1. Introduction
Cancer is a leading cause of mortality worldwide, with both incidence and death rates increasing each year. According to the World Health Organization (WHO), approximately 20 million new cancer cases and 10 million cancer-related deaths occurred globally in 2020. These numbers are projected to rise by 1.5-fold by 2040 [
1]. The development of cancer is influenced by various factors, among which the abnormal activation of proto-oncogenes plays a pivotal role [
2]. The
c-myb proto-oncogene was originally identified through reverse transcription of retroviral sequences from AMV and E26, with its encoded C-MYB protein regulating essential cellular processes, such as differentiation, proliferation, and maintenance of homeostasis [
3]. Aberrant expression of
c-myb is closely associated with several malignancies, such as acute myeloid leukemia, breast cancer, and colorectal cancer [
4,
5]. The C-MYB protein exerts its oncogenic effects by modulating cell cycle progression, differentiation, apoptosis, and metastasis through interactions with transcription factors, cell cycle regulators, microRNAs (miRNAs), and non-coding RNAs (ncRNAs) [
6,
7,
8,
9].
Targeting
c-myb gene expression with small molecules has emerged as a promising anti-tumor strategy. Some small molecules inhibiting the C-MYB protein have been reported and showed therapeutic potential [
10,
11]. In addition, anti-sense oligonucleotides (ASOs) targeting
c-myb have been introduced into clinical trials for hematological malignancies [
12]. Therefore, direct modulation of
c-myb transcription offers an alternative approach for effective cancer therapy. Conventional cancer treatments, such as surgery, radiotherapy, and chemotherapy, have shown some therapeutic effects; however, these effects are limited due to incomplete tumor eradication, systemic toxicity, and poor specificity [
13]. The importance of gene-level regulation in cancer therapy has attracted significant attention; in particular, non-canonical DNA secondary structures, including G-quadruplexes (G4s) and i-motifs (also named as IMs or C-quadruplexes) on gene promoters, play crucial roles in transcriptional control [
14]. Under near-physiological conditions, various G-rich DNA oligomers can form G-quadruplexes, while complementary C-rich oligomers can form i-motif structures, especially under mild acidic or molecular crowding conditions [
15]. It should be noted that these two types of structures appear differently within the cell cycle, and G-quadruplexes are enriched in the S phase, while i-motifs are prominent in the G1 phase [
16]. These quadruplex structures can act as “molecular switches” regulating gene transcription via conformational transitions. G-quadruplexes are widely recognized as transcriptional repressors, and some ligands targeting G4 structures, such as CX-3543, CX-5461, and APTO-253, have been introduced into clinical trials [
17]. In contrast, small molecules targeting i-motif structures have received much less attention, with their biological roles remaining unclear and controversial. For example, acridone derivative
B19 can stabilize
c-myc promoter i-motif and down-regulate its gene transcription [
18], while acridone derivative
B14 can stabilize
BCL-2 promoter i-motif and up-regulate its gene transcription [
19]. To date, no i-motif-targeting compound has progressed to clinical trials.
c-myb gene promoter contains both G-rich and C-rich sequences, which can form G-quadruplex (G4) and i-motif structures, respectively [
20,
21]. Dual-targeting ligands that simultaneously interact with both G4 (primarily in S phase) and i-motif (primarily in G1 phase) can offer great potential for regulating gene expression very effectively across multiple stages of the cell cycle. Our previous research has identified bisacridine derivative
A06 as a dual-targeting ligand capable of disrupting both G4 and i-motif structures on the promoter of tumor suppressor gene retinoblastoma (RB), consequently increasing RB expression significantly and inhibiting tumor proliferation and metastasis [
22]. This dual-modulation approach has allowed for reduced dosage of drug administration with decreased toxicity, resulting in an increased anti-tumor therapeutic index. Encouraged by these findings, we further screened our compound libraries for dual-targeting other oncogene promoter quadruplex structures and identified carbazole derivative
G51 as a promising candidate for the
c-myb gene promoter. While
A06 disrupts both G-quadruplex and i-motif structures of the RB promoter, in the present study, it was found that
G51 showed opposing effects on different quadruplex structures, which could stabilize G-quadruplex while disassembling i-motif on the
c-myb gene promoter. In comparison, a structurally similar compound
G50 specifically disrupted G-quadruplex without significant interaction with i-motif on the
c-myb gene promoter. Our molecular- and cellular-level experiments showed that
G51 significantly down-regulated
c-myb gene transcription and translation with very strong anti-tumor activity in comparison to
G50. Furthermore, using a colorectal cancer xenograft model, our in vivo experiment showed that
G51 had potent anti-tumor activity without significant toxicity to major organs. These results increased our understanding of
c-myb promoter quadruplex structures and their potential as therapeutic targets. This study also provided a promising approach for the practical development of highly specific anti-cancer agents targeting quadruplex structures of oncogene promoters.
2. Results
The
c-myb promoter region contains G/C-rich sequences, which have been found to be critical regulators of promoter activity [
20,
21]. The C-rich strand, capable of forming the i-motif (IM) structure, has the following sequence: 5′-TCCTCCTCCTCCTCCTTCTCCTCCTCCTCCGTGACCTCCTCCTCCTCC-3′. Its complementary G-rich strand, capable of forming the G-quadruplex (G4) structure, has the following sequence: 5′-GGAGGAGGAGGAGGTCACGGAGGAGGAGGAGGAGAAGGAGGAGGAGGA-3′ (
Table S1). In the present study, circular dichroism (CD) spectroscopy was employed to verify the formation of these secondary structures under our experimental conditions. As shown in
Figure S1A, the C-rich sequence exhibited a characteristic positive CD peak at 285–288 nm and a negative peak near 260 nm in the pH range of 5.0–6.25, consistent with i-motif formation [
23]. The transitional pH (pH
T), defined as the pH at which 50% of the C-rich sequences form i-motifs, was determined to be 6.4 through curve fitting (
Figure S1B), indicating its relatively high structural stability. Accordingly, a buffer with pH 5.5 was used in our subsequent experiments to ensure complete i-motif formation. In contrast, the CD spectrum of the G-rich sequence exhibited typical features of a hybrid G4 structure (
Figure S1C), including positive peaks at 295 nm and 260 nm, as well as a negative peak at 245 nm, indicating that the
c-myb G4 formed a stable hetero-quadruplex structure at a physiological pH of 7.4 under our experimental conditions [
24].
2.1. Screening for Binding of Small Molecules to c-myb Promoter Quadruplex Structures
In order to find dual-targeting ligands capable of binding to
c-myb promoter G-quadruplex and i-motif structures, we screened various compounds, including bisacridine derivatives, acridone derivatives, carbazole derivatives, acridone–naphthalimide hybrid derivatives, and quinoline derivatives, by using surface plasmon resonance (SPR); the data obtained are shown in
Table S2. Although most of these compounds had poor binding affinity to
c-myb i-motif (
KD > 50 μM), we found that carbazole derivatives
G49 and
G51 showed good binding affinity, with their
KD values determined to be less than 5 μM. SPR was also performed to assess the binding affinity of these compounds to the
c-myb G-quadruplex. As shown in
Figure 1A,B,
G49 showed moderate binding affinity, with
KD values determined to be 3.34 μM and 5.15 μM for the i-motif and G4, respectively. In comparison, compound
G50 showed preferential binding to the
c-myb G-quadruplex only, with the
KD value determined to be 3.03 μM, without significant interaction with
c-myb i-motif (
Figure 1C,D). Importantly,
G51 exhibited strong binding affinity to both i-motif and G4 structures, with their
KD values determined to be 0.58 μM and 1.10 μM (
Figure 1E,F), respectively. These data suggest that both
G49 and
G51 are potential dual-targeting ligands.
In order to further verify the above data, we studied the binding affinities of
G49,
G50, and
G51 to quadruplex structures using microscale thermophoresis (MST). As shown in
Figure S2,
G51 exhibited the strongest binding affinity to both i-motif and G-quadruplex structures, with
KD values determined to be 1.04 ± 0.40 μM for the
c-myb i-motif and 1.43 ± 0.64 μM for the G4 structure. In comparison,
G49 had a weak binding affinity, with
KD values determined to be 2.11 ± 0.71 μM for the i-motif and 8.53 ± 3.22 μM for the G4.
