Recombinant Production of a TRAF-Domain Lectin from Cauliflower: A Soluble Expression Strategy for Functional Protein Recovery in E. coli
Abstract
1. Introduction
2. Results
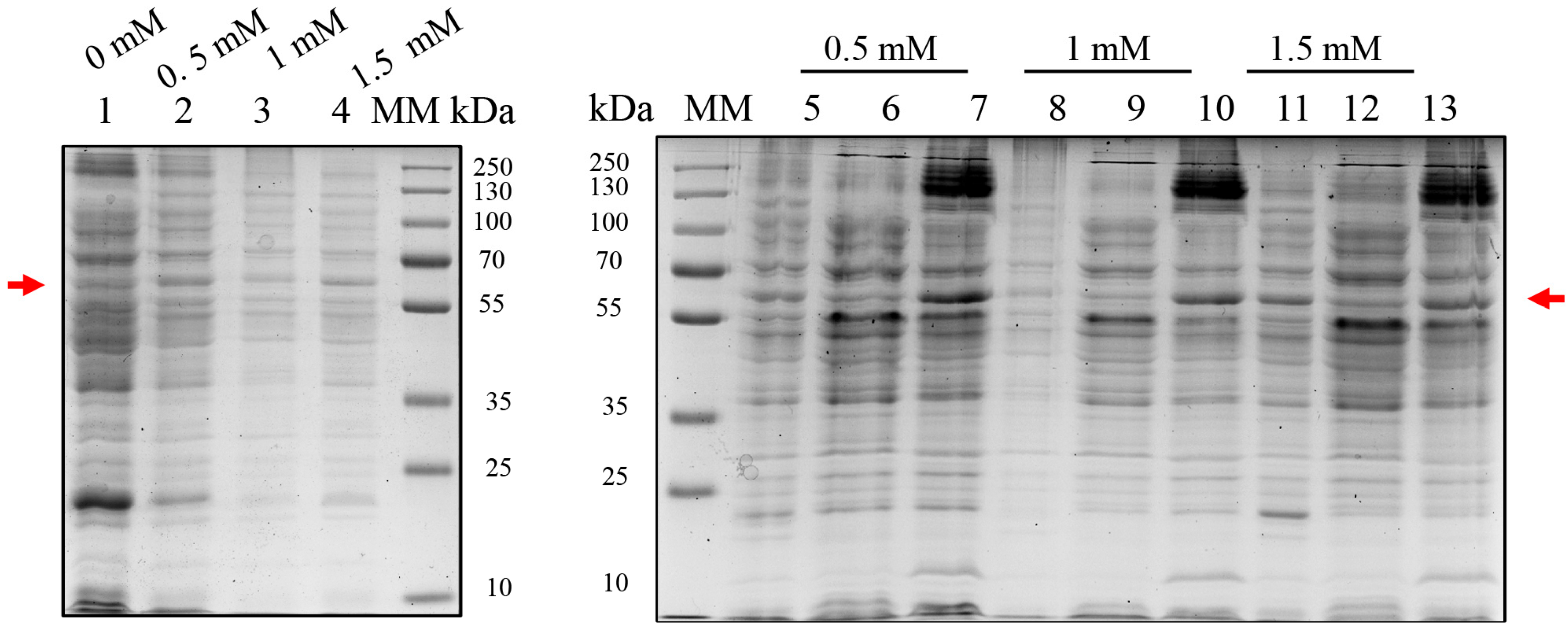
2.1. Evaluation of BOL Expression in E. coli BL21(DE3) and C41(DE3) Using pET28a
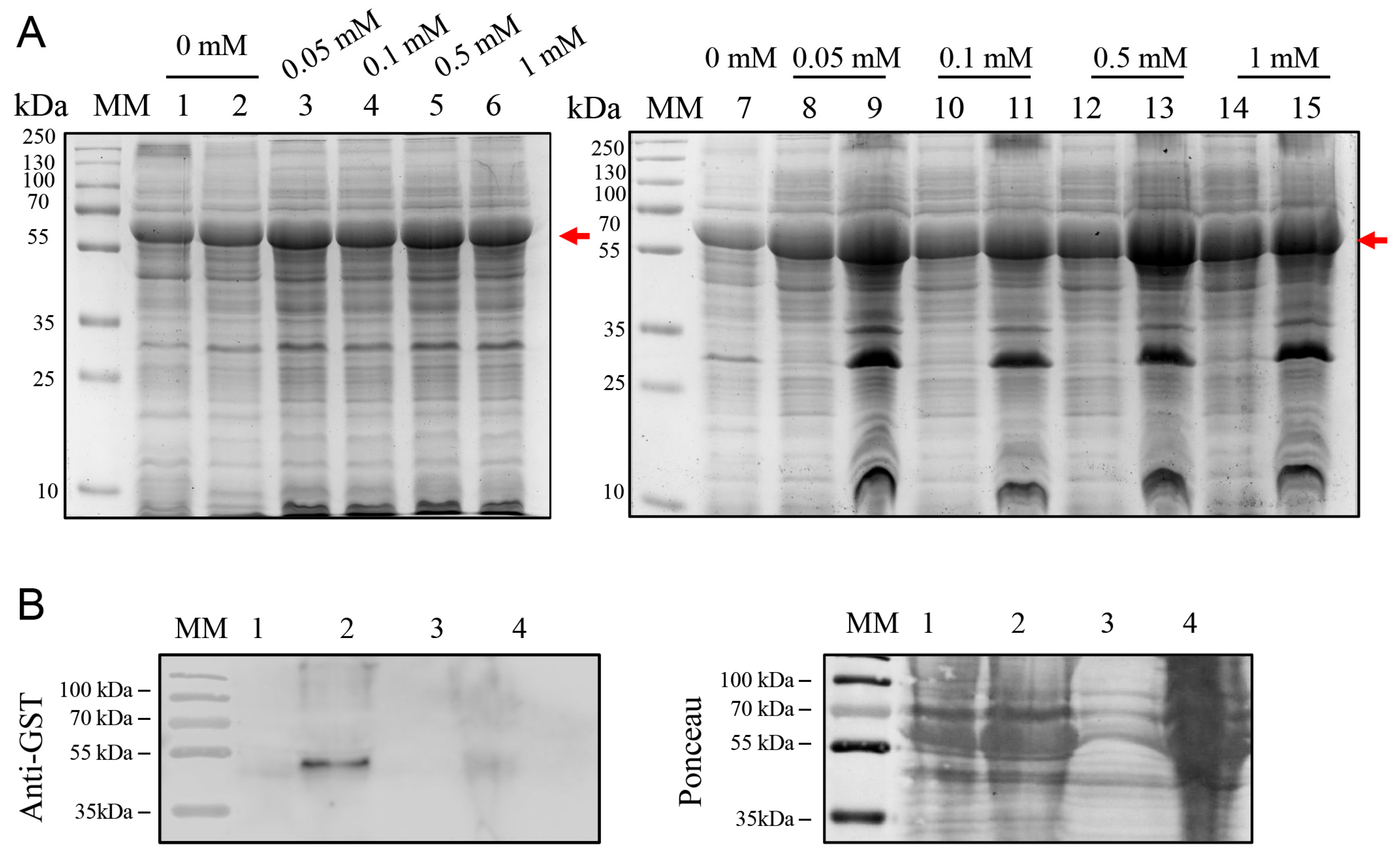
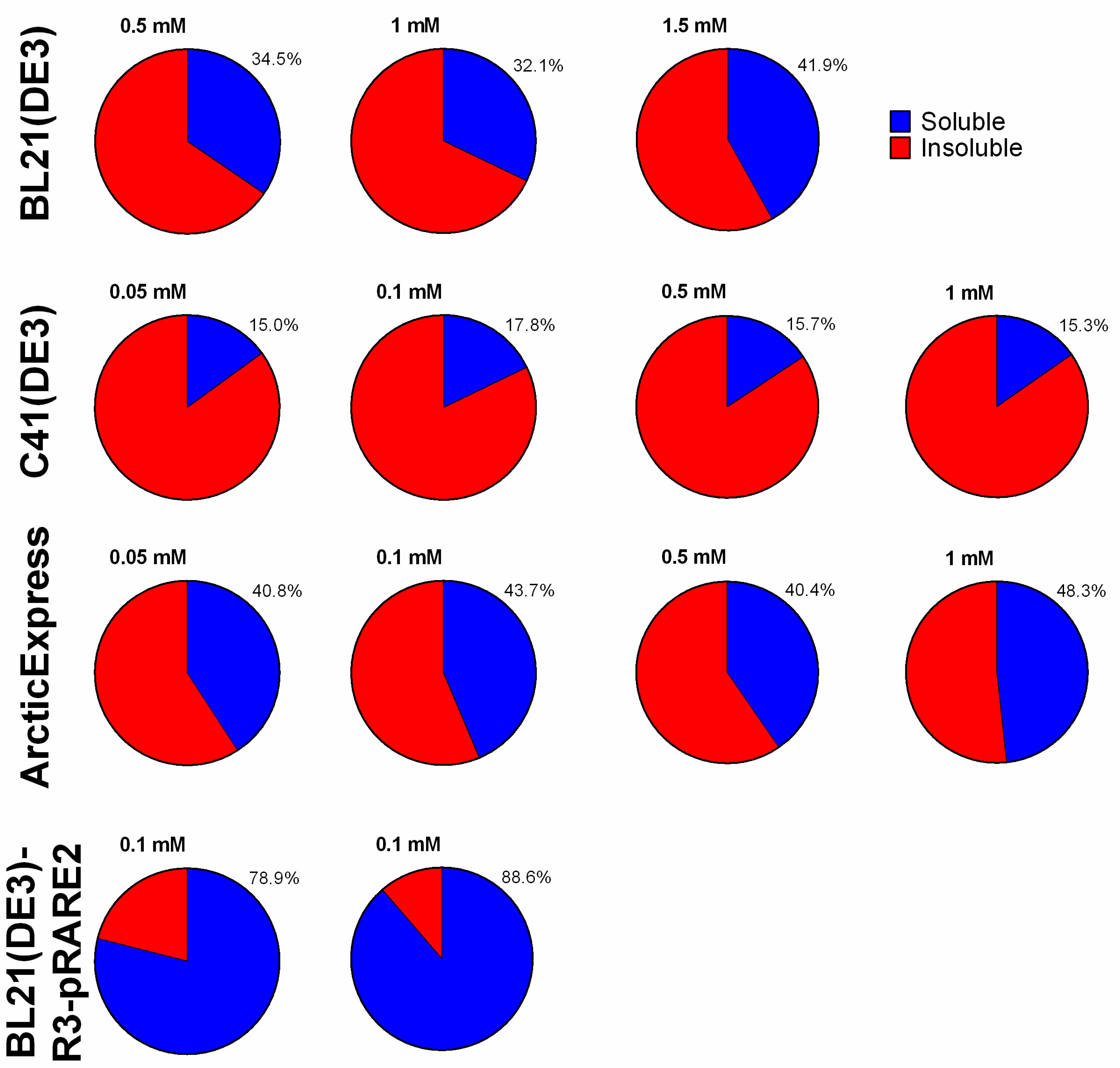
2.2. Expression of BOL-GST in E. coli BL21(DE3)
2.3. Expression of BOL-GST in E. coli C41(DE3)
2.4. Evaluation of ArcticExpress(DE3) for Low-Temperature Expression
2.5. High-Yield Expression of BOL-GST in E. coli BL21-R3-pRARE2
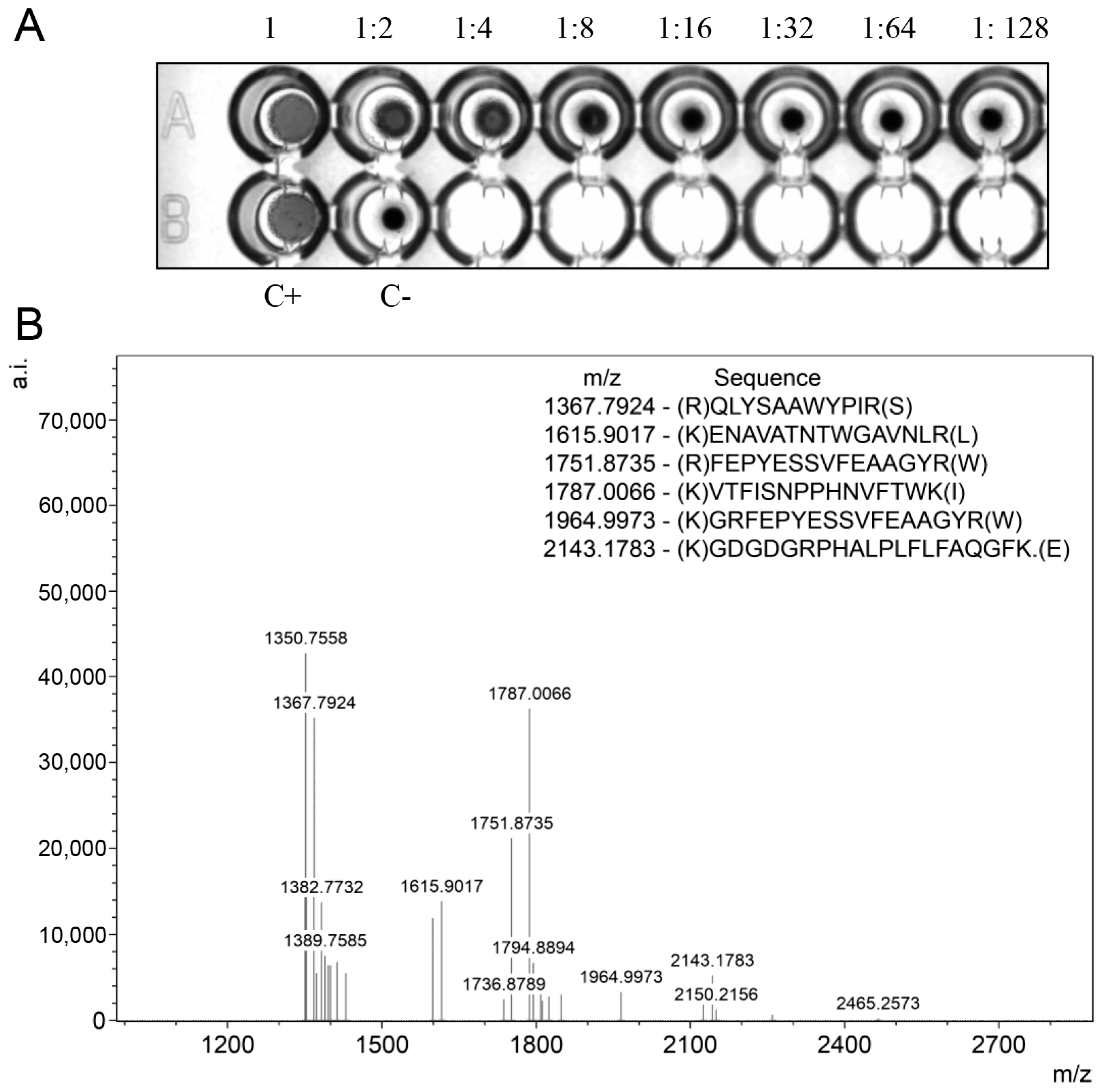
2.6. Functional Activity and Identity Confirmation of Recombinant BOL-GST
3. Discussion
4. Materials and Methods
4.1. Plasmid Synthesis and Propagation
4.2. Transformation of Escherichia coli by Heat Shock
4.3. Recombinant Expression of BOL Lectin
4.3.1. Expression Using pET28a Vector
4.3.2. Expression Using pGEX-4T-1 Vector
4.4. Sample Collection and Analysis
4.5. SDS-PAGE and Densitometry Analysis
4.6. Western Blot
4.7. Purification of the BOL-GST Fusion Protein
4.8. Mass Spectrometry and Protein Identification
4.9. Hemagglutination Activity Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osterne, V.J.S.; Nascimento, K.S.; Cavada, B.S.; Van Damme, E.J.M. The Future of Plant Lectinology: Advanced Technologies and Computational Tools. BBA Adv. 2025, 7, 100145. [Google Scholar] [CrossRef]
