Abstract
Pyruvate is an alpha-keto acid that occurs naturally in living cells. It is a key metabolite in cellular respiration and a substrate for the synthesis of glucose (in gluconeogenesis) and certain amino acids. Exogenous pyruvate, for example in the form of sodium pyruvate or ethyl pyruvate, has potential therapeutic applications due to its antioxidant and anti-inflammatory properties. This review summarises cell culture and animal studies that report the cytoprotective effects of exogenous pyruvate compounds during exposure to environmental pollutants, drugs, UV radiation, and burns. These reports show that the main mechanisms through which exogenous pyruvate exerts its beneficial effects are the neutralisation of reactive oxygen species, protection and stabilisation of mitochondria, maintenance of ATP levels, and inhibition of inflammatory signalling pathways, including the nuclear factor-kappa B (NF-κB) pathway. The article also outlines potential challenges associated with the therapeutic use of exogenous pyruvate. These include the instability of inorganic pyruvate (sodium pyruvate) and the fact that the metabolism of ethyl pyruvate differs between humans and animals.
1. Introduction
Pyruvate (CH3COCOO−) is a simple three-carbon α-keto acid that is produced naturally in all living cells. It is primarily a product of glycolysis and an important intermediate in glucose metabolism and cellular energy production. Endogenous pyruvate, which is produced by glycolysis, enters the mitochondria, where it either feeds the Krebs cycle or is converted into lactate during periods of oxygen deprivation. It can also be used in the synthesis of alanine or to support gluconeogenesis [1,2] (Figure 1).
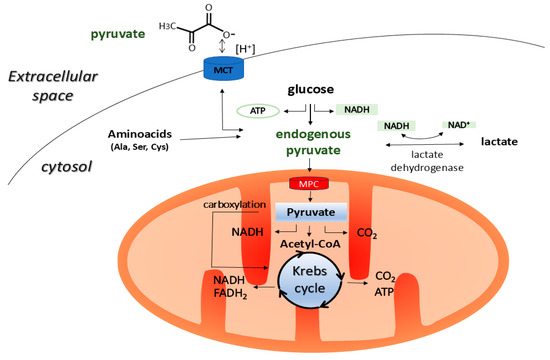
Figure 1.
Pyruvate is mainly produced in the cytoplasm of animal cells by glycolysis. It can also be formed in the cytoplasm through the metabolism of malate, lactate, and amino acids such as alanine, cysteine, serine, and glycine. The resulting pyruvate is then transported to the mitochondria via the MPC, which is located in the inner mitochondrial membrane, where it is incorporated into the Krebs cycle by being converted into acetyl-CoA. In addition, pyruvate can undergo carboxylation in the mitochondria to form oxaloacetate, which is a common intermediate in both the Krebs cycle and gluconeogenesis. In the cytoplasm, pyruvate can also be converted into lactate under oxygen-limited conditions, or it can be used for synthesising alanine or secreted from the cell into the intercellular space via the MCT transporter in symport with H+ [1,2]. MCT, monocarboxylate transporter; MPC, mitochondrial pyruvate carrier; NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide.
Furthermore, exogenous pyruvate (see Figure 2 for chemical structures), mainly in the form of ethyl pyruvate and sodium pyruvate supplements, is known for its antioxidant and anti-inflammatory properties [1,3]. Small amounts of pyruvate can also be found in food. Foods rich in pyruvate include milk (0.303 mM), red wine (0.446 mM), white wine (0.139 mM), and apple juice (0.75 mM) [4]. The beneficial effects of exogenous pyruvate have been demonstrated in animal models of phenylketonuria [5], epilepsy [6], and burns [7,8], among others. In human studies, inhaled pyruvate improved parameters in patients with COPD [9,10] and in patients with COVID-19 [11], while oral or intravenous pyruvate had beneficial effects in patients with mitochondrial diseases [12], liver disease [13], diabetes [14], and glaucoma [15]. In addition, pyruvate compounds, which include both organic and inorganic derivatives, have a variety of applications in areas such as cosmetics, food production, and the chemical industry (see Table 1). Pyruvate is also an optional ingredient in culture media used for growing cell lines in vitro, where it serves as an additional energy substrate. Depending on the formulation, culture media typically contain 0.5–1 mM (55–110 mg/L) of sodium pyruvate.
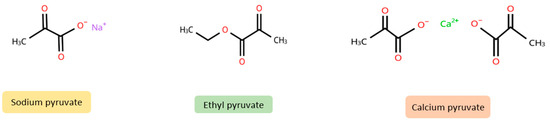
Figure 2.
Chemical structures of sodium pyruvate, ethyl pyruvate, and calcium pyruvate (created using chemspider) (https://www.chemspider.com/StructureSearch, accessed on 15 April 2025).

Table 1.
Main applications of pyruvate compounds in various fields.
The first reports on the cytoprotective and antioxidant properties of exogenous pyruvate in cultured mammalian cells were published in 1985 [24] and 1987 [25]. These studies demonstrated the ability of pyruvate to act as an antioxidant against hydrogen peroxide (H2O2)-induced cytotoxicity in cultures of the Chinese hamster V79 cell line, as well as in mouse cell lines (P388 lymphoma and P815 mastocytoma). These reports also suggested that pyruvate may have a similar protective function against H2O2 generated in blood plasma in vivo [24,25]. Subsequent studies in experimental models in vitro [26,27,28] and in vivo [29] have confirmed pyruvate’s protective capacity against pro-oxidant conditions in cells and tissues. Since then, studies describing the antioxidant and anti-inflammatory properties of pyruvate compounds have been published regularly.
Oxidative stress is a common pathological condition that occurs when cells and tissues are exposed to toxic agents, such as UV radiation or environmental pollutants. This condition is characterised by the excessive production of reactive oxygen species (ROS) and a reduction in antioxidant defences, which leads to oxidative damage to cellular structures and cell death. At the same time, these changes activate pro-inflammatory pathways, including those related to nuclear factor kappa B (NF-κB). This leads to inflammation and further tissue damage, thereby promoting the development of various diseases [30,31]. It is well known that enhancing the body’s antioxidant defences protects cells exposed to toxic agents and prevents pathological changes. In this review, we will: (1) summarise preclinical studies (in vivo and in vitro) that highlight the protective effects of exogenous pyruvate against damage induced by physical and chemical toxicants; (2) discuss the antioxidant and anti-inflammatory mechanisms involved in pyruvate’s cytoprotective effects; (3) present issues that hinder the use of exogenous pyruvate as an antioxidant.
2. Pyruvate Effects on Physical and Chemical Toxicants
2.1. In Vivo Studies
Table 2 summarises the results of 24 studies that investigated the effects of exogenous pyruvate against toxic agents in vivo. Most of these studies used rat or mouse models. One study used guinea pigs as test subjects. The form of exogenous pyruvate primarily studied was ethyl pyruvate, which was administered to the animals in doses ranging from 40 to 100 mg/kg. In addition, some researchers analysed the effects of aromatic pyruvate derivatives [32] and sodium pyruvate [33]. Two papers did not specify the form of pyruvate used in the study [34,35]. Most of these studies found that the administration of exogenous pyruvate significantly reduced the adverse health effects caused by heat stress [36], ionising radiation [37,38], UV radiation [34], analgesics [39], chemotherapeutic agents [40,41], alcohol [42] as well as other substances. The protective effects of pyruvate have been observed in various organs of animals, including the skin, lungs, liver, and brain. These effects included the normalisation of parameters related to oxidative stress, such as reduced lipid peroxidation and increased antioxidant defences [43,44]. Anti-apoptotic and anti-inflammatory effects were also observed, including inhibition of necrotic lesions and release of pro-inflammatory factors from tissues [36,38,45]. The signalling pathways affected by pyruvate included those related to the molecules NF-κB, Toll-like receptor 4 (TLR4), and High Mobility Group Box 1 (HMGB1) [36,43,46].

