Biglycan Alleviates Age-Related Muscle Atrophy and Hepatocellular Senescence
Abstract
1. Introduction
2. Results
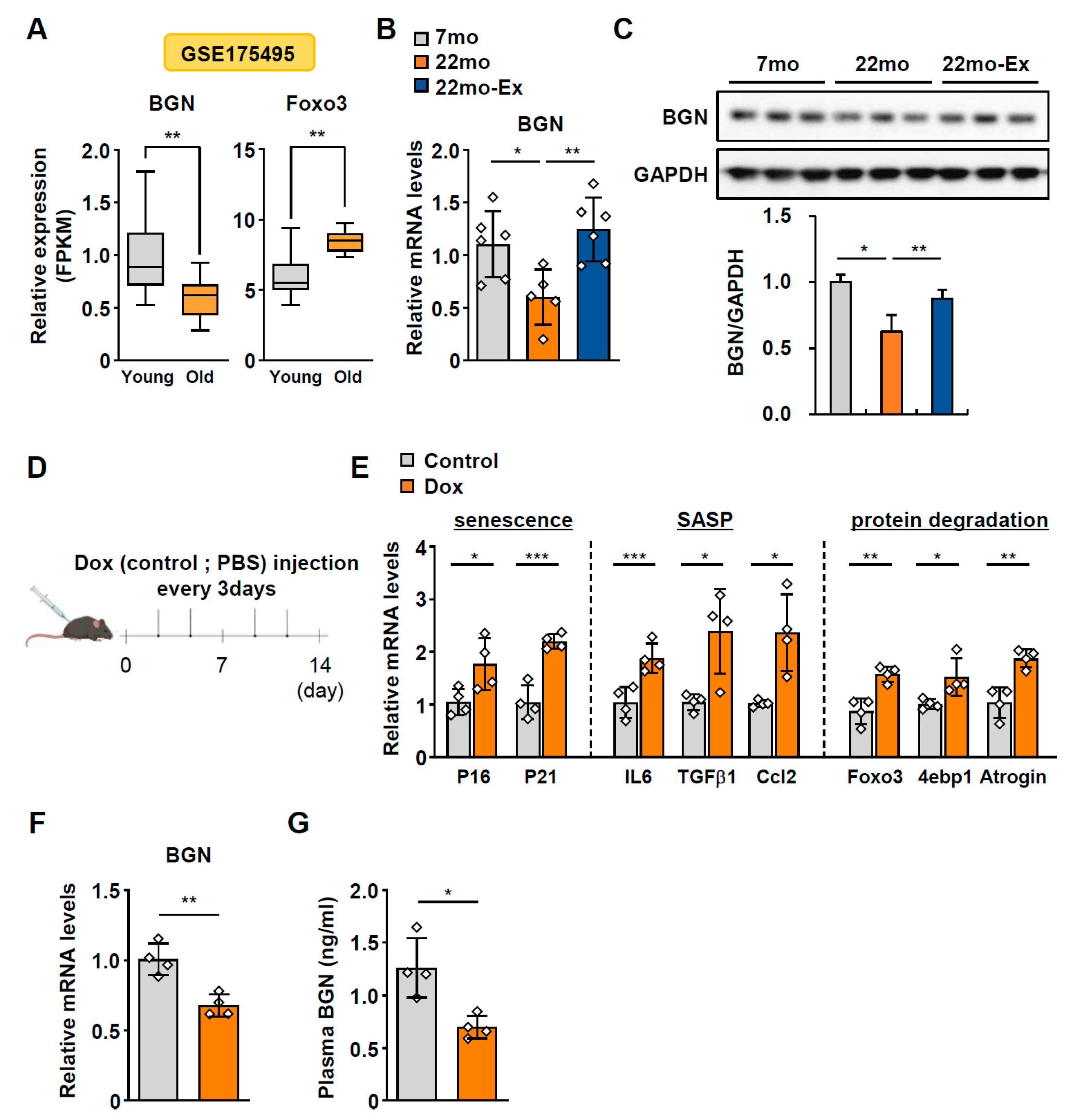
2.1. BGN Expression Progressively Declines with Age
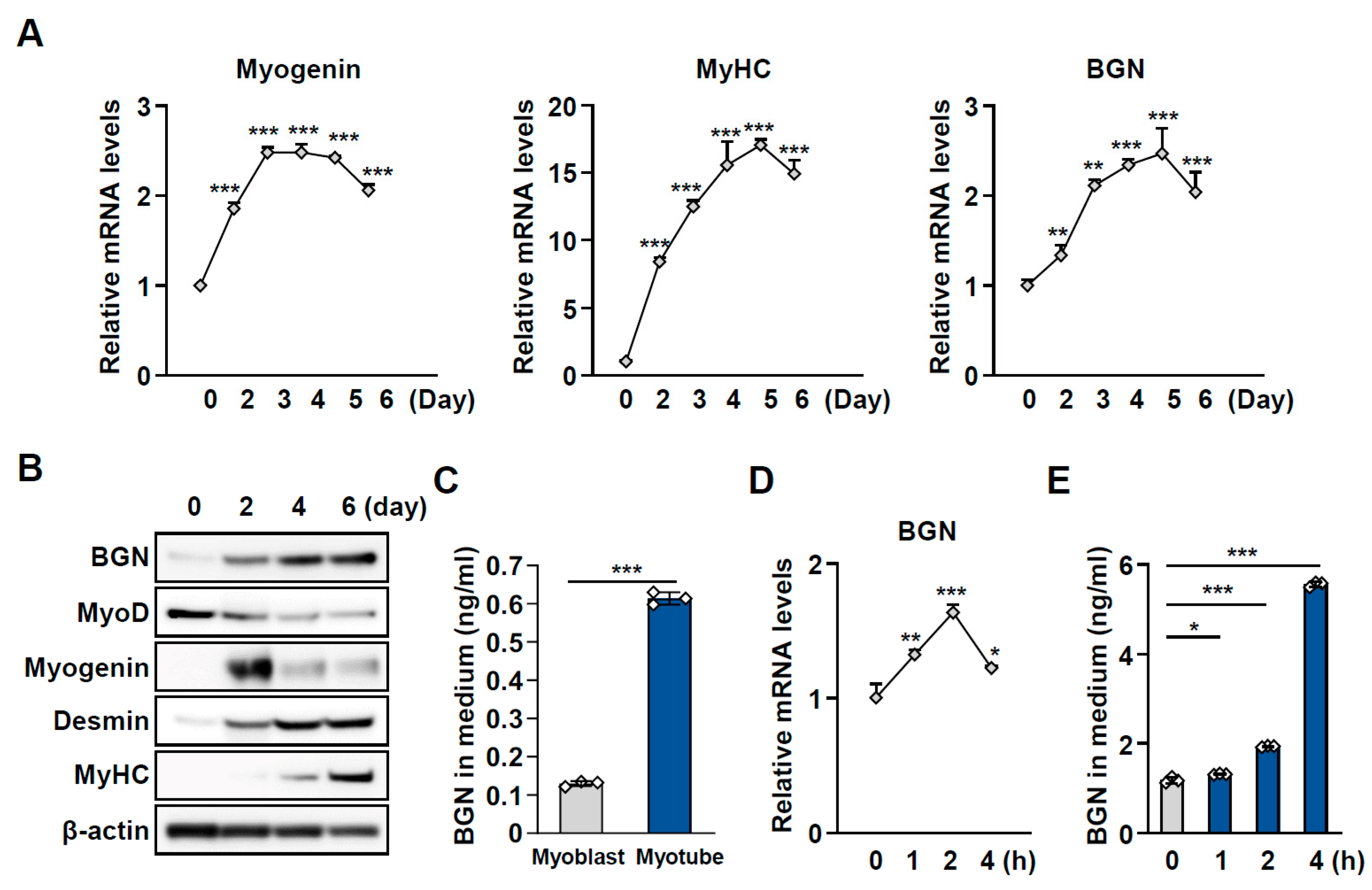
2.2. BGN Is a Secreted Factor Associated with Muscle Function
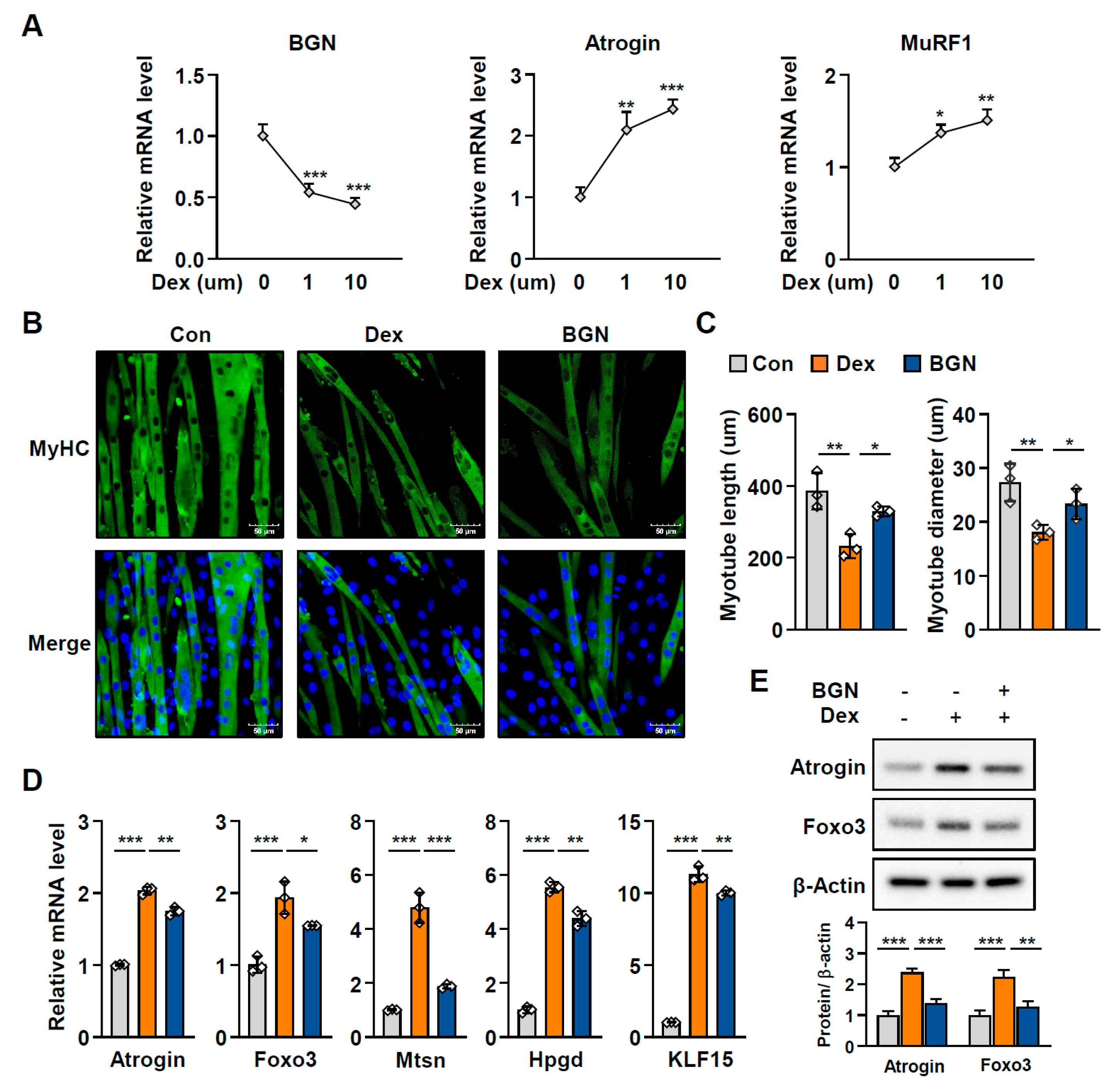
2.3. BGN Has a Protective Effect Against Muscle Atrophy
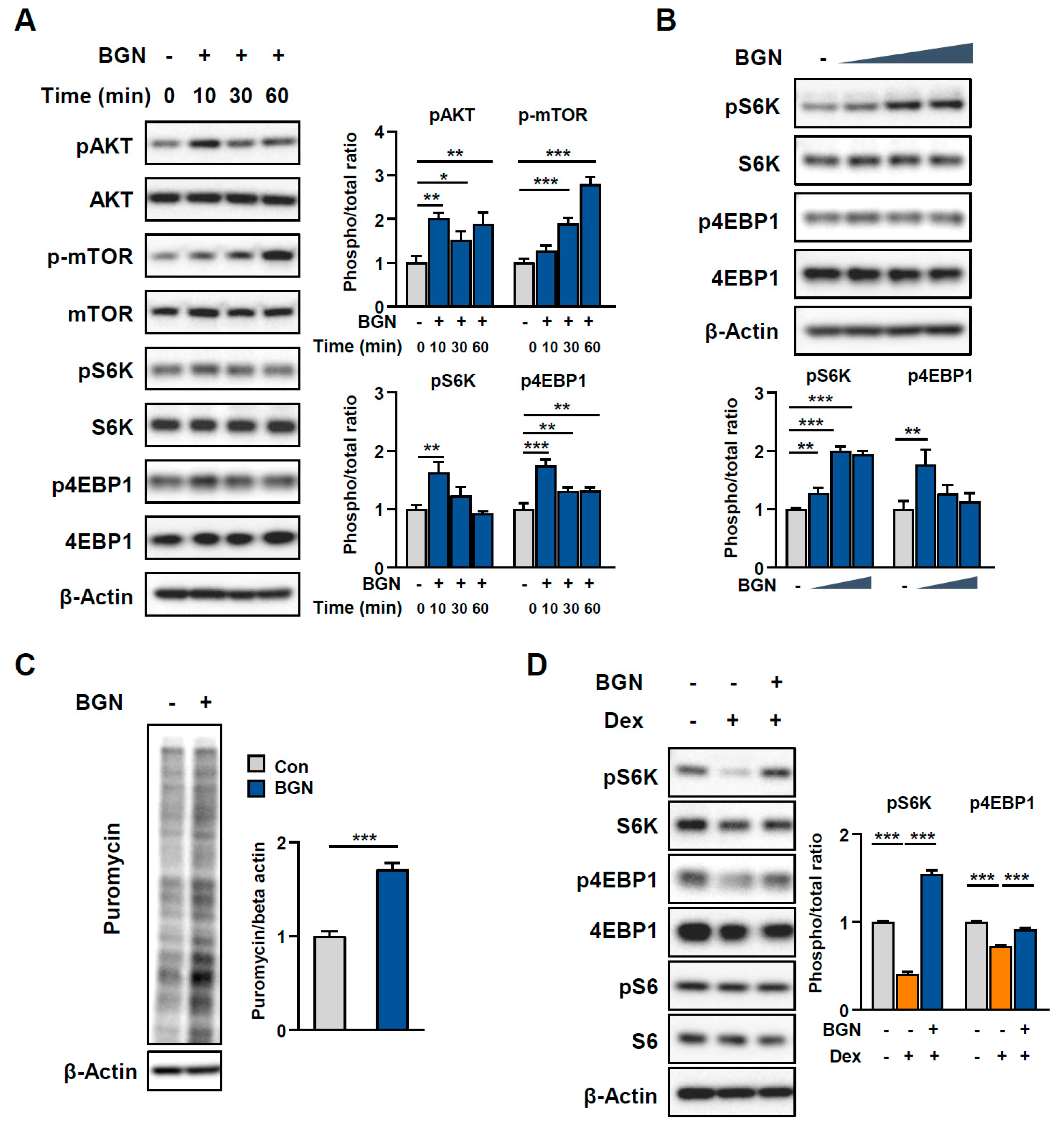
2.4. BGN Promotes the AKT/mTOR Pathway to Increase Protein Synthesis
2.5. Dox-Induced Hepatocellular Senescence Is Restored by BGN
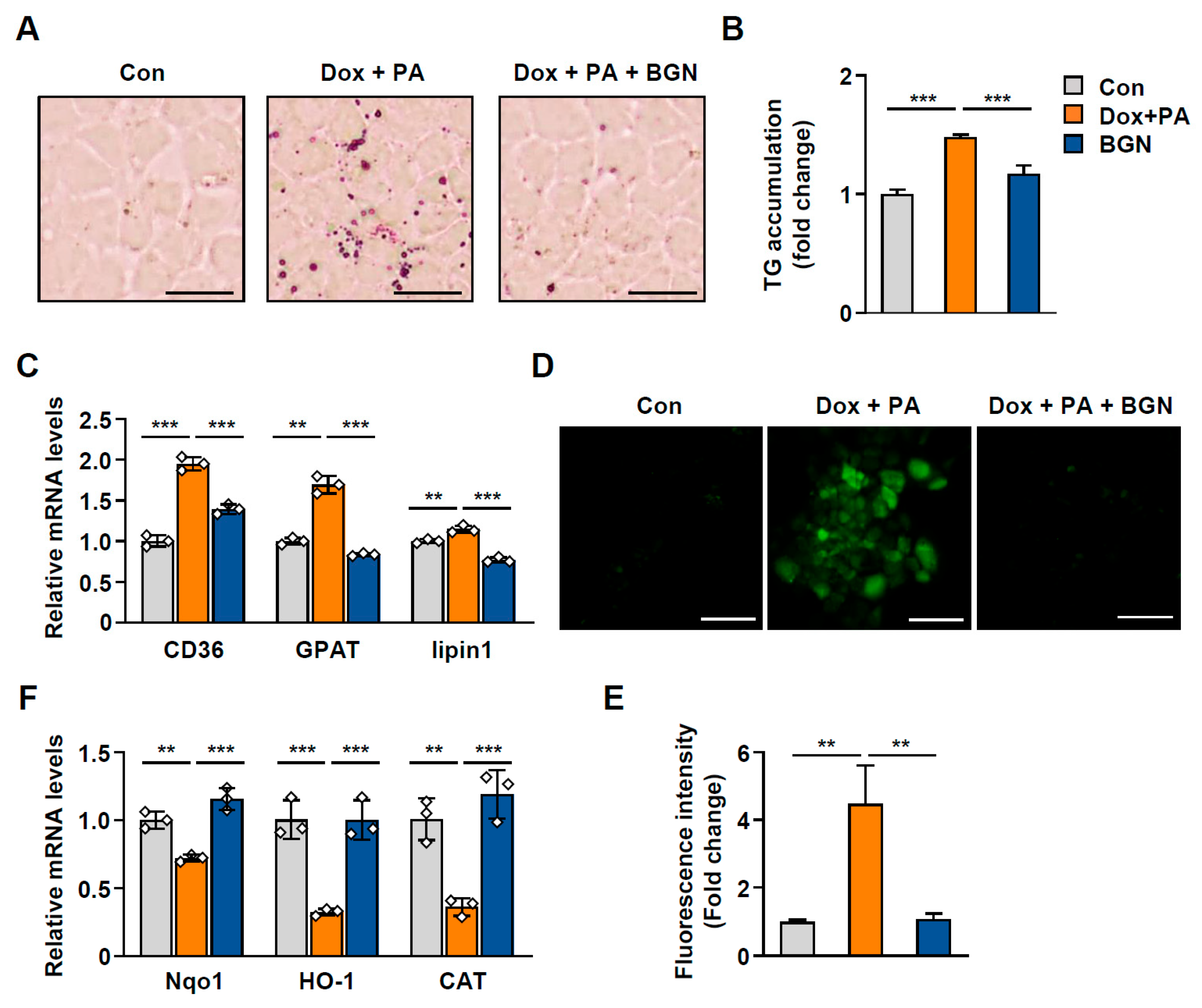
2.6. BGN Inhibits Lipid Accumulation During Hepatic Senescence
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Cell Culture and Reagents
4.3. Quantitative PCR
4.4. Immunoblotting
4.5. Measurement of BGN
4.6. Immunofluorescence Staining
4.7. SUnSET Assay
4.8. β-Galactosidase Staining
4.9. ROS Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BGN | biglycan |
| SASP | senescence-associated secretory phenotype |
| ECM | extracellular matrix |
| Dox | doxorubicin |
| Fsk | forskolin |
| Dex | dexamethasone |
| AKT | serine-threonine kinase, protein kinase B |
| mTOR | mammalian target of rapamycin |
| IL6 | interleukin 6 |