- Kobayashi, M.; Utsushi, H.; Fujisaki, K.; Takeda, T.; Yamashita, T.; Terauchi, R. A Jacalin-like Lectin Domain-Containing Protein of Sclerospora graminicola Acts as an Apoplastic Virulence Effector in Plant–Oomycete Interactions. Mol. Plant Pathol. 2022, 23, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dugan, A.E.; Peiffer, A.L.; Kiessling, L.L. Advances in Glycoscience to Understand Viral Infection and Colonization. Nat. Methods 2022, 19, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Amera, G.M.; Muthukumaran, J.; Singh, A.K. Insights into Biological Role of Plant Defense Proteins: A Review. Biocatal. Agric. Biotechnol. 2022, 40, 102293. [Google Scholar] [CrossRef]
- Cohen, L.J.; Han, S.M.; Lau, P.; Guisado, D.; Liang, Y.; Nakashige, T.G.; Ali, T.; Chiang, D.; Rahman, A.; Brady, S.F. Unraveling Function and Diversity of Bacterial Lectins in the Human Microbiome. Nat. Commun. 2022, 13, 3101. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 Years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Podder, M.K.; Hossain, M.M.; Kabir, S.R.; Asaduzzaman, A.K.M.; Hasan, I. Antimicrobial, Antioxidant and Antiproliferative Activities of a Galactose-Binding Seed Lectin from Manilkara zapota. Heliyon 2024, 10, e24592. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Blanco-Labra, A.; García-Gasca, T. Expression of Lectins in Heterologous Systems. Int. J. Mol. Sci. 2018, 19, 616. [Google Scholar] [CrossRef]
- Duarte, C.E.M.; Abranches, M.V.; Silva, P.F.; de Paula, S.O.; Cardoso, S.A.; Oliveira, L.L. A New TRAF-Like Protein from Brassica oleracea ssp. botrytis with Lectin Activity and Its Effect on Macrophages. Int. J. Biol. Macromol. 2017, 94, 508–514. [Google Scholar] [CrossRef]