Table 2.
Summary of animal studies evaluating pyruvate effects on toxicity of physical and chemical agents.
2.2. In Vitro Studies
Table 3 lists in vitro studies investigating the effectiveness of pyruvate in combatting physical and chemical cytotoxicity in various animal cell models. The in vitro models employed in these studies included RAW264.7 macrophages [38,53], human fibroblasts [56], human bronchial epithelial cells [57], and primary neuronal cell lines [58]. Studies have mostly used sodium pyruvate or ethyl pyruvate at millimolar concentrations, with some studies using ethyl pyruvate at micromolar doses. In studies, pyruvate showed protective effects against toxic effects induced by radiation (ionising and UV) [34,37], H2O2 [59], metals (Cd, V, Al, Zn) [43,60,61,62], paracetamol metabolites [39], organic dusts, and cigarette smoke [57,63]. The actions of pyruvate included antioxidant effects (reduction of ROS levels, increase in GSH levels) [63,64], stabilisation of mitochondrial function [61], increase in ATP levels [26], increase in expression of proteins associated with the Nrf2 pathway [63], reduction of inflammatory mediators (e.g., HMGB1, NF-κB) [38,57], anti-apoptotic, and anti-necrotic effects [39].

Table 3.
Summary of cell culture studies evaluating pyruvate effects on toxicity of physical and chemical agents.
3. Cytoprotective Modes Activated by Exogenous Pyruvate in Animal Cells
3.1. Direct ROS Scavenging Activity
Alpha-keto acids undergo rapid decarboxylation in the presence of H2O2, resulting in the formation of a carboxylic acid, water, and carbon dioxide according to the reaction: R-COCOOH + H2O2 → R-COOH + H2O + CO2. First described in 1904, this reaction is the primary mechanism responsible for the antioxidant (reactive oxygen species (ROS) scavenging) properties of pyruvate compounds. Due to the relatively high concentration of pyruvate in tissues (in the micromolar range), early reports considered it to be one of the main elements of the cellular antioxidant defence, alongside catalase and glutathione peroxidase, which regulate the level of H2O2 in the cellular environment. One clear advantage of the reaction of pyruvate with H2O2 over enzymatic reactions for neutralising H2O2 is that non-enzymatic decarboxylation of pyruvate does not involve the formation of toxic by-products [67,68]. Furthermore, the scavenging activities of ethyl pyruvate and sodium pyruvate against superoxide anion and hypochlorous acid were detected using chemiluminescence and spectrophotometry, respectively [55,69]. Vasquez-Vivar et al. [70] found that pyruvate can also react with peroxynitrite according to the scheme CH3COCOO− + ONOO− → CH3COO− + NO2− + CO2. This reaction proceeds with an apparent second-order rate constant of 88 M−1 s−1 and is one order faster than the reaction of pyruvate with H2O2 (2.2 M−1 s−1) [70]. Nagatome et al. [39] also reported the ability of 1 and 10 mM ethyl pyruvate to scavenge peroxynitrite, using dihydrorhodamine as a peroxynitrite activity probe. The ability of ethyl pyruvate to scavenge free radicals was confirmed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays [71]. In these assays, the ability of ethyl pyruvate to scavenge the synthetic DPPH radical and reduce Fe3+ to Fe2+ ions (FRAP assay) increased proportionally with the concentration of ethyl pyruvate [71].
As mentioned above, pyruvate can be considered part of the cellular antioxidant defence system because it can directly neutralise H2O2 (and other ROS). However, according to a study by Guarino et al. [72], the role of intracellular pyruvate in H2O2 elimination may be insignificant. In fact, the authors of the study calculated that the elimination time of intracellular H2O2 in the presence of physiological levels of pyruvate is far too long (95% of 1 µM H2O2 is removed in approximately 150 min), compared to the rate at which H2O2 is neutralised by cytosolic glutathione peroxidase (95% of 1 µM H2O2 is removed in just 0.06 s). However, it was noted that pyruvate may play an important role in the elimination of H2O2 in extracellular spaces where the concentration of H2O2 is much higher and peroxidase activity is minimal, considering that pyruvate concentrations can rise to 1 mM [72]. Approximately, this concentration of pyruvate, i.e., 0.84 mM, was physiologically achievable in the blood of human volunteers 20 min after intravenous administration of sodium pyruvate [73].
3.2. Effects on Mitochondrial Functions
In vitro studies of this α-ketoacid have reported beneficial effects of pyruvate on mitochondrial function, such as mitochondrial membrane potential, against chemical and physical agents [37,60,61,63,74]. In support of this, research by Kang et al. [75] on cultured bovine pulmonary artery endothelial cells showed that blocking the transport of pyruvate into the mitochondria using the specific inhibitor α-cyano-3-hydroxycinnamate significantly reduced pyruvate’s protective effect against the pro-apoptotic properties of H2O2. This suggests that pyruvate metabolism within the mitochondria is involved in the cytoprotective effects of this compound.
Pyruvate affects mitochondria in several ways. Firstly, it stimulates pyruvate dehydrogenase activity by strongly inhibiting pyruvate dehydrogenase kinase. This increases the conversion of pyruvate to acetyl-CoA, which is a key molecule in the mitochondrial Krebs cycle [76]. Secondly, pyruvate can replenish Krebs cycle intermediates by converting directly to oxaloacetate in a reaction catalysed by pyruvate carboxylase [76,77]. This helps to maintain the Krebs cycle and the production of NADH and FADH, which are required for oxidative phosphorylation and ATP synthesis. Additionally, NADH derived from the Krebs cycle can also transfer electrons to nicotinamide adenine dinucleotide phosphate (NADP) via nicotinamide nucleotide transhydrogenase (NNT), which is a protein located in the inner mitochondrial membrane. The NADPH, via the activities of glutathione reductase and thioredoxin reductase, is used to reconstitute cellular reductants, i.e., reduced glutathione (GSH) and thioredoxin (Trx), respectively. This enhances mitochondrial antioxidant defences [78,79]. It is worth noting that clinical studies in people with incurable mitochondrial diseases also suggest that exogenous pyruvate plays a supportive role in maintaining mitochondrial function. Administration of pyruvate to patients alleviated some of the symptoms of these diseases [1].
In addition, cells can use exogenous pyruvate to synthesise aspartate, bypassing the mitochondrial-dependent synthesis of this amino acid. This is particularly important when the electron transport chain (ETC) in the mitochondria is damaged, as this chain plays an essential role in the synthesis of aspartate. Notably, aspartate is essential for cellular protein, purine, and pyrimidine synthesis. Therefore, inhibition of aspartate synthesis by impairment of ETC inhibits cell proliferation. When mitochondrial function related to the electron transport chain is impaired, pyruvate supplementation allows mitochondrial-independent synthesis of aspartate and preservation of cell proliferation despite mitochondrial damage [80,81]. In this way, exogenous pyruvate may protect the ability of cells to proliferate in the presence of mitochondrial toxic agents, such as mitotoxic antibiotics [82].
3.3. Anti-Inflammatory Activity of Pyruvate
Inflammation is the body’s defence response to a toxic agent of biological, chemical, or physical origin. However, chronic and excessive inflammation can exacerbate cell and tissue damage. Various in vitro and in vivo studies, summarised in Table 2 and Table 3, have reported that pyruvate inhibits the release of inflammation-related cytokines, such as COX2, HMGB1, and TNF-α, under conditions of physical and chemical challenge. The anti-inflammatory properties of pyruvate are probably partly due to its antioxidant effects, as oxidative stress and inflammation are known to be mutually supportive. However, as Fink [83] points out, some in vitro studies have shown that ethyl pyruvate exhibits stronger anti-inflammatory effects than known antioxidants (e.g., N-acetylcysteine). This suggests that the anti-inflammatory properties of ethyl pyruvate are not solely due to ROS scavenging. Therefore, below is a brief summary of main anti-inflammatory mechanisms activated by pyruvate, which may contribute to its cytoprotective effects.
