Hepatic AhR Activation by TCDD Induces Obesity and Steatosis via Hepatic Plasminogen Activator Inhibitor-1 (PAI-1)
Abstract
1. Introduction
2. Results
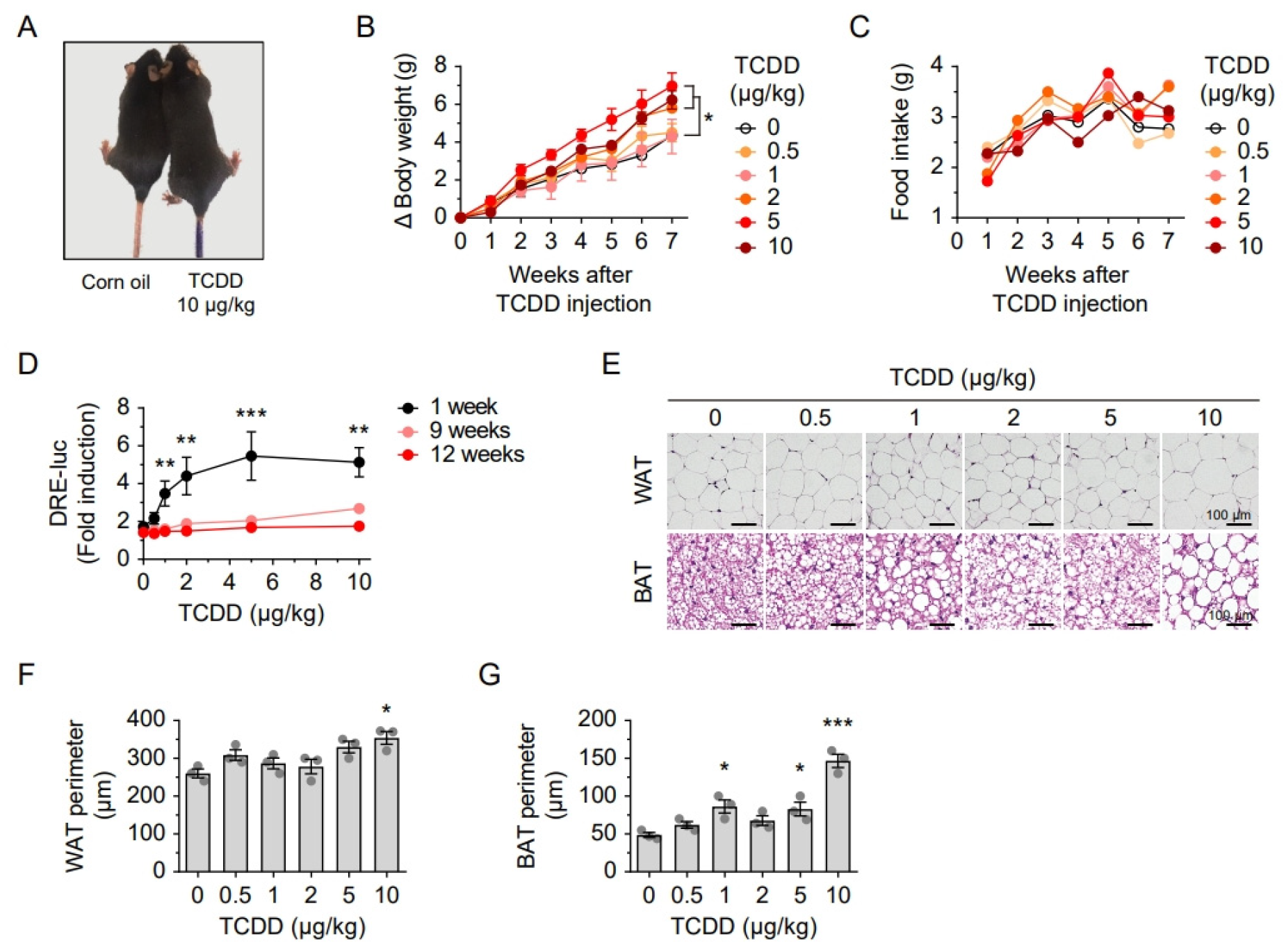
2.1. Lipid Accumulation and Downregulation of Mitochondrial Proteins Following a Single TCDD Injection
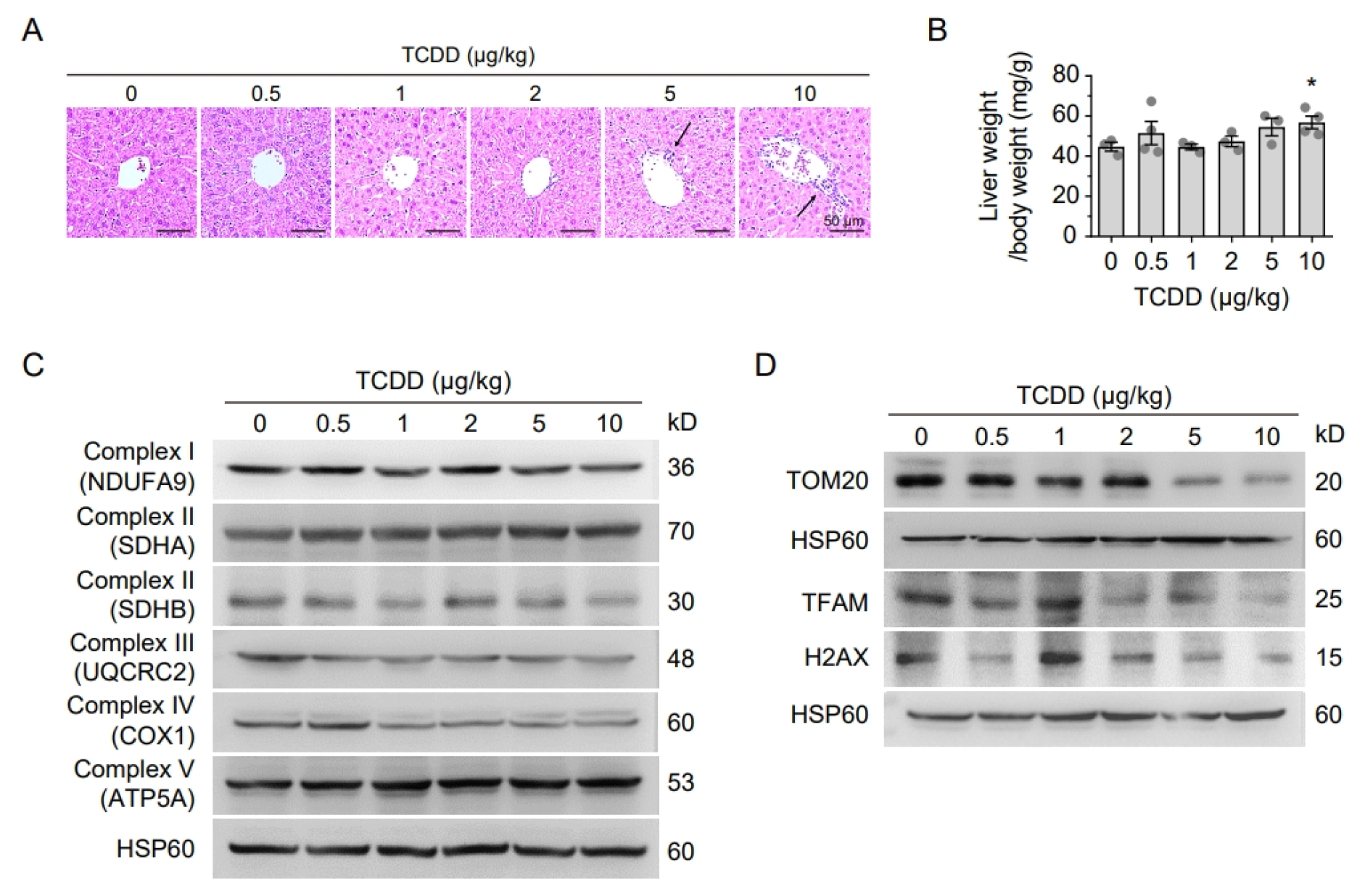
2.2. TCDD Promotes Liver Injury and Mitochondria Dysfunction
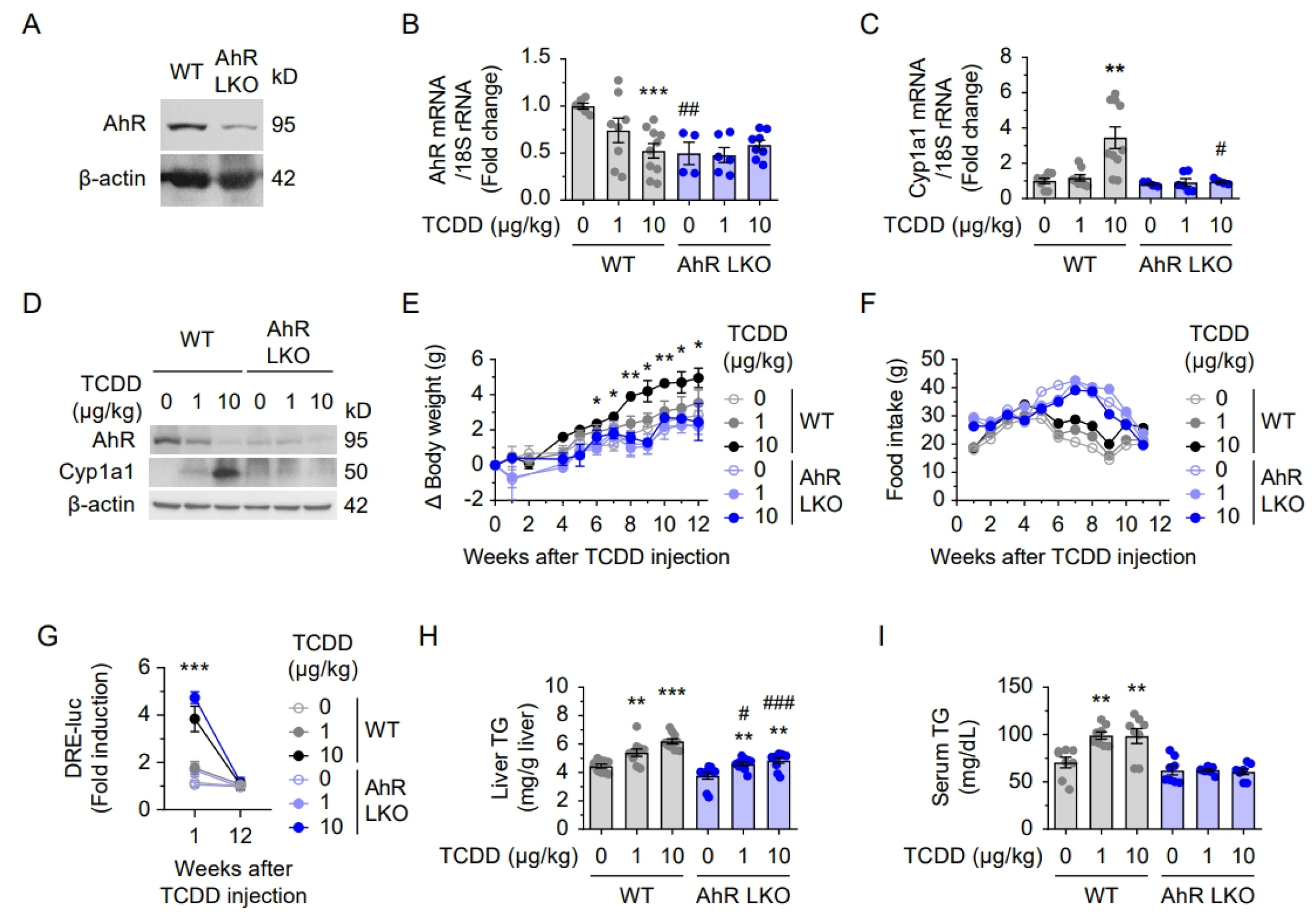
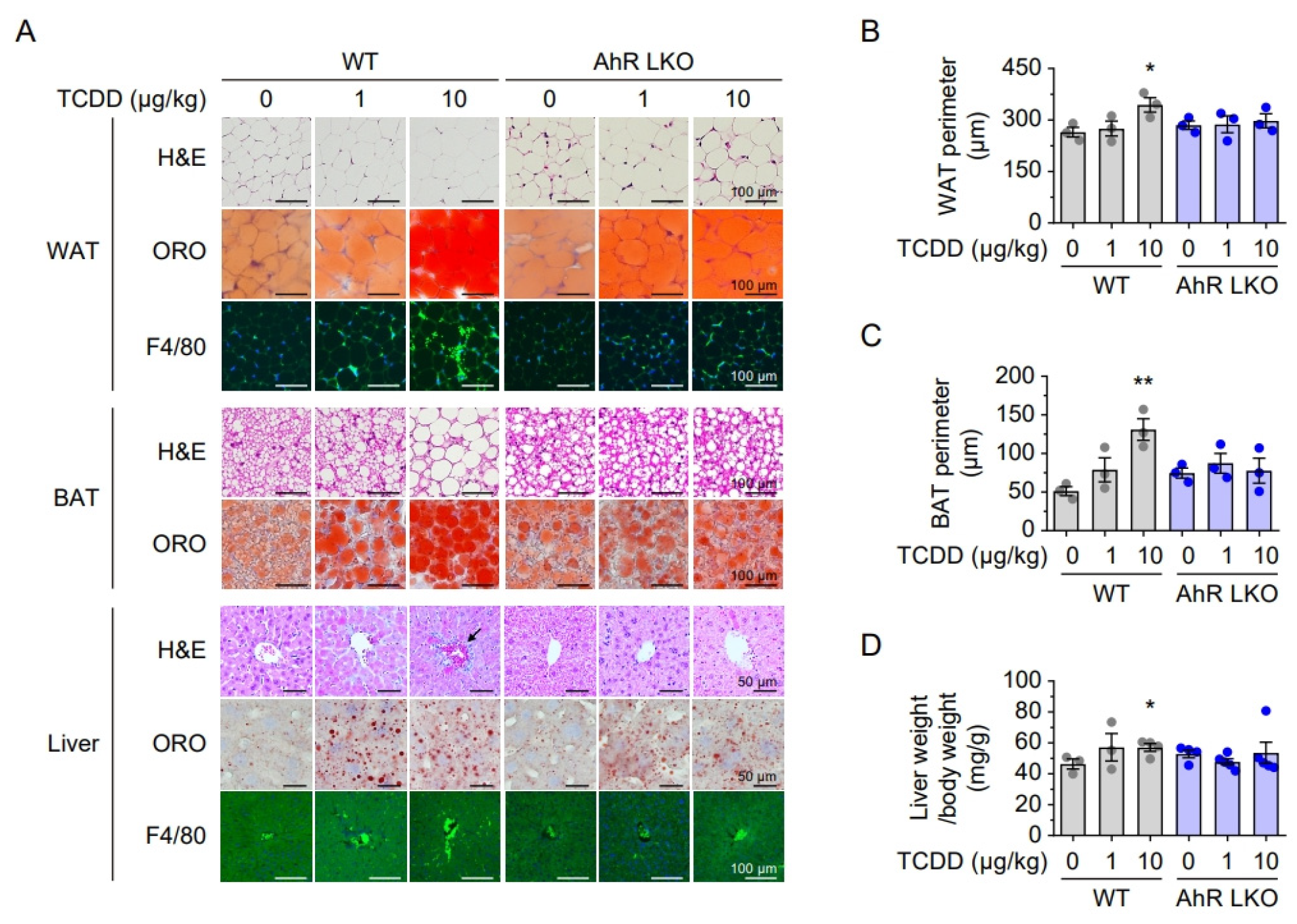
2.3. Attenuated Lipid Accumulation and Inflammation in AhR LKO Mice
2.4. Hepatic AhR Deficiency Alleviates TCDD-Induced Lipid Accumulation and Inflammation
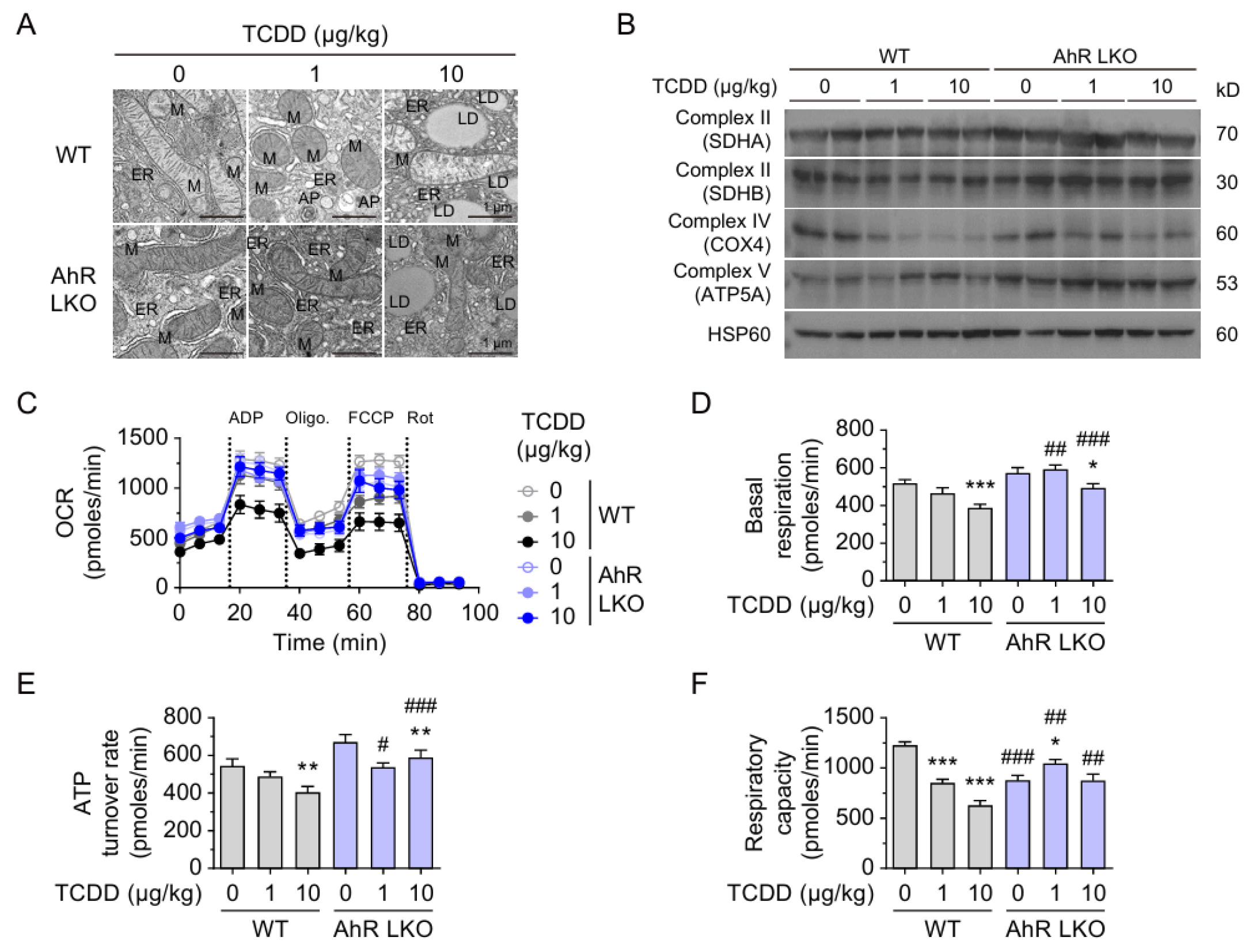
2.5. Hepatic AhR-Dependent Mitochondrial Dysfunction After TCDD Injection
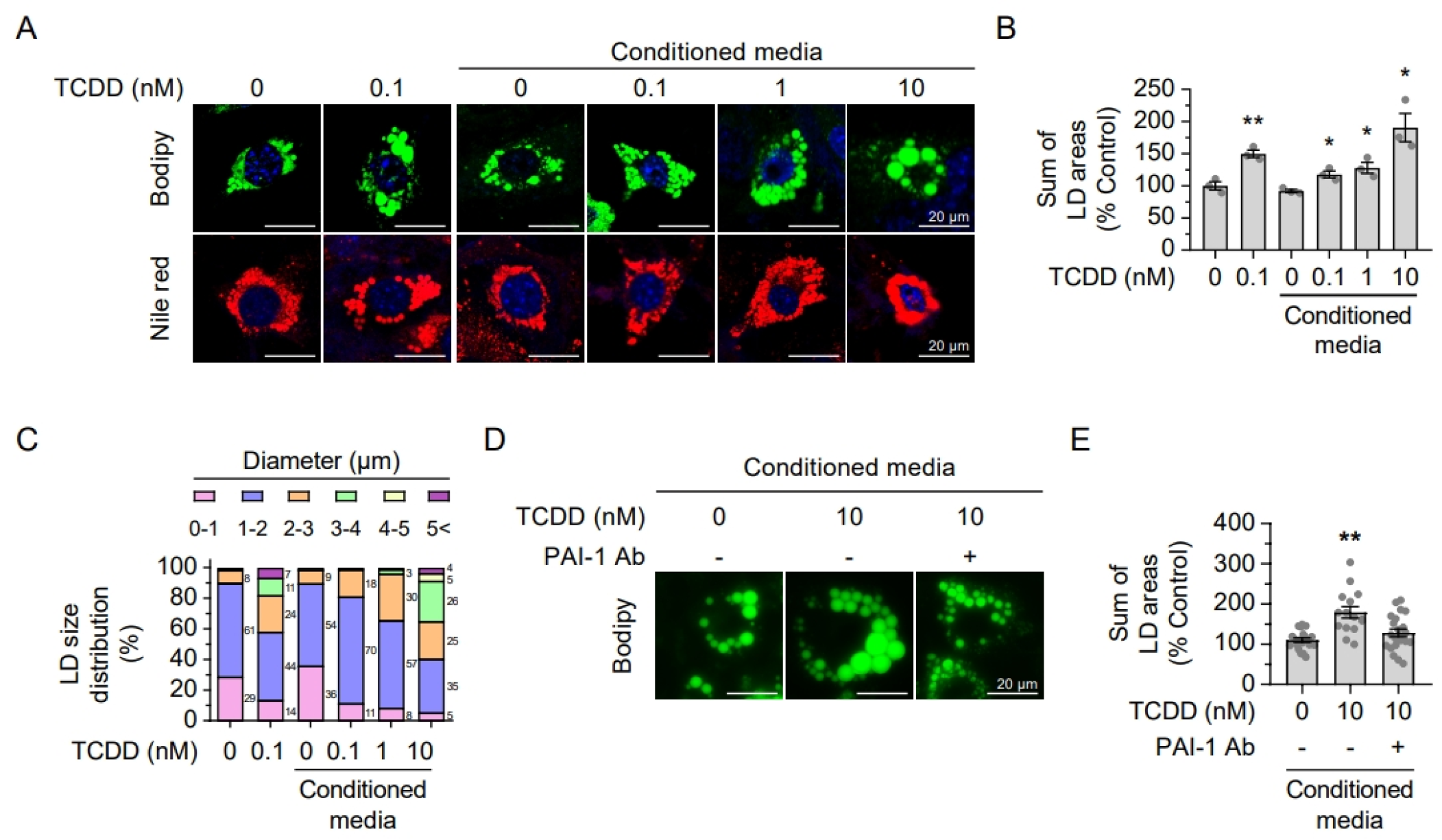
2.6. Secreted Hepatic PAI-1 Promotes Adipocyte Hypertrophy
2.7. TCDD-Induced PAI-1 Expression Impairs Hepatic tPA–ApoB Binding
3. Discussion
4. Materials and Methods
4.1. Animal and Experimental Design
4.2. TCDD Injection
4.3. Cell Culture and Treatment
4.4. Measurement of AhR Ligands in Serum
4.5. Histochemistry
4.6. Real Time Quantitative Reverse Transcription-PCR (qRT-PCR)
4.7. Western Blot Analysis of Mitochondrial Proteins
4.8. Triglyceride Assay
4.9. Transmission Electron Microscopy
4.10. Oxygen Consumption Rate
4.11. Adipogenesis Assay
4.12. Proximity Ligation Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| AhRL | Aryl hydrocarbon receptor ligand |
| ApoB | apolipoprotein B |
| BAT | Brown adipose tissue |
| CALA | cell-based AhR ligand activity |
| COX1 | cytochrome C oxidase subunit 1 |
| LD | Lipid droplet |
| LKO | Liver specific knock-out |