- Duarte, C.E.M.; Alamillo, J.M.; Koehler, A.D.; Pineda, M.; Otoni, W.C.; de Oliveira, L.L. Molecular and Biochemical Analyses of a Novel Lectin with MATH Domains from Brassica oleracea. Acta Physiol. Plant. 2020, 42, 60. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Azizi-Dargahlou, S. Factors Involved in Heterologous Expression of Proteins in E. coli Host. Arch. Microbiol. 2023, 205, 5. [Google Scholar] [CrossRef]
- Sandomenico, A.; Sivaccumar, J.P.; Ruvo, M. Evolution of Escherichia coli Expression System in Producing Antibody Recombinant Fragments. Int. J. Mol. Sci. 2020, 21, 6324. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Nong, F.T.; Wang, Y.Z.; Yan, C.X.; Gu, Y.; Song, P.; Sun, X.M. Strategies for Efficient Production of Recombinant Proteins in Escherichia coli: Alleviating the Host Burden and Enhancing Protein Activity. Microb. Cell Fact. 2022, 21, 191. [Google Scholar] [CrossRef]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.Z.; Zhou, T. Challenges Associated with the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 630551. [Google Scholar] [CrossRef]
- Sørensen, H.P.; Mortensen, K.K. Advanced Genetic Strategies for Recombinant Protein Expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Farahani, N.; Golichenari, B.; Sahebkar, A. Recombinant Protein Expression in Escherichia coli (E. coli): What We Need to Know. Curr. Pharm. Des. 2018, 24, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Atroshenko, D.L.; Sergeev, E.P.; Golovina, D.I.; Pometun, A.A. Additivities for Soluble Recombinant Protein Expression in Cytoplasm of Escherichia coli. Fermentation 2024, 10, 120. [Google Scholar] [CrossRef]