3.3.1. Reduction of NF-κB Activity
Nuclear factor-κB (NF-κB) is a family of inducible transcription factors that occur as homodimers or heterodimers of Rel/NF-κB family proteins (p50/p105, p52/p100, RelB, c-Rel, and p65). NF-κB is found in the cytoplasm of animal cells in an inactive form, associated with inhibitory IκB proteins. The NF-κB proteins are released when activated by stress factors such as cytokines, UV irradiation, cigarette smoke, and heavy metals. They then translocate to the nucleus, where they regulate the transcription of genes associated with the inflammatory response [84]. Some studies suggest that the inhibition of the NF-κB pathway is important for the anti-inflammatory mechanism of exogenous pyruvate when the body is exposed to toxic chemicals or physical agents [36,46,57]. Pyruvate may act on the NF-κB pathway by a variety of mechanisms. Hu et al. [36] reported that the inhibitory effect of pyruvate on NF-κB activation induced by heat stroke (as measured by p65 phosphorylation) in rat livers is mediated by the induction of the antioxidant enzyme heme oxygenase-1 (HO-1) and the preservation of autophagy. Furthermore, another proposed mechanism may be the induction of heat shock protein (HSP) 70 synthesis by pyruvate. This can inhibit the phosphorylation and degradation of IκB, thus blocking the activation of NF-κB proteins [36]. Bhat et al. [57] observed that ethyl pyruvate blocked the translocation of NF-κB p65 from the cytoplasm to the nucleus when induced by organic dust. In addition, Han et al. [85] proposed that there is a direct interaction between pyruvate and the NF-κB factor. The researchers proposed that ethyl pyruvate inhibits NF-κB signalling in human embryonic kidney 293 cells by directly modifying the NF-κB structural protein subunit p65, thereby blocking the binding of the NF-κB factor to DNA [85].
3.3.2. Blocking HMGB1 Secretion
High mobility group box 1 (HMGB1) is a nuclear protein that plays an important role in cellular processes such as transcription, DNA replication and repair, and the regulation of chromatin structure under physiological conditions. In contrast, when cellular injury occurs, HMGB1 is released into the cytoplasm and then into the extracellular environment, where it acts as a damage-associated molecular pattern molecule (DAMP). Excessive amounts of secreted HMGB1 aggravate inflammation [86,87]. Pyruvate, especially in the form of ethyl pyruvate, is a known HMGB1 antagonist and reduces HMGB1 secretion in infections and inflammatory diseases (see Yang et al. [3] for a review). Similarly, inhibition of HMGB1 by ethyl pyruvate may contribute to its anti-inflammatory and cytoprotective effects in inflammation induced by chemical or physical toxicants. As summarised in Table 2 and Table 3, ethyl pyruvate inhibits the radiation-induced release of HMGB1 into the extracellular environment in both RAW264.7 murine macrophage cells and HBE human bronchial epithelial cells [38]. Another study found that ethyl pyruvate inhibited the nuclear-to-cytoplasmic transport of HMGB1 induced by organic dust extract in an airway epithelial cell model [57] and reduced the overexpression of this protein in the liver of CCl4-treated rats [46].
There are several possible mechanisms by which ethyl pyruvate inhibits HMGB1 secretion. For example, Seo et al. [88] demonstrated that ethyl pyruvate inhibited ischaemia-reperfusion-related HMGB1 release from renal cells in mice by inducing heme oxygenase-1 (HO-1) expression; this effect was abolished by zinc protoporphyrin, an HO-1 inhibitor. In another study, ethyl pyruvate was found to inhibit the secretion of HMGB1 by lipopolysaccharide (LPS)-activated BV2 microglia cells. This was achieved by blocking the phosphorylation of HMGB1 induced by LPS, which is likely due to the inhibition of protein kinase C alpha (PKCα) and calcium/calmodulin-dependent protein kinase (CaMK) IV activity by ethyl pyruvate. These two enzymes are required for HMGB1 phosphorylation [89]. Another study by the same research group showed that ethyl pyruvate reduces Ca2+ levels in BV2 cells, presumably through chelation. This inhibits the activation of PKCα and CaMKIV kinase, as well as the phosphorylation and secretion of HMGB1 from cells [90]. In addition, other authors [91] demonstrated that ethyl pyruvate inhibited HMGB1 acetylation and secretion by LPS-stimulated RAW 264.7 macrophages by modulating the SIRT1/STAT pathway, i.e., induction of the deacetylase Sirtuin 1 (SIRT1) and inhibition of LPS-induced STAT1 protein phosphorylation.
3.3.3. Nrf2 Induction
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that plays a key role in defending cells against various stress factors, including xenobiotics, inflammation, and the accumulation of abnormal proteins. Under physiological conditions, Nrf2 is present at low levels in an inactive form in the cytoplasm, where it is constantly being degraded. However, when activated by oxidative, electrophilic or proteotoxic stress, Nrf2 evades proteasome-mediated degradation. Instead, it translocates to the nucleus, where it binds to antioxidant response element (ARE) sequences in the promoter region of certain genes, thereby stimulating their transcription. The Nrf2 factor targets genes that encode antioxidant enzymes, drug-metabolising enzymes, and metabolic enzymes [92]. Nrf2 plays a role in preventing inflammation in part by regulating the expression of genes associated with the ARE sequence [93]. Studies report that exogenous pyruvate can positively affect Nrf2 signalling [94], leading to Nrf2 activation via several mechanisms. For instance, ethyl pyruvate has been shown to induce a cytoprotective effect against H2O2 toxicity by stimulating Nrf2, which then leads to Nrf2-mediated HO-1 induction in primary cultures of astrocytes. Further analysis revealed the involvement of the protein kinase B (Akt) signalling pathway and mitogen-activated protein kinases (MAPK), including extracellular signal-regulated kinase (ERK), in ethyl pyruvate-induced Nrf2 nuclear translocation [95]. In addition, the involvement of p38 MAPK, but not ERK and Jun N-terminal kinase (JNK) MAPKs, in the ethyl pyruvate-dependent activation of Nrf2 has been reported in RAW 264.7 cells [96]. Kim et al. [97] investigated the role of the interaction of Nrf2 with its transcriptional co-activator p300 in the induction of anti-inflammatory effects by ethyl pyruvate in a BV2 microglial cell line. In this study, ethyl pyruvate was found to stimulate Nrf2 transcriptional activity by enhancing the interaction between Nrf2 and P300, while reducing the interaction between P300 and p65. This attenuation of the interaction between P300 and p65 (where P300 acts as a co-activator for p65, a NF-κB subunit) has anti-inflammatory effects [97].
3.4. Other Possible Protective Mechanisms of Pyruvate
Exogenous pyruvate increased intracellular citrate and isocitrate levels in mouse cortical neuronal cells while providing neuroprotection against zinc-induced cytotoxicity. In this case, pyruvate’s indirect neuroprotective mechanism was related to the ability of citrate and isocitrate to directly chelate free zinc ions, thereby reducing their neurotoxicity [58]. In addition, Kim et al. [62] suggested that the protective effect of pyruvate against zinc (Zn) toxicity in mouse cortical neurons may be related to the regeneration of nicotinamide adenine dinucleotide (NAD) through the reduction of pyruvate to lactate in a reaction catalysed by lactate dehydrogenase (LDH). They based this conclusion on the observation that inhibiting NAD synthesis through LDH-competitive inhibition reduced the protective effect of pyruvate on these cells [62]. Finally, it has been suggested that exogenous pyruvate may be effective in treating metabolic acidosis, which can occur in cases of alcohol poisoning, for example. In this case, pyruvate’s possible mechanism of action is the result of the metabolic reactions that the compound undergoes in cells. These reactions include the LDH-catalysed reduction of pyruvate to lactate, and the oxidation of pyruvate to acetyl-CoA (by pyruvate dehydrogenase), which is then converted to water and carbon dioxide in the Krebs cycle. These reactions are associated with intracellular proton consumption and the correction of cellular acidosis (see Zhou [2] for a review).
