Epigallocatechin-3-Gallate Enhances Antioxidant Activity and Improves Testicular and Epididymal Histology in Cadmium-Exposed Prepubertal Rats
Abstract
1. Introduction
2. Results
2.1. Effects of Cd, EGCG, and Cd+EGCG on the Weight and Concentration of Cd in Blood, Testes, and Epididymides
2.2. Effects of Cd, EGCG, and Cd+EGCG on Testosterone Concentrations
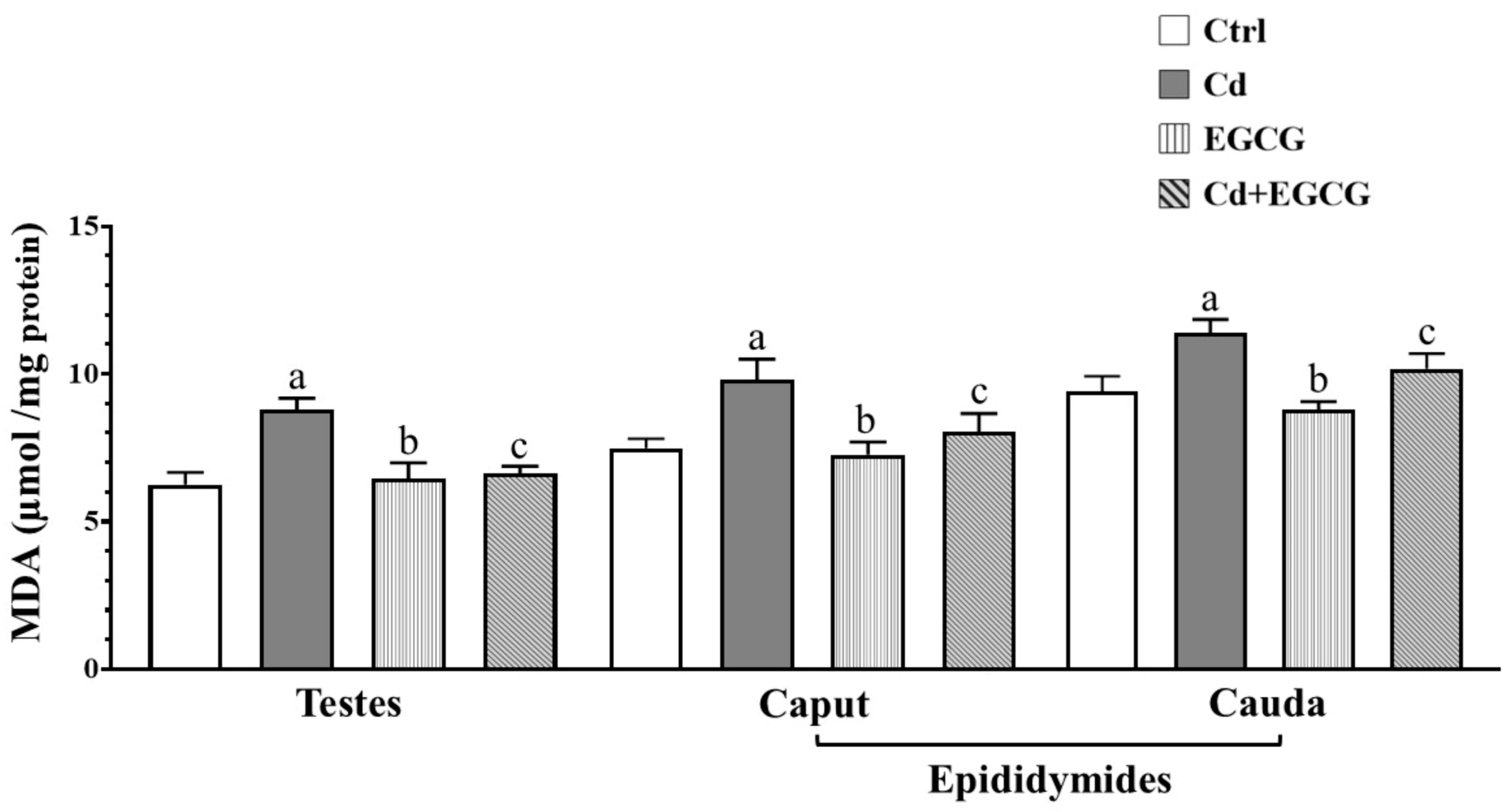
2.3. Effects of Cd, EGCG, and Cd+EGCG on Lipoperoxidation
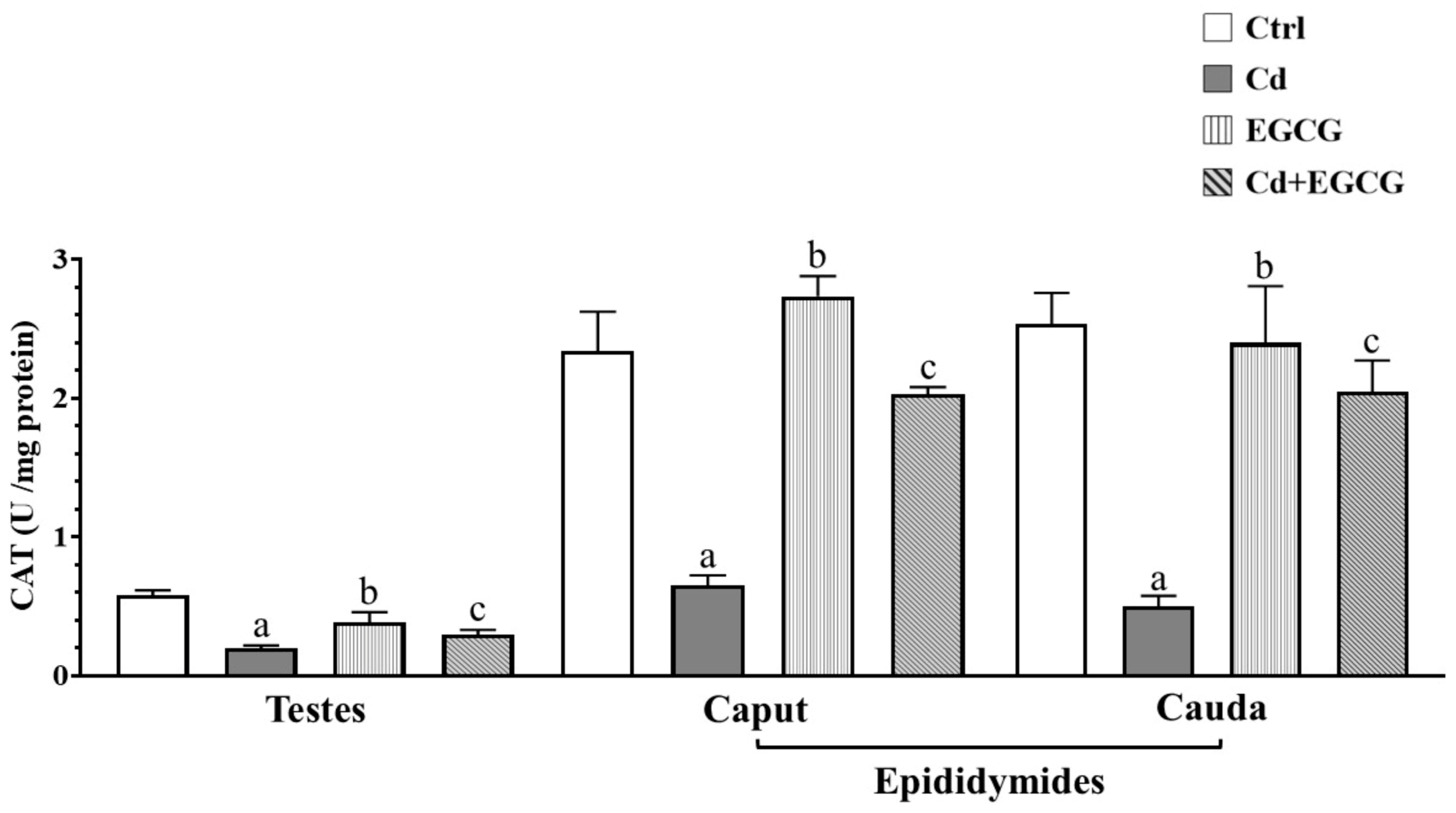
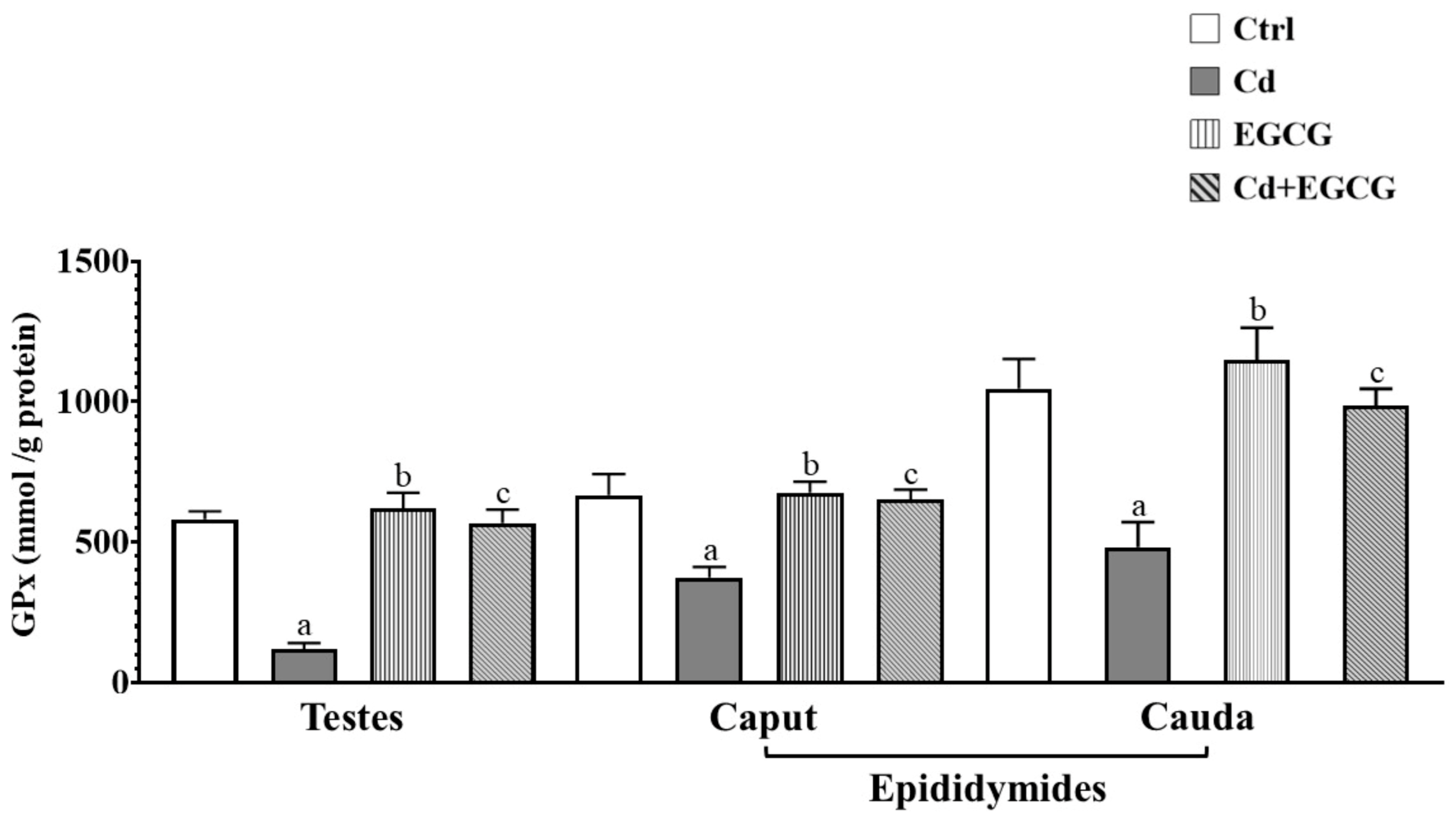
2.4. Effects of Cd, EGCG, and Cd+EGCG on Antioxidant Activity
