Functional Food Ingredients Enhancing Immune Health: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction and Synthesis
2.5. Risk of Bias and Certainty of Evidence
2.6. PRISMA Documentation
3. Results and Discussion
3.1. Historical Evolution of Functional Food Ingredients in Immune Modulation (1925–2025)
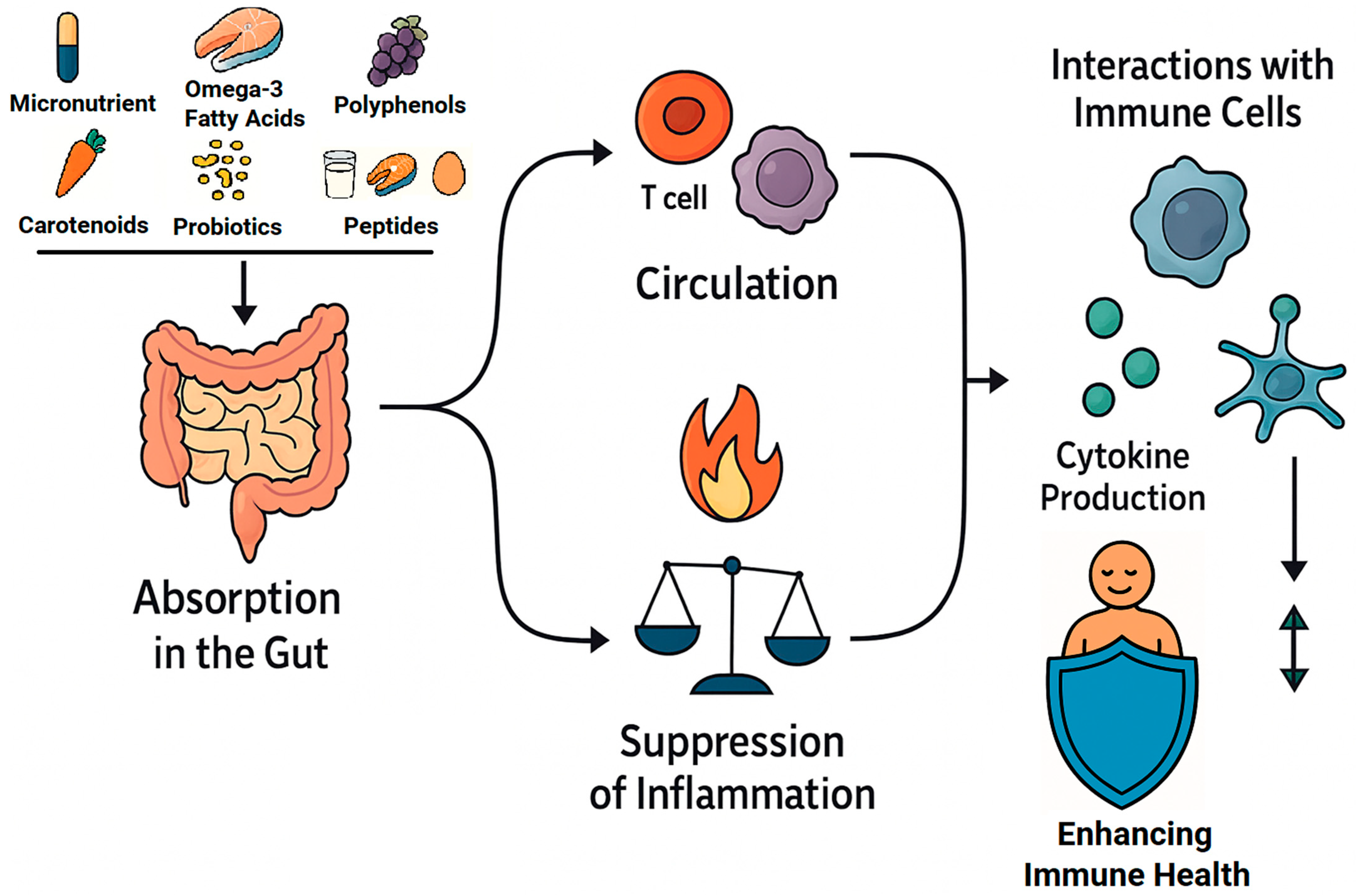
3.2. Bioactive Compounds in Functional Foods and Their Impact on Immunity
3.2.1. Micronutrients in Immune Modulation
3.2.2. Polyphenols and Flavonoids: Molecular Mechanisms and Immune Modulation
3.2.3. Carotenoids: Antioxidant Defense and Immune Enhancement
3.2.4. Omega-3 Fatty Acids and Immune Resolution
3.2.5. Food-Derived Bioactive Peptides: Molecular Mechanisms and Immunomodulatory Potential
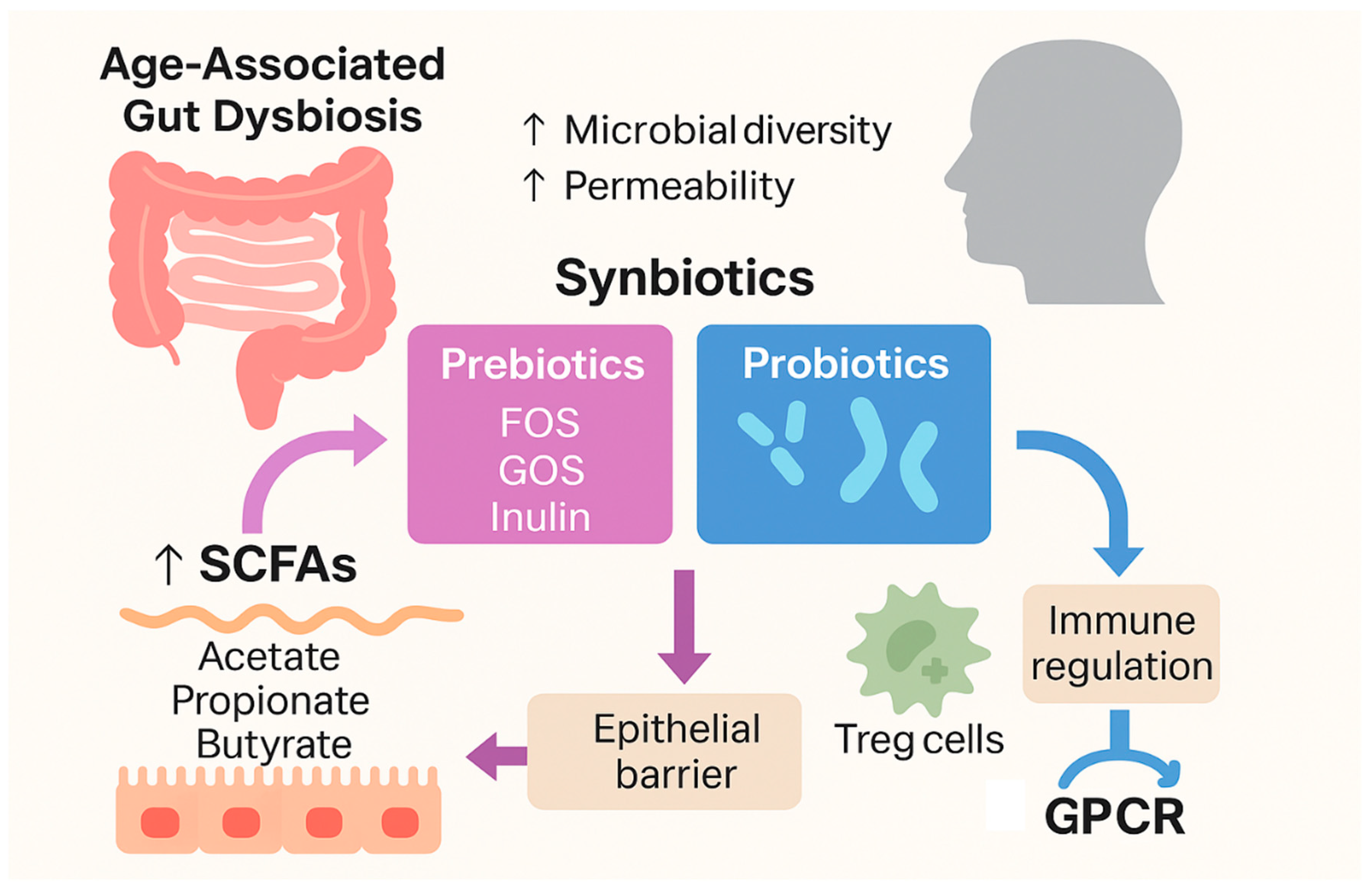
3.2.6. Probiotics and Gut–Immune Axis Modulation
| Bioactive Compound | Dietary Source | Immune Effects | Evidence Type | Reference |
|---|---|---|---|---|
| Vitamin D | Fortified dairy, fish oil | ↑ Cathelicidin, ↑ Tregs, ↓ IL-6, TNF-α, IFN-γ | Clinical trial, review | [42,43,44,45,46] |
| Vitamin C | Citrus fruits, kiwifruit, bell peppers | ↓ ROS, ↑ phagocytosis, ↑ lymphocyte proliferation, ↓ NF-κB activity | Clinical studies, mechanistic | [47,48,49,50] |
| Vitamin E | Nuts, seeds, plant oils, spinach | ↑ T cell response, ↓ oxidative damage | RCTs in elderly, mechanistic | [51,52,53] |
| Zinc | Shellfish, red meat, legumes | ↑ T cell maturation, ↓ oxidative stress, ↑ NK and macrophage activity | RCTs, aging populations | [54,55,56,57,58,59] |
| Selenium | Brazil nuts, seafood | ↑ NK cell function, ↑ antioxidant selenoproteins, ↓ ROS | Mechanistic studies, reviews | [60,61,62,63] |
| Iron | Red meat, liver, lentils, fortified cereals | Essential for T/B cell proliferation, ↑ phagocytosis, modulates redox balance | Clinical trials, supplementation studies | [64,65,66] |
| Polyphenols | Quercetin-Apples, onions, berries EGCG-Green tea Resveratrol Red grapes, wine | ↓ Mast cell activation, ↑ antiviral interferon response ↑ Treg differentiation, ↓ NF-κB pathway ↑ SIRT1, ↓ age-associated inflammation | In vitro, animal model Review, in vivo Animal model | [67,68,69,70,71,72,73,74,75,76,77,78,79] |
| Curcumin | Turmeric (Curcuma longa) | ↓ COX-2, ↓ iNOS, modulation of JAK/STAT and NLRP3 inflammasome, ↓ pro-inflammatory mediators, ↑ macrophage phagocytosis and antigen presentation, ↑ NK cytotoxicity, antioxidant properties | In vitro, in vivo, clinical potential | [80,81,82,83,84] |
| Flavonoids (Quercetin, Hesperidin) | Fruits, onions, apples, citrus | ↓ viral entry/replication, ↓ mast cell activation and histamine release, ↓ MAPK and NF-κB signaling, ↓ cytokine storm. Hesperidin: ↑ endothelial function, ↑Nitric oxide (NO) synthesis, ↓ ICAM-1/VCAM-1, ↓ vascular inflammation | In vitro, animal models, mechanistic studies | [11,85,87] |
| Carotenoids (β-carotene, lycopene, lutein) | Carrots, tomatoes, leafy greens | ↑ Retinoic acid signaling, ↓ IL-8, ↓ CRP, ↑ lymphocyte activity | Observational studies, mechanistic data | [88,89,90,91,92,93] |
| Omega-3 (EPA/DHA) | Fatty fish, algae oil | ↑ Specialized pro-resolving mediators (SPMs), ↓ IL-6, ↑ macrophage efferocytosis | Meta-analysis, RCT | [94,95,96,97,98,99,100,101,102] |
| Peptides | Milk, fish, eggs, nuts, legumes | ↓ TNF-α, ↓ IL-6, ↑ IL-10 Modulation of Th1/Th2 balance, ↓ pro-inflammatory cytokines ↑ NK cytotoxicity, ↑ IFN-γ ↓ IL-1β, ↑ Treg, improved gut barrier ↑ CD4+ T cells, ↑ Gut mucosal immunity, ↓ infections ↑ Macrophage activity, ↑ phagocytosis | Review, in vivo | [9,105,106,107,108,109,110,111,112,113] |
