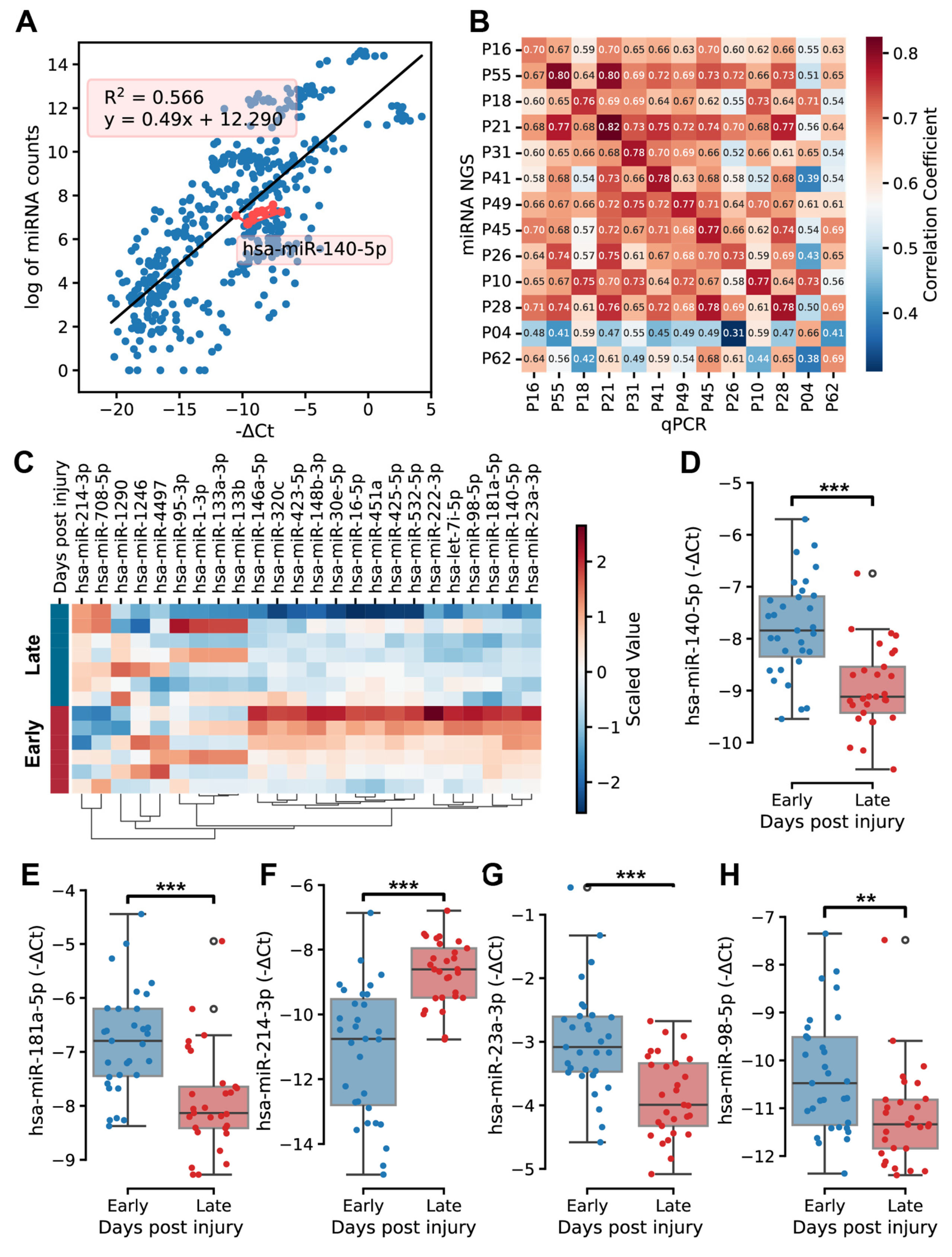
2.4. Expression Profiles of miRNAs in Fracture Hematoma
2.5. Quantitative PCR Validation and Function Analysis of Key MicroRNAs
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Sample Processing
4.3. MiRNA Isolation and Sequencing
4.4. Quantitative PCR
4.5. Cytokine Profiles
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoff, P.; Gaber, T.; Strehl, C.; Schmidt-Bleek, K.; Lang, A.; Huscher, D.; Burmester, G.R.; Schmidmaier, G.; Perka, C.; Duda, G.N.; et al. Immunological characterization of the early human fracture hematoma. Immunol. Res. 2016, 64, 1195–1206. [Google Scholar] [CrossRef]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Vanderkarr, M.F.; Ruppenkamp, J.W.; Vanderkarr, M.; Holy, C.E.; Blauth, M. Risk factors and healthcare costs associated with long bone fracture non-union: A retrospective US claims database analysis. J. Orthop. Surg. Res. 2023, 18, 745. [Google Scholar] [CrossRef] [PubMed]
- Steppe, L.; Megafu, M.; Tschaffon-Muller, M.E.A.; Ignatius, A.; Haffner-Luntzer, M. Fracture healing research: Recent insights. Bone Rep. 2023, 19, 101686. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Postacchini, F.; Gumina, S.; De Santis, P.; Albo, F. Epidemiology of clavicle fractures. J. Shoulder Elb. Surg. 2002, 11, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Kihlstrom, C.; Moller, M.; Lonn, K.; Wolf, O. Clavicle fractures: Epidemiology, classification and treatment of 2 422 fractures in the Swedish Fracture Register; an observational study. BMC Musculoskelet. Disord. 2017, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Zlowodzki, M.; Zelle, B.A.; Cole, P.A.; Jeray, K.; McKee, M.D. Treatment of Acute Midshaft Clavicle Fractures: Systematic Review of 2144 Fractures: On behalf of the Evidence-Based Orthopaedic Trauma Working Group. J. Orthop. Trauma 2005, 19, 504–507. [Google Scholar] [CrossRef]
- McKee, R.C.; Whelan, D.B.; Schemitsch, E.H.; McKee, M.D. Operative versus nonoperative care of displaced midshaft clavicular fractures: A meta-analysis of randomized clinical trials. J. Bone Joint Surg. Am. 2012, 94, 675–684. [Google Scholar] [CrossRef]
- Wang, S.; He, W.; Wang, H.; Liu, D.; Wang, M.; Yang, H.; Pan, G.; Li, B. Hematoma-like dynamic hydrogelation through natural glycopeptide molecular recognition for infected bone fracture repair. Bioact. Mater. 2023, 30, 73–84. [Google Scholar] [CrossRef]
- Walters, G.; Pountos, I.; Giannoudis, P.V. The cytokines and micro-environment of fracture haematoma: Current evidence. J. Tissue Eng. Regen. Med. 2018, 12, e1662–e1677. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Maschmeyer, P.; Gaber, T.; Schutze, T.; Raue, T.; Schmidt-Bleek, K.; Dziurla, R.; Schellmann, S.; Lohanatha, F.L.; Rohner, E.; et al. Human immune cells’ behavior and survival under bioenergetically restricted conditions in an in vitro fracture hematoma model. Cell Mol. Immunol. 