Exogenous Pyruvate in Defense Against Human-Exposure Toxicants: A Review of In Vitro and In Vivo Evidence
Abstract
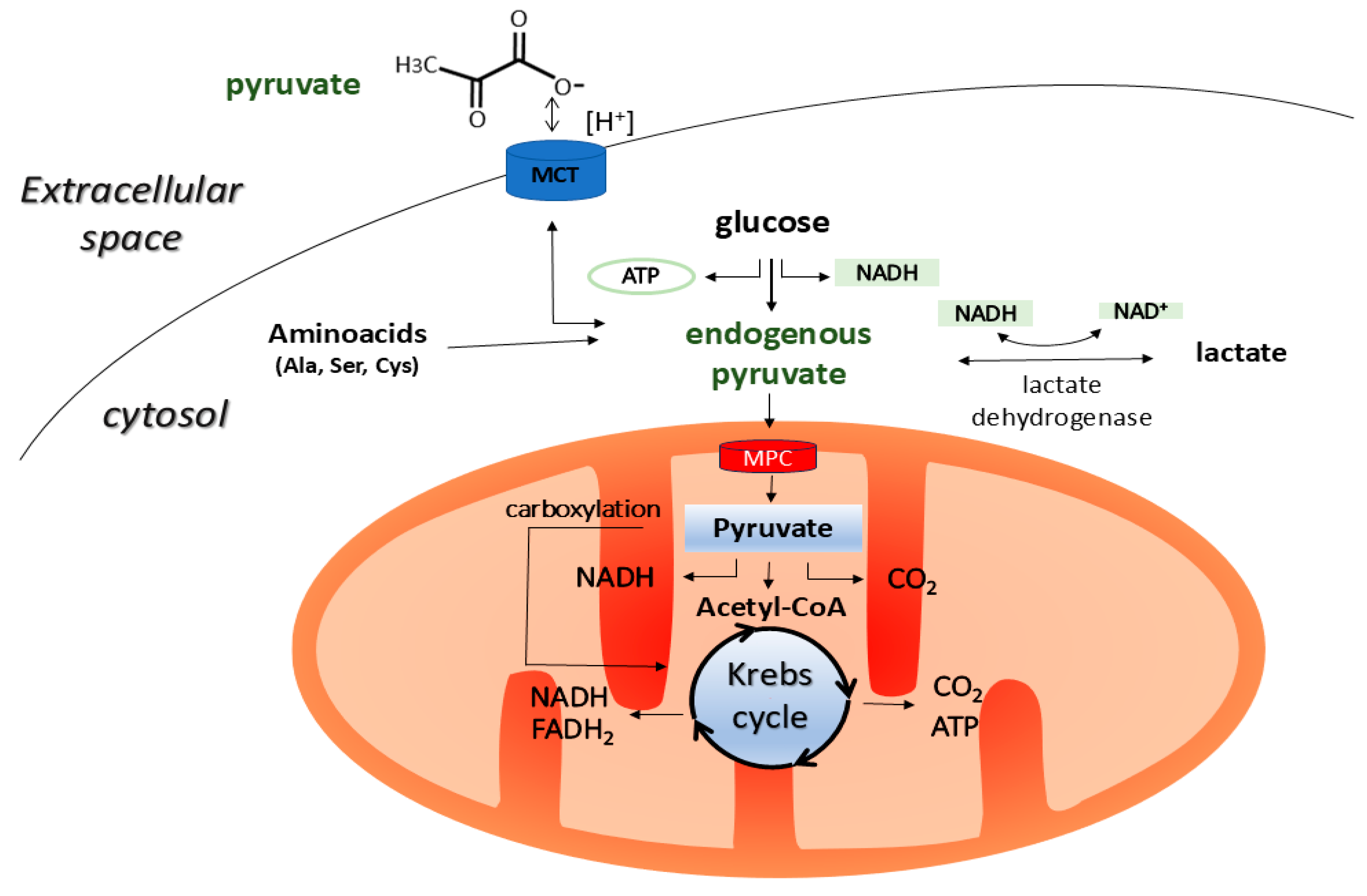
1. Introduction
2. Pyruvate Effects on Physical and Chemical Toxicants
2.1. In Vivo Studies
2.2. In Vitro Studies
3. Cytoprotective Modes Activated by Exogenous Pyruvate in Animal Cells
3.1. Direct ROS Scavenging Activity
3.2. Effects on Mitochondrial Functions
3.3. Anti-Inflammatory Activity of Pyruvate
3.3.1. Reduction of NF-κB Activity
3.3.2. Blocking HMGB1 Secretion
3.3.3. Nrf2 Induction
3.4. Other Possible Protective Mechanisms of Pyruvate
4. Potential Barriers to the Use of Pyruvate as a Protective Agent
5. Conclusions
- 1.
- Existing research on the effects of exogenous pyruvate in the context of physical and chemical toxicants has mainly focused on the ethyl derivative of pyruvate, particularly in animal models. As previously mentioned, however, this derivative may not be effectively converted to free pyruvate in humans. Therefore, in vivo studies using sodium pyruvate could provide a more accurate assessment of metabolism and protective effect of exogenous pyruvate, particularly at the level of individual organs.
- 2.
- In addition, consideration should be given to the potential side effects associated with the formation of toxic pyruvate dimers and the risk of hypernatraemia that may accompany the administration of higher doses of sodium pyruvate.
- 3.
- An important area of research is the role of mitochondria in the protective mechanisms of exogenous pyruvate, given their key importance in the cellular metabolism of this compound.
- 4.
- Finally, the development of stable forms of pyruvate could offer additional opportunities for the therapeutic use of this α-keto acid.