| MTP | Microsomal triglyceride transfer protein |
| OCR | oxygen consumption rate |
| ORO | Oil red O |
| OXPHOS | oxidative phosphorylation |
| PAI-1 | plasminogen activator inhibitor-1 |
| PLA | Proximity ligation assay |
| POPs, | persistent organic pollutants |
| TCDD | 2,3,7,8-tetrachlorodi-benzodioxin |
| TFAM | transcription factor A, mitochondrial |
| TG | triglyceride |
| tPA | tissue-type plasminogen activator |
| TOM20 | translocase outer mitochondrial membrane 20 |
| VLDL | very-low-density lipoprotein |
| WAT | white adipose tissue |
| WT | wild type |
References
- Bray, G.A.; Champagne, C.M. Beyond energy balance: There is more to obesity than kilocalories. J. Am. Diet. Assoc. 2005, 105, S17–S23. [Google Scholar] [CrossRef]
- Eisenmann, J.C. Insight into the causes of the recent secular trend in pediatric obesity: Common sense does not always prevail for complex, multi-factorial phenotypes. Prev. Med. 2006, 42, 329–335. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Warembourg, C.; Guil, N.; Stratakis, N.; Lertxundi, A.; Irizar, A.; Llop, S.; Lopez-Espinosa, M.J.; Basagana, X.; Gonzalez, J.R.; et al. Nutritional Modulation of Associations between Prenatal Exposure to Persistent Organic Pollutants and Childhood Obesity: A Prospective Cohort Study. Environ. Health Perspect. 2023, 131, 37011. [Google Scholar] [CrossRef]
- Kelishadi, R.; Poursafa, P.; Jamshidi, F. Role of environmental chemicals in obesity: A systematic review on the current evidence. J. Environ. Public Health 2013, 2013, 896789. [Google Scholar] [CrossRef]
- Mohanto, N.C.; Ito, Y.; Kato, S.; Kamijima, M. Life-Time Environmental Chemical Exposure and Obesity: Review of Epidemiological Studies Using Human Biomonitoring Methods. Front. Endocrinol. 2021, 12, 778737. [Google Scholar] [CrossRef]