| Ccl2 | CC motif chemokine ligand 2 |
| Tgfβ1 | transforming growth factor beta 1 |
| TNFα | palmitic acid |
| p21 | cyclin-dependent kinase inhibitor 1A (CDKN1A) |
| p16 | cyclin-dependent kinase inhibitor 2A (CDKN2A) |
| ROS | reactive oxygen species |
| MyoD | myoblast determination protein |
| MyHC | myosin heavy chain |
| MuRF1 | muscle RING finger 1 |
| Atrogin | muscle atrophy F-box (MAFbx) |
| Foxo3 | forkhead box O3 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| Hprt | hypoxanthine phosphoribosyltransferase |
| L32 | ribosomal protein L32 |
References
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Stern, M.M.; Myers, R.L.; Hammam, N.; Stern, K.A.; Eberli, D.; Kritchevsky, S.B.; Soker, S.; Van Dyke, M. The Influence of Extracellular Matrix Derived from Skeletal Muscle Tissue on the Proliferation and Differentiation of Myogenic Progenitor Cells Ex Vivo. Biomaterials 2009, 30, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Casar, J.C.; McKechnie, B.A.; Fallon, J.R.; Young, M.F.; Brandan, E. Transient up-Regulation of Biglycan During Skeletal Muscle Regeneration: Delayed Fiber Growth Along with Decorin Increase in Biglycan-Deficient Mice. Dev. Biol. 2004, 268, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Mercado, M.L.; Amenta, A.R.; Hagiwara, H.; Rafii, M.S.; Lechner, B.E.; Owens, R.T.; McQuillan, D.J.; Froehner, S.C.; Fallon, J.R. Biglycan Regulates the Expression and Sarcolemmal Localization of Dystrobrevin, Syntrophin, and Nnos. FASEB J. 2006, 20, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Kumar, A.; Beason, D.P.; Iozzo, R.V.; Birk, D.E.; Soslowsky, L.J. The Injury Response of Aged Tendons in the Absence of Biglycan and Decorin. Matrix Biol. 2014, 35, 232–238. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Beach, Z.M.; Weiss, S.N.; Eekhoff, J.D.; Soslowsky, L.J. Knockdown of Biglycan Reveals an Important Role in Maintenance of Structural and Mechanical Properties During Tendon Aging. J. Orthop. Res. 2023, 41, 2287–2294. [Google Scholar] [CrossRef]
- Florin, A.; Lambert, C.; Sanchez, C.; Zappia, J.; Durieux, N.; Tieppo, A.M.; Mobasheri, A.; Henrotin, Y. The Secretome of Skeletal Muscle Cells: A Systematic Review. Osteoarthr. Cartil. Open 2020, 2, 100019. [Google Scholar] [CrossRef]
- Gueugneau, M.; d’Hose, D.; Barbé, C.; de Barsy, M.; Lause, P.; Maiter, D.; Bindels, L.B.; Delzenne, N.M.; Schaeffer, L.; Gangloff, Y.G.; et al. Increased Serpina3n Release into Circulation During Glucocorticoid-Mediated Muscle Atrophy. J. Cachexia Sarcopenia Muscle 2018, 9, 929–946. [Google Scholar] [CrossRef]