G50 exhibited strong binding affinity to the G4 structure, with the
KD value determined to be 1.11 ± 0.52 μM, without significant interaction with the i-motif (
KD = 10.06 ± 2.67 μM). Our above data from MST are consistent with our SPR result; therefore, we selected carbazole derivative
G51 (synthetic route as shown in
Scheme S1) as the primary compound for further investigation as a dual-targeting ligand for
c-myb i-motif and G4, with the structurally similar carbazole derivative
G50, targeting
c-myb G4 only, as a control for comparison.
2.2. Interactions of Selected Carbazole Derivatives with c-myb Promoter Quadruplex Structures
Circular dichroism (CD) spectroscopy, a sensitive technique for monitoring biomolecular conformational changes, was employed to characterize the interactions of our carbazole derivatives with the quadruplex structures [
25,
26]. As shown in
Figure S3, the
c-myb i-motif alone exhibited characteristic spectral features with a positive peak at 288 nm and a negative peak at 260 nm [
23]. Upon the addition of compound
G49 or
G51, these peaks gradually changed in dose-dependent manners, indicating their good interactions [
25,
27]. In comparison, compound
G50 had no significant effect on these peaks, indicating their weak interactions. On the other hand, all three compounds affected the positive peak intensity of the
c-myb G-quadruplex (G4) in dose-dependent manners, indicating their good interactions. These CD experimental data are consistent with our above SPR and MST results.
CD melting experiments were then carried out to evaluate the effects of our carbazole derivatives on the thermal stability of the quadruplex structures [
28]. As shown in
Figure S4, the intrinsic melting temperatures (T
m) of our
c-myb i-motif and G-quadruplex were determined to be 58.9 °C and 80.3 °C, respectively. Upon the addition of
G49,
G50, or
G51, the melting temperature (T
m) of the i-motif was reduced to 49.3 °C, 55.0 °C, or 46.0 °C, while the melting temperature (T
m) of the G-quadruplex was increased to 87.5 °C, 87.2 °C, or 92.7 °C, respectively (
Figure 2A,B). Therefore,
∆Tm values for carbazole derivatives with the i-motif were determined to be −9.6 °C for
G49, −3.9 °C for
G50, and −12.9 °C for
G51, while ∆
Tm values for carbazole derivatives with the G-quadruplex were determined to be 7.2 °C for
G49, 6.9 °C for
G50, and 12.4 °C for
G51, respectively. These data showed that
G51 could significantly destabilize the i-motif (Δ
Tm = −12.9 °C) while stabilizing G-quadruplex (Δ
Tm = +12.4 °C), which was a better dual-targeting ligand than
G49 (Δ
Tm = −9.6 °C for i-motif and +7.2 °C for G-quadruplex). In contrast, compound
G50 could stabilize G-quadruplex (Δ
Tm = +6.9 °C) without a significant effect on the i-motif (Δ
Tm = −3.9 °C). These CD melting experimental data are also consistent with our above binding interaction studies.
In order to further verify the above results, fluorescence resonance energy transfer (FRET) experiments were performed using 5′-FAM and 3′-TAMRA dual-labeled
c-myb oligomers. The formation of the quadruplex structure can bring the fluorophores into proximity, yielding high FRET efficiency with an emission at 585 nm, while dissociation can reduce this signal [
29,
30]. As shown in
Figure S5C–E, the fluorescence response at 585 nm decreased significantly with increasing concentrations of compounds
G49 and
G51, indicating that the i-motif structure could be unfolded by these two compounds. In comparison,
G50 showed a very weak unfolding effect on the i-motif. The quantitative analysis (
Figure 2C) showed that
G51 could significantly destabilize the i-motif with an increased ratio of fluorescence response values at 518 and 585 nm. On the other hand, as shown in
Figure S5F–H, all three compounds, namely,
G49,
G50, and
G51, could stabilize the G-quadruplex with a decreased ratio of fluorescence response values at 518 and 585 nm. Our FRET data are consistent with our above experimental results. All the above results demonstrate that
G51, as a dual-targeting ligand, could significantly destabilize
c-myb promoter i-motif while stabilizing
c-myb promoter G-quadruplex, and in comparison,
G50 could stabilize only
c-myb promoter G-quadruplex without a significant effect on
c-myb promoter i-motif.
2.3. Carbazole Derivative G51 Could Specifically Interact with c-myb Promoter Quadruplex Structures Through Destabilization of Its i-Motif and Stabilization of Its G-Quadruplex
Ligand specificity represents a crucial parameter for evaluating small-molecule therapeutics. To assess the binding specificity of compound
G51, we studied its effect on promoter quadruplex structures from various other oncogenes, including
c-jun,
HIF-1α,
Rb,
Kras, and
Ret, by using SPR and MST experiments. As shown in
Tables S3 and S4,
G51 exhibited much higher binding affinity to
c-myb i-motif and G-quadruplex (
KD = 0.5−1.5 μM) than its binding affinity to other tested secondary structures (
KD > 7.7 μM), suggesting its excellent specificity. This remarkable specificity was further confirmed by using thiazole orange (TO) displacement assays [
31,
32], and
G51 showed DC
50 values of 0.8 μM and 1.0 μM for
c-myb i-motif and G-quadruplex, respectively (
Figure S6). In comparison,
G51 displayed much lower displacement activity on quadruplex structures from other gene promoters, demonstrating its strong binding specificity to
c-myb promoter quadruplex structures.
CD titration experiments were also performed for the binding of
G51 to some other gene quadruplex structures (
Figure S7), which showed that
G51 had no significant effect on these tested quadruplex structures, reinforcing its specific interaction with
c-myb promoter quadruplex structures. In addition, FRET analysis showed that
G51 had no significant effect on different types of fluorescently labeled DNA secondary structures from promoters of various oncogenes (e.g.,
c-Jun,
Kras,
VEGF, and
Bcl-2), as shown in
Figure 2D. We also studied the effect of
G51 on the thermal stability of some other secondary structures from promoters of various oncogenes, including
Hras,
Bcl-2,
HIF-1α,
Ret,
Rb,
and c-Jun, by using a CD melting experiment. As shown in
Figure S8,
G51 exhibited no significant impact on these structures, with
ΔTm values ranging from −4.5 to +4.1 °C. These results were further corroborated by using FRET melting experiments, as shown in
Figure S9.
G51 had the strongest effect on
c-myb quadruplex structures, with
ΔTm values determined to be −10.0 °C for the i-motif and +9.3 °C for the G-quadruplex, without a significant impact on other gene quadruplex structures. All the above data suggest that
G51, as a dual-targeting ligand, had specific binding to
c-myb promoter quadruplexes, with the destabilization of
c-myb promoter i-motif and concurrent stabilization of
c-myb promoter G-quadruplex. This opposing effect could provide a good opportunity for specific gene regulation, with great potential for the development of a precision therapeutic agent.
2.4. Further Studies of Interactions Between Carbazole Derivative G51 and c-myb Promoter Quadruplex Structures
An ultraviolet–visible (UV) experiment was employed to characterize the binding interactions between
G51 and
c-myb quadruplex structures. Ligand intercalation between DNA base pairs typically manifests through hypochromic effects (reduced absorption intensity) and bathochromic shifts (red shift of absorption maxima), and these phenomena are attributed to π-π stacking interactions [
33,
34]. As shown in
Figure S10, the titration of
G51 with
c-myb i-motif or G-quadruplex DNA produced hypochromicity, accompanied by red shifts. These spectral perturbations suggest intercalative binding of
G51 to both quadruplex forms, with π-π stacking between the carbazole moiety and nucleobases.