4. Potential Barriers to the Use of Pyruvate as a Protective Agent
The instability of pyruvate, particularly in its inorganic form (e.g., sodium pyruvate), is often cited as an argument against its use as a drug. Indeed, sodium pyruvate is unstable in solutions with a pH above 6.0 at room temperature, undergoing spontaneous aldol condensation reactions and producing a potentially toxic by-product: pyruvate dimer (parapyruvate, 4-hydroxy-4-methyl-2-ketoglutaric acid). Parapyruvate has been found in industrial sodium pyruvate preparations and commercial calcium pyruvate supplements. The latter contain parapyruvate concentrations ranging from 1.4% to 10.6%, which could potentially have harmful biological effects [98,99]. However, Zhou [100] reported that one way to prevent parapyruvate formation in aqueous sodium pyruvate solutions is to maintain a pH of around 4.5. This renders the pyruvate solution stable for two years at room temperature (U.S. patent: 8,835,508 B2, 2014). The shelf life of pyruvate solutions can also be extended by using relatively dilute solutions (lower concentrations of pyruvate slow the formation of parapyruvate). Additionally, commercially available sodium pyruvate solutions (at a concentration of 100 mM) for cell culture supplementation have a recommended storage temperature of 2–8 °C, which maintains a shelf life of 1 year [101]. Furthermore, animal studies have shown that administration of sodium pyruvate can lead to transient hypernatraemia [102]. This effect does not occur when N-1-methylnicotinamide pyruvate (MNA pyruvate) is used instead. MNA pyruvate retains the protective properties characteristic of pyruvate [103]
Some studies report that ethyl pyruvate, an aliphatic ester, derived from pyruvic acid, is more stable in aqueous solutions and therefore more protective than sodium pyruvate. Ethyl pyruvate is also more hydrophobic, which makes it more likely that it can diffuse into cells more rapidly than the pyruvate anion [104,105]. For example, Shin et al. [95] compared the protective effects of ethyl pyruvate and sodium pyruvate against H2O2 toxicity in a primary astrocyte culture. They demonstrated that preincubation with ethyl pyruvate (1–10 mM) provided protection, whereas preincubation with the same concentrations of sodium pyruvate had no protective effect. The same study also found that sodium pyruvate was significantly less effective than ethyl pyruvate in inducing antioxidant genes such as heme oxygenase 1 (HO-1), glutathione S-transferase (GST), and NAD(P)H:quinone oxidoreductase (NQO1) in this cell model [95]. Another study showed that ethyl pyruvate had significantly higher antioxidant activity against H2O2 and superoxide anion in chemiluminescence measurements than sodium pyruvate [55]. In addition, protective effects of ethyl pyruvate against inflammation-induced multi-organ damage have been demonstrated in animal models (see Yang et al. [3] for a review). However, in a placebo-controlled phase II study evaluating the safety and efficacy of intravenously administered ethyl pyruvate in patients undergoing cardiac surgery, ethyl pyruvate failed to demonstrate a protective effect against postoperative complications, markers of inflammation, or organ dysfunction [106]. One of the suggested reasons for the significant differences in the effects of ethyl pyruvate between animal models and human studies is the difference in the metabolism of this compound in the two groups. Specifically, ethyl pyruvate in animal plasma is hydrolysed by the enzyme carboxylesterase, releasing the pyruvate anion [107]. However, unlike most rodents, human blood plasma does not contain this enzyme [108,109]. The absence of carboxylesterase activity in human plasma, and conversely its high activity in rodent plasma, may explain the differences in the effects of ethyl pyruvate between human and rodent studies [72,107]. In fact, the activity of certain classes of esterase enzymes (including the aforementioned carboxylesterase) in human tissues differs from that in other species such as mouse, rat, rabbit, or dog. This makes it difficult to find a suitable human surrogate in esterified prodrug studies [108,109].
The safety of pyruvate therapy has been addressed in a review article by Zhou [110]. The reviewed studies of pyruvate administration in clinical settings indicated that pyruvate, when administered intravenously to patients with diabetes or liver disease, or intracoronary to patients with heart disease, is well tolerated, with no major side effects occurring during treatment. Only gastrointestinal disturbances were observed following oral administration [110]. For example, the oral administration of 15–20 g of sodium pyruvate (in single doses, three times a day for seven days) to diabetic patients caused diarrhoea. However, no gastrointestinal reactions were observed at lower doses of pyruvate (less than 10 g) [14]. Minor gastrointestinal complaints were also observed in patients with glaucoma during a 3-week period of oral pyruvate therapy at an ascending dose of 1.5 to 3 g (in combination with nicotinamide treatment). These complaints did not require intervention, however, and resolved with continued treatment [15]. In a study by Petkova et al. [13], 21 patients with chronic liver disease were administered sodium pyruvate intravenously at doses of 54–86.6 g/day for 10 days or 50–54 g/day for 15 days. The majority of patients (approximately 94.4%) tolerated the treatment well or very well, while approximately 5.5% experienced satisfactory or poor clinical tolerability (adverse effects included changes in blood pressure, pulse rate, and clinical appearance). Additionally, no adverse effects were observed when sodium pyruvate nasal spray was administered to patients infected with SARS-CoV-2 [16].
5. Conclusions
This review highlights the often-overlooked pyruvate molecule’s cytoprotective properties, exploring its potential to protect against environmental challenges. In vitro and in vivo studies demonstrate that during exposure to toxic chemicals and physical agents, exogenous pyruvate directly neutralises ROS (primarily H2O2), protects and stabilises mitochondrial function, increases antioxidant defences, and maintains ATP levels. Pyruvate also exhibits anti-inflammatory effects. Furthermore, a key advantage of using pyruvate as a supplement is its broad therapeutic index, which has been confirmed in clinical investigations. This makes pyruvate safe to use without risking unacceptable side effects.
However, the review also identified several areas that require further clarification in future scientific research.
- 1.
- Existing research on the effects of exogenous pyruvate in the context of physical and chemical toxicants has mainly focused on the ethyl derivative of pyruvate, particularly in animal models. As previously mentioned, however, this derivative may not be effectively converted to free pyruvate in humans. Therefore, in vivo studies using sodium pyruvate could provide a more accurate assessment of metabolism and protective effect of exogenous pyruvate, particularly at the level of individual organs.
- 2.
- In addition, consideration should be given to the potential side effects associated with the formation of toxic pyruvate dimers and the risk of hypernatraemia that may accompany the administration of higher doses of sodium pyruvate.
- 3.
- An important area of research is the role of mitochondria in the protective mechanisms of exogenous pyruvate, given their key importance in the cellular metabolism of this compound.
- 4.