| Probiotics (L. plantarum) | Fermented dairy, kefir | ↑ Mucosal IgA, ↑ IL-10, ↑ TGF-β, ↓ LPS-induced inflammation | Clinical trial, meta-analysis | [113,114,115,116,117,118,119,120,121,122,123] |
| Prebiotics (inulin, FOS) | Chicory root, onions | ↑ SCFA (butyrate), ↑ gut Treg activity | Human intervention |
3.2.7. Synergistic Interactions and Challenges in Functional Food Development
3.2.8. Heterogeneity of Clinical Evidence: Challenges and Opportunities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NF-κB | Nuclear Factor-kappa B |
| MAPK | Mitogen-Activated Protein Kinases |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| FOS | Fructooligosaccharides |
| SCFA | Short-Chain Fatty Acids |
| Treg | Regulatory T Cells |
| EPA | Eicosapentaenoic Acid |
| DHA | Docosahexaenoic Acid |
| SPMs | Specialized Pro-Resolving Mediators |
| VDR | Vitamin D Receptor |
| IL | Interleukin |
| TNF-α | Tumor Necrosis Factor-Alpha |
| ROS | Reactive Oxygen Species |
| NK | Natural Killer (Cells) |
| HO-1 | Like Heme Oxygenase-1 |
| GPx | Glutathione Peroxidase |
| EGCG | Epigallocatechin-3-Gallate |
| AMPK | AMP-Activated Protein Kinase |
| SIRT1 | Sirtuin 1 |
| mTOR | Mechanistic Target of Rapamycin |
| SASP | Senescence-Associated Secretory Phenotype |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible Nitric Oxide Synthase |
| JAK/STAT | Janus Kinase / Signal Transducer and Activator of Transcription |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| RARs | Retinoic Acid Receptors |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| LC-PUFAs | Long-Chain Polyunsaturated Fatty Acids |
| LOX | Lipoxygenase |
| ChemR23 | Chemerin Receptor 23 |
| GPR32 | G-Protein-Coupled Receptor 32 |
| ALX/FPR2 | Lipoxin A4 Receptor / Formyl Peptide Receptor 2 |
| CRP | C-Reactive Protein |
| URTIs | Upper Respiratory Tract Infections |
| RCTs | Randomized Controlled Trials |
| GALT | Gut-Associated Lymphoid Tissue |
| TGF-β | Transforming Growth Factor-Beta |
| Th1 | T Helper Type 1 (Cells) |
| sIgA | Secretory Immunoglobulin A |
| IFN-γ | Interferon Gamma |
References
- Soni, S.; Paari, K.A. A Review on the Immunomodulatory Properties of Functional Nutraceuticals as Dietary Interventions for Children to Combat COVID-19 Related Infections. Food Prod. Process. Nutr. 2023, 5, 17. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Dey, P.; Mukherjee, S.K.; Parai, D. Association of Probiotics and Prebiotics with Human Microbiome and the Functioning of Immune System. In Probiotics, Prebiotics, Synbiotics, and Postbiotics; Springer Nature Singapore: Singapore, 2023; pp. 101–115. [Google Scholar]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Ferreira, C.; Vieira, P.; Sá, H.; Malva, J.; Castelo-Branco, M.; Reis, F.; Viana, S. Polyphenols: Immunonutrients Tipping the Balance of Immunometabolism in Chronic Diseases. Front. Immunol. 2024, 15, 1360065. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the Therapeutic Symphony of Probiotics, Prebiotics, and Postbiotics in Gut-Immune Harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, Immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved During an Episode of Common Colds—Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in Order to Prevent or Treat Common Colds. Evid.-Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Villanueva, Á.; Montserrat-de-la-Paz, S.; Sanchez-Fidalgo, S.; Millán-Linares, M.C. Evidence of Immunomodulatory Food-Protein Derived Peptides in Human Nutritional Interventions: Review on the Outcomes and Potential Limitations. Nutrients 2023, 15, 2681. [Google Scholar] [CrossRef] [PubMed]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali-Schöll, I.; et al. Nutrient Supplementation for Prevention of Viral Respiratory Tract Infections in Healthy Subjects: A Systematic Review and Meta-analysis. Allergy 2022, 77, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, Y.; Fei, Y.; Zhang, J.; Yin, S.; Zou, H.; Zhu, F. Efficacy of Probiotic, Prebiotic, and Synbiotics Supplements in Individuals with Anemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Gastroenterol. 2024, 24, 472. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Yin, J.; Peng, X.; King, L.; Li, L.; Xu, Z.; Zhou, L.; Peng, Z.; Ze, X.; et al. Effect of Synbiotic Supplementation on Immune Parameters and Gut Microbiota in Healthy Adults: A Double-Blind Randomized Controlled Trial. Gut Microbes 2023, 15, 2247025. [Google Scholar] [CrossRef]
- Minihane, A.M. Omega-3 Fatty Acids, Brain Health and the Menopause. Post Reprod. Health 2025, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hajzer, Z.E.; Alibrahem, W.; Kharrat Helu, N.; Oláh, C.; Prokisch, J. Functional Foods in Clinical Trials and Future Research Directions. Foods 2025, 14, 2675. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.; Barabási, A.-L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research Gaps and Opportunities in Precision Nutrition: An NIH Workshop Report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Academy of Sciences. Elmer Verner McCollum. In Biographical Memoirs; National Academies Press: Washington, DC, USA, 1974; ISBN 978-0-309-02239-2. [Google Scholar]
- McCullough, F.S.W.; Northrop-Clewes, C.A.; Thurnham, D.I. The Effect of Vitamin A on Epithelial Integrity. Proc. Nutr. Soc. 1999, 58, 289–293. [Google Scholar] [CrossRef]
- Jungeblut, C.W. A Further Contribution to Vitamin C Therapy in Experimental Poliomyelitis. J. Exp. Med. 1939, 70, 315–332. [Google Scholar] [CrossRef]
- Chandra, R.K.; Dayton, D.H. Trace Element Regulation of Immunity and Infection. Nutr. Res. 1982, 2, 721–733. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Needleman, P.; Raz, A.; Minkes, M.S.; Ferrendelli, J.A.; Sprecher, H. Triene Prostaglandins: Prostacyclin and Thromboxane Biosynthesis and Unique Biological Properties. Proc. Natl. Acad. Sci. USA 1979, 76, 944–948, Correction in Proc. Natl. Acad. Sci. USA 1979, 76, 3040. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Gado, F.; Ferrario, G.; Della Vedova, L.; Zoanni, B.; Altomare, A.; Carini, M.; Aldini, G.; D’Amato, A.; Baron, G. Targeting Nrf2 and NF-ΚB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules 2023, 28, 1356. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-Based Prebiotics and Synbiotics: Potential for Cancer Chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef]
- Lotfi, F.; Akbarzadeh-Khiavi, M.; Lotfi, Z.; Rahbarnia, L.; Safary, A.; Zarredar, H.; Baghbanzadeh, A.; Naghili, B.; Baradaran, B. Micronutrient Therapy and Effective Immune Response: A Promising Approach for Management of COVID-19. Infection 2021, 49, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida, A.; Alshammari, M.K.; Kamal, M.; Diwan, A.; Asdaq, S.M.B.; Alshehri, S. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Oniani, D.; Shao, Z.; Arciero, P.; Sivarajkumar, S.; Hilsman, J.; Mohr, A.E.; Ibe, S.; Moharir, M.; Li, L.-J.; et al. A Scoping Review of Artificial Intelligence for Precision Nutrition. Adv. Nutr. 2025, 16, 100398. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. ‘Multi-Omics’ Data Integration: Applications in Probiotics Studies. npj Sci. Food 2023, 7, 25. [Google Scholar] [CrossRef]
