The OvarianTag™ Biomarker Panel Emerges as a Prognostic Tool to Guide Clinical Decisions in Cisplatin-Based Treatment of Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Results
2.1. Individuals
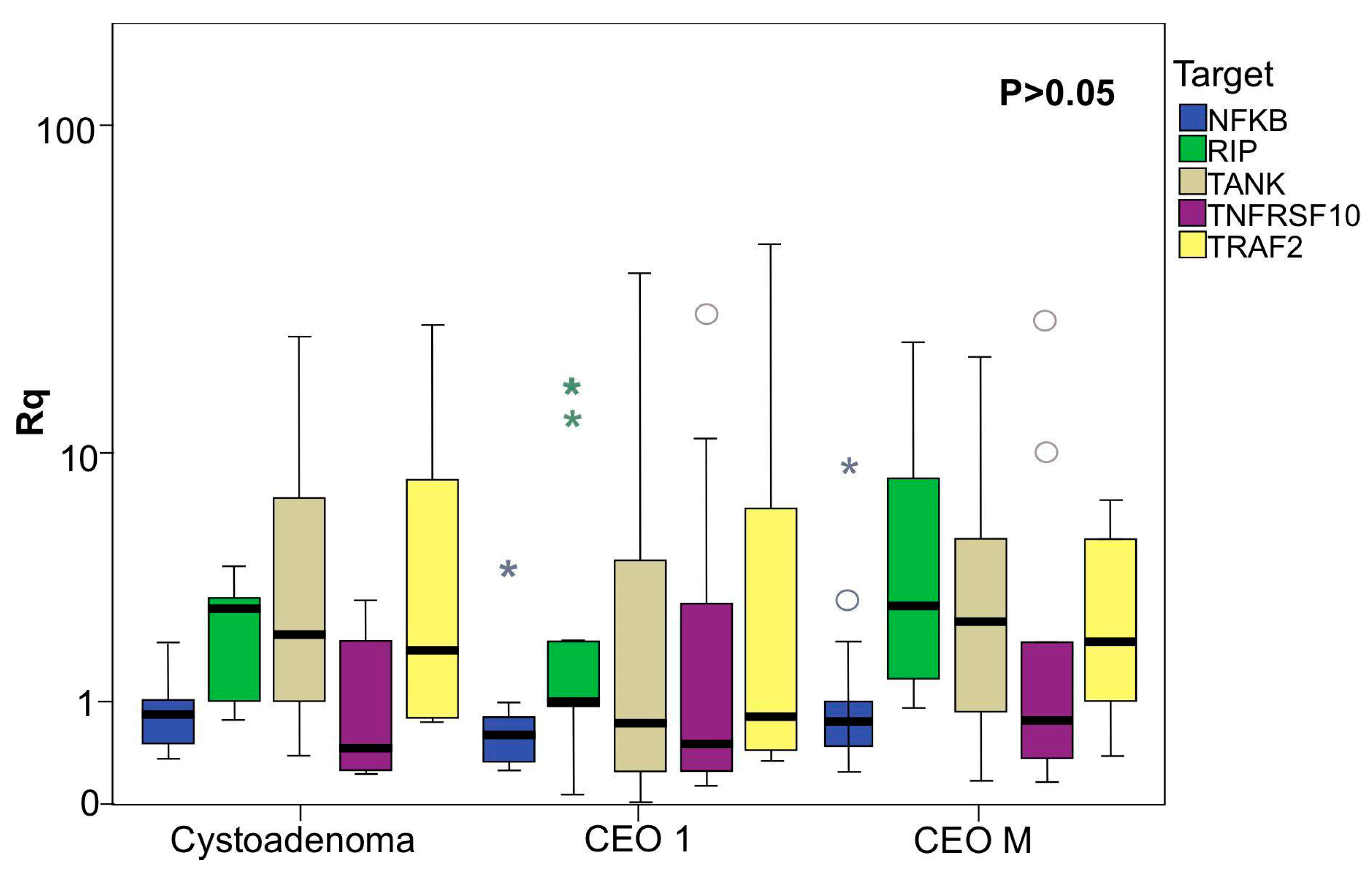
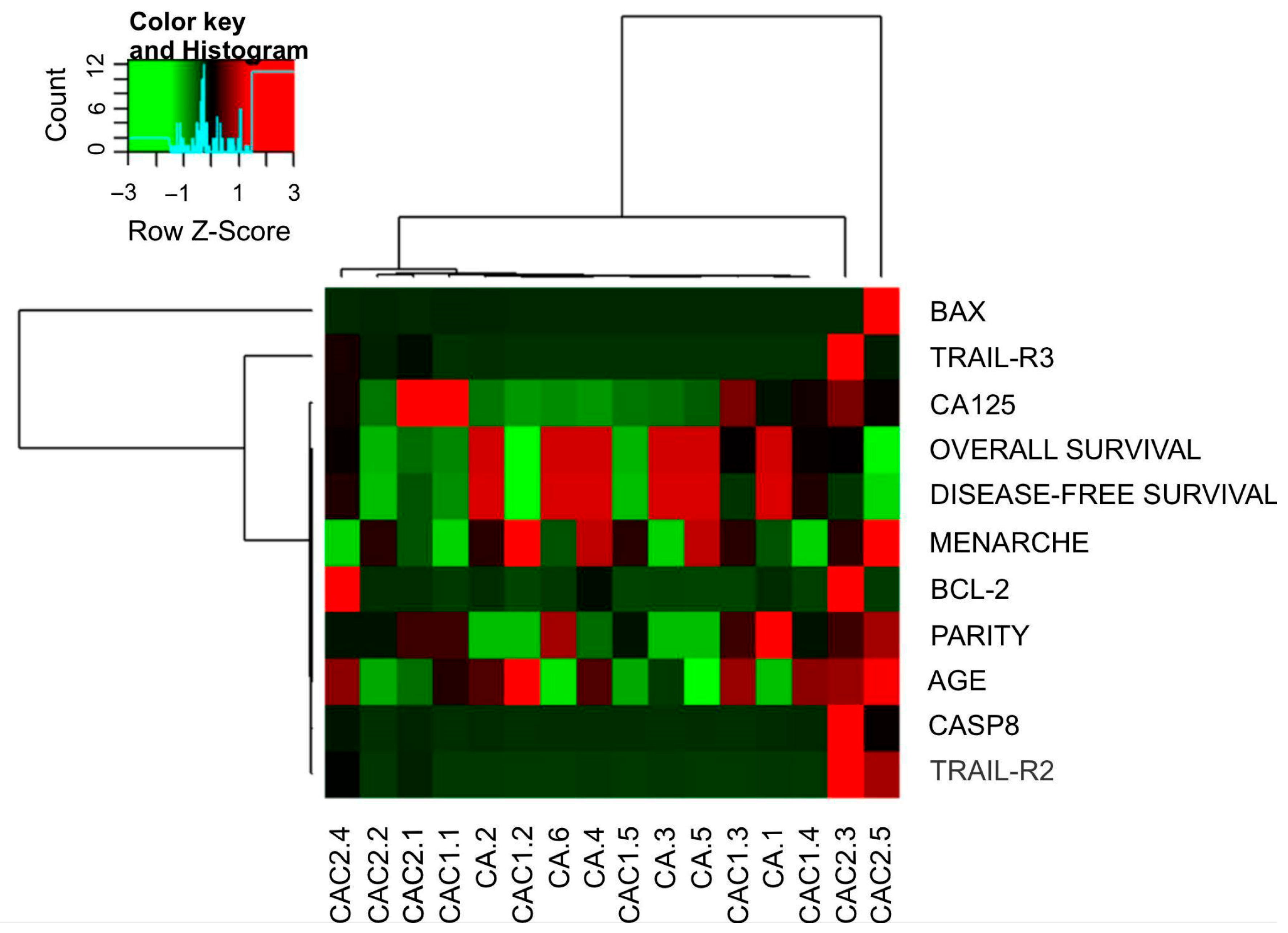