Funding
Conflicts of Interest
References
- Li, M.; Zhou, S.; Chen, C.; Ma, L.; Luo, D.; Tian, X.; Dong, X.; Zhou, Y.; Yang, Y.; Cui, Y. Therapeutic Potential of Pyruvate Therapy for Patients with Mitochondrial Diseases: A Systematic Review. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938240. [Google Scholar] [CrossRef]
- Zhou, F.Q. Pyruvate in the Correction of Intracellular Acidosis: A Metabolic Basis as a Novel Superior Buffer. Am. J. Nephrol. 2005, 25, 55–63. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, S.; Tonnessen, T.I. Ethyl Pyruvate Is a Novel Anti-Inflammatory Agent to Treat Multiple Inflammatory Organ Injuries. J. Inflamm. 2016, 13, 37. [Google Scholar] [CrossRef]
- Shapiro, F.; Silanikove, N. Rapid and Accurate Determination of Malate, Citrate, Pyruvate and Oxaloacetate by Enzymatic Reactions Coupled to Formation of a Fluorochromophore: Application in Colorful Juices and Fermentable Food (Yogurt, Wine) Analysis. Food Chem. 2011, 129, 608–613. [Google Scholar] [CrossRef]
- Bortoluzzi, V.T.; Brust, L.; Preissler, T.; de Franceschi, I.D.; Wannmacher, C.M.D. Creatine plus Pyruvate Supplementation Prevents Oxidative Stress and Phosphotransfer Network Disturbances in the Brain of Rats Subjected to Chemically-Induced Phenylketonuria. Metab. Brain Dis. 2019, 34, 1649–1660. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Yi, J.-S.; Chung, S.-J.; Kim, D.-K.; Byun, H.-R.; Lee, J.-Y.; Koh, J.-Y. Pyruvate Protects against Kainate-Induced Epileptic Brain Damage in Rats. Exp. Neurol. 2007, 208, 159–167. [Google Scholar] [CrossRef]
- Hu, S.; Liu, W.; Zhao, Y.; Lin, Z.; Luo, H.; Bai, X.; Sheng, Z.; Zhou, F. Pyruvate-Enriched Oral Rehydration Solution Improved Intestinal Absorption of Water and Sodium during Enteral Resuscitation in Burns. Burns J. Int. Soc. Burn Inj. 2014, 40, 693–701. [Google Scholar] [CrossRef]
- Liu, R.; Hu, X.-H.; Wang, S.-M.; Guo, S.-J.; Li, Z.-Y.; Bai, X.-D.; Zhou, F.-Q.; Hu, S. Pyruvate in Oral Rehydration Salt Improves Hemodynamics, Vasopermeability and Survival after Burns in Dogs. Burns J. Int. Soc. Burn Inj. 2016, 42, 797–806. [Google Scholar] [CrossRef]
- Martin, A.; Lupfer, C.; Amen, R. Inhaled Sodium Pyruvate Reduces Oxygen Radicals and Inflammatory Cytokines in COPD Patients. Eur. J. Respir. Med. 2022, 4, 320–326. [Google Scholar] [CrossRef]
- Votto, J.J.; Bowen, J.B.; Barton, R.W.; Thrall, R.S. Inhaled Sodium Pyruvate Improved FEV1 and Decreased Expired Breath Levels of Nitric Oxide in Patients with Chronic Obstructive Pulmonary Disease. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 329–334. [Google Scholar] [CrossRef]
- Nadler, R.A. Sodium Pyruvate Inhalation for COVID-19 Long Hauler Symptoms—An Effective and Inexpensive Treatment. J. Immunol. 2022, 208, 125.25. [Google Scholar] [CrossRef]
- Komaki, H.; Nishigaki, Y.; Fuku, N.; Hosoya, H.; Murayama, K.; Ohtake, A.; Goto, Y.-I.; Wakamoto, H.; Koga, Y.; Tanaka, M. Pyruvate Therapy for Leigh Syndrome Due to Cytochrome c Oxidase Deficiency. Biochim. Biophys. Acta 2010, 1800, 313–315. [Google Scholar] [CrossRef]
- Petkova, I.; Mateva, L.; Petrov, K.; Beniozef, D.; Thorn, W. Effects of Sodium Pyruvate Applied in Patients with Chronic Liver Diseases (CLD) of Different Etiology. Comptes Rendus Acad. Bulg. Sci. 2003, 56, 107. [Google Scholar]
- Petkova, I.; Hristov, V.; Petrov, K.; Thorn, W. Oral Application of Sodium Pyruvate in Healthy Persons and Patients with Diabetes Mellitus Type I. Comptes Rendus Acad. Bulg. Sci. 2007, 60, 579–584. [Google Scholar]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 11–18. [Google Scholar] [CrossRef]
- Lupfer, C.R.; Nadler, R.; Amen, R.; Martin, A. Inhalation of Sodium Pyruvate to Reduce the Symptoms and Severity of Respiratory Diseases Including COVID-19, Long COVID, and Pulmonary Fibrosis. Eur. J. Respir. Med. 2021, 3, 229–237. [Google Scholar] [CrossRef]
- Kgi-Admin. N-115 by EmphyCorp for Idiopathic Pulmonary Fibrosis: Likelihood of Approval. Pharmaceutical Technology. Available online: https://www.pharmaceutical-technology.com/data-insights/n-115-emphycorp-idiopathic-pulmonary-fibrosis-likelihood-of-approval/ (accessed on 16 April 2025).