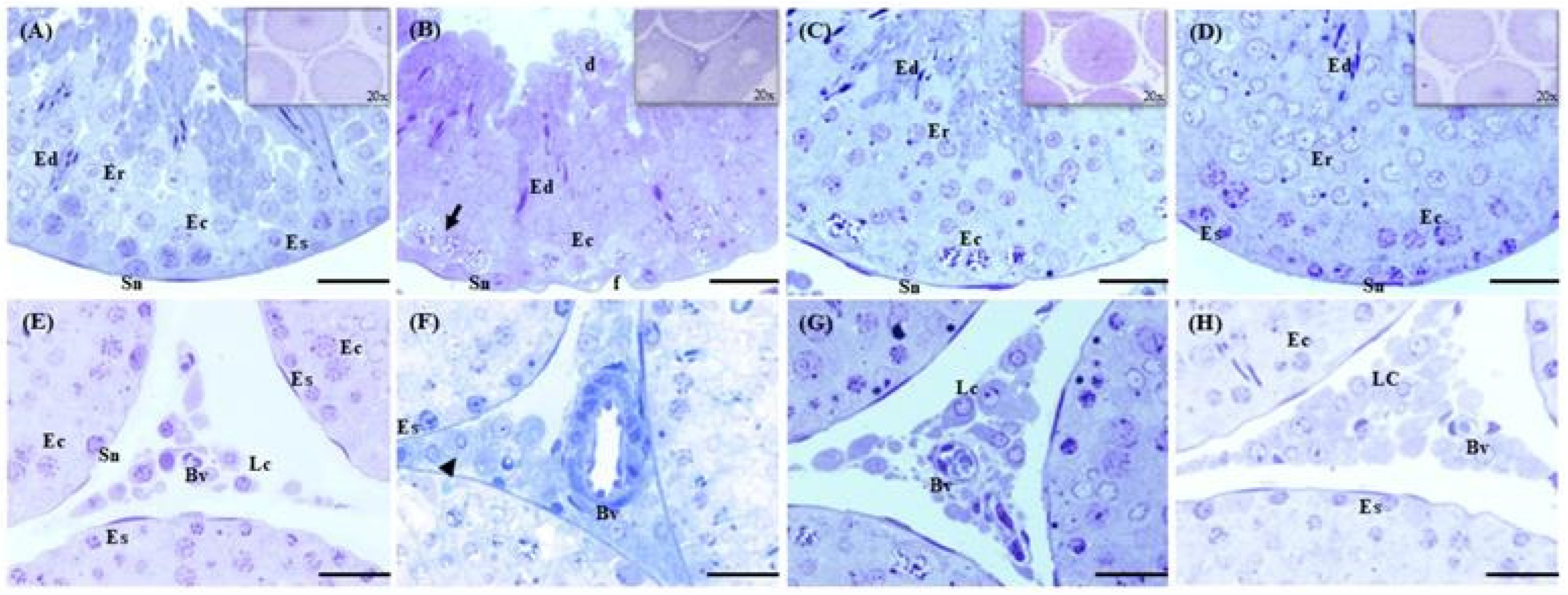
2.5. Effects of Cd, EGCG, and Cd+EGCG on Testicular Epithelium
2.6. Effects of Cd, EGCG, and Cd+EGCG on Epididymal Epithelium
3. Discussion
4. Materials and Methods
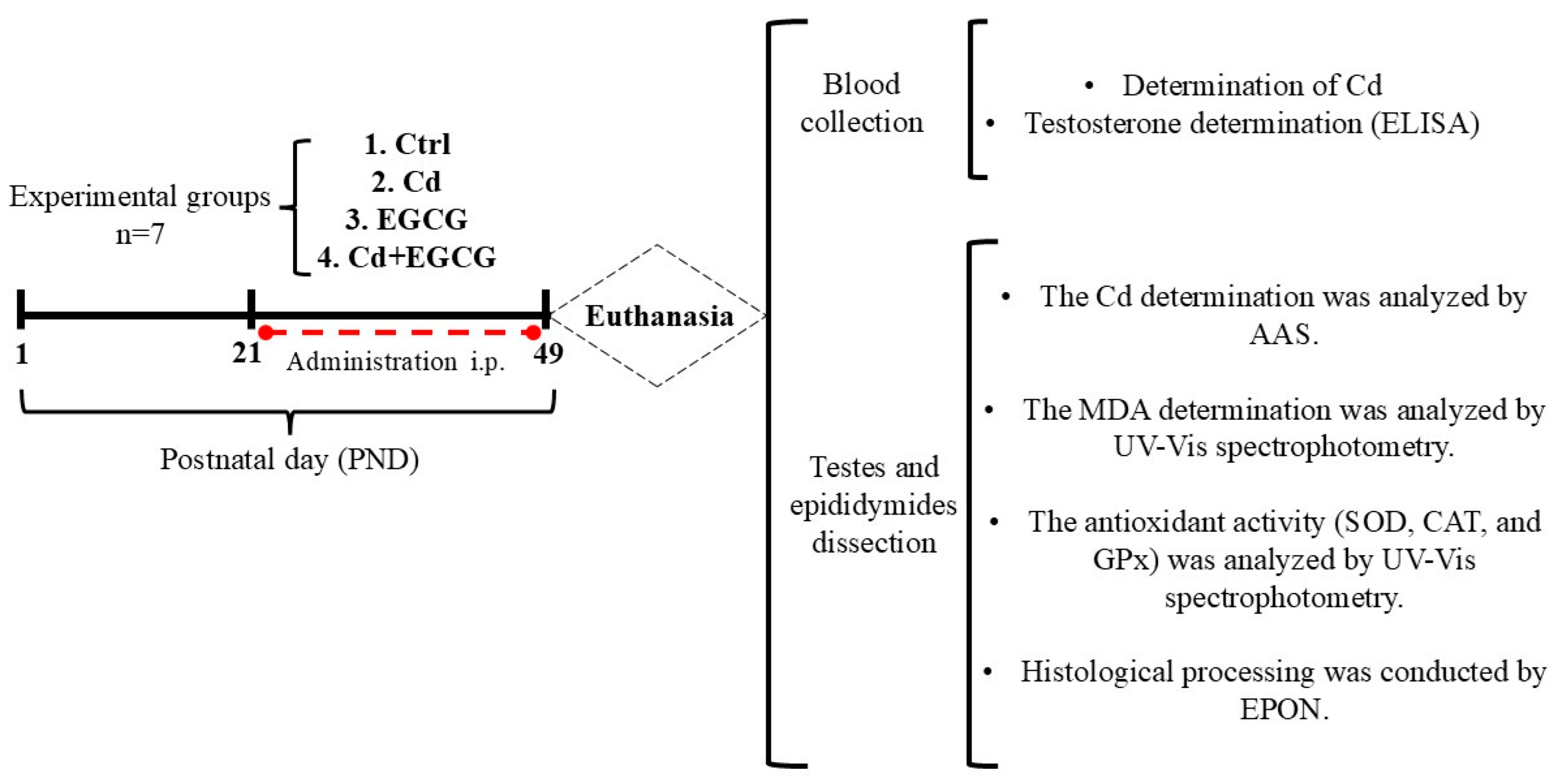
4.1. Experimental Animals
4.2. Treatments
4.3. Experimental Procedure
4.4. Biochemical Analysis
4.4.1. Cadmium Determination in Blood, Testes, and Epididymides
4.4.2. Quantification of Serum Testosterone
4.4.3. Quantification of Malondialdehyde (MDA)
4.5. Determination of Antioxidant Enzyme Activity
4.5.1. Superoxide Dismutase
4.5.2. Catalase
4.5.3. Glutathione Peroxidase
4.6. Histological Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| EGCG | (-)-Epigallocatechin-3-gallate |
| ip | Intraperitoneal |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| GnRH | Gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| HPG | Hypothalamic–pituitary–gonadal |
| PND | Postnatal day |
| AAS | Atomic absorption spectrophotometry |
| EPON | Epoxy resin |
| StAR | Steroidogenic acute regulatory protein |
| P450 | Cytochrome P450 |
| Nrf2 | Nuclear erythroid factor 2 |
| BTB | Blood–testis barrier |
| Mn | Manganese |
| Cu | Copper |
| Zn | Zinc |
| Ni | Nickel |
| As | Arsenic |
| Pb | Lead |
| Hg | Mercury |
| GTS | Total glutathione transferase |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B |
| MDA | Malondialdehyde |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Tena-Sempere, M. Kisspeptins and Puberty. Rev. Española Endocrinol. Pediatr. 2017, 8, 8–14. [Google Scholar] [CrossRef]
- Krishna, K.B.; Witchel, S.F. Normal and Abnormal Puberty. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279024 (accessed on 9 April 2024).