- Stratakis, N.; Rock, S.; La Merrill, M.A.; Saez, M.; Robinson, O.; Fecht, D.; Vrijheid, M.; Valvi, D.; Conti, D.V.; McConnell, R.; et al. Prenatal exposure to persistent organic pollutants and childhood obesity: A systematic review and meta-analysis of human studies. Obes. Rev. 2022, 23 (Suppl. S1), e13383. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.T.; Consonni, D.; Patterson, D.G., Jr.; Needham, L.L.; Lucier, G.; Brambilla, P.; Cazzaniga, M.A.; Mocarelli, P.; Pesatori, A.C.; Bertazzi, P.A.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environ. Health Perspect. 1998, 106, 273–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, J.; Xu, Y.; Zhong, T.; Yu, X.; Wang, L.; Xiao, Y.; Peng, Y.; Sun, Q. A review of food contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin and its toxicity associated with metabolic disorders. Curr. Res. Food Sci. 2023, 7, 100617. [Google Scholar] [CrossRef]
- Salihovic, S.; Mattioli, L.; Lindstrom, G.; Lind, L.; Lind, P.M.; van Bavel, B. A rapid method for screening of the Stockholm Convention POPs in small amounts of human plasma using SPE and HRGC/HRMS. Chemosphere 2012, 86, 747–753. [Google Scholar] [CrossRef]
- Brulport, A.; Le Corre, L.; Chagnon, M.-C. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 2017, 390, 43–52. [Google Scholar] [CrossRef]
- Orlowska, K.; Nault, R.; Ara, J.; LaPres, J.J.; Harkema, J.; Demireva, E.Y.; Xie, H.; Wilson, R.H.; Bradfield, C.A.; Yap, D.; et al. Disruption of canonical AHR-mediated induction of hepatocyte PKM2 expression compromises antioxidant defenses and increases TCDD-induced hepatotoxicity. Redox Biol. 2024, 77, 103405. [Google Scholar] [CrossRef]