- Kumar, P.; Mahapatra, D.K.; Kumar, D.; Taleuzzaman, M.; Borikar, S.; Gulecha, V.S.; Zalte, A.G.; Dadure, K.M.; Puranik, M.; Das, M.; et al. Liposomal Delivery System for the Effective Delivery of Nutraceuticals and Functional Foods. In Nutraceutical Delivery Systems; Apple Academic Press: New York, NY, USA, 2022; pp. 173–184. [Google Scholar]
- Coppens, P.; da Silva, M.F.; Pettman, S. European Regulations on Nutraceuticals, Dietary Supplements and Functional Foods: A Framework Based on Safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Montserrat-de la Paz, S. The Role of Bioactive Compounds in Immunonutrition. Nutrients 2024, 16, 3432. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of Selected Vitamins and Trace Elements to Immune Function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Younes, S. The Role of Nutrition on the Treatment of COVID-19. Human Nutr. Metab. 2024, 36, 200255. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C, Respiratory Infections and the Immune System. Trends Immunol. 2003, 24, 579–580. [Google Scholar] [CrossRef]
- Mussa, A.; Afolabi, H.A.; Syed, N.H.; Talib, M.; Murtadha, A.H.; Hajissa, K.; Mokhtar, N.F.; Mohamud, R.; Hassan, R. The NF-ΚB Transcriptional Network Is a High-Dose Vitamin C-Targetable Vulnerability in Breast Cancer. Biomedicines 2023, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, J. Role of Vitamin E in Immunity and Inflammation. Nutrients. 2018, 10, 1614. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chang, H.-S.; Yang, Y.-P.; Lin, T.-W.; Lai, W.-Y.; Lin, Y.-Y.; Chang, C.-C. The Role of Micronutrient and Immunomodulation Effect in the Vaccine Era of COVID-19. J. Chin. Med. Assoc. 2021, 84, 821–826. [Google Scholar] [CrossRef]
- Meydani, S.N.; Han, S.N.; Wu, D. Vitamin E and Immune Response in the Aged: Molecular Mechanisms and Clinical Implications. Immunol. Rev. 2005, 205, 269–284. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Sharma, A. A Review on Role of Zinc as a Potent Immunity Boosting Agent. Mater. Today Proc. 2022, 68, 880–885. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Ibs, K.-H.; Rink, L. Zinc-Altered Immune Function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Moin, A.; Bhutta, Z.A. Zinc Supplementation for the Prevention of Pneumonia in Children Aged 2 Months to 59 Months. Cochrane Database Syst. Rev. 2016, 2017, CD005978. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Fairweather-Tait, S.; Vinceti, M. Selenium and Immune Function: A Systematic Review and Meta-Analysis of Experimental Human Studies. Am. J. Clin. Nutr. 2023, 117, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, S.J. Iron and Its Relation to Immunity and Infectious Disease. J. Nutr. 2001, 131, 616S–635S. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the Iron-Infection Axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the Antioxidant and Antimutagenic Activity of Extracts from Goji Berry of Greek Cultivation. Toxicol. Rep. 2018, 5, 251–257, Erratum in Toxicol. Rep. 2020, 25, 62–63. https://doi.org/10.1016/j.toxrep.2020.12.008. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Olivieri, F.; Mazzucchelli, R.; Togni, L.; Santarelli, A.; Marzioni, D.; Rippo, M.R. Effect of Natural Compounds on NRF2/KEAP1 Signaling in Periodontitis: A Potential Use to Prevent Age-Related Disorders. Mol. Biol. Rep. 2025, 52, 771. [Google Scholar] [CrossRef]