The ESI-MS experiment is a highly sensitive technique capable of detecting non-covalent interactions [
35]. As shown in
Figure S11, binding of
G51 to
c-myb i-motif could be detected with the observation of a stable
c-myb i-motif–
G51 complex (
m/
z 13,119.8) in BPES buffer at pH 5.5. In comparison, this complex formation was not observed in BPES buffer at pH 7.0, which indicates that
G51 could bind to the i-motif structure without significant binding to its corresponding C-rich oligomer. Similarly, the binding of
G51 to
c-myb G4 could also be detected with the observation of a stable
c-myb G4–
G51 complex (
m/
z 14,156.4). These experiments provide direct evidence for the dual binding of
G51 to
c-myb i-motif and G-quadruplex structures.
We further investigated the interaction of
G51 with
c-myb promoter quadruplex structures by using an electrophoretic mobility shift (EMSA) experiment [
36]. As shown in
Figure S12, for the pre-formed i-motif structure, the addition of an increasing amount of
G51 progressively diminished the i-motif band intensity while enhancing the single-strand DNA band, indicating the unfolding of the i-motif structure by
G51. Conversely, with the unannealed G-rich oligomer, the addition of an increasing amount of
G51 gradually slowed down the migration of the DNA band, indicating G-quadruplex formation induced by
G51. The above results demonstrate that
G51 could unfold
c-myb promoter i-motif while inducing G4 formation in concentration-dependent manners. These results provide an in-depth understanding of the opposing effect of
G51 on i-motif and G-quadruplex structures.
2.5. G51 Down-Regulated c-myb Transcription and Translation in HCT116 Cells
Since
G51 could specifically stabilize
c-myb promoter G-quadruplex while unfolding its corresponding i-motif structure, we evaluated its regulatory effect on
c-myb gene transcription and translation. We assessed
c-myb promoter activity by using a dual-luciferase reporter assay [
37] with three engineered recombinant plasmids containing the
c-myb promoter sequence, including a wild-type
c-myb plasmid (WT), a deleted
c-myb plasmid (Del) lacking a quadruplex-forming sequence, and a mutated
c-myb plasmid (Mut) with a quadruplex-forming sequence mutated to disrupt quadruplex structure formation. These
c-myb promoter sequences were cloned upstream of firefly luciferase (FLuc) in pGL vectors to construct recombinant plasmids pGL-WT
c-myb, pGL-Del
c-myb, and pGL-Mut
c-myb (
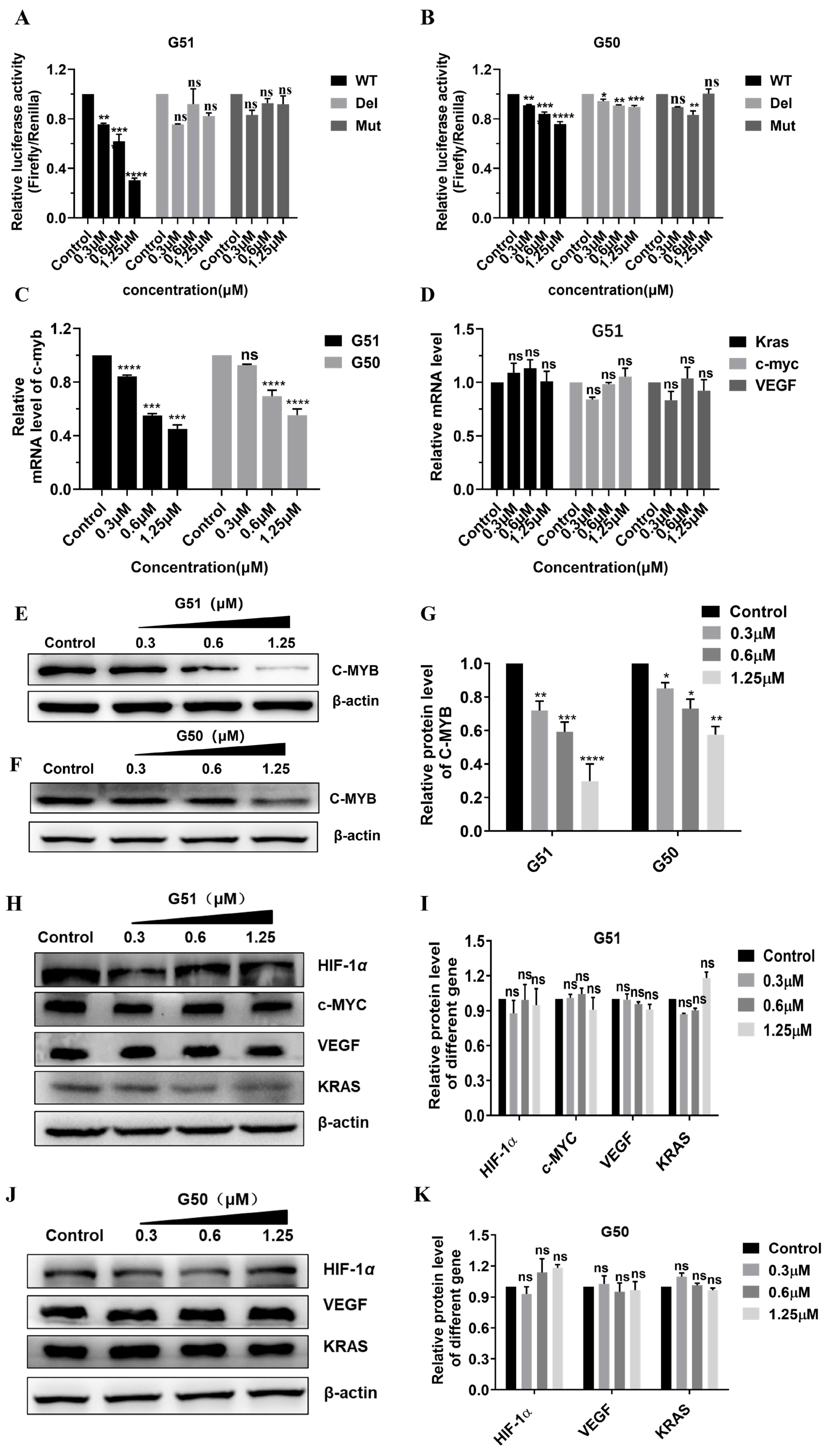
Table S6). The plasmid was then co-transfected with Renilla luciferase (RLuc) control plasmid pRL-TK into HCT116 cells. Following 48 h incubation with
G50 or
G51, dose-dependent inhibition of WT promoter activity was observed, as shown in
Figure 3A,B. Notably,
G51 exhibited significantly higher activity than
G50, indicating that the stabilization of G-quadruplex and destabilization of its corresponding i-motif both contributed positively to the gene regulation. Both compounds had no significant effect on the regulation of the Del or Mut plasmid, indicating their critical interaction with quadruplex structures for regulatory activity.
In order to quantitatively assess the effect of
G51 on
c-myb transcription, we performed real-time quantitative PCR (qPCR) analysis (primer sequences as shown in
Table S7) on HCT116 cells incubated with increasing concentrations of
G50 or
G51 for 48 h. As shown in
Figure 3C,D, both compounds exhibited concentration-dependent down-regulation of
c-myb transcription, and
G51 showed higher regulatory activity than
G50. Notably,
G51 had no significant effect on the transcription of some other tested genes, including
c-myc,
VEGF, and
Kras, indicating that its regulatory effect on
c-myb transcription was highly specific. These results also showed that
G51 had higher regulatory activity than
G50, indicating that the stabilization of G-quadruplex and destabilization of its corresponding i-motif both made positive contributions to the gene regulation.
Next, we further investigated the regulatory effect of
G51 on C-MYB protein translation by using a Western blot (WB) and immunofluorescence (IF) experiments [
38]. As shown in
Figure 3E–K, both
G50 and
G51 down-regulated C-MYB protein translation in concentration-dependent manners, and
G51 showed much higher regulatory activity than
G50. In addition,
G51 had no significant effect on the expressions of other proteins, including HIF-1α, C-MYC, VEGF, and KRAS, further confirming its regulatory specificity at the protein translation level. Our immunofluorescence (IF) experiment also showed that the fluorescence intensity of C-MYB protein in HCT116 cells decreased substantially upon incubation with increasing concentrations of
G51 (
Figure S13). All these results showed that
G51 could specifically down-regulate
c-myb expression much more effectively through dual interactions with opposing effects on gene promoter i-motif and G-quadruplex structures.