- Nault, R.; Fader, K.A.; Ammendolia, D.A.; Dornbos, P.; Potter, D.; Sharratt, B.; Kumagai, K.; Harkema, J.R.; Lunt, S.Y.; Matthews, J.; et al. Dose-Dependent Metabolic Reprogramming and Differential Gene Expression in TCDD-Elicited Hepatic Fibrosis. Toxicol. Sci. 2016, 154, 253–266. [Google Scholar] [CrossRef]
- Cholico, G.N.; Fling, R.R.; Zacharewski, N.A.; Fader, K.A.; Nault, R.; Zacharewski, T.R. Thioesterase induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin results in a futile cycle that inhibits hepatic beta-oxidation. Sci. Rep. 2021, 11, 15689. [Google Scholar] [CrossRef]
- Riaz, F.; Pan, F.; Wei, P. Aryl hydrocarbon receptor: The master regulator of immune responses in allergic diseases. Front. Immunol. 2022, 13, 1057555. [Google Scholar] [CrossRef] [PubMed]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, A.G.; Im, S.; Oh, S.J.; Yoon, H.J.; Park, J.H.; Pak, Y.K. A Novel Aryl Hydrocarbon Receptor Antagonist HBU651 Ameliorates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 14871. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Baillie-Hamilton, P.F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 2002, 8, 185–192. [Google Scholar] [CrossRef]
- Fader, K.A.; Nault, R.; Ammendolia, D.A.; Harkema, J.R.; Williams, K.J.; Crawford, R.B.; Kaminski, N.E.; Potter, D.; Sharratt, B.; Zacharewski, T.R. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters Lipid Metabolism and Depletes Immune Cell Populations in the Jejunum of C57BL/6 Mice. Toxicol. Sci. 2015, 148, 567–580. [Google Scholar] [CrossRef]