- Finally, the development of stable forms of pyruvate could offer additional opportunities for the therapeutic use of this α-keto acid.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Li, M.; Zhou, S.; Chen, C.; Ma, L.; Luo, D.; Tian, X.; Dong, X.; Zhou, Y.; Yang, Y.; Cui, Y. Therapeutic Potential of Pyruvate Therapy for Patients with Mitochondrial Diseases: A Systematic Review. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938240. [Google Scholar] [CrossRef]
- Zhou, F.Q. Pyruvate in the Correction of Intracellular Acidosis: A Metabolic Basis as a Novel Superior Buffer. Am. J. Nephrol. 2005, 25, 55–63. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, S.; Tonnessen, T.I. Ethyl Pyruvate Is a Novel Anti-Inflammatory Agent to Treat Multiple Inflammatory Organ Injuries. J. Inflamm. 2016, 13, 37. [Google Scholar] [CrossRef]
- Shapiro, F.; Silanikove, N. Rapid and Accurate Determination of Malate, Citrate, Pyruvate and Oxaloacetate by Enzymatic Reactions Coupled to Formation of a Fluorochromophore: Application in Colorful Juices and Fermentable Food (Yogurt, Wine) Analysis. Food Chem. 2011, 129, 608–613. [Google Scholar] [CrossRef]
- Bortoluzzi, V.T.; Brust, L.; Preissler, T.; de Franceschi, I.D.; Wannmacher, C.M.D. Creatine plus Pyruvate Supplementation Prevents Oxidative Stress and Phosphotransfer Network Disturbances in the Brain of Rats Subjected to Chemically-Induced Phenylketonuria. Metab. Brain Dis. 2019, 34, 1649–1660. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Yi, J.-S.; Chung, S.-J.; Kim, D.-K.; Byun, H.-R.; Lee, J.-Y.; Koh, J.-Y. Pyruvate Protects against Kainate-Induced Epileptic Brain Damage in Rats. Exp. Neurol. 2007, 208, 159–167. [Google Scholar] [CrossRef]
- Hu, S.; Liu, W.; Zhao, Y.; Lin, Z.; Luo, H.; Bai, X.; Sheng, Z.; Zhou, F. Pyruvate-Enriched Oral Rehydration Solution Improved Intestinal Absorption of Water and Sodium during Enteral Resuscitation in Burns. Burns J. Int. Soc. Burn Inj. 2014, 40, 693–701. [Google Scholar] [CrossRef]
- Liu, R.; Hu, X.-H.; Wang, S.-M.; Guo, S.-J.; Li, Z.-Y.; Bai, X.-D.; Zhou, F.-Q.; Hu, S. Pyruvate in Oral Rehydration Salt Improves Hemodynamics, Vasopermeability and Survival after Burns in Dogs. Burns J. Int. Soc. Burn Inj. 2016, 42, 797–806. [Google Scholar] [CrossRef]
- Martin, A.; Lupfer, C.; Amen, R. Inhaled Sodium Pyruvate Reduces Oxygen Radicals and Inflammatory Cytokines in COPD Patients. Eur. J. Respir. Med. 2022, 4, 320–326. [Google Scholar] [CrossRef]
- Votto, J.J.; Bowen, J.B.; Barton, R.W.; Thrall, R.S. Inhaled Sodium Pyruvate Improved FEV1 and Decreased Expired Breath Levels of Nitric Oxide in Patients with Chronic Obstructive Pulmonary Disease. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 329–334. [Google Scholar] [CrossRef]
- Nadler, R.A. Sodium Pyruvate Inhalation for COVID-19 Long Hauler Symptoms—An Effective and Inexpensive Treatment. J. Immunol. 2022, 208, 125.25. [Google Scholar] [CrossRef]
- Komaki, H.; Nishigaki, Y.; Fuku, N.; Hosoya, H.; Murayama, K.; Ohtake, A.; Goto, Y.-I.; Wakamoto, H.; Koga, Y.; Tanaka, M. Pyruvate Therapy for Leigh Syndrome Due to Cytochrome c Oxidase Deficiency. Biochim. Biophys. Acta 2010, 1800, 313–315. [Google Scholar] [CrossRef]
- Petkova, I.; Mateva, L.; Petrov, K.; Beniozef, D.; Thorn, W. Effects of Sodium Pyruvate Applied in Patients with Chronic Liver Diseases (CLD) of Different Etiology. Comptes Rendus Acad. Bulg. Sci. 2003, 56, 107. [Google Scholar]
- Petkova, I.; Hristov, V.; Petrov, K.; Thorn, W. Oral Application of Sodium Pyruvate in Healthy Persons and Patients with Diabetes Mellitus Type I. Comptes Rendus Acad. Bulg. Sci. 2007, 60, 579–584. [Google Scholar]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 11–18. [Google Scholar] [CrossRef]
- Lupfer, C.R.; Nadler, R.; Amen, R.; Martin, A. Inhalation of Sodium Pyruvate to Reduce the Symptoms and Severity of Respiratory Diseases Including COVID-19, Long COVID, and Pulmonary Fibrosis. Eur. J. Respir. Med. 2021, 3, 229–237. [Google Scholar] [CrossRef]
- Kgi-Admin. N-115 by EmphyCorp for Idiopathic Pulmonary Fibrosis: Likelihood of Approval. Pharmaceutical Technology. Available online: https://www.pharmaceutical-technology.com/data-insights/n-115-emphycorp-idiopathic-pulmonary-fibrosis-likelihood-of-approval/ (accessed on 16 April 2025).
- Jaffary, F.; Faghihi, G.; Saraeian, S.; Hosseini, S.M. Comparison the Effectiveness of Pyruvic Acid 50% and Salicylic Acid 30% in the Treatment of Acne. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 31. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Qiu, J.-Q.; Wang, M.-Y.; Feng, L.; Luo, D.; Gao, R.-R.; Zhou, F.-Q.; Che, K.-X. Effects of Sodium Pyruvate Supplementation on Repeated Sprint Exercise Performance and Recovery in Male College Soccer Players: A Randomized Controlled Trial. Ann. Palliat. Med. 2022, 11, 598–610. [Google Scholar] [CrossRef]
- Jäger, R.; Metzger, J.; Lautmann, K.; Shushakov, V.; Purpura, M.; Geiss, K.-R.; Maassen, N. The Effects of Creatine Pyruvate and Creatine Citrate on Performance during High Intensity Exercise. J. Int. Soc. Sports Nutr. 2008, 5, 4. [Google Scholar] [CrossRef]
- Olek, R.A.; Kujach, S.; Radak, Z. Current Knowledge about Pyruvate Supplementation: A Brief Review. Sports Med. Health Sci. 2024, 6, 295–301. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Y.; Ding, N.; Fang, Y. Recent Advances in Metabolic Engineering for the Biosynthesis of Phosphoenol Pyruvate-Oxaloacetate-Pyruvate-Derived Amino Acids. Molecules 2024, 29, 2893. [Google Scholar] [CrossRef]
- Durak, M.Z.; Churey, J.J.; Gates, M.; Sacks, G.L.; Worobo, R.W. Decontamination of Green Onions and Baby Spinach by Vaporized Ethyl Pyruvate. J. Food Prot. 2012, 75, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Andrae, U.; Singh, J.; Ziegler-Skylakakis, K. Pyruvate and Related Alpha-Ketoacids Protect Mammalian Cells in Culture against Hydrogen Peroxide-Induced Cytotoxicity. Toxicol. Lett. 1985, 28, 93–98. [Google Scholar] [CrossRef]
- O’Donnell-Tormey, J.; Nathan, C.F.; Lanks, K.; DeBoer, C.J.; de la Harpe, J. Secretion of Pyruvate. An Antioxidant Defense of Mammalian Cells. J. Exp. Med. 1987, 165, 500–514. [Google Scholar] [CrossRef]