2013, 10, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Walters, G.; Panteli, M.; Einhorn, T.A.; Giannoudis, P.V. Inflammatory Profile and Osteogenic Potential of Fracture Haematoma in Humans. J. Clin. Med. 2019, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, S.; Sun, K.; Wang, J.; Liu, X.; Zhao, Y.; Yang, J.; Zhao, D.; Xue, C.; Tao, Y.; et al. Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet. Disord. 2021, 22, 494. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Bucher, C.H.; Berkmann, J.C.; Burkhardt, L.M.; Paschke, C.; Schlundt, C.; Lang, A.; Wolter, A.; Damerau, A.; Geissler, S.; Volk, H.D.; et al. Local immune cell contributions to fracture healing in aged individuals—A novel role for interleukin 22. Exp. Mol. Med. 2022, 54, 1262–1276. [Google Scholar] [CrossRef]
- Dimmeler, S.; Nicotera, P. MicroRNAs in age-related diseases. EMBO Mol. Med. 2013, 5, 180–190. [Google Scholar] [CrossRef]
- Nugent, M. MicroRNAs and Fracture Healing. Calcif. Tissue Int. 2017, 101, 355–361. [Google Scholar] [CrossRef]
- Foessl, I.; Kotzbeck, P.; Obermayer-Pietsch, B. miRNAs as novel biomarkers for bone related diseases. J. Lab. Precis. Med. 2019, 4, 2. [Google Scholar] [CrossRef]
- Hackl, M.; Heilmeier, U.; Weilner, S.; Grillari, J. Circulating microRNAs as novel biomarkers for bone diseases—Complex signatures for multifactorial diseases? Mol. Cell Endocrinol. 2016, 432, 83–95. [Google Scholar] [CrossRef]
- Feichtinger, X.; Muschitz, C.; Heimel, P.; Baierl, A.; Fahrleitner-Pammer, A.; Redl, H.; Resch, H.; Geiger, E.; Skalicky, S.; Dormann, R.; et al. Bone-related Circulating MicroRNAs miR-29b-3p, miR-550a-3p, and miR-324-3p and their Association to Bone Microstructure and Histomorphometry. Sci. Rep. 2018, 8, 4867. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Longaker, M.T.; Quarto, N. Circulating and extracellular vesicle-derived microRNAs as biomarkers in bone-related diseases. Front. Endocrinol. 2023, 14, 1168898. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—Types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018, 42, 247–258. [Google Scholar] [CrossRef]
- Nauth, A.; Lee, M.; Gardner, M.J.; Brinker, M.R.; Warner, S.J.; Tornetta, P., 3rd; Leucht, P. Principles of Nonunion Management: State of the Art. J. Orthop. Trauma 2018, 32 (Suppl. S1), S52–S57. [Google Scholar] [CrossRef]
- Alam, A.; Thelin, E.P.; Tajsic, T.; Khan, D.Z.; Khellaf, A.; Patani, R.; Helmy, A. Cellular infiltration in traumatic brain injury. J. Neuroinflamm. 2020, 17, 328. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Prosser, I.; Lawson, Z.; Evans, A.; Harrison, S.; Morris, S.; Maguire, S.; Kemp, A.M. A timetable for the radiologic features of fracture healing in young children. AJR Am. J. Roentgenol. 2012, 198, 1014–1020. [Google Scholar] [CrossRef]
- Marongiu, G.; Leinardi, L.; Congia, S.; Frigau, L.; Mola, F.; Capone, A. Reliability and reproducibility of the new AO/OTA 2018 classification system for proximal humeral fractures: A comparison of three different classification systems. J. Orthop. Traumatol. 2020, 21, 4. [Google Scholar] [CrossRef]