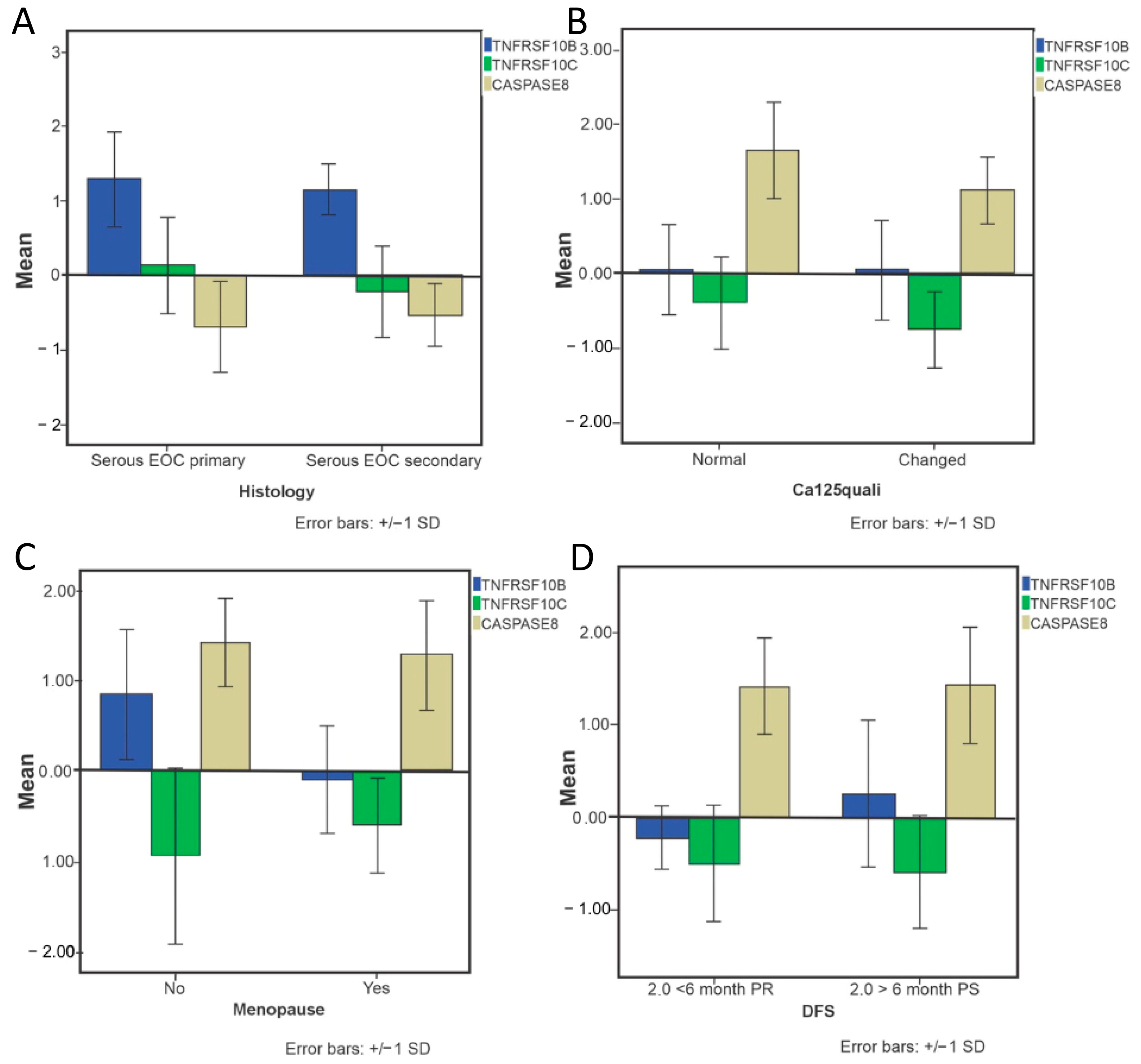
2.2. Data of Gene Expression and Clinical Features Related
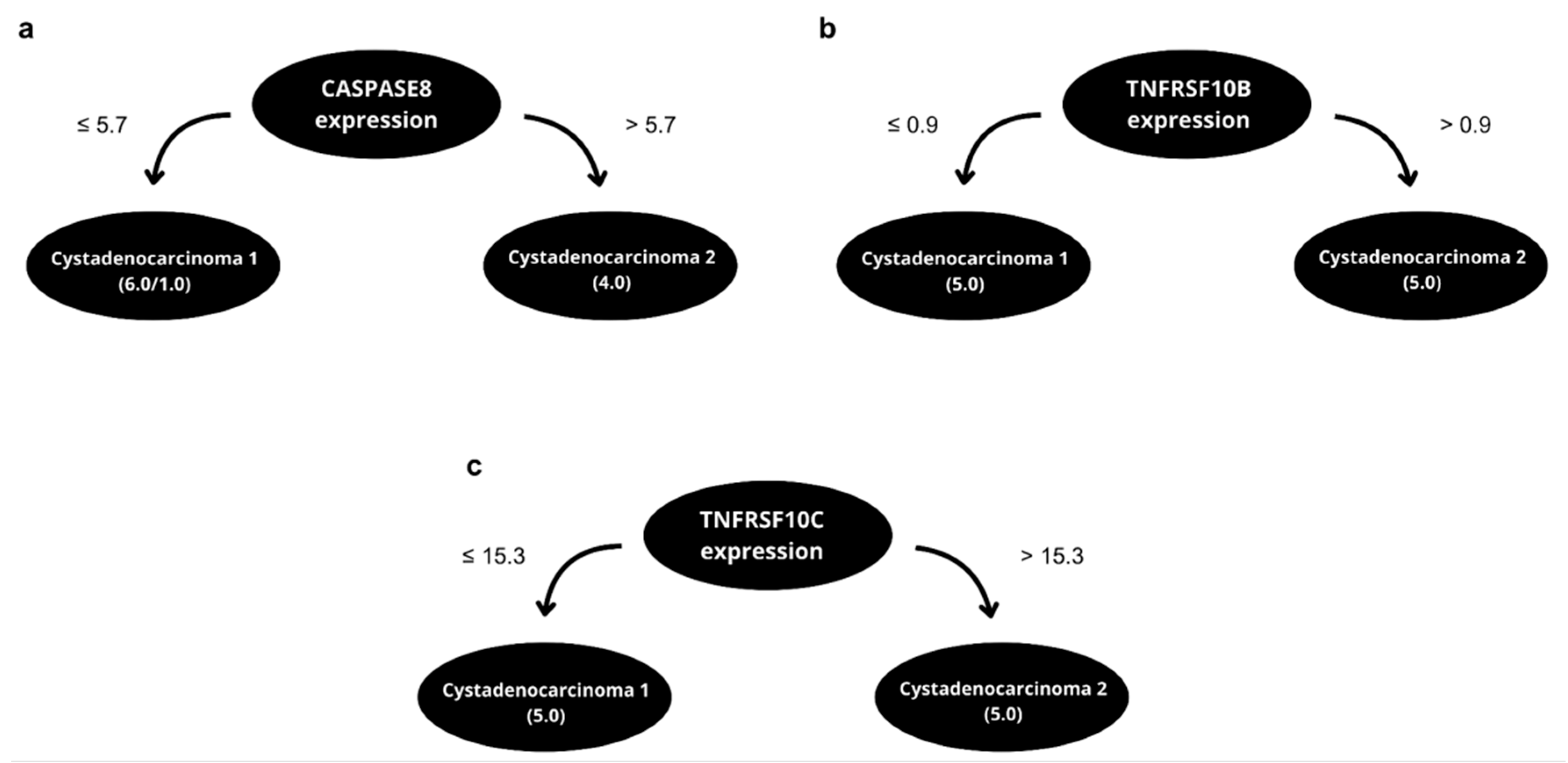
2.3. Predictive Algorithm Designer to Clinical Outcomes Prediction in EOC Patients and Biological Relevance of Attributes Selected
3. Discussion
4. Methods and Materials
4.1. Study Design and Participants
4.2. Total RNA Extraction and Quality Analysis
4.3. Gene Expression Analysis
4.4. Data Analysis and Developing an Intelligent System for Clinical Outcomes Prediction in EOC Patients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Neoplasia Maligna Do Corpo Do Útero e Do Ovário (Taxas Ajustadas)—Instituto Nacional de Câncer—INCA. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/estimativa/por-neoplasia-taxas-ajustadas/corpo-utero-ovario (accessed on 10 February 2025).