2.6. G51 Could Induce Cancer Cell Apoptosis with Inhibition of Cell Proliferation and Metastasis
The anti-proliferative effects of
G49,
G50, and
G51 were evaluated across eight human cancer cell lines (HCT116, SW620, RKO, SW480, DLD1, A549, HGC-27, and HeLa) using an MTT assay, as shown in
Table S5. Among these three compounds,
G51 showed the most potent cytotoxicity against almost all tested cancer cell lines, with particular efficacy against HCT116 colorectal cancer cells (IC
50 = 0.6 ± 0.1 μM). Then, we tested its effect on NCM460 cells (human normal colonic mucosal epithelial cells) for comparison. Our results revealed that
G51 had good selectivity on HCT116 colorectal cancer cells, with only moderate cytotoxicity against NCM460 normal cells (IC
50 = 12.8 ± 0.5 μM). On the other hand, the effects of
G50 on HCT116 colorectal cancer cells (IC
50 = 4.7 ± 0.8 μM) and NCM460 normal cells (IC
50 = 19.8 ± 0.8 μM) were also determined for comparison. These data indicate that
G51 had about five times better selectivity than
G50 on the inhibition of cancer cells versus normal cells, suggesting that the dual-targeting mechanism of
G51 could also decrease off-target side effects. Based on the above data, we selected
G51 as the primary focus for our following in-depth cellular-level studies. To further evaluate the inhibitory effect of
G51 on cell growth, we performed colony formation experiments on HCT116 cells. Our data show that
G51 could reduce the colony number and size, with much higher activity than
G50 (
Figure 4). Compound
G51 could significantly reduce the colony number in a dose-dependent manner based on our quantitative analysis, suggesting that
G51 could much more effectively inhibit cell growth, possibly through dual interactions with opposing effects on gene promoter i-motif and G-quadruplex structures.
Then, the effect of
G51 on the apoptosis of HCT116 cells was quantitatively assessed by using the Annexin V-FITC/PI dual-staining method. As shown in
Figure S14, dose-dependent increases in apoptosis were observed for HCT116 cells incubated with
G50 or
G51. Upon treatment with 1.25 μM
G51, the HCT116 cell apoptosis ratio was increased from 2.65% to 38.32%. In comparison, upon treatment with the same concentration of
G50, the HCT116 cell apoptosis ratio was increased from 1.81% to 26.61% only. In order to understand the mechanism of HCT116 cell apoptosis induced by
G51, a Western blot experiment was performed, with the data shown in
Figure S15. Our results showed that
G51 also down-regulated BCL-2 expression and up-regulated BAX expression in a dose-dependent manner, which could possibly cause an increase in the permeability of the cell membrane and, consequently, promote the release of cytochrome C from mitochondria. This could trigger the activation of downstream caspase-3 and caspase-9, which could then induce apoptosis. These data are consistent with those for the mitochondrial apoptosis pathway reported previously [
39]. Notably,
G51 had a much stronger effect than
G50 on the expression levels of BCL-2 and BAX. Following this experiment, we examined the effect of
G51 on the HCT116 cell cycle by using the propidium bromide (PI) staining method. As shown in
Figure S16, upon treatment with
G51, HCT116 cells in the G
0/G
1 phase were significantly increased in a dose-dependent manner, exhibiting a clear G
0/G
1 phase block. In comparison,
G50 caused only a slight G
0/G
1 phase block. These results demonstrated that
G51, as a dual-targeting ligand, had potent anti-cancer activity, possibly through the activation of the intrinsic apoptotic pathway and induction of cell cycle arrest at the G
0/G
1 phase.
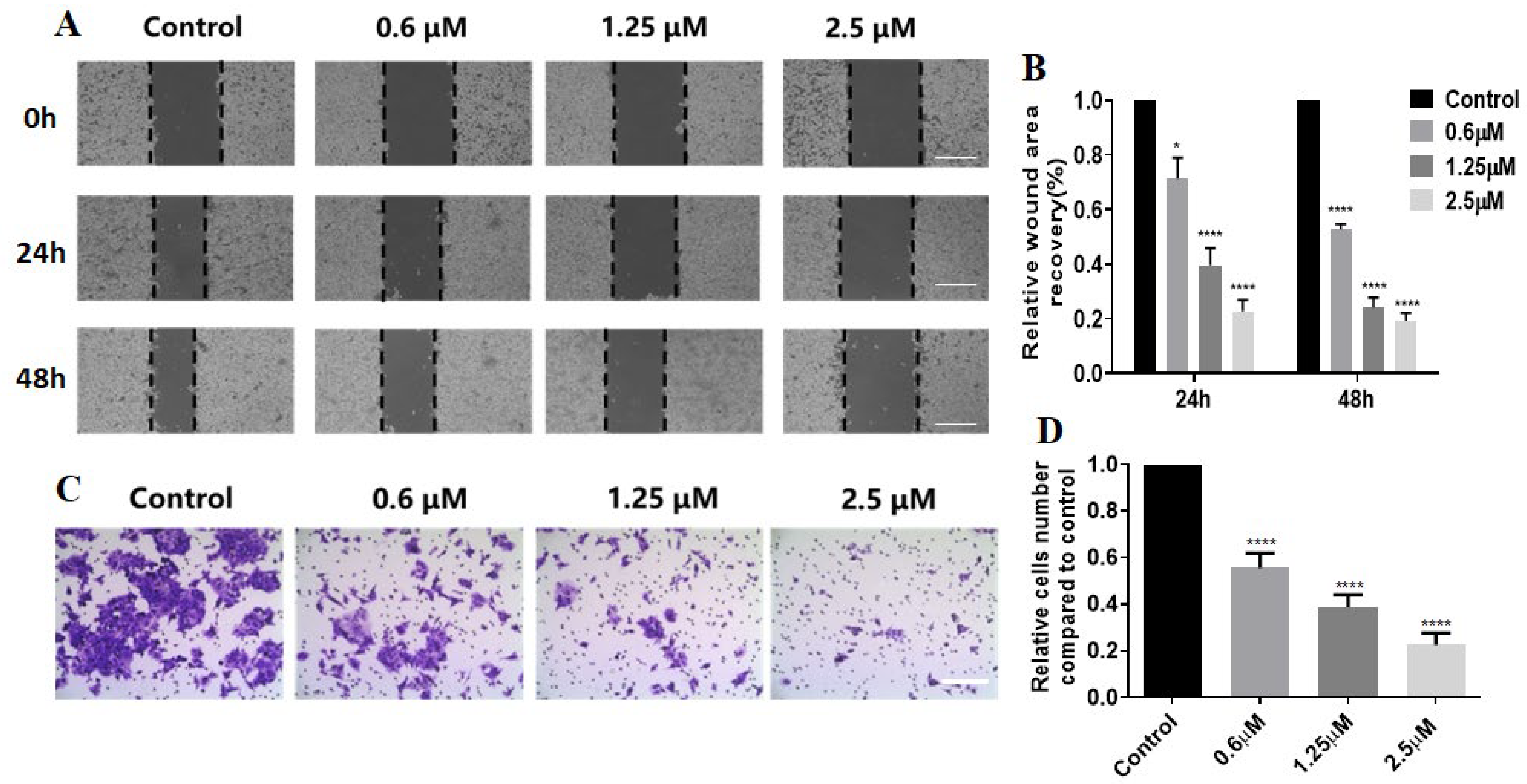
Next, the effect of
G51 on the migration ability of HCT116 cells was studied through a scratching experiment. As shown in
Figure 5A,B,
G51 significantly slowed down the migration of HCT116 cells in a dose-dependent manner upon incubation for 24 h and 48 h, in comparison with the control group. After incubation with
G51 at a 2.5 μM concentration, the scratch area remained almost unchanged, indicating that
G51 could effectively inhibit HCT116 cell migration. We also performed a transwell experiment, as shown in
Figure 5C,D.