- Ahmed, M. Targeting Aging Pathways with Natural Compounds: A Review of Curcumin, Epigallocatechin Gallate, Thymoquinone, and Resveratrol. Immun. Ageing 2025, 22, 28. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L.; et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Yao, J.; Wei, C.; Wang, J.Y.; Zhang, R.; Li, Y.X.; Wang, L.S. Effect of Resveratrol on Treg/Th17 Signaling and Ulcerative Colitis Treatment in Mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4+ T Cell Activation by Enhancing the Expression and Activity of Sirt1. PLoS ONE 2013, 8, e75139. [Google Scholar] [CrossRef]
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol Regulates the Expression of LXR-α in Human Macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-H.; Jeong, H.-J.; Kim, S.-Y.; Shin, T.-Y.; Um, J.-Y.; Hong, S.-H.; Kim, H.-M. Epigallocatechin-3-Gallate Inhibits Secretion of TNF-α, IL-6 and IL-8 through the Attenuation of ERK and NF-ΚB in HMC-1 Cells. Int. Arch. Allergy Immunol. 2007, 142, 335–344. [Google Scholar] [CrossRef] [PubMed]
- XU, Z.; WEI, C.; ZHANG, R.; YAO, J.; ZHANG, D.; WANG, L. Epigallocatechin-3-Gallate-Induced Inhibition of Interleukin-6 Release and Adjustment of the Regulatory T/T Helper 17 Cell Balance in the Treatment of Colitis in Mice. Exp. Ther. Med. 2015, 10, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Lee, J.; Lee, S.-Y.; Kim, E.-K.; Moon, Y.-M.; Jung, Y.O.; Park, S.-H.; Cho, M.-L. EGCG Attenuates Autoimmune Arthritis by Inhibition of STAT3 and HIF-1α with Th17/Treg Control. PLoS ONE 2014, 9, e86062. [Google Scholar] [CrossRef]
- Hong, Q.; Lyu, W.; Zhang, C.; Yao, W.; Han, Y.; Chen, N. Research Trajectory and Future Trends in Curcumin Related to Immunity: A Bibliometric Analysis of Publications from Last Two Decades. Front. Immunol. 2025, 16, 1559670. [Google Scholar] [CrossRef]
- Parker, J.M.; Zhao, L.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy. Cancers 2025, 17, 2491. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Mirabile, G.; Ettari, R.; Pioggia, G.; Gangemi, S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int. J. Mol. Sci. 2022, 23, 14710. [Google Scholar] [CrossRef]
- Campagna, R.; Cecati, M.; Vignini, A. The Multifaceted Role of the Polyphenol Curcumin: A Focus on Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2025, 21, e15733998313402. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and Its Analogues in Oral Squamous Cell Carcinoma: State-of-the-Art and Therapeutic Potential. Anticancer. Agents Med. Chem. 2025, 25, 313–329. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Wang, C.; Guo, B.; Zhang, T. Quercetin Attenuates MRGPRX2-Mediated Mast Cell Degranulation via the MyD88/IKK/NF-ΚB and PI3K/AKTRac1/Cdc42 Pathway. J. Inflamm. Res. 2024, 17, 7099–7110. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Sibel Kılıç, C.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. Am. Chem. Soc. (ACS) Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells While Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A. Dietary Carotenoids and Human Immune Function. Nutrition 2001, 17, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, Infection, and Immune Function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Hall, J.A.; Grainger, J.R.; Spencer, S.P.; Belkaid, Y. The Role of Retinoic Acid in Tolerance and Immunity. Immunity 2011, 35, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of Lycopene as Antioxidant Carotenoid in the Prevention of Chronic Diseases: A Review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef]