- Natoli, R.; Rutar, M.; Lu, Y.-Z.; Chu-Tan, J.A.; Chen, Y.; Saxena, K.; Madigan, M.; Valter, K.; Provis, J.M. The Role of Pyruvate in Protecting 661W Photoreceptor-Like Cells Against Light-Induced Cell Death. Curr. Eye Res. 2016, 41, 1473–1481, Correction in Curr. Eye Res. 2025, 50, 460. [Google Scholar] [CrossRef]
- Kładna, A.; Marchlewicz, M.; Piechowska, T.; Kruk, I.; Aboul-Enein, H.Y. Reactivity of Pyruvic Acid and Its Derivatives towards Reactive Oxygen Species. Lumin. J. Biol. Chem. Lumin. 2015, 30, 1153–1158. [Google Scholar] [CrossRef]
- Zwolak, I.; Gołębiowska, D. Protective Activity of Pyruvate against Vanadium-Dependent Cytotoxicity in Chinese Hamster Ovary (CHO-K1) Cells. Toxicol. Ind. Health 2018, 34, 283–292. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Yang, Y.; Takata, T.; Sakurai, T. Systemic Pyruvate Administration Markedly Reduces Neuronal Death and Cognitive Impairment in a Rat Model of Alzheimer’s Disease. Exp. Neurol. 2015, 271, 145–154. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of Reactive Oxygen Species in Ultraviolet-Induced Photodamage of the Skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Z.; Liu, C.; Nie, Y.; Cui, L. Climate Change, Air Pollution and Chronic Respiratory Diseases: Understanding Risk Factors and the Need for Adaptive Strategies. Environ. Health Prev. Med. 2025, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Protective Effect of Indole-3-Pyruvate against Ultraviolet B-Induced Damage to Cultured HaCaT Keratinocytes and the Skin of Hairless Mice. PLoS ONE 2014, 9, e96804, Correction in PLoS ONE 2015, 10, e0128054. [Google Scholar] [CrossRef]
- Gupta, S.K.; Awor, L.; Rastogi, S.; Prakash, J.; Gupta, Y.K.; Varma, S.D.; Velpandian, T. Delayed Manifestation of Ultra Violet Radiation Induced Erythema in Guinea Pigs by Sodium Pyruvate—A Free Radical Scavenger. Indian J. Physiol. Pharmacol. 1998, 42, 315–318. [Google Scholar]
- Han, C.; Yang, H.; Li, B.; Wang, Z. Exogenous Pyruvate Facilitates Ultraviolet B-Induced DNA Damage Repair by Promoting H3K9 Acetylation in Keratinocytes and Melanocytes. Biomed. Pharmacother. 2020, 126, 110082. [Google Scholar] [CrossRef]
- Ullah, N.; Naseer, M.I.; Ullah, I.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Neuroprotective Profile of Pyruvate against Ethanol-Induced Neurodegeneration in Developing Mice Brain. Neurol. Sci. 2013, 34, 2137–2143. [Google Scholar] [CrossRef]
- Hu, J.-M.; Hsu, C.-H.; Lin, Y.-C.; Kung, C.-W.; Chen, S.-Y.; Lin, W.-T.; Cheng, P.-Y.; Shen, H.-H.; Lee, Y.-M. Ethyl Pyruvate Ameliorates Heat Stroke-Induced Multiple Organ Dysfunction and Inflammatory Responses by Induction of Stress Proteins and Activation of Autophagy in Rats. Int. J. Hyperth. 2021, 38, 862–874. [Google Scholar] [CrossRef]
- Epperly, M.; Jin, S.; Nie, S.; Cao, S.; Zhang, X.; Franicola, D.; Wang, H.; Fink, M.P.; Greenberger, J.S. Ethyl Pyruvate, a Potentially Effective Mitigator of Damage after Total-Body Irradiation. Radiat. Res. 2007, 168, 552–559. [Google Scholar] [CrossRef]
- Chen, B.; Na, F.; Yang, H.; Li, R.; Li, M.; Sun, X.; Hu, B.; Huang, G.; Lan, J.; Xu, H.; et al. Ethyl Pyruvate Alleviates Radiation-Induced Lung Injury in Mice. Biomed. Pharmacother. 2017, 92, 468–478. [Google Scholar] [CrossRef]
- Nagatome, M.; Kondo, Y.; Kadowaki, D.; Saishyo, Y.; Irikura, M.; Irie, T.; Ishitsuka, Y. Ethyl Pyruvate Attenuates Acetaminophen-Induced Liver Injury and Prevents Cellular Injury Induced by N-Acetyl-p-Benzoquinone Imine. Heliyon 2018, 4, e00521. [Google Scholar] [CrossRef]
- Najafi, G.; Atashfaraz, E.; Farokhi, F. Attenuation of Methotrexate-Induced Embryotoxicity and Oxidative Stress by Ethyl Pyruvate. Int. J. Fertil. Steril. 2016, 10, 232–238. [Google Scholar]
- Choi, S.S.; Koh, W.U.; Nam, J.S.; Shin, J.W.; Leem, J.G.; Suh, J.H. Effect of Ethyl Pyruvate on Paclitaxel-Induced Neuropathic Pain in Rats. Korean J. Pain 2013, 26, 135–141. [Google Scholar] [CrossRef]
- Shen, F.; Wang, Z.; Liu, W.; Liang, Y. Ethyl Pyruvate Can Alleviate Alcoholic Liver Disease through Inhibiting Nrf2 Signaling Pathway. Exp. Ther. Med. 2018, 15, 4223. [Google Scholar] [CrossRef]
- Chavali, V.D.; Agarwal, M.; Vyas, V.K.; Saxena, B. Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer’s Disease in Rats via Inhibiting Toll-Like Receptor 4. J. Mol. Neurosci. MN 2020, 70, 836–850. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Shahrooz, R.; Ahmadi, A.; Malekinjad, H.; Mardani, K. Protective Effect of Ethyl Pyruvate on Mice Sperm Parameters in Phenylhydrazine Induced Hemolytic Anemia. Vet. Res. Forum Int. Q. J. 2016, 7, 63–68. [Google Scholar]
- Bakdemir, M.; Çetin, E. Hepatoprotective Effects of Ethyl Pyruvate against Carbon Tetrachloride-Induced Oxidative Stress, Biochemical and Histological Alterations in Rats. Arch. Physiol. Biochem. 2021, 127, 359–366. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Li, S.; Lu, C.; Li, J.; Zong, Y.; Qi, W.; Yang, H. Hepatoprotective Effects of Ethyl Pyruvate against CCl4-Induced Hepatic Fibrosis via Inhibition of TLR4/NF-κB Signaling and up-Regulation of MMPs/TIMPs Ratio. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 72–81. [Google Scholar] [CrossRef]
- Karabeyoglu, M.; Kocer, B.; Ozel, U.; Atasay, F.O.; Ustun, H.; Dolapci, M.; Karabeyoglu, I.; Cengiz, O. The Protective Effect of Ethyl Pyruvate on Lung Injury after Burn in Rats. Saudi Med. J. 2007, 28, 1489–1492. [Google Scholar]
- Bakhtiary, Z.; Shahrooz, R.; Ahmadi, A.; Soltanalinejad, F. Ethyl Pyruvate Ameliorates the Damage Induced by Cyclophosphamide on Adult Mice Testes. Int. J. Fertil. Steril. 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Dedeoğlu, S.; Ayral, M. Protective Effect of Ethyl Pyruvate on Amikacin-Induced Ototoxicity in Rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2460–2466. [Google Scholar] [CrossRef]
- Kelle, I.; Eriman, H.; Erdinc, M.; Uyar, E. Risperidone and Ethyl Pyruvate Have Protective Effects against Ketamine-Induced Cognitive Impairments in Mice. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1708–1716. [Google Scholar] [CrossRef]
- Nourani, M.R.; Kalantari Hesari, A.; Shahrooz, R.; Asadi, M.R. Effect of Sodium Cyanide-Induced Tissue Hypoxia on Reproductive Capability of Male Mice and the Protective Effect of Ethyl Pyruvate. Arch. Razi Inst. 2021, 76, 323–333. [Google Scholar] [CrossRef]