- Köppl, C.; Lingg, N.; Fischer, A.; Kröß, C.; Loibl, J.; Buchinger, W.; Schneider, R.; Jungbauer, A.; Striedner, G.; Cserjan-Puschmann, M.; et al. Fusion Tag Design Influences Soluble Recombinant Protein Production in Escherichia coli. Int. J. Mol. Sci. 2022, 23, 7678. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.; Speicher, D.W. Purification of Proteins Fused to Glutathione S-Transferase. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2011; Volume 681, pp. 259–280. [Google Scholar]
- Shendge, A.A.; D’Souza, J.S. Strategic Optimization of Conditions for the Solubilization of GST-Tagged Amphipathic Helix-Containing Ciliary Proteins Overexpressed as Inclusion Bodies in E. coli. Microb. Cell Fact. 2022, 21, 258. [Google Scholar] [CrossRef]
- Zapata, J.M.; Martínez-García, V.; Lefebvre, S. Phylogeny of the TRAF/MATH Domain. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 597, pp. 1–24. [Google Scholar]
- Qi, H.; Xia, F.N.; Xiao, S.; Li, J. TRAF Proteins as Key Regulators of Plant Development and Stress Responses. J. Integr. Plant Biol. 2022, 64, 431–448. [Google Scholar] [CrossRef]
- Miroux, B.; Walker, J.E. Over-Production of Proteins in Escherichia coli: Mutant Hosts that Allow Synthesis of Some Membrane Proteins and Globular Proteins at High Levels. J. Mol. Biol. 1996, 260, 289–298. [Google Scholar] [CrossRef]
- Carmignotto, G.P.; Azzoni, A.R. On the Expression of Recombinant Cas9 Protein in E. coli BL21(DE3) and BL21(DE3) Rosetta Strains. J. Biotechnol. 2019, 306, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Saribas, A.S.; Gruenke, L.; Waskell, L. Overexpression and Purification of the Membrane-Bound Cytochrome P450 2B4. Protein Expr. Purif. 2001, 21, 303–309. [Google Scholar] [CrossRef]