- Rembe, J.D.; Garabet, W.; Augustin, M.; Dissemond, J.; Ibing, W.; Schelzig, H.; Stuermer, E.K. Immunomarker profiling in human chronic wound swabs reveals IL-1 beta/IL-1RA and CXCL8/CXCL10 ratios as potential biomarkers for wound healing, infection status and regenerative stage. J. Transl. Med. 2025, 23, 407. [Google Scholar] [CrossRef]
- Evans, A.R.; Giannoudis, P.V.; Leucht, P.; McKinley, T.O.; Gaski, G.E.; Frey, K.P.; Wenke, J.C.; Lee, C. The local and systemic effects of immune function on fracture healing. OTA Int. 2024, 7, e328. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. S4), S3–S6. [Google Scholar] [CrossRef]
- Bazzocchi, A.; Gazzotti, S.; Santarpia, L.; Madeddu, C.; Petroni, M.L.; Aparisi Gomez, M.P. Editorial: Importance of body composition analysis in clinical nutrition. Front. Nutr. 2022, 9, 1080636. [Google Scholar] [CrossRef]
| Days Post Injury | Early (n = 34) | Late (n = 30) |
|---|---|---|
| Age (median (min–max)) | 37 (18–75) | 30 (18–80) |
| Gender | ♂ 26, ♀ 8 | ♂ 26, ♀ 4 |
| BMI (Mean ± SD) | 23.44 ± 2.95 | 24.38 ± 3.63 |
| Smoking | 11 Yes | 6 Yes |
| Type of fracture | ||
| A | 10 (29.41%) | 10 (33.33%) |
| B | 9 (26.47%) | 9 (30.00%) |
| C | 15 (44.12%) | 11 (36.67%) |
| Hb (Mean ± SD, g/dL) | 14.41 ± 1.18 | 14.74 ± 1.34 |
| Leukocytes (Mean ± SD, g/L) | 9.08 ± 2.99 | 7.28 ± 2.66 |
| Erythrocytes (Mean ± SD, T/l) | 5.04 ± 1.98 | 4.9 ± 0.52 |
| Haematocrit (Mean ± SD, %) | 42.19 ± 3.22 | 43.21 ± 3.96 |
| Thrombocytes (Mean ± SD, G/l) | 228.62 ± 47.23 | 225.27 ± 49.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Failer, S.; Muehlhaupt, N.; Schwenk, C.; Biberthaler, P.; Ketzer, C.; Roemmermann, G.; Bohe, O.; Hanschen, M. Temporal Molecular Signatures of Early Human Clavicle Fracture Healing: Characterization of Hematological, Cytokine, and miRNA Profiles. Int. J. Mol. Sci. 2025, 26, 8825. https://doi.org/10.3390/ijms26188825
Wan L, Failer S, Muehlhaupt N, Schwenk C, Biberthaler P, Ketzer C, Roemmermann G, Bohe O, Hanschen M. Temporal Molecular Signatures of Early Human Clavicle Fracture Healing: Characterization of Hematological, Cytokine, and miRNA Profiles. International Journal of Molecular Sciences. 2025; 26(18):8825. https://doi.org/10.3390/ijms26188825
Chicago/Turabian StyleWan, Li, Sandra Failer, Nadja Muehlhaupt, Christina Schwenk, Peter Biberthaler, Conrad Ketzer, Gregor Roemmermann, Olivia Bohe, and Marc Hanschen. 2025. "Temporal Molecular Signatures of Early Human Clavicle Fracture Healing: Characterization of Hematological, Cytokine, and miRNA Profiles" International Journal of Molecular Sciences 26, no. 18: 8825. https://doi.org/10.3390/ijms26188825
APA StyleWan, L., Failer, S., Muehlhaupt, N., Schwenk, C., Biberthaler, P., Ketzer, C., Roemmermann, G., Bohe, O., & Hanschen, M. (2025). Temporal Molecular Signatures of Early Human Clavicle Fracture Healing: Characterization of Hematological, Cytokine, and miRNA Profiles. International Journal of Molecular Sciences, 26(18), 8825. https://doi.org/10.3390/ijms26188825