- Arrotia, K.; Garcia, P.; Barbieri, M.; Justino, M.; Violin, L. The Epididymis: Embryology, Structure, Function and Its Role in Fertilization and Infertility. In Embryology-Updates and Highlights on Classic Topics; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Euling, S.Y.; Selevan, S.G.; Pescovitz, O.H.; Skakkebaek, N.E. Role of environmental factors in the timing of puberty. Pediatrics 2008, 121 (Suppl. S3), S167–S171. [Google Scholar] [CrossRef] [PubMed]
- Picut, C.A.; Remick, A.K.; de Rijk, E.P.; Simons, M.L.; Stump, D.G.; Parker, G.A. Postnatal development of the testis in the rat: Morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol. Pathol. 2015, 43, 326–342. [Google Scholar] [CrossRef]
- Arteaga-Silva, M.; Vigueras-Villaseñor, R.M.; Retana-Márquez, S.; Hernández -González, M.; Bonilla-Jaime, H.; Guzmán-García, X.; Contreras-Montiel, J.L. Testosterone Levels and Development of the Penile Spines and Testicular Tissue during the Postnatal Growth in Wistar Rats. Adv. Sex. Med. 2013, 3, 9. [Google Scholar] [CrossRef]
- Zemunik, T.; Peruzovic, M.; Capkun, V.; Zekan, L.; Tomic, S.; Milkovic, K. Reproductive ability of pubertal male and female rats. Braz. J. Med. Biol. Res. 2003, 36, 871–877. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Preventing Disease Through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019.
- Das, S.; Kar, I.; Patra, A.K. Cadmium induced bioaccumulation, histopathology, gene regulation in fish and its amelioration—A review. J. Trace Elem. Med. Biol. 2023, 79, 127202. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Vanbuel, I.; Iven, V.; Kunnen, K.; Vandionant, S.; Huybrechts, M.; Hendrix, S. Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free Radic. Biol. Med. 2023, 199, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of thermal stress-induced lead (Pb) toxicity on apoptotic cell death, inflammatory response, oxidative defense, and DNA methylation in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2020, 224, 105479. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; López, A.L.; Montes, S.; Bonilla-Jaime, H.; Morales, I.; Limón-Morales, O.; Ríos, C.; Hernández-González, M.; Vigueras-Villaseñor, R.M.; Arteaga-Silva, M. Delay in puberty indices of Wistar rats caused by Cadmium. Focus on the redox system in reproductive organs. Reprod. Toxicol. 2021, 99, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C.A.; Gollücke, A.P.; Dolder, H. Grape juice concentrate (G8000(®)) intake mitigates testicular morphological and ultrastructural damage following cadmium intoxication. Int. J. Exp. Pathol. 2015, 96, 301–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Djuric, A.; Begic, A.; Gobeljic, B.; Stanojevic, I.; Ninkovic, M.; Vojvodic, D.; Pantelic, A.; Zebic, G.; Prokic, V.; Dejanovic, B.; et al. Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem. Toxicol. 2015, 86, 25–33. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C.A.; Cuquetto-Leite, L.; do Nascimento da Silva, E.; Thomazini, B.F.; Cordeiro, G.D.S.; Predes, F.S.; Gollücke, A.P.B.; Dolder, H. Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats. Int. J. Exp. Pathol. 