- Huang, C.J.; Peng, H.L.; Patel, A.K.; Singhania, R.R.; Di Dong, C.; Cheng, C.Y. Effects of Lower Temperature on Expression and Biochemical Characteristics of HCV NS3 Antigen Recombinant Protein. Catalysts 2021, 11, 1297. [Google Scholar] [CrossRef]
- Guo, X.; Wang, R.; Ma, R.; Fan, X.; Gao, Y.; Zhang, X.; Yuchi, Z.; Wu, H. Facile Purification of Active Recombinant Mouse Cytosolic Carboxypeptidase 6 from Escherichia coli. Protein Expr. Purif. 2022, 197, 106112. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, T.; Dani, N.; Cheng, B.; Tse-Dinh, Y.C. Analysis of DNA Relaxation and Cleavage Activities of Recombinant Mycobacterium tuberculosis DNA Topoisomerase I from a New Expression and Purification Protocol. BMC Biochem. 2009, 10, 18. [Google Scholar] [CrossRef]
- Hershberg, R.; Petrov, D.A. Selection on Codon Bias. Annu. Rev. Genet. 2008, 42, 287–299. [Google Scholar] [CrossRef]
- Novy, R. Overcoming the Codon Bias of E. coli for Enhanced Protein Expression. Innovations 2001, 12, 1–3. [Google Scholar]
- Reiss, R.; Faccio, G.; Thöny-Meyer, L.; Richter, M. Cloning, Expression and Biochemical Characterization of the Cholesterol Oxidase CgChoA from Chryseobacterium gleum. BMC Biotechnol. 2014, 14, 46. [Google Scholar] [CrossRef]
- Burgess-Brown, N.A.; Sharma, S.; Sobott, F.; Loenarz, C.; Oppermann, U.; Gileadi, O. Codon Optimization Can Improve Expression of Human Genes in Escherichia coli: A Multi-Gene Study. Protein Expr. Purif. 2008, 59, 94–102. [Google Scholar] [CrossRef]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of Active Eukaryotic Proteins Through Bacterial Expression Systems: A Review of the Existing Biotechnology Strategies. Mol. Cell. Biochem. 