- Jaffary, F.; Faghihi, G.; Saraeian, S.; Hosseini, S.M. Comparison the Effectiveness of Pyruvic Acid 50% and Salicylic Acid 30% in the Treatment of Acne. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 31. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Qiu, J.-Q.; Wang, M.-Y.; Feng, L.; Luo, D.; Gao, R.-R.; Zhou, F.-Q.; Che, K.-X. Effects of Sodium Pyruvate Supplementation on Repeated Sprint Exercise Performance and Recovery in Male College Soccer Players: A Randomized Controlled Trial. Ann. Palliat. Med. 2022, 11, 598–610. [Google Scholar] [CrossRef]
- Jäger, R.; Metzger, J.; Lautmann, K.; Shushakov, V.; Purpura, M.; Geiss, K.-R.; Maassen, N. The Effects of Creatine Pyruvate and Creatine Citrate on Performance during High Intensity Exercise. J. Int. Soc. Sports Nutr. 2008, 5, 4. [Google Scholar] [CrossRef]
- Olek, R.A.; Kujach, S.; Radak, Z. Current Knowledge about Pyruvate Supplementation: A Brief Review. Sports Med. Health Sci. 2024, 6, 295–301. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Y.; Ding, N.; Fang, Y. Recent Advances in Metabolic Engineering for the Biosynthesis of Phosphoenol Pyruvate-Oxaloacetate-Pyruvate-Derived Amino Acids. Molecules 2024, 29, 2893. [Google Scholar] [CrossRef]
- Durak, M.Z.; Churey, J.J.; Gates, M.; Sacks, G.L.; Worobo, R.W. Decontamination of Green Onions and Baby Spinach by Vaporized Ethyl Pyruvate. J. Food Prot. 2012, 75, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Andrae, U.; Singh, J.; Ziegler-Skylakakis, K. Pyruvate and Related Alpha-Ketoacids Protect Mammalian Cells in Culture against Hydrogen Peroxide-Induced Cytotoxicity. Toxicol. Lett. 1985, 28, 93–98. [Google Scholar] [CrossRef]
- O’Donnell-Tormey, J.; Nathan, C.F.; Lanks, K.; DeBoer, C.J.; de la Harpe, J. Secretion of Pyruvate. An Antioxidant Defense of Mammalian Cells. J. Exp. Med. 1987, 165, 500–514. [Google Scholar] [CrossRef]
- Natoli, R.; Rutar, M.; Lu, Y.-Z.; Chu-Tan, J.A.; Chen, Y.; Saxena, K.; Madigan, M.; Valter, K.; Provis, J.M. The Role of Pyruvate in Protecting 661W Photoreceptor-Like Cells Against Light-Induced Cell Death. Curr. Eye Res. 2016, 41, 1473–1481, Correction in Curr. Eye Res. 2025, 50, 460. [Google Scholar] [CrossRef]
- Kładna, A.; Marchlewicz, M.; Piechowska, T.; Kruk, I.; Aboul-Enein, H.Y. Reactivity of Pyruvic Acid and Its Derivatives towards Reactive Oxygen Species. Lumin. J. Biol. Chem. Lumin. 2015, 30, 1153–1158. [Google Scholar] [CrossRef]
- Zwolak, I.; Gołębiowska, D. Protective Activity of Pyruvate against Vanadium-Dependent Cytotoxicity in Chinese Hamster Ovary (CHO-K1) Cells. Toxicol. Ind. Health 2018, 34, 283–292. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Yang, Y.; Takata, T.; Sakurai, T. Systemic Pyruvate Administration Markedly Reduces Neuronal Death and Cognitive Impairment in a Rat Model of Alzheimer’s Disease. Exp. Neurol. 2015, 271, 145–154. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of Reactive Oxygen Species in Ultraviolet-Induced Photodamage of the Skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Z.; Liu, C.; Nie, Y.; Cui, L. Climate Change, Air Pollution and Chronic Respiratory Diseases: Understanding Risk Factors and the Need for Adaptive Strategies. Environ. Health Prev. Med. 2025, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Protective Effect of Indole-3-Pyruvate against Ultraviolet B-Induced Damage to Cultured HaCaT Keratinocytes and the Skin of Hairless Mice. PLoS ONE 2014, 9, e96804, Correction in PLoS ONE 2015, 10, e0128054. [Google Scholar] [CrossRef]
- Gupta, S.K.; Awor, L.; Rastogi, S.; Prakash, J.; Gupta, Y.K.; Varma, S.D.; Velpandian, T. Delayed Manifestation of Ultra Violet Radiation Induced Erythema in Guinea Pigs by Sodium Pyruvate—A Free Radical Scavenger. Indian J. Physiol. Pharmacol. 1998, 42, 315–318. [Google Scholar]
- Han, C.; Yang, H.; Li, B.; Wang, Z. Exogenous Pyruvate Facilitates Ultraviolet B-Induced DNA Damage Repair by Promoting H3K9 Acetylation in Keratinocytes and Melanocytes. Biomed. Pharmacother. 2020, 126, 110082. [Google Scholar] [CrossRef]
- Ullah, N.; Naseer, M.I.; Ullah, I.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Neuroprotective Profile of Pyruvate against Ethanol-Induced Neurodegeneration in Developing Mice Brain. Neurol. Sci. 2013, 34, 2137–2143. [Google Scholar] [CrossRef]
- Hu, J.-M.; Hsu, C.-H.; Lin, Y.-C.; Kung, C.-W.; Chen, S.-Y.; Lin, W.-T.; Cheng, P.-Y.; Shen, H.-H.; Lee, Y.-M. Ethyl Pyruvate Ameliorates Heat Stroke-Induced Multiple Organ Dysfunction and Inflammatory Responses by Induction of Stress Proteins and Activation of Autophagy in Rats. Int. J. Hyperth. 2021, 38, 862–874. [Google Scholar] [CrossRef]
- Epperly, M.; Jin, S.; Nie, S.; Cao, S.; Zhang, X.; Franicola, D.; Wang, H.; Fink, M.P.; Greenberger, J.S. Ethyl Pyruvate, a Potentially Effective Mitigator of Damage after Total-Body Irradiation. Radiat. Res. 2007, 168, 552–559. [Google Scholar] [CrossRef]
- Chen, B.; Na, F.; Yang, H.; Li, R.; Li, M.; Sun, X.; Hu, B.; Huang, G.; Lan, J.; Xu, H.; et al. Ethyl Pyruvate Alleviates Radiation-Induced Lung Injury in Mice. Biomed. Pharmacother. 2017, 92, 468–478. [Google Scholar] [CrossRef]