G51 significantly reduced the number of invaded cells in a dose-dependent manner in comparison with the control group. These experiments show that
G51 could effectively inhibit the migration and invasion of HCT116 cells.
2.7. G51 Inhibited Tumor Growth in a Human Colorectal Cancer Xenograft
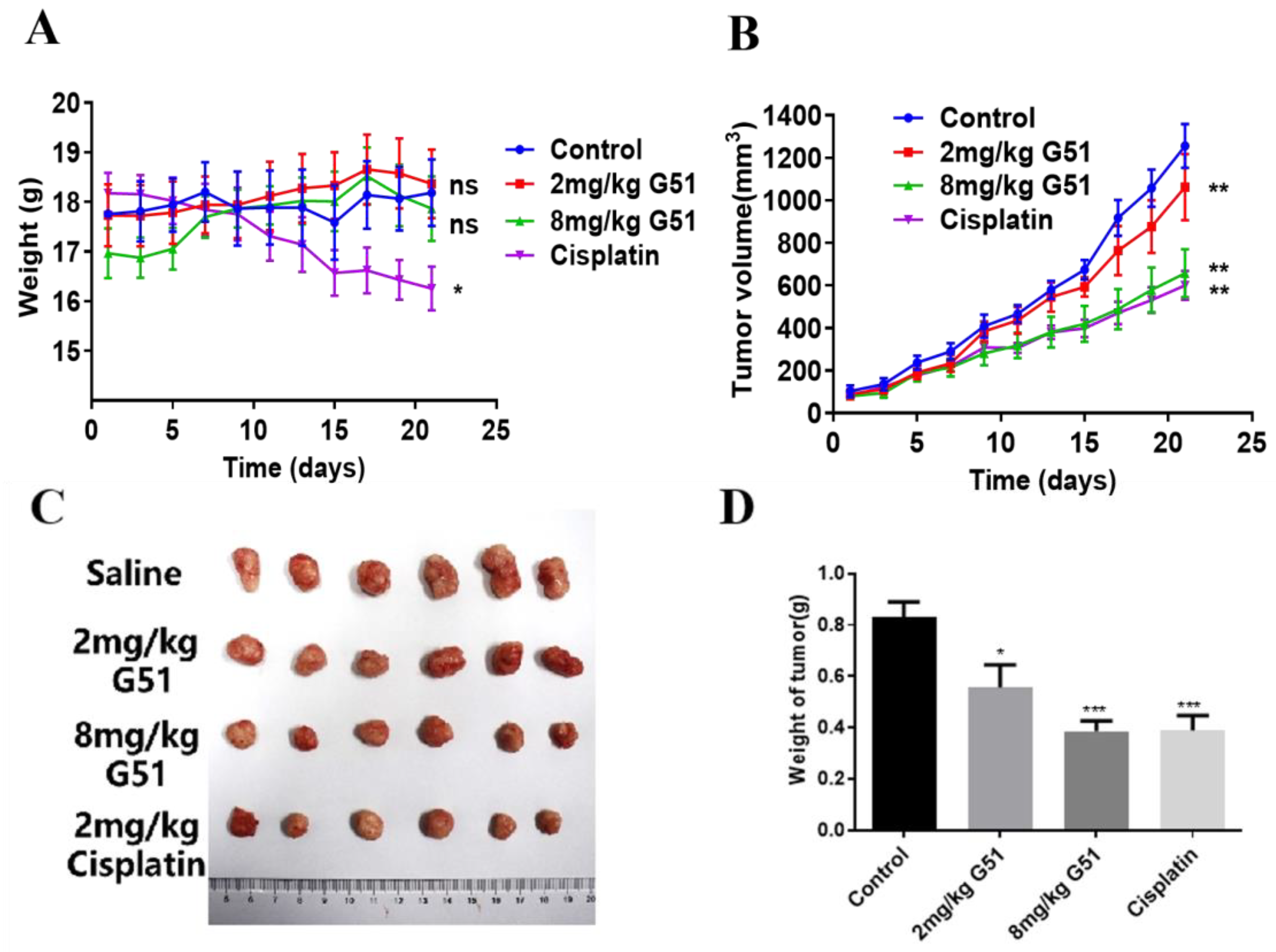
Based on the above results, we further evaluated the anti-tumor activity of
G51 in vivo by using an HCT116 xenograft model in nude mice. Tumor establishment was achieved through serial transplantation. The primary tumors grown from 8 × 10
6 cells were dissected into fragments and re-implanted subcutaneously using a trocar needle. When the tumor volume reached 50–100 mm
3, the nude mice were randomly divided into four groups (n = 6/group), including a control group (saline), a low-dose group (2 mg/kg
G51), a high-dose group (8 mg/kg
G51), and a positive control group (2 mg/kg cisplatin, a platinum-based chemotherapy drug routinely used as a positive control in xenograft models). The drugs were administered intraperitoneally every 48 h for 21 days. Nude mice’s body weight and tumor volume were recorded prior to each administration, as shown in
Figure 6A,B. The tumors of the control group continued to grow, while the tumors of the
G51 and cisplatin groups grew at a significantly slower rate. As shown in
Figure 6C,D, the tumor growth inhibition ratio (TGI) was calculated using the equation (1—tumor weight in drug-treated group/tumor weight in saline group) to evaluate the inhibitory effect of the drug. The TGI values were determined to be 31% for the low-dose
G51 group, 54% for the high-dose
G51 group, and 56% for the cisplatin group, which indicates that
G51 could inhibit tumor growth in a dose-dependent manner. TGI analysis revealed comparable efficacy between high-dose
G51 (54%) and cisplatin (56%), demonstrating the potent in vivo anti-tumor activity of
G51. Importantly, no significant body weight changes were observed in
G51 treatment groups in comparison to the cisplatin group, which had obvious body weight loss (
Figure 6A), suggesting no significant side effect for
G51 treatment at therapeutic doses.