- Hartwig, S.; Raschke, S.; Knebel, B.; Scheler, M.; Irmler, M.; Passlack, W.; Muller, S.; Hanisch, F.G.; Franz, T.; Li, X.; et al. Secretome Profiling of Primary Human Skeletal Muscle Cells. Biochim. Biophys. Acta 2014, 1844, 1011–1017. [Google Scholar] [CrossRef]
- Giatagana, E.M.; Berdiaki, A.; Gaardløs, M.; Samsonov, S.A.; Tzanakakis, G.N.; Nikitovic, D. Biglycan Interacts with Type I Insulin-Like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy. Cancers 2022, 14, 1196. [Google Scholar] [CrossRef]
- Chung, I.; Kim, S.A.; Kim, S.; Lee, J.O.; Park, C.Y.; Lee, J.; Kang, J.; Lee, J.Y.; Seo, I.; Lee, H.J.; et al. Biglycan Reduces Body Weight by Regulating Food Intake in Mice and Improves Glucose Metabolism through Ampk/Akt Dual Pathways in Skeletal Muscle. FASEB J. 2021, 35, e21794. [Google Scholar] [CrossRef]
- Trim, W.V.; Walhin, J.P.; Koumanov, F.; Bouloumié, A.; Lindsay, M.A.; Chen, Y.C.; Travers, R.L.; Turner, J.E.; Thompson, D. Divergent Immunometabolic Changes in Adipose Tissue and Skeletal Muscle with Ageing in Healthy Humans. J. Physiol. 2022, 600, 921–947. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating Changes in Human Plasma Proteome Profiles across the Lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, G.H.; Lee, D.S.; Jeong, H.J.; Lim, J.H. Activation of the Apelin/Apj System by Vitamin D Attenuates Age-Related Muscle Atrophy. Life Sci. 2024, 359, 123205. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, Y.; Duan, C.; Wang, S.; He, W.; Zhang, Y.; An, X.; He, M. Emerging Role of Aging in the Progression of NAFLD to HCC. Ageing Res. Rev. 2023, 84, 101833. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Duseja, A.; Das, A.; Dhiman, R.K.; Chawla, Y.K.; Kohli, K.K.; Bhansali, A. Patients with Nonalcoholic Fatty Liver Disease (NAFLD) Have Higher Oxidative Stress in Comparison to Chronic Viral Hepatitis. J. Clin. Exp. Hepatol. 2013, 3, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Pei, X.; Chen, Y.; Zhang, J.; Zhang, Y.; Du, Y.; Hao, S.; et al. Mitochondrial Dysfunction in the Regulation of Aging and Aging-Related Diseases. Cell Commun. Signal. 2025, 23, 290. [Google Scholar] [CrossRef]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jörgensen, J.A.; Moonen-Kornips, E.; et al. Impact of Aging and Exercise on Skeletal Muscle Mitochondrial Capacity, Energy Metabolism, and Physical Function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef] [PubMed]