- Duval, C.; Teixeira-Clerc, F.; Leblanc, A.F.; Touch, S.; Emond, C.; Guerre-Millo, M.; Lotersztajn, S.; Barouki, R.; Aggerbeck, M.; Coumoul, X. Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model. Environ. Health Perspect. 2017, 125, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Domenech, O. Cytotoxicity and mitochondrial dysfunction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in isolated rat hepatocytes. Toxicol. Lett. 2009, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Jun, D.W.; Kim, J.T.; Jeong, J.H.; Park, H.; Chang, Y.S.; Park, K.S.; Lee, H.K.; Pak, Y.K. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors 2013, 39, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Monica Lind, P.; Salihovic, S.; van Bavel, B.; Lind, L.; Ingelsson, E. Influence of persistent organic pollutants on oxidative stress in population-based samples. Chemosphere 2014, 114, 303–309. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, W.H.; Kang, Y.C.; Kang, S.; Im, S.; Park, S.; Kim, J.T.; Lee, M.; Seok, J.; Oh, M.S.; et al. Serum biomarkers from cell-based assays for AhRL and MIS strongly predicted the future development of diabetes in a large community-based prospective study in Korea. Sci. Rep. 2020, 10, 6339. [Google Scholar] [CrossRef]
- Im, S.; Kang, S.; Son, W.J.; Son, M.; Oh, S.J.; Yoon, H.J.; Pak, Y.K. Dioxin-Induced PAI-1 Expression: A Novel Pathway to Pancreatic β-Cell Failure in Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 11974. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, H.; Lund, H.; Zhang, Z.; Castleberry, M.; Rodriguez, M.; Kuriakose, G.; Gupta, S.; Lewandowska, M.; Powers, H.R.; et al. Intracellular tPA-PAI-1 interaction determines VLDL assembly in hepatocytes. Science 2023, 381, eadh5207. [Google Scholar] [CrossRef]
