A Bifunctional SARS-CoV-2 Entry Inhibitor Targeting the Host Protease TMPRSS2 and Viral Spike Protein HR1 Region
Abstract
1. Introduction
2. Results and Discussion
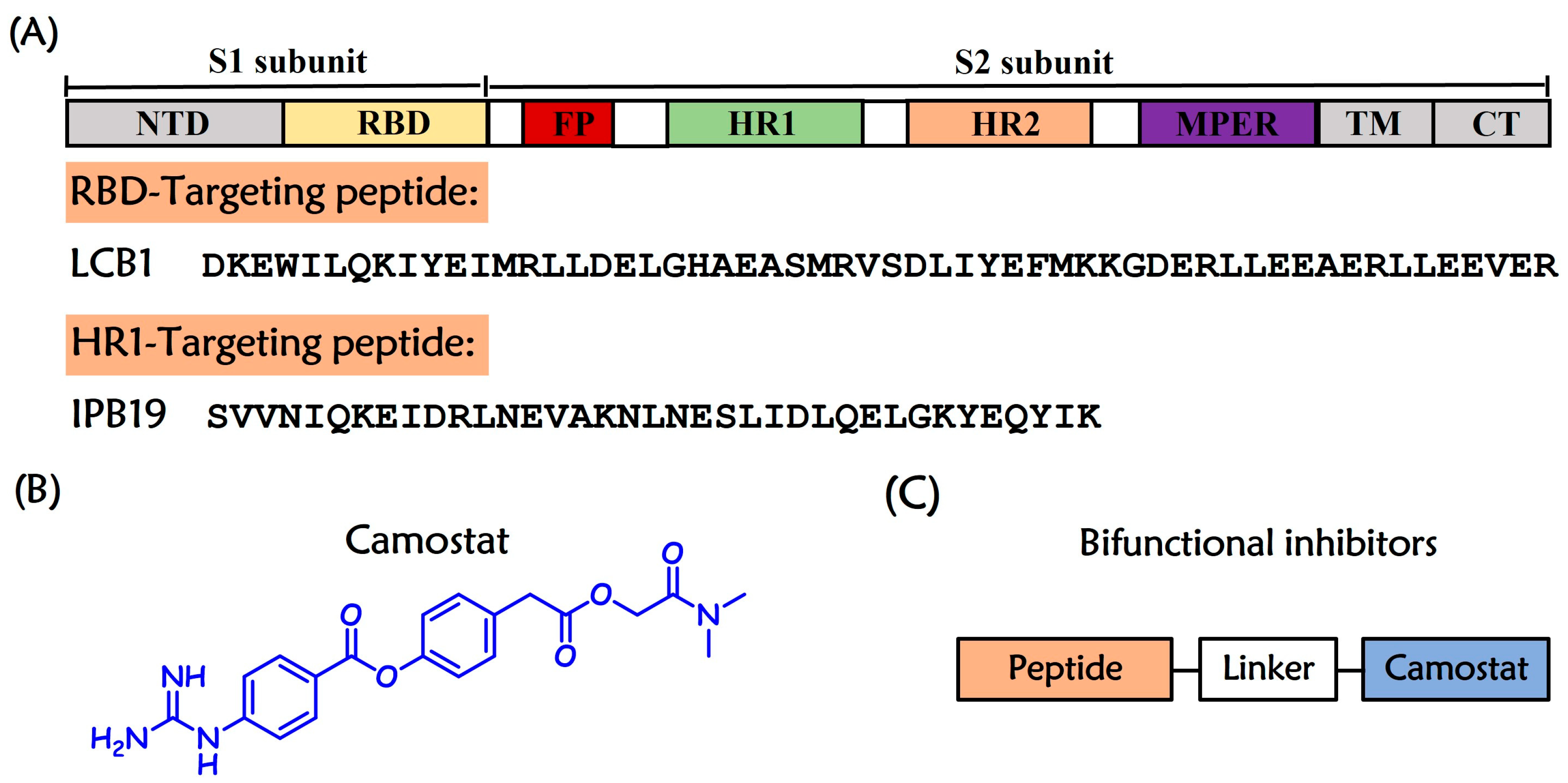
2.1. Design and Chemistry
2.2. Bifunctional Entry Inhibitors Demonstrated Excellent Anti-SARS-CoV-2 Activity
2.3. Bifunctional Entry Inhibitors Could Interact with HR1 Region of SARS-CoV-2 S Protein
2.4. Bifunctional Entry Inhibitors Could Bind to Host Protease TMPRSS2
3. Materials and Methods
3.1. Chemistry
3.2. General Protocol for the Synthesis of Small-Molecule Compounds
3.3. Peptide Synthesis
3.4. Synthesis of Bifunctional Chimeras
3.5. Inhibition of SARS-CoV-2 PsV Infection
3.6. CD Spectroscopy
3.7. Native-PAGE
3.8. Evaluation of Inhibition of TMPRSS2 Catalytic Activity
3.9. SPR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Data. COVID-19 Cases Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 10 February 2025).