- Nagatome, M.; Kondo, Y.; Kadowaki, D.; Saishyo, Y.; Irikura, M.; Irie, T.; Ishitsuka, Y. Ethyl Pyruvate Attenuates Acetaminophen-Induced Liver Injury and Prevents Cellular Injury Induced by N-Acetyl-p-Benzoquinone Imine. Heliyon 2018, 4, e00521. [Google Scholar] [CrossRef]
- Najafi, G.; Atashfaraz, E.; Farokhi, F. Attenuation of Methotrexate-Induced Embryotoxicity and Oxidative Stress by Ethyl Pyruvate. Int. J. Fertil. Steril. 2016, 10, 232–238. [Google Scholar]
- Choi, S.S.; Koh, W.U.; Nam, J.S.; Shin, J.W.; Leem, J.G.; Suh, J.H. Effect of Ethyl Pyruvate on Paclitaxel-Induced Neuropathic Pain in Rats. Korean J. Pain 2013, 26, 135–141. [Google Scholar] [CrossRef]
- Shen, F.; Wang, Z.; Liu, W.; Liang, Y. Ethyl Pyruvate Can Alleviate Alcoholic Liver Disease through Inhibiting Nrf2 Signaling Pathway. Exp. Ther. Med. 2018, 15, 4223. [Google Scholar] [CrossRef]
- Chavali, V.D.; Agarwal, M.; Vyas, V.K.; Saxena, B. Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer’s Disease in Rats via Inhibiting Toll-Like Receptor 4. J. Mol. Neurosci. MN 2020, 70, 836–850. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Shahrooz, R.; Ahmadi, A.; Malekinjad, H.; Mardani, K. Protective Effect of Ethyl Pyruvate on Mice Sperm Parameters in Phenylhydrazine Induced Hemolytic Anemia. Vet. Res. Forum Int. Q. J. 2016, 7, 63–68. [Google Scholar]
- Bakdemir, M.; Çetin, E. Hepatoprotective Effects of Ethyl Pyruvate against Carbon Tetrachloride-Induced Oxidative Stress, Biochemical and Histological Alterations in Rats. Arch. Physiol. Biochem. 2021, 127, 359–366. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Li, S.; Lu, C.; Li, J.; Zong, Y.; Qi, W.; Yang, H. Hepatoprotective Effects of Ethyl Pyruvate against CCl4-Induced Hepatic Fibrosis via Inhibition of TLR4/NF-κB Signaling and up-Regulation of MMPs/TIMPs Ratio. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 72–81. [Google Scholar] [CrossRef]
- Karabeyoglu, M.; Kocer, B.; Ozel, U.; Atasay, F.O.; Ustun, H.; Dolapci, M.; Karabeyoglu, I.; Cengiz, O. The Protective Effect of Ethyl Pyruvate on Lung Injury after Burn in Rats. Saudi Med. J. 2007, 28, 1489–1492. [Google Scholar]
- Bakhtiary, Z.; Shahrooz, R.; Ahmadi, A.; Soltanalinejad, F. Ethyl Pyruvate Ameliorates the Damage Induced by Cyclophosphamide on Adult Mice Testes. Int. J. Fertil. Steril. 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Dedeoğlu, S.; Ayral, M. Protective Effect of Ethyl Pyruvate on Amikacin-Induced Ototoxicity in Rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2460–2466. [Google Scholar] [CrossRef]
- Kelle, I.; Eriman, H.; Erdinc, M.; Uyar, E. Risperidone and Ethyl Pyruvate Have Protective Effects against Ketamine-Induced Cognitive Impairments in Mice. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1708–1716. [Google Scholar] [CrossRef]
- Nourani, M.R.; Kalantari Hesari, A.; Shahrooz, R.; Asadi, M.R. Effect of Sodium Cyanide-Induced Tissue Hypoxia on Reproductive Capability of Male Mice and the Protective Effect of Ethyl Pyruvate. Arch. Razi Inst. 2021, 76, 323–333. [Google Scholar] [CrossRef]
- Kelle, I.; Akkoc, H.; Tunik, S.; Nergiz, Y.; Erdinc, M.; Erdinc, L. Protective Effects of Ethyl Pyruvate in Cisplatin-Induced Nephrotoxicity. Biotechnol. Biotechnol. Equip. 2014, 28, 674–680. [Google Scholar] [CrossRef]
- Chen, H.; Bai, H.; Xi, M.; Liu, R.; Qin, X.; Liang, X.; Zhang, W.; Zhang, X.; Li, W.; Hai, C. Ethyl Pyruvate Protects Rats from Phosgene-Induced Pulmonary Edema by Inhibiting Cyclooxygenase2 and Inducible Nitric Oxide Synthase Expression. J. Appl. Toxicol. JAT 2013, 33, 71–77. [Google Scholar] [CrossRef]
- Yang, R.; Zou, X.; Koskinen, M.-L.; Tenhunen, J. Ethyl Pyruvate Reduces Liver Injury at Early Phase but Impairs Regeneration at Late Phase in Acetaminophen Overdose. Crit. Care 2012, 16, R9. [Google Scholar] [CrossRef]
- Fedeli, D.; Falcioni, G.; Olek, R.A.; Massi, M.; Cifani, C.; Polidori, C.; Gabbianelli, R. Protective Effect of Ethyl Pyruvate on msP Rat Leukocytes Damaged by Alcohol Intake. J. Appl. Toxicol. JAT 2007, 27, 561–570. [Google Scholar] [CrossRef]
- Ivanova, I.; Bogner, C.; Gronwald, W.; Kreutz, M.; Kurz, B.; Maisch, T.; Kamenisch, Y.; Berneburg, M. UVA-Induced Metabolic Changes in Non-Malignant Skin Cells and the Potential Role of Pyruvate as Antioxidant. Photochem. Photobiol. Sci. 2023, 22, 1889–1899. [Google Scholar] [CrossRef]
- Bhat, S.M.; Massey, N.; Karriker, L.A.; Singh, B.; Charavaryamath, C. Ethyl Pyruvate Reduces Organic Dust-Induced Airway Inflammation by Targeting HMGB1-RAGE Signaling. Respir. Res. 2019, 20, 27. [Google Scholar] [CrossRef]
- Sul, J.-W.; Kim, T.-Y.; Yoo, H.J.; Kim, J.; Suh, Y.-A.; Hwang, J.J.; Koh, J.-Y. A Novel Mechanism for the Pyruvate Protection against Zinc-Induced Cytotoxicity: Mediation by the Chelating Effect of Citrate and Isocitrate. Arch. Pharm. Res. 2016, 39, 1151–1159. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Barandalla, M.; Colleoni, S.; Lazzari, G. Pyruvate Antioxidant Roles in Human Fibroblasts and Embryonic Stem Cells. Mol. Cell. Biochem. 2017, 429, 137–150. [Google Scholar] [CrossRef]