- Jeong, J.H.; Cheol Kang, Y.; Piao, Y.; Kang, S.; Pak, Y.K. miR-24-mediated knockdown of H2AX damages mitochondria and the insulin signaling pathway. Exp. Mol. Med. 2017, 49, e313. [Google Scholar] [CrossRef]
- Hoyeck, M.P.; Merhi, R.C.; Blair, H.L.; Spencer, C.D.; Payant, M.A.; Martin Alfonso, D.I.; Zhang, M.; Matteo, G.; Chee, M.J.; Bruin, J.E. Female mice exposed to low doses of dioxin during pregnancy and lactation have increased susceptibility to diet-induced obesity and diabetes. Mol. Metab. 2020, 42, 101104. [Google Scholar] [CrossRef]
- Pak, Y.K.; Choi, H.S.; Park, W.H.; Im, S.; Lind, P.M.; Lind, L.; Lee, H.K. High Serum-Induced AhRL Is Associated with Prevalent Metabolic Syndrome and Future Impairment of Glucose Tolerance in the Elderly. Endocrinol. Metab. 2021, 36, 436–446. [Google Scholar] [CrossRef]
- He, J.; Lee, J.H.; Febbraio, M.; Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. 2011, 236, 1116–1121. [Google Scholar] [CrossRef]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 2010, 139, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shirakawa, H.; Tomita, S.; Ohsaki, Y.; Haketa, K.; Tooi, O.; Santo, N.; Tohkin, M.; Furukawa, Y.; Gonzalez, F.J.; et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008, 229, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Capetillo, O.; Lee, A.; Nussenzweig, M.; Nussenzweig, A. H2AX: The histone guardian of the genome. DNA Repair 2004, 3, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Hoon Jeong, J.; Min, H.K.; Jung, H.J.; Hwang, D.; Lee, S.W.; Kim Pak, Y. Shot-gun proteomic analysis of mitochondrial D-loop DNA binding proteins: Identification of mitochondrial histones. Mol. Biosyst. 2011, 7, 1523–1536. [Google Scholar] [CrossRef]