- Ovary Statistics|American Cancer Society—Cancer Facts & Statistics. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Ovary (accessed on 8 October 2023).
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef] [PubMed]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel Approaches to Ovarian Cancer Screening. Curr. Oncol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular Subtypes of Endometrial Cancer: Implications for Adjuvant Treatment Strategies. Int. J. Gynecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Global Epidemiology of Epithelial Ovarian Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. Pathogenesis of Ovarian Cancer: Lessons from Morphology and Molecular Biology and Their Clinical Implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Matulonis, U.A. Clinical and Translational Advances in Ovarian Cancer Therapy. Nat. Cancer 2023, 4, 1239–1257. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus Paclitaxel Once a Week versus Every 3 Weeks in Patients with Advanced Ovarian Cancer (MITO-7): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in Ovarian Cancer Therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef]
- Colombo, P.E.; Fabbro, M.; Theillet, C.; Bibeau, F.; Rouanet, P.; Ray-Coquard, I. Sensitivity and Resistance to Treatment in the Primary Management of Epithelial Ovarian Cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 207–216. [Google Scholar] [CrossRef]
- Gebhart, P.; Singer, C.; Gschwantler-Kaulich, D. CA125 Levels in BRCA Mutation Carriers—A Retrospective Single Center Cohort Study. BMC Cancer 2023, 23, 610. [Google Scholar] [CrossRef] [PubMed]
- Sölétormos, G.; Duffy, M.J.; Othman Abu Hassan, S.; Verheijen, R.H.M.; Tholander, B.; Bast, R.C.; Gaarenstroom, K.N.; Sturgeon, C.M.; Bonfrer, J.M.; Petersen, P.H.; et al. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int. J. Gynecol. Cancer 2016, 26, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; D’Andrea, A.D.; Kozono, D. A DNA Repair Pathway-Focused Score for Prediction of Outcomes in Ovarian Cancer Treated with Platinum-Based Chemotherapy. J. Natl. Cancer Inst. 2012, 104, 670–681. [Google Scholar] [CrossRef]
- Qin, Y.; Sheng, Y.; Ren, M.; Hou, Z.; Xiao, L.; Chen, R. Identification of necroptosis-related gene signatures for predicting the prognosis of ovarian cancer. Sci. Rep. 2024, 14, 11133. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.; Hu, T.; Chen, Y. Two novel prognostic models for ovarian cancer respectively based on ferroptosis and necroptosis. BMC Cancer 2022, 22, 65. [Google Scholar] [CrossRef]
- Wilczyński, J.; Paradowska, E.; Wilczyńska, J.; Wilczyński, M. Prediction of Chemoresistance—How Preclinical Data Could Help to Modify Therapeutic Strategy in High-Grade Serous Ovarian Cancer. Curr. Oncol. 2024, 31, 229–249. [Google Scholar] [CrossRef]
- Braga, L.D.C.; Álvares Da Silva Ramos, A.P.; Traiman, P.; Silva, L.M.; Lopes Da Silva-Filho, A. TRAIL-R3-Related Apoptosis: Epigenetic and Expression Analyses in Women with Ovarian Neoplasia. Gynecol. Oncol. 2012, 126, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Da Conceição Braga, L.; Silva, L.M.; Piedade, J.B.; Traiman, P.; Da Silva Filho, A.L. Epigenetic and Expression Analysis of TRAIL-R2 and BCL2: On the TRAIL to Knowledge of Apoptosis in Ovarian Tumors. Arch. Gynecol. Obs. 2014, 289, 1061–1069. [Google Scholar] [CrossRef]