- Kelle, I.; Akkoc, H.; Tunik, S.; Nergiz, Y.; Erdinc, M.; Erdinc, L. Protective Effects of Ethyl Pyruvate in Cisplatin-Induced Nephrotoxicity. Biotechnol. Biotechnol. Equip. 2014, 28, 674–680. [Google Scholar] [CrossRef]
- Chen, H.; Bai, H.; Xi, M.; Liu, R.; Qin, X.; Liang, X.; Zhang, W.; Zhang, X.; Li, W.; Hai, C. Ethyl Pyruvate Protects Rats from Phosgene-Induced Pulmonary Edema by Inhibiting Cyclooxygenase2 and Inducible Nitric Oxide Synthase Expression. J. Appl. Toxicol. JAT 2013, 33, 71–77. [Google Scholar] [CrossRef]
- Yang, R.; Zou, X.; Koskinen, M.-L.; Tenhunen, J. Ethyl Pyruvate Reduces Liver Injury at Early Phase but Impairs Regeneration at Late Phase in Acetaminophen Overdose. Crit. Care 2012, 16, R9. [Google Scholar] [CrossRef]
- Fedeli, D.; Falcioni, G.; Olek, R.A.; Massi, M.; Cifani, C.; Polidori, C.; Gabbianelli, R. Protective Effect of Ethyl Pyruvate on msP Rat Leukocytes Damaged by Alcohol Intake. J. Appl. Toxicol. JAT 2007, 27, 561–570. [Google Scholar] [CrossRef]
- Ivanova, I.; Bogner, C.; Gronwald, W.; Kreutz, M.; Kurz, B.; Maisch, T.; Kamenisch, Y.; Berneburg, M. UVA-Induced Metabolic Changes in Non-Malignant Skin Cells and the Potential Role of Pyruvate as Antioxidant. Photochem. Photobiol. Sci. 2023, 22, 1889–1899. [Google Scholar] [CrossRef]
- Bhat, S.M.; Massey, N.; Karriker, L.A.; Singh, B.; Charavaryamath, C. Ethyl Pyruvate Reduces Organic Dust-Induced Airway Inflammation by Targeting HMGB1-RAGE Signaling. Respir. Res. 2019, 20, 27. [Google Scholar] [CrossRef]
- Sul, J.-W.; Kim, T.-Y.; Yoo, H.J.; Kim, J.; Suh, Y.-A.; Hwang, J.J.; Koh, J.-Y. A Novel Mechanism for the Pyruvate Protection against Zinc-Induced Cytotoxicity: Mediation by the Chelating Effect of Citrate and Isocitrate. Arch. Pharm. Res. 2016, 39, 1151–1159. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Barandalla, M.; Colleoni, S.; Lazzari, G. Pyruvate Antioxidant Roles in Human Fibroblasts and Embryonic Stem Cells. Mol. Cell. Biochem. 2017, 429, 137–150. [Google Scholar] [CrossRef]
- Poteet, E.; Winters, A.; Xie, L.; Ryou, M.-G.; Liu, R.; Yang, S.-H. In Vitro Protection by Pyruvate against Cadmium-Induced Cytotoxicity in Hippocampal HT-22 Cells. J. Appl. Toxicol. JAT 2014, 34, 903–913. [Google Scholar] [CrossRef]
- Zwolak, I.; Wnuk, E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants 2022, 11, 909. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-K.; Kim, H.-J.; Yoon, S.-H.; Lee, J.-K. Neuroprotective Effect of Ethyl Pyruvate against Zn2+ Toxicity via NAD Replenishment and Direct Zn2+ Chelation. Neuropharmacology 2016, 105, 411–419. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Z.; Chang, J.; He, L.; Zhang, Z.; Song, X.; Hou, X.; Fan, F.; Jiang, Z. Sodium Pyruvate Exerts Protective Effects against Cigarette Smoke Extract-Induced Ferroptosis in Alveolar and Bronchial Epithelial Cells through the GPX4/Nrf2 Axis. J. Inflamm. 2023, 20, 28, Correction in J. Inflamm. 2025, 22, 1–3. [Google Scholar] [CrossRef]
- Harmon, R.C.; Kiningham, K.K.; Valentovic, M.A. Pyruvate Reduces 4-Aminophenol in Vitro Toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 179–186. [Google Scholar] [CrossRef]
- Hegde, K.R.; Kovtun, S.; Varma, S.D. Inhibition of Glycolysis in the Retina by Oxidative Stress: Prevention by Pyruvate. Mol. Cell. Biochem. 2010, 343, 101–105. [Google Scholar] [CrossRef]
- Hinoi, E.; Takarada, T.; Tsuchihashi, Y.; Fujimori, S.; Moriguchi, N.; Wang, L.; Uno, K.; Yoneda, Y. A Molecular Mechanism of Pyruvate Protection against Cytotoxicity of Reactive Oxygen Species in Osteoblasts. Mol. Pharmacol. 2006, 70, 925–935. [Google Scholar] [CrossRef]
- Giandomenico, A.R.; Cerniglia, G.E.; Biaglow, J.E.; Stevens, C.W.; Koch, C.J. The Importance of Sodium Pyruvate in Assessing Damage Produced by Hydrogen Peroxide. Free Radic. Biol. Med. 1997, 23, 426–434. [Google Scholar] [CrossRef]
- Nath, K.A.; Ngo, E.O.; Hebbel, R.P.; Croatt, A.J.; Zhou, B.; Nutter, L.M. Alpha-Ketoacids Scavenge H2O2 in Vitro and in Vivo and Reduce Menadione-Induced DNA Injury and Cytotoxicity. Am. J. Physiol. 1995, 268 Pt 1, C227–C236. [Google Scholar] [CrossRef]
- Olek, R.A.; Ziolkowski, W.; Kaczor, J.J.; Wierzba, T.H.; Antosiewicz, J. Higher Hypochlorous Acid Scavenging Activity of Ethyl Pyruvate Compared to Its Sodium Salt. Biosci. Biotechnol. Biochem. 2011, 75, 500–504. [Google Scholar] [CrossRef][Green Version]
- Vásquez-Vivar, J.; Denicola, A.; Radi, R.; Augusto, O. Peroxynitrite-Mediated Decarboxylation of Pyruvate to Both Carbon Dioxide and Carbon Dioxide Radical Anion. Chem. Res. Toxicol. 1997, 10, 786–794. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, A.; Pathak, M.; Kaur, L.; Kumar, V.; Roy, B.G.; Ojha, H. DNA Binding and Antiradical Potential of Ethyl Pyruvate: Key to the DNA Radioprotection. Chem. Biol. Interact. 2020, 332, 109313. [Google Scholar] [CrossRef]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.-Y. Reaction Rate of Pyruvate and Hydrogen Peroxide: Assessing Antioxidant Capacity of Pyruvate under Biological Conditions. Sci. Rep. 2019, 9, 19568. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Simpson, E.J.; Greenhaff, P.L. The Importance of Pyruvate Availability to PDC Activation and Anaplerosis in Human Skeletal Muscle. Am. J. Physiol. 1999, 276, E472–E478. [Google Scholar] [CrossRef]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.-J.; Mallet, R.T.; Yang, S.-H. Pyruvate Protects Mitochondria from Oxidative Stress in Human Neuroblastoma SK-N-SH Cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Chung, S.J.; Kang, I.J.; Park, J.H.; Bünger, R. Intramitochondrial Pyruvate Attenuates Hydrogen Peroxide-Induced Apoptosis in Bovine Pulmonary Artery Endothelium. Mol. Cell. Biochem. 2001, 216, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nishigaki, Y.; Fuku, N.; Ibi, T.; Sahashi, K.; Koga, Y. Therapeutic Potential of Pyruvate Therapy for Mitochondrial Diseases. Mitochondrion 2008, 7, 399–401. [Google Scholar] [CrossRef]