- Poteet, E.; Winters, A.; Xie, L.; Ryou, M.-G.; Liu, R.; Yang, S.-H. In Vitro Protection by Pyruvate against Cadmium-Induced Cytotoxicity in Hippocampal HT-22 Cells. J. Appl. Toxicol. JAT 2014, 34, 903–913. [Google Scholar] [CrossRef]
- Zwolak, I.; Wnuk, E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants 2022, 11, 909. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-K.; Kim, H.-J.; Yoon, S.-H.; Lee, J.-K. Neuroprotective Effect of Ethyl Pyruvate against Zn2+ Toxicity via NAD Replenishment and Direct Zn2+ Chelation. Neuropharmacology 2016, 105, 411–419. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Z.; Chang, J.; He, L.; Zhang, Z.; Song, X.; Hou, X.; Fan, F.; Jiang, Z. Sodium Pyruvate Exerts Protective Effects against Cigarette Smoke Extract-Induced Ferroptosis in Alveolar and Bronchial Epithelial Cells through the GPX4/Nrf2 Axis. J. Inflamm. 2023, 20, 28, Correction in J. Inflamm. 2025, 22, 1–3. [Google Scholar] [CrossRef]
- Harmon, R.C.; Kiningham, K.K.; Valentovic, M.A. Pyruvate Reduces 4-Aminophenol in Vitro Toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 179–186. [Google Scholar] [CrossRef]
- Hegde, K.R.; Kovtun, S.; Varma, S.D. Inhibition of Glycolysis in the Retina by Oxidative Stress: Prevention by Pyruvate. Mol. Cell. Biochem. 2010, 343, 101–105. [Google Scholar] [CrossRef]
- Hinoi, E.; Takarada, T.; Tsuchihashi, Y.; Fujimori, S.; Moriguchi, N.; Wang, L.; Uno, K.; Yoneda, Y. A Molecular Mechanism of Pyruvate Protection against Cytotoxicity of Reactive Oxygen Species in Osteoblasts. Mol. Pharmacol. 2006, 70, 925–935. [Google Scholar] [CrossRef]
- Giandomenico, A.R.; Cerniglia, G.E.; Biaglow, J.E.; Stevens, C.W.; Koch, C.J. The Importance of Sodium Pyruvate in Assessing Damage Produced by Hydrogen Peroxide. Free Radic. Biol. Med. 1997, 23, 426–434. [Google Scholar] [CrossRef]
- Nath, K.A.; Ngo, E.O.; Hebbel, R.P.; Croatt, A.J.; Zhou, B.; Nutter, L.M. Alpha-Ketoacids Scavenge H2O2 in Vitro and in Vivo and Reduce Menadione-Induced DNA Injury and Cytotoxicity. Am. J. Physiol. 1995, 268 Pt 1, C227–C236. [Google Scholar] [CrossRef]
- Olek, R.A.; Ziolkowski, W.; Kaczor, J.J.; Wierzba, T.H.; Antosiewicz, J. Higher Hypochlorous Acid Scavenging Activity of Ethyl Pyruvate Compared to Its Sodium Salt. Biosci. Biotechnol. Biochem. 2011, 75, 500–504. [Google Scholar] [CrossRef][Green Version]
- Vásquez-Vivar, J.; Denicola, A.; Radi, R.; Augusto, O. Peroxynitrite-Mediated Decarboxylation of Pyruvate to Both Carbon Dioxide and Carbon Dioxide Radical Anion. Chem. Res. Toxicol. 1997, 10, 786–794. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, A.; Pathak, M.; Kaur, L.; Kumar, V.; Roy, B.G.; Ojha, H. DNA Binding and Antiradical Potential of Ethyl Pyruvate: Key to the DNA Radioprotection. Chem. Biol. Interact. 2020, 332, 109313. [Google Scholar] [CrossRef]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.-Y. Reaction Rate of Pyruvate and Hydrogen Peroxide: Assessing Antioxidant Capacity of Pyruvate under Biological Conditions. Sci. Rep. 2019, 9, 19568. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Simpson, E.J.; Greenhaff, P.L. The Importance of Pyruvate Availability to PDC Activation and Anaplerosis in Human Skeletal Muscle. Am. J. Physiol. 1999, 276, E472–E478. [Google Scholar] [CrossRef]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.-J.; Mallet, R.T.; Yang, S.-H. Pyruvate Protects Mitochondria from Oxidative Stress in Human Neuroblastoma SK-N-SH Cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Chung, S.J.; Kang, I.J.; Park, J.H.; Bünger, R. Intramitochondrial Pyruvate Attenuates Hydrogen Peroxide-Induced Apoptosis in Bovine Pulmonary Artery Endothelium. Mol. Cell. Biochem. 2001, 216, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nishigaki, Y.; Fuku, N.; Ibi, T.; Sahashi, K.; Koga, Y. Therapeutic Potential of Pyruvate Therapy for Mitochondrial Diseases. Mitochondrion 2008, 7, 399–401. [Google Scholar] [CrossRef]
- Cappel, D.A.; Deja, S.; Duarte, J.A.G.; Kucejova, B.; Iñigo, M.; Fletcher, J.A.; Fu, X.; Berglund, E.D.; Liu, T.; Elmquist, J.K.; et al. Pyruvate-Carboxylase-Mediated Anaplerosis Promotes Antioxidant Capacity by Sustaining TCA Cycle and Redox Metabolism in Liver. Cell Metab. 2019, 29, 1291–1305.e8. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Ramos, M.; Ruiz, F.; Satrústegui, J.; Bogónez, E. Pyruvate Protection against Beta-Amyloid-Induced Neuronal Death: Role of Mitochondrial Redox State. J. Neurosci. Res. 2003, 73, 260–269. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating Mitochondrial Redox State Using NADH and NADPH Autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Van Vranken, J.G.; Rutter, J. You Down With ETC? Yeah, You Know D! Cell 2015, 162, 471–473. [Google Scholar] [CrossRef]
- Battaglia, S.; De Santis, S.; Rutigliano, M.; Sallustio, F.; Picerno, A.; Frassanito, M.A.; Schaefer, I.; Vacca, A.; Moschetta, A.; Seibel, P.; et al. Uridine and Pyruvate Protect T Cells’ Proliferative Capacity from Mitochondrial Toxic Antibiotics: A Clinical Pilot Study. Sci. Rep. 2021, 11, 12841. [Google Scholar] [CrossRef]