- da Conceição Braga, L.; Silva, L.M.; da Silva Ramos, A.P.Á.; Piedade, J.B.; Vidigal, P.V.T.; Traiman, P.; da Silva Filho, A.L. Single CpG Island Methylation Is Not Sufficient to Maintain the Silenced Expression of CASPASE-8 Apoptosis-Related Gene among Women with Epithelial Ovarian Cancer. Biomed. Pharmacother. 2014, 68, 87–91. [Google Scholar] [CrossRef]
- Salzberg, S.L. C4.5: Programs for Machine Learning by J. Ross Quinlan. Morgan Kaufmann Publishers, Inc., 1993. Mach. Learn. 1994, 16, 235–240. [Google Scholar] [CrossRef]
- Frank, E.; Hall, M.; Holmes, G.; Kirkby, R.; Pfahringer, B.; Witten, I.H.; Trigg, L. Weka—A Machine Learning Workbench for Data Mining. In Data Mining and Knowledge Discovery Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1269–1277. [Google Scholar] [CrossRef]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Cronin, A.M.; Sun, C.C.; Bixel, K.; Bookman, M.A.; Cristea, M.C.; Griggs, J.J.; Levenback, C.F.; Burger, R.A.; Mantia-Smaldone, G.; et al. Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Oncol. 2016, 34, 3854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in Diagnosis, Prediction, and Oncogenesis of Ovarian Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and Algorithms for Diagnosis of Ovarian Cancer: CA125, HE4, RMI and ROMA, a Review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B. Ovarian Cancer Biomarkers: Moving Forward in Early Detection. Adv. Exp. Med. Biol. 2020, 1219, 355–363. [Google Scholar] [CrossRef]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Falschlehner, C.; Emmerich, C.H.; Gerlach, B.; Walczak, H. TRAIL Signalling: Decisions between Life and Death. Int. J. Biochem. Cell Biol. 2007, 39, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-Related Apoptosis-Inducing Ligand (TRAIL) Receptor by Natural Products as a Potential Therapeutic Approach for Cancer Therapy. Exp. Biol. Med. 2015, 240, 760. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs Less Travelled: TRAIL in Cancer Biology and Therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Ion, G.N.D.; Nitulescu, G.M.; Popescu, C.I. Targeting TRAIL. Bioorg. Med. Chem. Lett. 2019, 29, 2527–2534. [Google Scholar] [CrossRef]
- Arts, H.J.G.; De Jong, S.; Hollema, H.; Ten Hoor, K.; Van Der Zee, A.G.J.; De Vries, E.G.E. Chemotherapy Induces Death Receptor 5 in Epithelial Ovarian Carcinoma. Gynecol. Oncol. 2004, 92, 794–800. [Google Scholar] [CrossRef]
- Hashemi Sheikhshabani, S.; Amini-Farsani, Z.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asgharzade, S. Oleuropein Reduces Cisplatin Resistance in Ovarian Cancer by Targeting Apoptotic Pathway Regulators. Life Sci. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Cho, Y.S. The Role of Necroptosis in the Treatment of Diseases. BMB Rep. 2018, 51, 219–224. [Google Scholar] [CrossRef]