2008, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludgero, A.K.d.M.; da Silva, A.L.A.; Cruz, L.H.; Brazão, C.A.C.; Taylor, K.M.H.; de Oliveira, L.L.; Bragança, C.R.S.; Duarte, C.E.M. Recombinant Production of a TRAF-Domain Lectin from Cauliflower: A Soluble Expression Strategy for Functional Protein Recovery in E. coli. Int. J. Mol. Sci. 2025, 26, 8287. https://doi.org/10.3390/ijms26178287
Ludgero AKdM, da Silva ALA, Cruz LH, Brazão CAC, Taylor KMH, de Oliveira LL, Bragança CRS, Duarte CEM. Recombinant Production of a TRAF-Domain Lectin from Cauliflower: A Soluble Expression Strategy for Functional Protein Recovery in E. coli. International Journal of Molecular Sciences. 2025; 26(17):8287. https://doi.org/10.3390/ijms26178287
Chicago/Turabian StyleLudgero, Ana Káren de Mendonça, Ana Luísa Aparecida da Silva, Luiz Henrique Cruz, Camila Aparecida Coelho Brazão, Kelly Maria Hurley Taylor, Leandro Licursi de Oliveira, Caio Roberto Soares Bragança, and Christiane Eliza Motta Duarte. 2025. "Recombinant Production of a TRAF-Domain Lectin from Cauliflower: A Soluble Expression Strategy for Functional Protein Recovery in E. coli" International Journal of Molecular Sciences 26, no. 17: 8287. https://doi.org/10.3390/ijms26178287
APA StyleLudgero, A. K. d. M., da Silva, A. L. A., Cruz, L. H., Brazão, C. A. C., Taylor, K. M. H., de Oliveira, L. L., Bragança, C. R. S., & Duarte, C. E. M. (2025). Recombinant Production of a TRAF-Domain Lectin from Cauliflower: A Soluble Expression Strategy for Functional Protein Recovery in E. coli. International Journal of Molecular Sciences, 26(17), 8287. https://doi.org/10.3390/ijms26178287