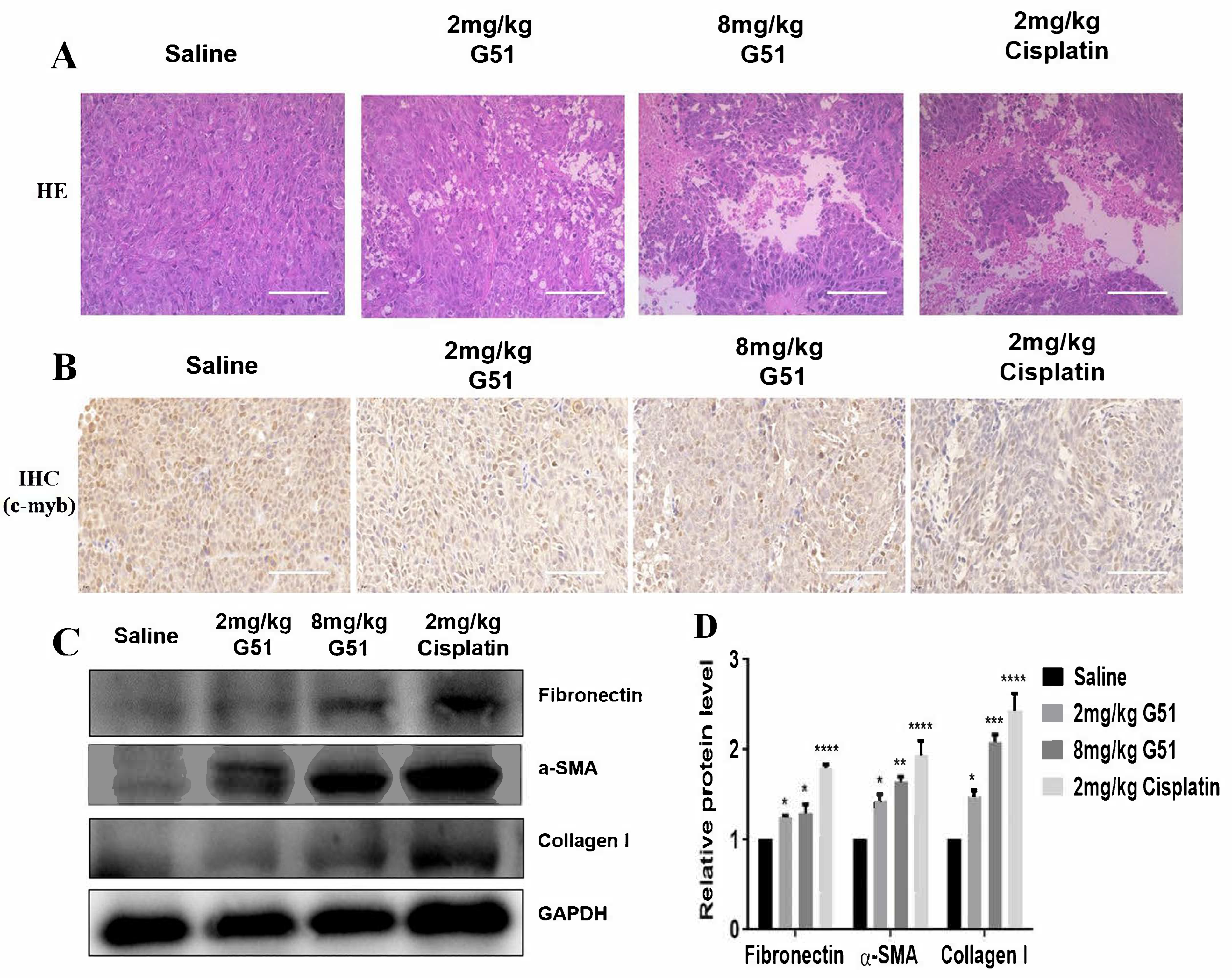
To further understand the anti-tumor mechanism of compound
G51, we performed hematoxylin–eosin (HE) staining and immunohistochemical (IHC) analysis of tumor tissues. As shown in
Figure 7A, HE staining revealed distinct morphological differences between the saline control group and treatment groups. The cells in the saline group exhibited tightly packed and uniform cells with intact morphology, while the
G51 treatment groups showed a dose-dependent increase in necrotic areas, characterized by cellular shrinkage, nuclear fragmentation, and loss of membrane integrity. These pathological changes were comparable to those observed in the cisplatin group, which indicates that
G51 could induce necrosis of tumor tissues with a similar effect to that of cisplatin. Meanwhile, IHC analysis, as shown in
Figure 7B, demonstrated that the expressions of C-MYB protein in the tumor cells, as indicated with brown dots for both
G51 treatment groups and the cisplatin group, were significantly decreased in comparison with the saline control group, confirming that
G51 was also able to down-regulate
c-myb in in vivo models, consistent with our in vitro Western blot experimental data.
In addition to its anti-tumor activity, we evaluated the toxicity of
G51 for safety reasons. As shown in
Figure 6A, the body weights of mice in the
G51 treatment groups remained stable throughout the experiment, while mice in the cisplatin group exhibited apparent weight loss, indicating that long-term cisplatin administration induced systemic side effects. To assess drug safety, we also performed HE staining to examine cellular morphology in major organs (heart, liver, spleen, lung, and kidney) of nude mice. As shown in
Figure S17B, no significant pathological lesions were observed in the
G51 treatment groups and the cisplatin group in comparison to the saline control group. Since hepatic fibrosis is a critical marker of liver injury and its irreversibility can lead to cirrhosis, we further evaluated the hepatic toxicity of
G51 by analyzing the expression of fibrosis markers, including Fibronectin, α-SMA, and Collagen I [
40]. As shown in
Figure 7C,D, both
G51 treatment groups and the cisplatin group exhibited elevated expressions of Fibronectin, α-SMA, and Collagen I in comparison to the saline control group. However, their increases in the
G51 treatment groups were much lower than those in the cisplatin group, indicating that
G51 did not induce significant liver toxicity. In addition, as shown in
Figure S17A, organ weight analysis showed reduced organ weights in the cisplatin group, while organ weights in the
G51 treatment groups had no significant changes, further supporting its favorable safety profile. All the above results demonstrate that
G51 exhibits lower toxicity than cisplatin, highlighting its potential advantage for further development for clinical application.
In summary, these results demonstrate that G51 exhibits potent anti-tumor activity in human colorectal cancer xenograft models at experimental doses, without apparent toxicity to major organs, highlighting its potential as a lead compound for further development of anti-cancer agents. Our findings reveal that compound G51, as a dual-targeting ligand, derives its anti-tumor effects through specific i-motif destabilization coupled with G-quadruplex stabilization. This discovery sets up a novel foundation for developing anti-cancer drugs through coordinated regulation of gene quadruplex structures.
3. Discussion
In this study, we identified and validated carbazole derivative G51 as a dual-targeting ligand simultaneously binding to both i-motif and G-quadruplex on the c-myb promoter region. G51 could significantly down-regulate c-myb transcription and translation through opposing effects on different quadruplex structures, with potent anti-tumor activity. In comparison, carbazole derivative G50, which only targets the G-quadruplex, showed very weak anti-tumor activity, highlighting the therapeutic advantage of dual-targeting ligands. Our comprehensive in vitro and in vivo experiments demonstrated that G51 could effectively inhibit tumor cell proliferation, migration, and invasion. G51 could also induce cancer cell apoptosis, according to our cell cycle analysis. Importantly, G51 exhibited potent anti-tumor activity in our HCT116 xenograft model, without significant side effects in comparison to the conventional chemotherapeutic agent cisplatin. This study not only expands the therapeutic potential of carbazole derivatives but also provides a novel example for developing anti-cancer drugs through coordinated opposing effects on different quadruplex structures for the specific regulation of gene expression.
Our present study demonstrated that G51 could become a promising lead compound for further development for colorectal cancer treatment. While this study provides promising results, some aspects could be further investigated in the future. The precise binding modes or interactions between G51 and quadruplex structures could be illustrated through structural studies of the binding complex using X-ray crystallography or cryo-electron microscopy for guiding rational ligand optimization. Due to the weak fluorescence activity of G51, direct visualization of its intracellular target engagement remains challenging. Its structural modifications through fluorophore conjugation or molecular optimization could enable real-time visualization of intracellular interactions to validate target specificity and mechanisms. The potential crosstalk between i-motif destabilization and G-quadruplex stabilization could also be studied for clarification. Systematic mutagenesis, combined with single-molecule imaging approaches, could determine whether these effects operate independently or synergistically. The comprehensive evaluation of the pharmacokinetic properties of G51, including its absorption, distribution, metabolism, and excretion (ADME), along with a detailed toxicological assessment in multiple animal models, could also be performed for future drug development.
In the present study, our primary focus was to investigate the effect of G51 on regulating c-myb transcription and translation through opposing effects on different quadruplex structures on the gene promoter for cancer treatment. The study of the effect of G51 could be further expanded to some other potential targets. Since G51 could stabilize c-myb promoter G-quadruplex, it would be interesting to further study its effect on telomeric G-quadruplex, which could possibly induce DNA damage, especially for telomeres. This aspect could be investigated by performing immunofluorescence combined with fluorescence in situ hybridization (IF/IF-FISH) analysis to assess the induction of DNA damage using gH2AX as a DNA damage marker for foci formation and its localization at telomeres. G51 could be compared to G50 for their DNA damage effects to investigate if the specificity of G51 for c-myb alters the capability of the compound to induce telomeric DNA damage.
In addition, direct evidence confirming i-motif interaction within a cellular context could be studied in the future. For instance, chromatin immunoprecipitation with quadruplex-specific antibodies or structure-specific chemical foot-printing in cells could further substantiate the proposed mechanism. However, the lack of reliable sources for i-motif-specific antibodies and the technical challenges of probing transient non-canonical structures in intact cells currently prevent these experiments from being carried out by our group. In addition, the selectivity of G51 for the c-myb promoter over other genomic quadruplex-forming sequences could also be investigated in the future. Although quadruplex structures are prevalent in multiple oncogene promoters, the observed low in vivo toxicity suggests high specificity with tolerable off-target effects.