- Weyemi, U.; Paul, B.D.; Bhattacharya, D.; Malla, A.P.; Boufraqech, M.; Harraz, M.M.; Bonner, W.M.; Snyder, S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 7471–7476. [Google Scholar] [CrossRef]
- Tijet, N.; Boutros, P.C.; Moffat, I.D.; Okey, A.B.; Tuomisto, J.; Pohjanvirta, R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol. Pharmacol. 2006, 69, 140–153. [Google Scholar] [CrossRef]
- Im, S.; Kang, S.; Kim, J.H.; Oh, S.J.; Pak, Y.K. Low-Dose Dioxin Reduced Glucose Uptake in C2C12 Myocytes: The Role of Mitochondrial Oxidative Stress and Insulin-Dependent Calcium Mobilization. Antioxidants 2022, 11, 2109. [Google Scholar] [CrossRef]
- Nault, R.; Doskey, C.M.; Fader, K.A.; Rockwell, C.E.; Zacharewski, T. Comparison of Hepatic NRF2 and Aryl Hydrocarbon Receptor Binding in 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Treated Mice Demonstrates NRF2-Independent PKM2 Induction. Mol. Pharmacol. 2018, 94, 876–884. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Oleaga, C.; Eren, M.; Amaral, A.P.; Shang, M.; Lux, E.; Khan, S.S.; Shah, S.J.; Omura, Y.; Pamir, N.; et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci. Rep. 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saeidi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2019, 28, 3285. [Google Scholar] [CrossRef]
- Boverhof, D.R.; Burgoon, L.D.; Tashiro, C.; Chittim, B.; Harkema, J.R.; Jump, D.B.; Zacharewski, T.R. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol. Sci. 2005, 85, 1048–1063. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kang, S.; Piao, Y.; Kang, Y.C.; Lim, S.; Pak, Y.K. Qi-activating quercetin alleviates mitochondrial dysfunction and neuroinflammation in vivo and in vitro. Arch. Pharm. Res. 2020, 43, 553–566. [Google Scholar] [CrossRef]