- Pechinskii, S.V.; Kuregyan, A.G. The Impact of Carotenoids on Immunity (Review). Pharm. Chem. J. 2014, 47, 509–513. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Specialized Pro-Resolving Mediator Network: An Update on Production and Actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nano-Proresolving Medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, Specialized Proresolving Lipid Mediators, and Their Potential Roles in Metabolic Diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Patted, P.G.; Masareddy, R.S.; Patil, A.S.; Kanabargi, R.R.; Bhat, C.T. Omega-3 Fatty Acids: A Comprehensive Scientific Review of Their Sources, Functions and Health Benefits. Futur. J. Pharm. Sci. 2024, 10, 94. [Google Scholar] [CrossRef]
- Wu, S.; Li, C. Influence of Maternal Fish Oil Supplementation on the Risk of Asthma or Wheeze in Children: A Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2022, 10, 817110. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, S.C.M.; Brigham, E.P.; Woo, H.; Hanson, C.K.; McCormack, M.C.; Koch, A.; Putcha, N.; Hansel, N.N. Omega-3 Fatty Acid Intake and Prevalent Respiratory Symptoms among U.S. Adults with COPD. BMC Pulm. Med. 2019, 19, 97. [Google Scholar] [CrossRef]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C. Bioactive Peptides and Protein Hydrolysates: Research Trends and Challenges for Application as Nutraceuticals and Functional Food Ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Elass, E.; Masson, M.; Mazurier, J.; Legrand, D. Lactoferrin Inhibits the Lipopolysaccharide-Induced Expression and Proteoglycan-Binding Ability of Interleukin-8 in Human Endothelial Cells. Infect. Immun. 2002, 70, 1860–1866. [Google Scholar] [CrossRef]
- Woodford, K.B. Casomorphins and Gliadorphins Have Diverse Systemic Effects Spanning Gut, Brain and Internal Organs. Int. J. Environ. Res. Public Health 2021, 18, 7911. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and Bioactive Properties of Peptides Derived from Marine Side Streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Jegani, K.T.; Balde, A.; Nazeer, R.A. A Review on Anti-Inflammatory and Antioxidant Peptides Derived from Marine Organisms: Mechanism of Action and Therapeutic Applications. Food Biosci. 2025, 63, 105745. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J. Egg White Protein Ovotransferrin-Derived IRW (Ile-Arg-Trp) Inhibits LPS-Induced Barrier Integrity Dysfunction and Inflammation in Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 14170–14178. [Google Scholar] [CrossRef]
- Ge, H.; Li, T.; Yang, Q.; Tang, Y.; Liu, J.; Yu, Y.; Zhang, T. Egg White Peptides Administration in Enhancing Pathological Immune Response and Regulating Intestinal Bacteria Abundance: A New Strategy for Relieving Young Mice Colitis. Food Front. 2023, 4, 782–794. [Google Scholar] [CrossRef]
- Ano, Y.; Kobayashi, K.; Koikeda, T.; Kawashima, R. β-Lactolin, a Whey-Derived Gly-Thr-Trp-Tyr Lactopeptide, Promotes Cerebral Blood Flow During Cognitive Tasks: A Randomized Controlled Trial. Curr. Dev. Nutr. 2021, 5, 889. [Google Scholar] [CrossRef]
- Cotoia, A.; Cantatore, L.P.; Beck, R.; Tullo, L.; Fortarezza, D.; Marchese, F.; Ferrara, G.; Cinnella, G. Immunological Effects of Glutamine Supplementation in Polytrauma Patients in Intensive Care Unit. J. Anesth. Analg. Crit. Care 2022, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics and Immune Health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674, Erratum in Front. Microbiol. 2024, 14, 1378225. https://doi.org/10.3389/fmicb.2023.1216674. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Li, Y.; Huang, S.; Wu, Y.; Cai, S.; Aipire, A.; Li, J. Dendritic Cell-Based Vaccine Prepared with Recombinant Lactococcus Lactis Enhances Antigen Cross-Presentation and Antitumor Efficacy through ROS Production. Front. Immunol. 2023, 14, 1208349. [Google Scholar] [CrossRef]