- Majumdar, P.; Niyogi, S. SARS-CoV-2 mutations: The biological trackway towards viral fitness. Epidemiol. Infect. 2021, 149, e110. [Google Scholar] [CrossRef]
- Sharma, A.; Farouk, I.A.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell Entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e286. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. Pöhlmann, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Kakizaki, M.; Shiwa-Sudo, N.; Okura, T.; Tahara, M.; Fukushi, S.; Maeda, K.; Kawase, M.; Asanuma, H.; Tomita, Y.; et al. Essential Role of TMPRSS2 in SARS-CoV-2 Infection in Murine Airways. Nat. Commun. 2022, 13, 6100. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Veesler, D. Structural Insights into Coronavirus Entry. Adv. Virus Res. 2019, 105, 93–116. [Google Scholar]
- Xing, L.; Liu, Z.; Wang, X.; Liu, Q.; Xu, W.; Mao, Q.; Zhang, X.; Hao, A.; Xia, S.; Liu, Z.; et al. Early Fusion Intermediate of ACE2-using Coronavirus Spike Acting as an Antiviral Target. Cell 2025, 188, 1297–1314.e24. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.; Park, Y.J.; et al. De Novo Design of Picomolar SARS-CoV-2 Miniprotein Inhibitors. Science 2020, 370, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Arora, P.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; et al. Camostat Mesylate Inhibits SARS-CoV-2 Activation by TMPRSS2-related Proteases and Its Metabolite GBPA Exerts Antiviral Activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef]
- Wong, J.P.; Damania, B. SARS-CoV-2 Dependence on Host Pathways. Science 2021, 371, 884–885. [Google Scholar] [CrossRef]
- Gunst, J.D.; Staerke, N.B.; Pahus, M.H.; Kristensen, L.H.; Bodilsen, J.; Lohse, N.; Dalgaard, L.S.; Brønnum, D.; Fröbert, O.; Hønge, B.; et al. Efficacy of the TMPRSS2 Inhibitor Camostat Mesilate in Patients Hospitalized with Covid-19-a Double-blind Randomized Controlled Trial. EClinicalMedicine 2021, 35, 100849. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned, Nature reviews. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) Infection by a Highly Potent Pan-coronavirus Fusion Inhibitor Targeting Its Spike Protein that Harbors a High Capacity to Mediate Membrane Fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, Y.; Jiao, T.; Wu, T.; Xiao, X.; Qin, B.; Chong, H.; Lei, X.; Ren, L.; Cui, S.; et al. Structure-based Design and Characterization of Novel Fusion-inhibitory Lipopeptides against SARS-CoV-2 and Emerging Variants. Emerg. Microbes Infect. 2021, 10, 1227–1240. [Google Scholar] [CrossRef]