- Degterev, A.; Ofengeim, D.; Yuan, J. Targeting RIPK1 for the Treatment of Human Diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 9714–9722. [Google Scholar] [CrossRef]
- Wu, W.; Liu, P.; Li, J. Necroptosis: An Emerging Form of Programmed Cell Death. Crit. Rev. Oncol. Hematol. 2012, 82, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ju, J.; Zhang, Z.; Zhao, C.; Li, Z.; Zheng, J.; Sheng, T.; Zhang, H.; Hu, L.; Yu, X.; et al. Discovery of Potent Necroptosis Inhibitors Targeting RIPK1 Kinase Activity for the Treatment of Inflammatory Disorder and Cancer Metastasis. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Oncotype DX Breast Recurrence Score®: A Review of Its Use in Early-Stage Breast Cancer. Mol. Diagn. Ther. 2020, 24, 621–632. [Google Scholar] [CrossRef]
- Brandão, M.; Pondé, N.; Piccart-Gebhart, M. MammaprintTM: A Comprehensive Review. Future Oncol. 2019, 15, 207–224. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Anton, C.; Carvalho, F.M.; Oliveira, E.I.; Maciel, G.A.R.; Baracat, E.C.; Carvalho, J.P. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (Sao Paulo) 2012, 67, 437–441. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG Inhibitors in Cancer Treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef]
- Kleemann, M.; Schneider, H.; Unger, K.; Bereuther, J.; Fischer, S.; Sander, P.; Marion Schneider, E.; Fischer-Posovszky, P.; Riedel, C.U.; Handrick, R.; et al. Induction of Apoptosis in Ovarian Cancer Cells by MiR-493-3p Directly Targeting AKT2, STK38L, HMGA2, ETS1 and E2F5. Cell. Mol. Life Sci. 2019, 76, 539–559. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Yaylim, I.; Ozkan, N.E.; Zaman, F.; Halim, T.A.; Chang, H.W. Restoring TRAIL Mediated Signaling in Ovarian Cancer Cells. Arch. Immunol. Ther. Exp. 2014, 62, 459–474. [Google Scholar] [CrossRef]
- Bhatla, N.; Denny, L. FIGO Cancer Report 2018. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 2–3. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 February 2025).
- Haykin, S. Redes Neurais: Princípios e Prática—Simon Haykin—Google Livros; Bookman Editora: Wellington, New Zealand, 2001; ISBN 0-13-273350-1. [Google Scholar]


| 1st Phase | 2nd Phase | ||||
|---|---|---|---|---|---|
| Normal ovary | Ovarian serous cystadenoma | Cystadenocarcinoma ovarian serous | Cystadenocarcinoma ovarian serous | p value | |
| n = 18 | n = 11 | n = 16 | n = 55 | ||
| Age (Years) | 50.61 ±2.13 | 54.3 ± 5.1 | 59.1 ± 2.7 | 58.7 ± 11.2 | |
| Parity | 2.83 ± 0.44 | 2.3 ± 0.8 | 1.9 ± 0.3 | 1.6 ± 1.3 | 0.128 |
| CA-125 (U/mL) | |||||
| CA < 35 (U/mL) | 11 (100%) | 4 (25%) | 12 (22.2%) | ||
| CA > 35(U/mL) | 0 | 12 (75%) | 42 (77.8%) | ||
| Cytoreduction | |||||
| Excellent (<1 cm) | 7 (43.8%) | 21 (46.7%) | |||
| Suboptimal (>1 cm) | 9 (56.33%) | 24 (53.33%) | |||
| Degree of tumor differentiation | |||||
| G2 | 6 (37.5%) | 8 (22.2%) | |||
| G3 | 10 (62.5%) | 28 (77.8%) | |||
| Staging | |||||
| I-II | 5 (31.8%) | 21 (41.2%) | |||
| III-IV | 11 (68.88%) | 30 (58.88%) | |||
| Ascites | |||||
| Yes | 10 (62.5%) | 30 (61.2%) | |||
| No | 6 (37.5%) | 19 (38.8%) | |||