- Cappel, D.A.; Deja, S.; Duarte, J.A.G.; Kucejova, B.; Iñigo, M.; Fletcher, J.A.; Fu, X.; Berglund, E.D.; Liu, T.; Elmquist, J.K.; et al. Pyruvate-Carboxylase-Mediated Anaplerosis Promotes Antioxidant Capacity by Sustaining TCA Cycle and Redox Metabolism in Liver. Cell Metab. 2019, 29, 1291–1305.e8. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Ramos, M.; Ruiz, F.; Satrústegui, J.; Bogónez, E. Pyruvate Protection against Beta-Amyloid-Induced Neuronal Death: Role of Mitochondrial Redox State. J. Neurosci. Res. 2003, 73, 260–269. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating Mitochondrial Redox State Using NADH and NADPH Autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Van Vranken, J.G.; Rutter, J. You Down With ETC? Yeah, You Know D! Cell 2015, 162, 471–473. [Google Scholar] [CrossRef]
- Battaglia, S.; De Santis, S.; Rutigliano, M.; Sallustio, F.; Picerno, A.; Frassanito, M.A.; Schaefer, I.; Vacca, A.; Moschetta, A.; Seibel, P.; et al. Uridine and Pyruvate Protect T Cells’ Proliferative Capacity from Mitochondrial Toxic Antibiotics: A Clinical Pilot Study. Sci. Rep. 2021, 11, 12841. [Google Scholar] [CrossRef]
- Fink, M.P. Ethyl Pyruvate: A Novel Anti-Inflammatory Agent. J. Intern. Med. 2007, 261, 349–362. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Han, Y.; Englert, J.A.; Yang, R.; Delude, R.L.; Fink, M.P. Ethyl Pyruvate Inhibits Nuclear Factor-kappaB-Dependent Signaling by Directly Targeting P65. J. Pharmacol. Exp. Ther. 2005, 312, 1097–1105. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High Mobility Group Box 1 (HMGB1): A Pivotal Regulator of Hematopoietic Malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, H.J.; Kim, H.; Park, S.W. Ethyl Pyruvate Directly Attenuates Active Secretion of HMGB1 in Proximal Tubular Cells via Induction of Heme Oxygenase-1. J. Clin. Med. 2019, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Lee, H.-K.; Lee, H.-B.; Jin, Y.; Lee, J.-K. Ethyl Pyruvate Inhibits HMGB1 Phosphorylation and Secretion in Activated Microglia and in the Postischemic Brain. Neurosci. Lett. 2014, 558, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Kim, I.-D.; Kim, S.-W.; Lee, H.-K.; Jin, Y.; Park, J.-H.; Kim, T.-K.; Suh, C.-K.; Kwak, J.; Lee, K.-H.; et al. Ethyl Pyruvate Inhibits HMGB1 Phosphorylation and Release by Chelating Calcium. Mol. Med. Camb. Mass 2015, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, E.J.; Kim, J.H.; Park, S.W.; Kim, H.J.; Chang, K.C. Ethyl Pyruvate Inhibits the Acetylation and Release of HMGB1 via Effects on SIRT1/STAT Signaling in LPS-Activated RAW264.7 Cells and Peritoneal Macrophages. Int. Immunopharmacol. 2016, 41, 98–105. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 Signaling by Natural Products-Can It Alleviate Diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Harvey, S.A.K.; Guerriero, E.; Charukamnoetkanok, N.; Piluek, J.; Schuman, J.S.; SundarRaj, N. Responses of Cultured Human Keratocytes and Myofibroblasts to Ethyl Pyruvate: A Microarray Analysis of Gene Expression. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2917–2927. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, S.-W.; Jin, Y.; Kim, I.-D.; Lee, J.-K. Ethyl Pyruvate-Mediated Nrf2 Activation and Hemeoxygenase 1 Induction in Astrocytes Confer Protective Effects via Autocrine and Paracrine Mechanisms. Neurochem. Int. 2012, 61, 89–99. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, Y.M.; Tsoyi, K.; Park, E.J.; Lee, Y.S.; Kim, H.J.; Lee, J.H.; Joe, Y.; Chung, H.T.; Chang, K.C. Ethyl Pyruvate Induces Heme Oxygenase-1 Through P38 Mitogen-Activated Protein Kinase Activation by Depletion of Glutathione in RAW 264.7 Cells and Improves Survival in Septic Animals. Antioxid. Redox Signal. 2012, 17, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Lee, H.-K.; Shin, J.-H.; Lee, J.-K. Up-down Regulation of HO-1 and iNOS Gene Expressions by Ethyl Pyruvate via Recruiting P300 to Nrf2 and Depriving It from P65. Free Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Lee, I.; Ting, H.; Chang, Y.-J.; Yang, N.-C. Parapyruvate, an Impurity in Pyruvate Supplements, Induces Senescence in Human Fibroblastic Hs68 Cells via Inhibition of the α-Ketoglutarate Dehydrogenase Complex. J. Agric. Food Chem. 2018, 66, 7504–7513. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.A.; Coxon, B. Identification and Quantitation of the Impurities in Sodium Pyruvate. Anal. Chem. 1986, 58, 2504–2510. [Google Scholar] [CrossRef]
- Zhou, F.-Q. Pyruvate as a Potential Beneficial Anion in Resuscitation Fluids. Front. Med. 2022, 9, 905978. [Google Scholar] [CrossRef]
- Lopalco, A.; Deeken, R.; Douglas, J.; Denora, N.; Stella, V.J. Some Preformulation Studies of Pyruvic Acid and Other α-Keto Carboxylic Acids in Aqueous Solution: Pharmaceutical Formulation Implications for These Peroxide Scavengers. J. Pharm. Sci. 2019, 108, 3281–3288. [Google Scholar] [CrossRef]
- Zhou, F.-Q. Advantages of Pyruvate-Based Fluids in Preclinical Shock Resuscitation-A Narrative Review. Front. Physiol. 2022, 13, 1027440. [Google Scholar] [CrossRef]
- Zabielska, M.A.; Adamus, J.; Kowalski, R.; Gebicki, J.; Slominska, E.M.; Khalpey, Z.; Smolenski, R.T. Cardioprotective Effect of N-Methylnicotinamide Salt of Pyruvate in Experimental Model of Cardiac Hypoxia. Pharmacol. Rep. 2018, 70, 378–384. [Google Scholar] [CrossRef]
- Kao, K.K.; Fink, M.P. The Biochemical Basis for the Anti-Inflammatory and Cytoprotective Actions of Ethyl Pyruvate and Related Compounds. Biochem. Pharmacol. 2010, 80, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Reade, M.C.; Fink, M.P. Bench-to-Bedside Review: Amelioration of Acute Renal Impairment Using Ethyl Pyruvate. Crit. Care 2005, 9, 556–560. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Swaminathan, M.; Grigore, A.M.; Roach, G.W.; Aberle, L.G.; Johnston, J.M.; Fink, M.P. A Phase II Multicenter Double-Blind Placebo-Controlled Study of Ethyl Pyruvate in High-Risk Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2009, 23, 324–329. [Google Scholar] [CrossRef]
- Zhou, F. Pyruvate Research and Clinical Application Outlooks A Revolutionary Medical Advance. Int. J. Nutr. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Bahar, F.G.; Ohura, K.; Ogihara, T.; Imai, T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.M.; Wollenberg, L.; Zhao, Z. Esterase Activities in the Blood, Liver and Intestine of Several Preclinical Species and Humans. Drug Metab. Lett. 2009, 3, 70–77. [Google Scholar] [CrossRef]
- Zhou, F. Pyruvate-Enriched Fluids as a Novel Medical Solution and Beverage. Food Sci. Nutr. Technol. 2021, 6, 000258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).