- Bocci, G.; Bradfute, S.B.; Ye, C.; Garcia, M.J.; Parvathareddy, J.; Reichard, W.; Surendranathan, S.; Bansal, S.; Bologa, C.G.; Perkins, D.J.; et al. Virtual and In Vitro Antiviral Screening Revive Therapeutic Drugs for COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 1278–1292. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and Opportunities in Drug Discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
- Cinar, R.; Iyer, M.R.; Kunos, G. Dual Inhibition of CB(1) Receptors and iNOS, as a Potential Novel Approach to the Pharmacological Management of Acute and Long COVID-19. Br. J. Pharmacol. 2022, 179, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational Design of Multitarget-Directed Ligands: Strategies and Emerging Paradigms. J. Med. Chem. 2019, 62, 8881–8914. [Google Scholar] [CrossRef]
- Simoni, E.; Daniele, S.; Bottegoni, G.; Pizzirani, D.; Trincavelli, M.L.; Goldoni, L.; Tarozzo, G.; Reggiani, A.; Martini, C.; Piomelli, D.; et al. Combining Galantamine and Memantine in Multitargeted, New Chemical Entities Potentially Useful in Alzheimer′s Disease. J. Med. Chem. 2012, 55, 9708–9721. [Google Scholar] [CrossRef] [PubMed]
- Ohshio, G.; Saluja, A.K.; Leli, U.; Sengupta, A.; Steer, M.L. Esterase Inhibitors Prevent Lysosomal Enzyme Redistribution in Two Noninvasive Models of Experimental Pancreatitis. Gastroenterology 1989, 96, 853–859. [Google Scholar] [CrossRef]
- Hempel, T.; Raich, L.; Olsson, S.; Azouz, N.P.; Klingler, A.M.; Hoffmann, M.; Pöhlmann, S.; Rothenberg, M.E.; Noé, F. Molecular mechanism of inhibiting the SARS-CoV-2 Cell entry facilitator TMPRSS2 with camostat and nafamostat. Chem. Sci. 2021, 12, 983–992. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(i)-catalyzed 1,3-dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by Copper(I)-catalyzed Azide-alkyne [3+2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. (Int. Ed. Engl.) 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Liu, F.; Li, S.; Shi, D. Rational Multitargeted Drug Design Strategy from the Perspective of a Medicinal Chemist. J. Med. Chem. 2021, 64, 10581–10605. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Dias, K.S.T.; Gontijo, V.S.; Ortiz, C.J.C.; Viegas, C., Jr. Multi-Target Directed Drugs as a Modern Approach for Drug Design Towards Alzheimer’s Disease: An Update. Curr. Med. Chem. 2018, 25, 3491–3525. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Pan, C.; Xu, L.; Shui, Y.; Liu, K.; Jiang, S. Interactions between different generation HIV-1 fusion inhibitors and the putative mechanism underlying the synergistic anti-HIV-1 effect resulting from their combination. FASEB J. 2012, 26, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.K.; Barney, S.; Guthrie, K.I.; Bucy, T.B.; Petteway, S.R., Jr.; Merutka, G. HIV-1 Membrane Fusion Mechanism: Structural Studies of the Interactions between Biologically-active Peptides from Gp41. Biochemistry 1996, 35, 13697–13708. [Google Scholar] [CrossRef] [PubMed]