- Fink, M.P. Ethyl Pyruvate: A Novel Anti-Inflammatory Agent. J. Intern. Med. 2007, 261, 349–362. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Han, Y.; Englert, J.A.; Yang, R.; Delude, R.L.; Fink, M.P. Ethyl Pyruvate Inhibits Nuclear Factor-kappaB-Dependent Signaling by Directly Targeting P65. J. Pharmacol. Exp. Ther. 2005, 312, 1097–1105. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High Mobility Group Box 1 (HMGB1): A Pivotal Regulator of Hematopoietic Malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, H.J.; Kim, H.; Park, S.W. Ethyl Pyruvate Directly Attenuates Active Secretion of HMGB1 in Proximal Tubular Cells via Induction of Heme Oxygenase-1. J. Clin. Med. 2019, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Lee, H.-K.; Lee, H.-B.; Jin, Y.; Lee, J.-K. Ethyl Pyruvate Inhibits HMGB1 Phosphorylation and Secretion in Activated Microglia and in the Postischemic Brain. Neurosci. Lett. 2014, 558, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Kim, I.-D.; Kim, S.-W.; Lee, H.-K.; Jin, Y.; Park, J.-H.; Kim, T.-K.; Suh, C.-K.; Kwak, J.; Lee, K.-H.; et al. Ethyl Pyruvate Inhibits HMGB1 Phosphorylation and Release by Chelating Calcium. Mol. Med. Camb. Mass 2015, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, E.J.; Kim, J.H.; Park, S.W.; Kim, H.J.; Chang, K.C. Ethyl Pyruvate Inhibits the Acetylation and Release of HMGB1 via Effects on SIRT1/STAT Signaling in LPS-Activated RAW264.7 Cells and Peritoneal Macrophages. Int. Immunopharmacol. 2016, 41, 98–105. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 Signaling by Natural Products-Can It Alleviate Diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Harvey, S.A.K.; Guerriero, E.; Charukamnoetkanok, N.; Piluek, J.; Schuman, J.S.; SundarRaj, N. Responses of Cultured Human Keratocytes and Myofibroblasts to Ethyl Pyruvate: A Microarray Analysis of Gene Expression. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2917–2927. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, S.-W.; Jin, Y.; Kim, I.-D.; Lee, J.-K. Ethyl Pyruvate-Mediated Nrf2 Activation and Hemeoxygenase 1 Induction in Astrocytes Confer Protective Effects via Autocrine and Paracrine Mechanisms. Neurochem. Int. 2012, 61, 89–99. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, Y.M.; Tsoyi, K.; Park, E.J.; Lee, Y.S.; Kim, H.J.; Lee, J.H.; Joe, Y.; Chung, H.T.; Chang, K.C. Ethyl Pyruvate Induces Heme Oxygenase-1 Through P38 Mitogen-Activated Protein Kinase Activation by Depletion of Glutathione in RAW 264.7 Cells and Improves Survival in Septic Animals. Antioxid. Redox Signal. 2012, 17, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Lee, H.-K.; Shin, J.-H.; Lee, J.-K. Up-down Regulation of HO-1 and iNOS Gene Expressions by Ethyl Pyruvate via Recruiting P300 to Nrf2 and Depriving It from P65. Free Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Lee, I.; Ting, H.; Chang, Y.-J.; Yang, N.-C. Parapyruvate, an Impurity in Pyruvate Supplements, Induces Senescence in Human Fibroblastic Hs68 Cells via Inhibition of the α-Ketoglutarate Dehydrogenase Complex. J. Agric. Food Chem. 2018, 66, 7504–7513. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.A.; Coxon, B. Identification and Quantitation of the Impurities in Sodium Pyruvate. Anal. Chem. 1986, 58, 2504–2510. [Google Scholar] [CrossRef]
- Zhou, F.-Q. Pyruvate as a Potential Beneficial Anion in Resuscitation Fluids. Front. Med. 2022, 9, 905978. [Google Scholar] [CrossRef]
- Lopalco, A.; Deeken, R.; Douglas, J.; Denora, N.; Stella, V.J. Some Preformulation Studies of Pyruvic Acid and Other α-Keto Carboxylic Acids in Aqueous Solution: Pharmaceutical Formulation Implications for These Peroxide Scavengers. J. Pharm. Sci. 2019, 108, 3281–3288. [Google Scholar] [CrossRef]
- Zhou, F.-Q. Advantages of Pyruvate-Based Fluids in Preclinical Shock Resuscitation-A Narrative Review. Front. Physiol. 2022, 13, 1027440. [Google Scholar] [CrossRef]
- Zabielska, M.A.; Adamus, J.; Kowalski, R.; Gebicki, J.; Slominska, E.M.; Khalpey, Z.; Smolenski, R.T. Cardioprotective Effect of N-Methylnicotinamide Salt of Pyruvate in Experimental Model of Cardiac Hypoxia. Pharmacol. Rep. 2018, 70, 378–384. [Google Scholar] [CrossRef]
- Kao, K.K.; Fink, M.P. The Biochemical Basis for the Anti-Inflammatory and Cytoprotective Actions of Ethyl Pyruvate and Related Compounds. Biochem. Pharmacol. 2010, 80, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Reade, M.C.; Fink, M.P. Bench-to-Bedside Review: Amelioration of Acute Renal Impairment Using Ethyl Pyruvate. Crit. Care 2005, 9, 556–560. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Swaminathan, M.; Grigore, A.M.; Roach, G.W.; Aberle, L.G.; Johnston, J.M.; Fink, M.P. A Phase II Multicenter Double-Blind Placebo-Controlled Study of Ethyl Pyruvate in High-Risk Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2009, 23, 324–329. [Google Scholar] [CrossRef]
- Zhou, F. Pyruvate Research and Clinical Application Outlooks A Revolutionary Medical Advance. Int. J. Nutr. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Bahar, F.G.; Ohura, K.; Ogihara, T.; Imai, T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.M.; Wollenberg, L.; Zhao, Z. Esterase Activities in the Blood, Liver and Intestine of Several Preclinical Species and Humans. Drug Metab. Lett. 2009, 3, 70–77. [Google Scholar] [CrossRef]