In summary, this study demonstrates that the carbazole derivative G51 significantly inhibits c-myb expression and exerts potent anti-tumor activity by simultaneously dismantling the i-motif and stabilizing the G-quadruplex in the c-myb promoter region. G51 represents a promising anti-tumor lead compound capable of bidirectionally modulating the stability of i-motif and G-quadruplex structures in the c-myb promoter. This bidirectional modulation capability is potentially significant because it could overcome the compensatory effects usually associated with single-target approaches, where the inhibition of one target structure could lead to up-regulation or compensation through the other. This study also provides a novel example for coordinated gene regulation through multiple non-canonical DNA structures, which reveals that the c-myb promoter i-motif and G-quadruplex can be co-targeted for synergistic effects. This discovery not only provides novel insights for developing anti-tumor drugs targeting nucleic acid secondary structures but also offers promising new strategies for cancer therapy through coordinated gene promoter quadruplex modulation. While further optimization and investigation are required, this work establishes an important theoretical and experimental foundation for developing dual-targeting ligands as potent anti-tumor lead compounds with low toxicity and few side effects.
4. Materials and Methods
4.1. Oligonucleotides and Compounds
The nucleotide oligomers used in this study were purchased from Sangong (Shanghai, China). Prior to use, the oligomers were centrifuged at 6000×
g rpm for 2 min, dissolved in ultra-pure water to a concentration of 100 μM with a vortex, and then stored at −20 °C. For the experiments, the oligomers were diluted with a buffer to the required concentrations. All oligomer sequences are listed as shown in
Table S1. The DNA oligonucleotides were subjected to thermal annealing (95 °C, 10 min) followed by a controlled cooling ramp (1 °C/min) to facilitate the formation of secondary structures, which were then stored at 4 °C for later usage. Their relevant working solutions were prepared through dilution with appropriate buffers, which are specified in
Table 1.
The compounds used for screening have been previously synthesized in our laboratory, including bisacridine derivatives, acridone derivatives, acridone–naphthimide derivatives, carbazole derivatives, and quinoline derivatives, with their structures shown in
Table S2. The compounds were dissolved in DMSO as a 10 mM reservoir solution and stored at −20 °C in a freezer.
4.2. Cell Culture
All types of cells were originally purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China) and were cultured in our laboratory using an ESCO constant-temperature incubator at 37 °C with 5% CO2. These included cervical adenocarcinoma cells (HeLa), non-small cell lung cancer cells (A549), gastric cancer cells (HGC-27), and human colon cancer cells (HCT-116, SW620, SW480, RKO, and DLD-1). The cell culture consisted of an RPMI-1640 (or DMEM or MEM) basic medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Specifically, HCT-116 and HGC-27 cells were cultured in an RPMI-1640 medium with 10% FBS, while Siha, A549, SW480, HepG2, and U2OS cells were cultured in a DMEM medium containing 10% FBS. HeLa cells were cultured in an MEM basic medium supplemented with 10% FBS.
4.3. Circular Dichroism (CD) Spectroscopy and CD Melting Experiment
The oligomers for i-motif and G-quadruplex (G4) were diluted to 2 μM with a BPES buffer (for i-motif) and Tris-HCl buffer at pH 7.4 (for G4), respectively, and stored at 4 °C after annealing. CD spectra from 230 to 350 nm were recorded on a Chirascan® circular dichroism spectrophotometer (Applied Photophysics, Leatherhead, UK) with a 10 mm path length quartz cuvette. The buffer was scanned at first, as a blank, followed by the scanning of the i-motif or G4-containing solution. After the addition of the compounds, the mixture was incubated with a vortex for 2–3 min, followed by scanning under the same conditions. For CD melting experiments, scanning was performed at a temperature range of 25–95 °C, with an increasing rate of 1 °C/min, and recorded at 5 °C intervals. The CD signals at 288 nm for i-motif and 265 nm for G4 were plotted against temperature using GraphPad Prism 9.0, and Tm values were determined. All spectra were blank-subtracted using corresponding buffer controls.
4.4. Surface Plasmon Resonance (SPR) Experiment
All buffers and regeneration reagents were filtered through a 0.22 μm membrane. Molecular interactions were characterized using a Biacore X100 system (Cytiva, Waltham, MA, USA) controlled by Biacore-8K Software v5.0.18. Streptavidin (1 mg/mL in sodium acetate, pH 5.0) was covalently immobilized on CM5 sensor chips. A biotin-labeled i-motif or G4 oligomer, with a sequence as shown in
Table S1, was diluted to 1 nM with MES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4, annealed, and stored at 4 °C overnight. The annealed oligomer with a certain secondary structure was then immobilized on the CM5 chip. The compound was serially diluted with the corresponding buffer to generate a concentration gradient, with the DMSO content fixed at 5%. The following instrument parameters were used, including a flow rate of 30 μL/min, binding time of 60 s, and dissociation time of 100 s. Regeneration was performed using 1.5 M glycine and 1 M KCl. The binding constant (K
D) was determined by fitting the data with Biacore Insight Evaluation 5.0.18 software.
4.5. Microscale Thermophoresis (MST) Experiment
A 5′-FAM-labeled DNA oligomer was diluted to 2 μM with MES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4, annealed to form a stable i-motif or G-quadruplex structure, and serially diluted (0.25–2 μM) using a two-fold dilution method. The following instrument settings were used: 40% LED blue intensity and 40% MST power. The compound was two-fold diluted to 16 concentrations starting from 2000 μM, mixed at a 1:1 volume ratio with DNA (5 μL each), and incubated for 10 min before MST analysis. The binding constant (KD) was derived using NTAnalysis 1.5.41 software.
4.6. TO Displacement Experiment
The i-motif/G4-forming oligonucleotides in 2 μM BPES at pH 5.5 and Tris-HCl at pH 7.4, respectively, were annealed and stored at 4 °C. Samples were mixed and stained with thiazole orange (TO) at a final concentration of 2 μM for 30 min without light. Fluorescence was measured (λex = 480 nm, λem = 500–700 nm) after the incremental addition of 1 equivalent compound per step until signal plateau. The displacement ratio was calculated based on the response at 530 nm using Origin and GraphPad 9.0.
4.7. Fluorescence Resonance Energy Transfer (FRET) Experiment
A 5′-FAM/3′-TAMRA-labeled oligomer for i-motif or G4 (0.1 μM in BPES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4) was annealed. Fluorescence profiles with excitation at 480 nm and an emission scan in the range of 500–700 nm were measured after the addition of the compound at 1–15 eq. FRET efficiency was determined with the ratio of 518 nm (FAM) to 585 nm (TAMRA) fluorescence data. For the FRET melting experiment, the temperature was ramped up from 25 to 95 °C at a rate of 1 °C/min, while fluorescence was measured at 1 °C intervals, with TM and ΔTM derived using GraphPad.
4.8. Electrophoretic Mobility Shift Assay (EMSA) Experiment
A c-myb promoter oligomer for i-motif or G4 was prepared at 5 μM with BPES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4, respectively, and then annealed. The oligomer was incubated with different equivalents of the compound for 24 h. Electrophoresis was carried out for 3–4 h on ice with a 10% non-denaturing gel at a voltage of 80 V and at pH 5.5 or 7.4. The gel was silver-stained and photographed after electrophoresis.
4.9. Ultraviolet–Visible (UV) Spectroscopy
A c-myb promoter oligomer for i-motif or G4 was prepared at 1000 μM with BPES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4, respectively, and then annealed. The G51 solution was prepared at a 1 μM concentration for subsequent UV scanning in the range of 350–550 nm. The c-myb oligomer for i-motif or G4 was added dropwise so that its final concentration was increased in increments of 5 μM. The solution was incubated for 2 min after each addition and then analyzed with UV spectroscopy.