- Thakur, R.; Kaur, S. Use of Postbiotics and Parabiotics from Lactobacilli in the Treatment of Infectious Diarrhea. Microb. Pathog. 2025, 204, 107580. [Google Scholar] [CrossRef]
- Langenneger, P.; List, S.R.; Rivas, A.P.; Tenorio, A.; Caseli, C.R.; Estrada, D.; Pires, D.; Acon, E.; Hernandez, F.; Pacheco, G.; et al. The Effect of Probiotics on Prevention of Respiratory Tract Infections in Children and Adolescents: A Systematic Review. Princ. Pract. Clin. Res. J. 2025, 10. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The Influence of Probiotics on Vaccine Responses—A Systematic Review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yue, M.; Wei, J.; Wang, Y.; Hong, D.; Wang, B.; Zhou, X.; Chen, T. Evaluation of the Anti-Aging Effects of a Probiotic Combination Isolated From Centenarians in a SAMP8 Mouse Model. Front. Immunol. 2021, 12, 792746. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy: Preventive and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 17573. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics Modulate the Microbiota–Gut–Brain Axis and Improve Memory Deficits in Aged SAMP8 Mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for Preventing Acute Upper Respiratory Tract Infections. Cochrane Database Syst. Rev. 2015, 21, CD006895. [Google Scholar] [CrossRef]
- Padayatty, S.; Levine, M. Vitamin C: The Known and the Unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Lynch, S.R.; Cook, J.D. Interaction of Vitamin C and Iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Rossander, L. Iron Absorption in Man: Ascorbic Acid and Dose-Dependent Inhibition by Phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Chen, L.; Huang, Y.; Christen, W.; Cook, N.R.; Copeland, T.; Mora, S.; Buring, J.E.; Lee, I.-M.; et al. Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial. Nutrients 2022, 14, 5307. [Google Scholar] [CrossRef]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient Synergy: Definition, Evidence, and Future Directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Rodrigues, C.E.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources. Foods 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; Assadpour, E.; Williams, L.; Jafari, S.M. Nano/Microencapsulated Natural Antimicrobials to Control the Spoilage Microorganisms and Pathogens in Different Food Products. Food Control 2021, 128, 108180. [Google Scholar] [CrossRef]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef]
- Calder, P.; Carr, A.; Gombart, A.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing Immunity in Viral Infections, with Special Emphasis on COVID-19: A Review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef]
- Galanakis, C.M. Introduction. In Nutraceutical and Functional Food Components; Academic Press: Cambridge, MA, USA, 2017; pp. 1–14. [Google Scholar]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation. Molecules 2021, 26, 1931. [Google Scholar] [CrossRef]
- Jin, D.; Wei, X.; He, Y.; Zhong, L.; Lu, H.; Lan, J.; Wei, Y.; Liu, Z.; Liu, H. The Nutritional Roles of Zinc for Immune System and COVID-19 Patients. Front. Nutr. 2024, 11, 1385591. [Google Scholar] [CrossRef]
- Niu, R.; Yang, Q.; Dong, Y.; Hou, Y.; Liu, G. Selenium Metabolism and Regulation of Immune Cells in Immune-associated Diseases. J. Cell. Physiol. 2022, 237, 3449–3464. [Google Scholar] [CrossRef]