- Tumasian, R.A., 3rd; Harish, A.; Kundu, G.; Yang, J.H.; Ubaida-Mohien, C.; Gonzalez-Freire, M.; Kaileh, M.; Zukley, L.M.; Chia, C.W.; Lyashkov, A.; et al. Skeletal Muscle Transcriptome in Healthy Aging. Nat. Commun. 2021, 12, 2014. [Google Scholar] [CrossRef]
- Takasugi, M.; Nonaka, Y.; Takemura, K.; Yoshida, Y.; Stein, F.; Schwarz, J.J.; Adachi, J.; Satoh, J.; Ito, S.; Tombline, G.; et al. An Atlas of the Aging Mouse Proteome Reveals the Features of Age-Related Post-Transcriptional Dysregulation. Nat. Commun. 2024, 15, 8520. [Google Scholar] [CrossRef]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Anand, V.; Khandelwal, M.; Sharma, A. Biglycan: An Emerging Small Leucine-Rich Proteoglycan (SLRP) Marker and Its Clinicopathological Significance. Mol. Cell. Biochem. 2021, 476, 3935–3950. [Google Scholar] [CrossRef]
- Pourteymour, S.; Eckardt, K.; Holen, T.; Langleite, T.; Lee, S.; Jensen, J.; Birkeland, K.I.; Drevon, C.A.; Hjorth, M. Global Mrna Sequencing of Human Skeletal Muscle: Search for Novel Exercise-Regulated Myokines. Mol. Metab. 2017, 6, 352–365. [Google Scholar] [CrossRef]
- Robbins, J.M.; Rao, P.; Deng, S.; Keyes, M.J.; Tahir, U.A.; Katz, D.H.; Beltran, P.M.J.; Marchildon, F.; Barber, J.L.; Peterson, B.; et al. Plasma Proteomic Changes in Response to Exercise Training Are Associated with Cardiorespiratory Fitness Adaptations. JCI Insight 2023, 8, e165867. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms Regulating Skeletal Muscle Growth and Atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. Mtor as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The Role of Mtor Signalling in the Regulation of Skeletal Muscle Mass in a Rodent Model of Resistance Exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kim, J.H.; Kim, M.J.; Min, B.; Lee, S.M.; Go, G.Y.; Kim, J.W.; Kim, S.; Kwak, J.Y.; Chun, S.W.; et al. Exercise-Induced Clcf1 Attenuates Age-Related Muscle and Bone Decline in Mice. Nat. Commun. 2025, 16, 4743. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef]
- Yao, X.; Mai, X.; Tian, Y.; Liu, Y.; Jin, G.; Li, Z.; Chen, S.; Dai, X.; Huang, L.; Fan, Z.; et al. Skeletal Muscle-Specific Bambi Deletion Induces Hypertrophy and Oxidative Switching Coupling with Adipocyte Thermogenesis against Metabolic Disorders. Sci. China Life Sci. 2025, 68, 1352–1368. [Google Scholar] [CrossRef]
- Shah, K.; Stufflebam, A.; Hilton, T.N.; Sinacore, D.R.; Klein, S.; Villareal, D.T. Diet and Exercise Interventions Reduce Intrahepatic Fat Content and Improve Insulin Sensitivity in Obese Older Adults. Obesity 