4.10. ESI-MS
A c-myb promoter oligomer for i-motif or G4 was prepared at 5 μM with BPES buffer at pH 5.5 or Tris-HCl buffer at pH 7.4, respectively, and then annealed. The oligomer was mixed with 10 eq G51, and the mixture was incubated for 24 h, which was then analyzed using an LCQ DECA PLUS XP mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
4.11. Dual-Luciferase Reporter Assay
For the dual-luciferase reporter assay, HCT116 cells were seeded in 96-well plates at a density of 5 × 103 cells per well (triplicate wells) and allowed to adhere overnight. Cells were transfected for 4–5 h with 100 ng each of pGL-WT c-myb (or pGL-Del c-myb or pGL-Mut c-myb) plasmid, along with the pRL-TK control plasmid, using the Lipo8000™ (Beyotime, Shanghai, China) transfection reagent. Following transfection, the cells were treated with the serially diluted compound G51 or G50 in an RPMI-1640 medium for 48 h. The cells were lysed, and R-Luc and F-Luc reaction solutions were added. Luciferase activity was detected, and the data were analyzed using Graphpad 9.0.
4.12. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
HCT116 cells were seeded in 6-well plates at a density of 1.6 × 105 cells per well and allowed to adhere overnight. The cells were treated with a serially diluted compound in an RPMI-1640 medium for 48 h. Total RNA was extracted from each well using 1 mL AG RNAex Pro Reagent (Acres Biological, Changsha, China, #AG21101). Reverse transcription was performed using Evo M-MLV (Acres Bio, Changsha, China, #AG11706), followed by qPCR using the SYBR Green Pro Taq HS Premix (GenStar, Guangzhou, China). Each experiment was performed in triplicate. Data analysis and visualization were conducted using GraphPad Prism 9.0.
4.13. Western Blot
HCT116 cells were seeded in 6-well plates at a density of 1.6 × 105 cells per well and allowed to adhere overnight. The cells were treated with a serially diluted compound in an RPMI-1640 medium for 48 h. Following treatment, the cells were lysed with RIPA (Bioteke, Wuxi, China) buffer on ice for 30 min. Protein quantification was performed using a BCA assay (Thermo Fisher, Waltham, MA, USA). Aliquots (30 μg/lane) were resolved by using SDS-PAGE and transferred to PVDF membranes (0.22 μm). After blocking, membranes were probed with primary antibody (1:1000, 4 °C, overnight) and HRP-conjugated secondary antibody (1:3000, RT, 50 min). Chemiluminescent signals were captured using a Tanon-4200SF imaging system (Tanon Science & Technology Co., Ltd., Shanghai, China).
4.14. Immunofluorescence
HCT116 cells (5 × 103/well) were cultured overnight in 96-well plates before treatment with serially diluted G51 in RPMI-1640 for 48 h. Fixed cells (4% PFA) were permeabilized (0.5% Triton X-100), blocked with 5% BSA, and immunostained with anti-c-myb (4 °C, overnight), followed by Alexa Fluor 488-conjugated secondary antibody (#A21206, Life Technologies, Carlsbad, CA, USA) at 37 °C for 30 min. Nuclei were counter-stained with DAPI (Invitrogen, Carlsbad, CA, USA) prior to imaging (Olympus FV3000 confocal microscope, Tokyo, Japan).
4.15. Cytotoxic Assay
Cell suspensions (HCT116 and other types of cells) were plated in 96-well microplates (5 × 103 cells/well) and cultured for 24 h to ensure attachment. Test compounds were prepared through logarithmic dilutions (20−0.3125 μM final concentration) in a medium and applied to cells for 48 h exposure. The activity was determined by using an MTT assay through 4 h incubation with 0.5 mg/mL methylthiazolyldiphenyl-tetrazolium bromide at 37 °C. Following the solubilization of formazan products in DMSO, optical density measurements were recorded at 570 nm using a Bio-Tek microplate reader (Winooski, VT, USA). Triplicate determinations were performed for each condition, with dose–response curves generated using nonlinear regression in GraphPad Prism (v9.0).
4.16. Colony Formation Assay
For the long-term proliferation assessment, cells were seeded at a low density (1 × 103 cells/well) in 6-well plates and exposed to G51/G50 (0.1−0.025 μM) for 7 days with medium replacement every 48 h. After methanol fixation and 0.1% crystal violet staining, colonies (>50 cells) were enumerated using automated image analysis.
4.17. FITC Annexin V/PI Cell Apoptosis Analysis
Using 6-well plates (1 × 105 cells/well), HCT116 cultures were treated with G51/G50 (1.25−0.3 μM) for 24 h. Dual staining with Annexin V-FITC and propidium iodide using an Apoptosis Kit (#AP101-100-kit, Multi Sciences, Hangzhou, China) was performed according to the manufacturer’s specifications. Quantitative analysis of apoptotic populations was conducted on a CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA, USA), with compensation controls using single-stained samples.
4.18. Cell Cycle Experiments
Following 24 h exposure to test compounds (0.6−0.15 μM), ethanol-fixed HCT116 cells were treated with RNase A (100 μg/mL) and propidium iodide (50 μg/mL) for 30 min at 37 °C. The CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA, USA) was used to analyze cell cycle distribution.
4.19. Cell Scrape Assay
HCT116 cells were seeded in 6-well plates at a density of 1 × 106 cells per well and allowed to adhere overnight. A uniform scratch wound was created using a 10 μL pipette tip, followed by replacement with a serum-free RPMI-1640 medium. The cells were treated with the serially diluted compound G51 at a concentration range of 2.5–0.6 μM in a serum-free medium for 48 h. Wound closure was monitored and photographed after 0, 24, and 48 h using phase-contrast microscopy.
4.20. Transwell Assay
Matrigel matrix was diluted 1:8 with a serum-free RPMI-1640 medium and coated onto the upper surface of transwell chambers. HCT116 cells (8 × 104 cells per chamber) were seeded in the coated chambers. The cells were treated with the serially diluted compound G51 at a concentration range of 2.5–0.6 μM in a serum-free medium for 24 h. Following incubation, the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Invaded cells were photographed using an EVOS FL Auto imaging system (Thermo Fisher Scientific, Waltham, MA, USA).
4.21. Evaluation of In Vivo Anti-Tumor Activity
SPF-grade male BALB/c-nu/nu nude mice (3–4 weeks old) were obtained from the Laboratory Animal Center of Sun Yat-Sen University. The mice were housed under controlled conditions (22 ± 1 °C, 60–70% humidity, and 12 h light/dark cycle). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (Approval No. SYSU-IACUC-2023-001980; Approval date: 19 September 2023) and complied with national guidelines for laboratory animal welfare.
For tumor xenografts, 200 μL of an HCT116 cell–Matrigel mixture (8 × 106 cells/mouse) was subcutaneously inoculated into the right shoulder using a sterile 1 mL syringe. Tumor volume was monitored, with data recorded every other day. When tumors reached 50–100 mm3 (approximately 8 days post-implantation), the mice were randomly allocated into four groups, including a control group (saline), a low-dose group (2 mg/kg G51), a high-dose group (8 mg/kg G51), and a positive control group (2 mg/kg cisplatin). Treatments were administered via intraperitoneal injection every other day, with body weights and tumor measurements recorded prior to each administration.
At the experimental endpoint (control tumor volume = 1400 mm3), the mice were euthanized, and their organs (including hearts, livers, spleens, lungs, and kidneys) and tumors were collected. Tissues were either bisected, followed by storage at −80 °C, or fixed in 4% paraformaldehyde. Tumor inhibition ratios were calculated as follows: IR = [1 − (mean experimental tumor weight/mean control tumor weight)] × 100%. Fixed tissues were processed for IHC and H&E staining by Servicebio company (Wuhan, China), with imaging obtained using a digital pathology system. Western blotting was performed to analyze Fibronectin, α-SMA, and Collagen I expressions in liver tissues.
4.22. Statistical Analysis
Data are presented as mean ± SEM. Statistical comparisons between groups were performed using either one-way ANOVA or unpaired Student’s t-tests in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). A p-value of ≤0.05 (*) was considered statistically significant.