- Sharma, M.; Boytard, L.; Hadi, T.; Koelwyn, G.; Simon, R.; Ouimet, M.; Seifert, L.; Spiro, W.; Yan, B.; Hutchison, S.; et al. Enhanced glycolysis and HIF-1alpha activation in adipose tissue macrophages sustains local and systemic interleukin-1beta production in obesity. Sci. Rep. 2020, 10, 5555. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.J.; Im, S.; Kang, S.; Lee, A.G.; Lee, B.C.; Pak, Y.K. Hepatic AhR Activation by TCDD Induces Obesity and Steatosis via Hepatic Plasminogen Activator Inhibitor-1 (PAI-1). Int. J. Mol. Sci. 2025, 26, 8452. https://doi.org/10.3390/ijms26178452
Oh SJ, Im S, Kang S, Lee AG, Lee BC, Pak YK. Hepatic AhR Activation by TCDD Induces Obesity and Steatosis via Hepatic Plasminogen Activator Inhibitor-1 (PAI-1). International Journal of Molecular Sciences. 2025; 26(17):8452. https://doi.org/10.3390/ijms26178452
Chicago/Turabian StyleOh, Seung Jun, Suyeol Im, Sora Kang, Aden Geonhee Lee, Byung Cheol Lee, and Youngmi Kim Pak. 2025. "Hepatic AhR Activation by TCDD Induces Obesity and Steatosis via Hepatic Plasminogen Activator Inhibitor-1 (PAI-1)" International Journal of Molecular Sciences 26, no. 17: 8452. https://doi.org/10.3390/ijms26178452
APA StyleOh, S. J., Im, S., Kang, S., Lee, A. G., Lee, B. C., & Pak, Y. K. (2025). Hepatic AhR Activation by TCDD Induces Obesity and Steatosis via Hepatic Plasminogen Activator Inhibitor-1 (PAI-1). International Journal of Molecular Sciences, 26(17), 8452. https://doi.org/10.3390/ijms26178452