- Zhou, F. Pyruvate-Enriched Fluids as a Novel Medical Solution and Beverage. Food Sci. Nutr. Technol. 2021, 6, 000258. [Google Scholar] [CrossRef]

| Application Area | Innovative and Established Uses | References |
|---|---|---|
| Drug industry | Oral sodium pyruvate: possible mitigation of mitochondrial diseases in human studies (insufficient data). Inhaled sodium pyruvate: anti-inflammatory effect in pulmonary diseases (N115, a drug containing sodium pyruvate, is in pre-registration for idiopathic pulmonary fibrosis). | [1,12,16,17] |
| Cosmetics | Pyruvic acid: exfoliating agent in dermatology | [18] |
| Diet supplements | Oral calcium pyruvate, sodium pyruvate, creatine pyruvate: possible improvement in exercise performance and weight loss aid (results inconclusive). | [19,20,21] |
| Nutraceutical and food industry | Pyruvate, phosphoenol pyruvate: starting materials for amino acid production. Ethyl pyruvate: a flavouring agent and preservative used in the food industry. | [22,23] |
| Pyruvate Compound | Type of Toxicant | Animal Model | Effects | Proposed Mechanism (s) | References |
|---|---|---|---|---|---|
| Physical Agents | |||||
| Ethyl pyruvate 60 mg/kg, iv | heat stroke (heating chamber, 42 °C) | rats | EP mitigated the damage caused by heat stress to the liver, heart, lungs, skeletal muscle, and intestinal epithelium. Increased rat survival was observed. |
| [36] |
| Ethyl pyruvate 40 mg/kg, ip | thermal injury (shaved dorsal skin exposed to boiling water for 12 s) | rats | EP alleviated the lung tissue damage caused by heat stress. |
| [47] |
| sodium pyruvate 250 mg/kg bw orally or 50 mg of 5% w/w sodium pyruvate ointment | UV-B, 280–315 nm (shaved dorsal skin exposed to UV-B for 2 min) | guinea pigs | Both oral and topical sodium pyruvate were found to have protective effects against UV-induced dermal erythema. | [33] | |
| Pyruvate 500 mg/kg, ip | UV-B, 280–320 nm 500 J/m2 on dorsal skin | mice | Pyruvate protected the epidermis of mice from UV-induced DNA damage, as measured by levels of cyclobutane pyrimidine dimers. | [34] | |
| Aromatic pyruvates (10 μM indole-3-pyruvate on dorsal skin) | UV-B, 290–320 nm 1 J/cm2 on dorsal skin | mice | Pyruvate significantly alleviated UV-B-induced erythema on the back of the skin, improved skin morphology, and prevented skin barrier damage. |
| [32] |
| Ethyl pyruvate 70 mg/kg/day for 5 days, ip | Ionizing radiation (gamma) Total body dose of 9.75 Gy | mice | EP has increased the survival rate of animals exposed to radiation | [37] | |
| Ethyl pyruvate 100 mg/kg/day for 28 days ip | Ionizing radiation (X-rays) Whole lung irradiation, 16 Gy | mice | EP partially relieved radiation-induced lung injury |
| [38] |
| Chemical Agents | |||||
| Ethyl pyruvate 50, 100, 200 mg/kg/day for 14 days, orally | AlCl3 50 mg/kg/day for 28 days, ip | rats | EP attenuated the histopathological changes induced by AlCl3 in the cerebral cortex (reduced β-amyloid plaque deposition). It also improved cognitive function. |
| [43] |
| Ethyl pyruvate 40 mg/kg/day for 35 days, ip | Phenylhydrazine 8 mg followed by 6 mg per 100 g every 48 h for 35 days, ip | mice | Mitigation of phenylhydrazine-induced sperm damage (number, morphology, motility, viability) |
| [44] |
| Ethyl pyruvate 50 and 100 mg, ip | Paracetamol 400 mg/kg, ip | mice | EP attenuated paracetamol-induced histopathological liver damage |
| [39] |
| Etyl pyruvate 40 mg/kg/day for 35 days, ip | Cyclophosphamide 15 mg/kg/week for 35 days, ip | mice | EP reduced the histopathological damage to the testes caused by cyclophosphamide. |
| [48] |
| Ethyl pyruvate 50 mg/kg/day for 14 days, ip | Amikacin 600 mg/kg/day for 14 days, im | rats | EP alleviated the damage to hearing caused by amikacin |
| [49] |
| Ethyl pyruvate 50 mg/kg for 2 weeks, ip | Ketamine 25 mg/kg for 2 weeks ip | mice | EP alleviated ketamine-induced cognitive impairment |
| [50] |
| Ethyl pyruvate 40 mg/kg (three doses), ip | Carbon tetrachloride (CCl4) 1.6 g/kg | rats | EP attenuated CCl4-induced histopathological liver damage |
| [45] |
| Ethyl pyruvate 40 mg/kg/day for 30 days, ip | Methotrexate 20 mg/kg once per week for 30 days, ip | mice | EP attenuated the negative effects of methotrexate on male mouse fertility (marker: in vitro fertilisation rate and embryonic development) |
| [40] |
| Ethyl pyruvate 40 mg/kg/day for 35 days ip | Sodium cyanide 2 mg/kg ip | mice | EP attenuated NaCN-induced histopathological changes in the testes and the negative effects of NaCN on fertility in male mice (markers: in vitro fertilisation rate and embryonic development). |
| [51] |
| Ethyl pyruvate (50 mg/kg for 5 days, ip) | Cisplatin (5 mg/kg for 5 days ip) | rats | EP attenuated cisplatin-induced histopathological changes in the kidney |
| [52] |
| ethyl pyruvate 40 mg/kg/day for up to 6 weeks, ip | CCl4 (CCl4:olive oil = 1:1) 3 mL/kg, twice a week for up to 6 weeks, sc | rats | EP attenuated the histopathological changes and liver fibrosis induced by CCl4 |
| [46] |