2009, 17, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Pundlik, S.S.; Barik, A.; Venkateshvaran, A.; Sahoo, S.S.; Jaysingh, M.A.; Math, R.G.H.; Lal, H.; Hashmi, M.A.; Ramanathan, A. Senescent Cells Inhibit Mouse Myoblast Differentiation Via the SASP-Lipid 15d-PGJ2 Mediated Modification and Control of Hras. eLife 2024, 13, RP95229. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.S.; Lim, J.H.; Lee, Y.J. Biglycan Alleviates Age-Related Muscle Atrophy and Hepatocellular Senescence. Int. J. Mol. Sci. 2025, 26, 8286. https://doi.org/10.3390/ijms26178286
Lee DS, Lim JH, Lee YJ. Biglycan Alleviates Age-Related Muscle Atrophy and Hepatocellular Senescence. International Journal of Molecular Sciences. 2025; 26(17):8286. https://doi.org/10.3390/ijms26178286
Chicago/Turabian StyleLee, Da Som, Joo Hyun Lim, and Yoo Jeong Lee. 2025. "Biglycan Alleviates Age-Related Muscle Atrophy and Hepatocellular Senescence" International Journal of Molecular Sciences 26, no. 17: 8286. https://doi.org/10.3390/ijms26178286
APA StyleLee, D. S., Lim, J. H., & Lee, Y. J. (2025). Biglycan Alleviates Age-Related Muscle Atrophy and Hepatocellular Senescence. International Journal of Molecular Sciences, 26(17), 8286. https://doi.org/10.3390/ijms26178286