| Menarche | 12.8 ± 0.4 | 12.8 ± 3.4 | 13.05 + 1.36 | 0.991 | |
| Menopause | |||||
| Yes | 18 (100%) | 6 (54.5%) | 13 (81.3%) * | 38 (76%) | 0.008 |
| No | 0 | 5 (45.5%) | 3 (18.8%) | 12 (24%) |
| Analysis | Full Training | LOOCV | Rule |
|---|---|---|---|
| 2017_10_16_Onco tag_histology_CA SP8_end | 90% | 80% | IF expression CASP8 <= 5.7 then early tumors else advanced tumors |
| 2017_10_16_Onco tag_histology_TN RF10B_end | 100% | 90% | IF expression TNRFS10B <= 0.9 then cystadenoma 1 else cystadenoma 2 |
| 2017_10_16_Onco tag_histology_TN RF10C_end | 100% | 90% | IF expression TNRFSF10C <= 15.3 then cystadenoma 1 else cystadenoma 2 |
| The Benefit of Platinum-Based Treatment | Risk of Tumor Recurrence in 12 Months | |||||
|---|---|---|---|---|---|---|
| Full Training | LOOCV | Full Training | LOOCV | Full Training | LOOCV | |
| Sensitivity | 100% | 100% | 88% | 78% | 100% | 88% |
| Specificity | 85% | 82% | 81% | 80% | 100% | 71% |
| Pos. Lik. Rat. | 67.5% | 56% | 46.7% | 38.9% | - | 31% |
| Neg. Lik. Rat | 0 | 0 | 15% | 28% | 0 | 1.6% |
| Pos. Pred. Va. | 42.9% | 28.6% | 70.0% | 70.0% | 100% | 92% |
| Neg. Pred. Va. | 100% | 100% | 92.9% | 85.7% | 100% | 62.5% |
| Accuracy | 86.7% | 83.3% | 83.3% | 79.2% | 100% | 84.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, L.d.C.; Amaral, L.R.d.; Villar Delfino, P.H.; Andrade, N.R.; de Oliveira Salles, P.G.; da Silva Filho, A.L.; Bertarini, P.L.L.; da Silva Ramos, A.P.Á.; Gomes, M.d.S.; Silva Lopes, L.M. The OvarianTag™ Biomarker Panel Emerges as a Prognostic Tool to Guide Clinical Decisions in Cisplatin-Based Treatment of Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2025, 26, 8393. https://doi.org/10.3390/ijms26178393
Braga LdC, Amaral LRd, Villar Delfino PH, Andrade NR, de Oliveira Salles PG, da Silva Filho AL, Bertarini PLL, da Silva Ramos APÁ, Gomes MdS, Silva Lopes LM. The OvarianTag™ Biomarker Panel Emerges as a Prognostic Tool to Guide Clinical Decisions in Cisplatin-Based Treatment of Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2025; 26(17):8393. https://doi.org/10.3390/ijms26178393
Chicago/Turabian StyleBraga, Letícia da Conceição, Laurence Rodrigues do Amaral, Pedro Henrique Villar Delfino, Nara Rosana Andrade, Paulo Guilherme de Oliveira Salles, Agnaldo Lopes da Silva Filho, Pedro Luiz Lima Bertarini, Ana Paula Álvares da Silva Ramos, Matheus de Souza Gomes, and Luciana Maria Silva Lopes. 2025. "The OvarianTag™ Biomarker Panel Emerges as a Prognostic Tool to Guide Clinical Decisions in Cisplatin-Based Treatment of Epithelial Ovarian Cancer" International Journal of Molecular Sciences 26, no. 17: 8393. https://doi.org/10.3390/ijms26178393
APA StyleBraga, L. d. C., Amaral, L. R. d., Villar Delfino, P. H., Andrade, N. R., de Oliveira Salles, P. G., da Silva Filho, A. L., Bertarini, P. L. L., da Silva Ramos, A. P. Á., Gomes, M. d. S., & Silva Lopes, L. M. (2025). The OvarianTag™ Biomarker Panel Emerges as a Prognostic Tool to Guide Clinical Decisions in Cisplatin-Based Treatment of Epithelial Ovarian Cancer. International Journal of Molecular Sciences, 26(17), 8393. https://doi.org/10.3390/ijms26178393