- Shrimp, J.H.; Kales, S.C.; Sanderson, P.E.; Simeonov, A.; Shen, M.; Hall, M.D. An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 997–1007. [Google Scholar] [CrossRef] [PubMed]
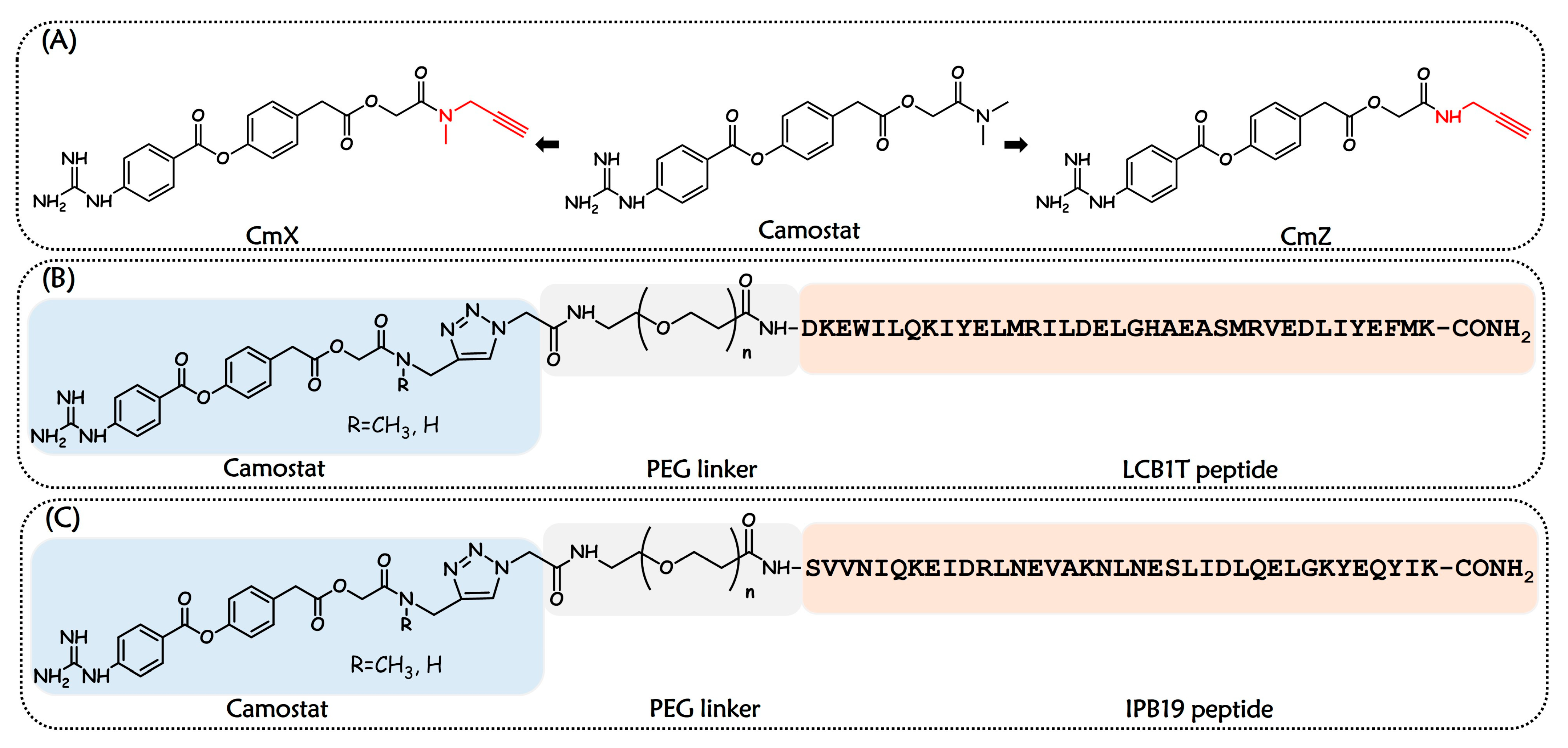



| Name | Sequence | IC50 (µM) |
|---|---|---|
| Class I chimeras | ||
| LP4X | CmX-PEG4--DKEWILQKIYELMRILDELGHAEASMRVEDLIYEFMK-CONH2 | 0.40 ± 0.06 |
| LP4Z | CmZ-PEG4--DKEWILQKIYELMRILDELGHAEASMRVEDLIYEFMK-CONH2 | 0.45 ± 0.20 |
| LP24X | CmX-PEG24-DKEWILQKIYELMRILDELGHAEASMRVEDLIYEFMK-CONH2 | 0.32 ± 0.03 |
| LP24Z | CmZ-PEG24-DKEWILQKIYELMRILDELGHAEASMRVEDLIYEFMK-CONH2 | 0.33 ± 0.01 |
| Class II chimeras | ||
| IP4X | CmX-PEG4--SVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK-CONH2 | 0.16 ± 0.07 |
| IP4Z | CmZ-PEG4--SVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK-CONH2 | 0.17 ± 0.09 |
| IP24X | CmX-PEG24-SVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK-CONH2 | 0.29 ± 0.10 |
| IP24Z | CmZ-PEG24-SVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK-CONH2 | 0.32 ± 0.18 |
| Control compounds | ||
| LCB1T | Ac-DKEWILQKIYELMRILDELGHAEASMRVEDLIYEFMK-CONH2 | 0.44 ± 0.04 |
| IPB19 | Ac-SVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK-CONH2 | 2.43 ± 0.61 |
| Camostat | - | 4.60 ± 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Q.; Yin, Z.; Du, S.; Zheng, L.; Du, X.; Shi, A.; Li, J.; Shi, W.; Yu, F.; et al. A Bifunctional SARS-CoV-2 Entry Inhibitor Targeting the Host Protease TMPRSS2 and Viral Spike Protein HR1 Region. Int. J. Mol. Sci. 2025, 26, 8289. https://doi.org/10.3390/ijms26178289
Wang H, Li Q, Yin Z, Du S, Zheng L, Du X, Shi A, Li J, Shi W, Yu F, et al. A Bifunctional SARS-CoV-2 Entry Inhibitor Targeting the Host Protease TMPRSS2 and Viral Spike Protein HR1 Region. International Journal of Molecular Sciences. 2025; 26(17):8289. https://doi.org/10.3390/ijms26178289
Chicago/Turabian StyleWang, Huan, Qing Li, Zhe Yin, Shu Du, Longbo Zheng, Xinmeng Du, Anqi Shi, Jichun Li, Weiguo Shi, Fei Yu, and et al. 2025. "A Bifunctional SARS-CoV-2 Entry Inhibitor Targeting the Host Protease TMPRSS2 and Viral Spike Protein HR1 Region" International Journal of Molecular Sciences 26, no. 17: 8289. https://doi.org/10.3390/ijms26178289
APA StyleWang, H., Li, Q., Yin, Z., Du, S., Zheng, L., Du, X., Shi, A., Li, J., Shi, W., Yu, F., Xiao, J., & Wang, C. (2025). A Bifunctional SARS-CoV-2 Entry Inhibitor Targeting the Host Protease TMPRSS2 and Viral Spike Protein HR1 Region. International Journal of Molecular Sciences, 26(17), 8289. https://doi.org/10.3390/ijms26178289