| Ethyl pyruvate 100 mg/kg for 15 days, orally | Ethanol 50% solution (10 mL) once, ip and 4 g/kg/day (52%) for 4 weeks, orally | mice | EP alleviated alcohol-induced liver histopathological changes |
| [42] |
| Ethyl pyruvate 50 mg/kg for 6 days, ip | Paclitaxel (Taxol) 2 mg/kg, every other day, ip | rats | EP did not significantly affect paclitaxel-induced allodynia | [41] | |
| Ethyl pyruvate 40 mg/kg, ip | phosgene gas 10% for 1 min in whole-body chamber | rats | EP alleviated phosgene gas-induced pulmonary oedema |
| [53] |
| Ethyl pyruvate 40 mg/kg every 8 h for up to 48 h, ip | Paracetamol (APAP) 350 mg/kg, ip | mice | Early phase in APAP overdose: EP attenuated paracetamol-induced histopathological changes in the liver Late phase in APAP overdose: EP increased paracetamol-induced liver histopathological changes | [54] | |
| 0.3–3% EP in drinking burette for 10 weeks | 10% ethanol solution in drinking burette for 10 weeks | rats | EP protected white blood cells from alcohol-induced damage |
| [55] |
| Pyruvate 500 mg/kg, sc | ethanol (2.5 g/kg), sc | mice | pyruvate attenuated ethanol-induced neurodegenerative changes in the brain |
| [35] |
| Pyruvate Compound | Type of Toxicant | Cell Culture Model | Effects | Proposed Mechanism (s) | References |
|---|---|---|---|---|---|
| Physical Agents | |||||
| Sodium pyruvate 1, 3, 5, 10 mM | Light-induced damage 15,000 lux light for 5 h | Murine photoreceptor-derived 66lW cells | SP improved morphology and significantly reduced light-induced cell death |
| [26] |
| Ethyl pyruvate 10 mM | Ionizing radiation (gamma), 0–8 Gy | 32Dcl3 mouse hematopoietic progenitor cell line | EP increased survival of irradiated cells |
| [37] |
| Sodium pyruvate 1 mM | UV-A, 340–420 nm single UV-A dose or 3 times/day (for 4 days) | Primary human skin fibroblasts |
| [56] | |
| Ethyl pyruvate 0.2–5 μM | Ionizing radiation (X-ray), 0–8 Gy | RAW264.7 macrophages, human bronchial epithelial cells | EP increased survival of irradiated cells |
| [38] |
| Pyruvate 1000 ng intracutaneously | UV-B, 280–320 nm, 500 J/m2 | Human skin | Reduced levels of cyclobutane pyrimidine dimer (DNA damage marker) | [34] | |
| Chemical Agents | |||||
| Ethyl pyruvate 5, 10, 20, 40 mM | AlCl3 1250 μM | Primary neuron–glial mixed cells | EP significantly reduced AlCl3-induced cell death | [43] | |
| Sodium pyruvate 4.5 mM | VOSO4 100 μM | Chinese hamster ovary K1 cells | SP improved morphology and significantly increased cell viability limited by VOSO4 |
| [61] |
| Sodium pyruvate 10 mM | Oxyradicals species (xanthine oxidase-induced) | Neural retina (from mice) | SP prevented ROS-induced inhibition of glycolysis and lactate formation in the retina |
| [65] |
| Ethyl pyruvate 0.01–1 mM | a reactive metabolite of APAP, N-acetyl-p-benzoquinone imine (NAPQI), 0.4 mM | human hepatoma cell line (HEPG2 cells) | EP improved cell viability limited by NAPQI |
| [39] |
| Sodium pyruvate 2.5–25 mM | H2O2 (0.128–32.768 mM) | Human fibroblasts (Hs27 cell line) | SP improved cell viability limited by H2O2 |
| [59] |
| Sodium pyruvate 8 mM | CdCl2 10, 30 and 100 μM | Murine hippocampal HT-22 cells | SP improved cell viability limited by CdCl2 |
| [60] |
| Ethyl pyruvate (1–15 mM) | ZnSO4 (40 or 400 μM) | Primary cortical cultures from mouse | EP improved cell viability limited by ZnSO4 |
| [62] |
| Pyruvate 5 mM | ZnCl2 (40, 200 or 300 μM depending on the measured endpoint) | Mix mouse cortical and pure hippocampal neuronal cells | Pyruvate reduced ZnCl2-induced cell death |
| [58] |
| Pyruvate 10 mM | p-aminophenol (0.1–0.5 mM) | Renal cortical slices from rats | Pyruvate reduced p-aminophenol-induced cell death |
| [64] |
| Pyruvate 1 mM | H2O2 10–1000 μM | Primary osteoblasts from rats | Pyruvate reduced H2O2-induced cell death |
| [66] |
| Ethyl pyruvate 2.5 μM | Organic dust | human bronchial epithelial cells (BEAS-2B) |
| [57] | |
| Ethyl pyruvate (2, 5 or 10μM) | phosgene 400 ppm | RAW 264.7 macrophages |
| [53] | |
| Sodium pyruvate (0.5, 1, 2 mM) | Cigarette smoke extract | A549 (human lung carcinoma epithelial cells) and BEAS-2B (bronchial epithelial cells) cell lines | Pyruvate improved cell viability limited by cigarette smoke |
| [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwolak, I. Exogenous Pyruvate in Defense Against Human-Exposure Toxicants: A Review of In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2025, 26, 8316. https://doi.org/10.3390/ijms26178316
Zwolak I. Exogenous Pyruvate in Defense Against Human-Exposure Toxicants: A Review of In Vitro and In Vivo Evidence. International Journal of Molecular Sciences. 2025; 26(17):8316. https://doi.org/10.3390/ijms26178316
Chicago/Turabian StyleZwolak, Iwona. 2025. "Exogenous Pyruvate in Defense Against Human-Exposure Toxicants: A Review of In Vitro and In Vivo Evidence" International Journal of Molecular Sciences 26, no. 17: 8316. https://doi.org/10.3390/ijms26178316
APA StyleZwolak, I. (2025). Exogenous Pyruvate in Defense Against Human-Exposure Toxicants: A Review of In Vitro and In Vivo Evidence. International Journal of Molecular Sciences, 26(17), 8316. https://doi.org/10.3390/ijms26178316