- Min, Y.-D.; Choi, C.-H.; Bark, H.; Son, H.-Y.; Park, H.-H.; Lee, S.; Park, J.-W.; Park, E.-K.; Shin, H.-I.; Kim, S.-H. Quercetin Inhibits Expression of Inflammatory Cytokines through Attenuation of NF-ΚB and P38 MAPK in HMC-1 Human Mast Cell Line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef]
- Balamurugan, B.S.; Marimuthu, M.M.C.; Sundaram, V.A.; Saravanan, B.; Chandrababu, P.; Chopra, H.; Malik, T. Micro Nutrients as Immunomodulators in the Ageing Population: A Focus on Inflammation and Autoimmunity. Immun. Ageing 2024, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Derbyshire, E.; Toribio-Mateas, M. Role of Fatty Acids and Micronutrients in Healthy Ageing: A Systematic Review of Randomised Controlled Trials Set in the Context of European Dietary Surveys of Older Adults. J. Human Nutr. Diet. 2016, 29, 308–324. [Google Scholar] [CrossRef]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium Animalis Subsp. Lactis BB-12 in Reducing the Risk of Infections in Early Childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Savilahti, E.; Pönkä, A.; Meurman, J.H.; Poussa, T.; Näse, L.; Saxelin, M.; Korpela, R. Effect of Long Term Consumption of Probiotic Milk on Infections in Children Attending Day Care Centres: Double Blind, Randomised Trial. BMJ 2001, 322, 1327. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef]
- Xu, R.; Molenaar, A.J.; Chen, Z.; Yuan, Y. Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases. Nutrients 2025, 17, 1540. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data from Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-L.; Shih, P.-C.; Liu, S.-J.; Lin, C.-H.; Liu, J.-M.; Lei, W.-T.; Lin, C.-Y. The Influence of Prebiotic or Probiotic Supplementation on Antibody Titers after Influenza Vaccination: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drug Des. Dev. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols Nanoencapsulation for Therapeutic Applications. J. Biomol. Res. Ther. 2016, 5, 1000139. [Google Scholar] [CrossRef]
- Favari, C.; de Alvarenga, J.F.R.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors Driving the Inter-Individual Variability in the Metabolism and Bioavailability of (Poly)Phenolic Metabolites: A Systematic Review of Human Studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef]
- Chaudhary, D.; Guleria, D.; Aggarwal, H.; Mishra, V.; Chauhan, A.; Dufossé, L.; Joshi, N.C. Nutrigenomics and Personalized Diets—Tailoring Nutrition for Optimal Health. Appl. Food Res. 2025, 5, 100980. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderidou, I.; Leontopoulos, S.; Skenderidis, P. Functional Food Ingredients Enhancing Immune Health: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8408. https://doi.org/10.3390/ijms26178408
Skenderidou I, Leontopoulos S, Skenderidis P. Functional Food Ingredients Enhancing Immune Health: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(17):8408. https://doi.org/10.3390/ijms26178408
Chicago/Turabian StyleSkenderidou, Irene, Stefanos Leontopoulos, and Prodromos Skenderidis. 2025. "Functional Food Ingredients Enhancing Immune Health: A Systematic Review" International Journal of Molecular Sciences 26, no. 17: 8408. https://doi.org/10.3390/ijms26178408
APA StyleSkenderidou, I., Leontopoulos, S., & Skenderidis, P. (2025). Functional Food Ingredients Enhancing Immune Health: A Systematic Review. International Journal of Molecular Sciences, 26(17), 8408. https://doi.org/10.3390/ijms26178408