2017, 98, 86–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, L.; Dai, J.; Chen, Y.; Tong, Y.; Li, Y.; Fu, G.; Teng, Z.; Huang, J.; Quan, C.; Zhang, Z.; et al. Reproductive toxicity of cadmium in pubertal male rats induced by cell apoptosis. Toxicol. Ind. Health 2021, 37, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Y.; Li, Z.; Hua, Q.; Wang, L.; Song, X.; Zou, B.; Ding, M.; Zhao, J.; Tang, C. Joint Toxicity of a Multi-Heavy Metal Mixture and Chemoprevention in Sprague Dawley Rats. Int. J. Environ. Res. Public Health 2020, 17, 1451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 96, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Rodríguez Mdel, C.; Montaño-Rodríguez, A.R.; Altamirano-Lozano, M.A. Modulation of hexavalent chromium-induced genotoxic damage in peripheral blood of mice by epigallocatechin-3-gallate (EGCG) and its relationship to the apoptotic activity. J. Toxicol. Environ. Health Part A 2016, 79, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [PubMed]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Siwach, A.; Sachdeva, D.; Sachdeva, S.N. Revisiting cadmium-induced toxicity in the male reproductive system: An update. Arch. Toxicol. 2024, 98, 3619–3639. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Nong, Q.; Guo, H.; Li, Y.; Tian, H.; Zhang, J.; Liu, L.; He, B.; Hu, L.; Jiang, G. pH-dependent cadmium binding to hemoglobin: Implications for human excretion. Sci. Total Environ. 2025, 958, 177700. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, M.; Pizzino, G.; Bitto, A.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; De Luca, F.; Santamaria, A.; et al. Cadmium delays puberty onset and testis growth in adolescents. Clin. Endocrinol. 2015, 83, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hastak, K.; Ahmad, N.; Lewin, J.S.; Mukhtar, H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. USA 2001, 98, 10350–10355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: A reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abib, R.T.; Peres, K.C.; Barbosa, A.M.; Peres, T.V.; Bernardes, A.; Zimmermann, L.M.; Quincozes-Santos, A.; Fiedler, H.D.; Leal, R.B.; Farina, M.; et al. Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem. Toxicol. 2011, 49, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Chen, S.T.; Liang, C.; Shi, Y.H.; Chen, Q.S. Effects of Cadmium Exposure on Leydig Cells and Blood Vessels in Mouse Testis. Int. J. Environ. Res. Public Health 2022, 19, 2416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Q.; Cheng, C.Y.; Liu, Y.X. Development, function and fate of fetal Leydig cells. Semin. Cell Dev. Biol. 2016, 59, 89–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biswas, N.M.; Sen Gupta, R.; Chattopadhyay, A.; Choudhury, G.R.; Sarkar, M. Effect of atenolol on cadmium-induced testicular toxicity in male rats. Reprod. Toxicol. 2001, 15, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Waites, G.M.; Gladwell, R.T. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol. Rev. 1982, 62, 624–671. [Google Scholar] [CrossRef] [PubMed]
- de Araújo-Ramos, A.T.; Basso, C.G.; Passoni, M.T.; Ribeiro, D.C.K.; Spercoski, K.M.; de Oliveira, J.M.; Romano, R.M.; Merino, C.; Sandri, J.M.M.; de Almeida, M.F.; et al. The endocrine disrupting effects of sodium arsenite in the rat testis is not mediated through macrophage activation. Reprod. Toxicol. 2021, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, X.; Gong, J.; Lv, J.; Yuan, X.; Shi, M.; Fu, C.; Tan, B.; Fan, Z.; Chen, L.; et al. Patterns of alteration in boar semen quality from 9 to 37 months old and improvement by protocatechuic acid. J. Anim. Sci. Biotechnol. 2024, 15, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lafuente, A. The hypothalamic-pituitary-gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem. Toxicol. 2013, 59, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol. Environ. Saf. 2021, 226, 112878. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.L.; Wang, H.; Liu, P.; Wang, Q.; Zhao, X.F.; Meng, X.H.; Yu, T.; Zhang, H.; Zhang, C.; Zhang, Y.; et al. Pubertal cadmium exposure impairs testicular development and spermatogenesis via disrupting testicular testosterone synthesis in adult mice. Reprod. Toxicol. 2010, 29, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Arenas-Ríos, E.; Jiménez-Morales, I.; Cortés-Barberena, E.; Montes, S.; Vigueras-Villaseñor, R.M.; Arteaga-Silva, M. Postnatal cadmium administration affects the presence and distribution of carbohydrates in the sperm membrane during maturation in the epididymis in adult Wistar rats. Reprod. Fertil. Dev. 2021, 33, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, X.; Wang, L.; Su, D.; He, Z.; Li, J.; Gong, J.; Zhang, W.; Ma, S.; Shi, M.; et al. Gut microbiota dysbiosis and oxidative damage in high-fat diet-induced impairment of spermatogenesis: Role of protocatechuic acid intervention. Food Front. 2024, 5, 2566–2578. [Google Scholar] [CrossRef]
- Fouad, A.A.; Qutub, H.O.; Fouad, A.E.A.; Audeh, A.M.; Al-Melhim, W.N. Epigallocatechin-3-gallate counters cisplatin toxicity of rat testes. Pharm. Biol. 2017, 55, 1710–1714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vigueras-Villaseñor, R.M.; Jiménez Cabrera, T.; Chávez Saldaña, M.; Jiménez Trejo, F.; Cuevas Alpuche, O.; Rojas-Castañeda, J.C. Epigallocatechin-3-gallate protects the testis from damage generated by experimental cryptorchidism in rabbits. Histol. Histopathol. 2019, 34, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fu, L. Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Dev. Ther. 2019, 13, 2811–2824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, N.A.; Mandal, A.K.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Shang, X.J.; Lv, L.; Wang, Y.; Zhang, J.; Quan, C.; Shi, Y.; Liu, Y.; Zhang, L. Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy. Cell Death Dis. 2022, 13, 928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robaire, B.; Hinton, B.T.; Orgebin-Crist, M.-C. CHAPTER 22-The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Academic Press: St Louis, MO, USA, 2006; pp. 1071–1148. [Google Scholar]
- Prozialeck, W.C.; Edwards, J.R.; Woods, J.M. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006, 79, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.P.; Predes, F.S.; Monteiro, J.C.; Freitas, K.M.; Wada, R.S.; Dolder, H. Advantage of Guaraná (Paullinia cupana Mart.) supplementation on cadmium-induced damages in testis of adult Wistar rats. Toxicol. Pathol. 2013, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, A.R.; Helal, G.K.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Bakheet, S.A. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxidative Med. Cell. Longev. 2009, 2, 73–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.; Guo, X.; Wang, H.; Zhou, S.; Li, L.; Chen, X.; Wang, G.; Liu, J.; Ge, H.S.; Ge, R.S. A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis. Sci. Rep. 2017, 7, 6337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, N.H.; Pei, H.; Huang, Y.P.; Li, Y.F. (-)-Epigallocatechin-3-Gallate Inhibits Arsenic-Induced Inflammation and Apoptosis through Suppression of Oxidative Stress in Mice. Cell Physiol. Biochem. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, N.; Namavar, M.R. Optimisation of ketamine-xylazine anaesthetic dose and its association with changes in the dendritic spine of CA1 hippocampus in the young and old male and female Wistar rats. Vet. Med. Sci. 2022, 8, 2545–2552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, R.P.; Street, J.C.; Shupe, J.L.; Bourcier, D.R. Accumulation and depletion of cadmium and lead in tissues and milk of lactating cows fed small amounts of these metals. J. Dairy Sci. 1982, 65, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Krall, J.; Norton, L.; McKay, K.; Kay, D.; Lynch, R.E. Catalase and superoxide dismutase in Escherichia coli. J. Biol. Chem. 1983, 258, 6277–6281. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. (JCB) 1965, 27, 1A–149A. [Google Scholar]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zúñiga, A.; Landero-Huerta, D.A.; Rojas-Castañeda, J.C.; Sánchez-Huerta, K.; Contreras-García, I.J.; Reynoso-Robles, R.; Arteaga-Silva, M.; Vigueras-Villaseñor, R.M. Methimazole-induced congenital hypothyroidism affects gonocytes differentiation and arrests meiosis: Role of Sertoli cells. Front. Cell Dev. Biol. 2024, 12, 1493872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vigueras-Villaseñor, R.M.; Molina-Ortiz, D.; Reyes-Torres, G.; del Angel, D.S.; Moreno-Mendoza, N.A.; Cruz, M.E.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Effect of allopurinol on damage caused by free radicals to cryptorchid testes. Acta Histochem. 2009, 111, 127–137. [Google Scholar] [CrossRef] [PubMed]

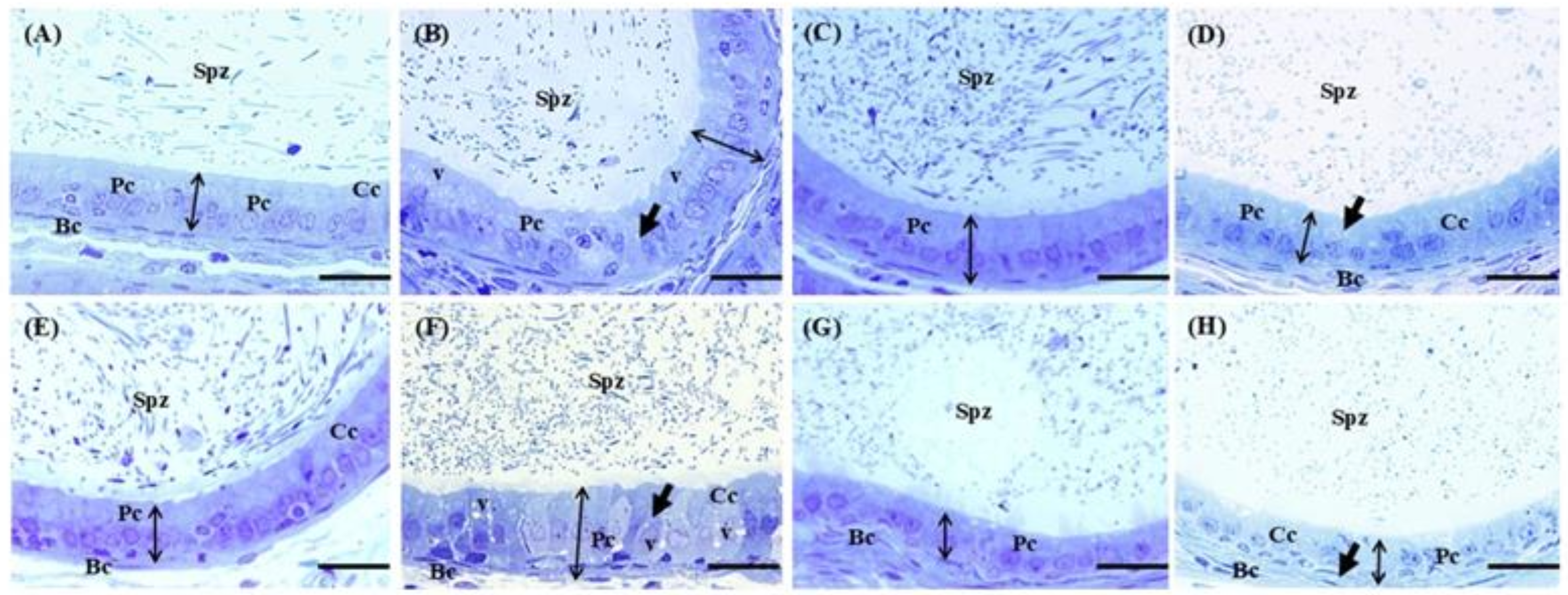
| Ctrl | Cd | EGCG | Cd+EGCG | |
|---|---|---|---|---|
| Testes weight (g) | 1.40 ± 0.003 | 1.27 ± 0.016 a | 1.41 ± 0.035 b | 1.41 ± 0.029 c |
| Testes Cd concentration (μg/g) | 0.84 ± 0.19 | 4.77 ± 0.62 a | 0.59 ± 0.16 b | 0.62 ± 0.15 c |
| Epididymides caput Cd concentration (μg/g) | 0.86 ± 0.046 | 2.18 ± 0.32 a | 0.73 ± 0.14 b | 0.82 ± 0.16 c |
| Caput weight (g) | 0.085 ± 0.005 | 0.062 ± 0.004 a | 0.087 ± 0.005 b | 0.081 ± 0.003 c |
| Epididymides cauda Cd concentration (μg/g) | 0.64 ± 0.086 | 2.44 ± 0.16 a | 0.55 ± 0.12 b | 0.56 ± 0.12 c |
| Cauda weight (g) | 0.065 ± 0.005 | 0.039 ± 0.004 a | 0.071 ± 0.005 b | 0.068 ± 0.003 c |
| Blood Cd concentration (μg/mL) | 0.04 ± 0.011 | 0.23 ± 0.007 a | 0.04 ± 0.008 b | 0.04 ± 0.008 c |
| Ctrl | Cd | EGCG | Cd+EGCG | |
|---|---|---|---|---|
| ng/mL | 2.39 ± 0.09 | 1.00 ± 0.07 a | 2.19 ± 0.21 b | 2.13 ± 0.21 c |
| Ctrl | Cd | EGCG | Cd+EGCG | |
|---|---|---|---|---|
| Seminiferous epithelium area (µm2) | 563.8 ± 10.7 | 474.9 ± 25.5 a | 572.9 ± 11.9 b | 521.5 ± 10.2 c |
| Seminiferous epithelium diameter (µm) | 329.4 ± 9.5 | 251.9 ± 3.7 a | 330.9 ± 11.2 b | 317.7 ± 11.4 c |
| Histopathological index | 1.53 ± 0.13 | 1.73 ± 0.11 | 1.46 ± 0.13 | 1.60 ± 0.13 |
| Maturation index | 8.6 ± 0.16 | 8.4 ± 0.12 | 8.7 ± 0.19 | 8.5 ± 0.18 |
| Ctrl | Cd | EGCG | Cd+EGCG | ||
|---|---|---|---|---|---|
| Caput | Height (µm) | 24.8 ± 0.68 | 33.2 ± 0.45 a | 24.6 ± 0.63 b | 23.4 ± 0.40 c |
| Area (µm2) | 73.2 ± 1.87 | 81.0 ± 1.33 a | 73.2 ± 1.56 b | 73.6 ± 1.26 c | |
| Cauda | Height (µm) | 23.4 ± 0.25 | 29.8 ± 0.48 a | 23.3 ± 0.24 b | 23.4 ± 0.40 c |
| Area (µm2) | 64.4 ± 0.93 | 79.4 ± 1.78 a | 68.0 ± 1.72 b | 68.5 ± 1.95 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Aguirre, S.G.; Vigueras-Villaseñor, R.M.; Bonilla-Jaime, H.; Montes, S.; Galván-Arzate, S.; Hernández-Rodríguez, J.; Marín de Jesús, S.; Carrizales-Yañez, L.; Rojas-Castañeda, J.C.; Arteaga-Silva, M. Epigallocatechin-3-Gallate Enhances Antioxidant Activity and Improves Testicular and Epididymal Histology in Cadmium-Exposed Prepubertal Rats. Int. J. Mol. Sci. 2025, 26, 8264. https://doi.org/10.3390/ijms26178264
Pérez-Aguirre SG, Vigueras-Villaseñor RM, Bonilla-Jaime H, Montes S, Galván-Arzate S, Hernández-Rodríguez J, Marín de Jesús S, Carrizales-Yañez L, Rojas-Castañeda JC, Arteaga-Silva M. Epigallocatechin-3-Gallate Enhances Antioxidant Activity and Improves Testicular and Epididymal Histology in Cadmium-Exposed Prepubertal Rats. International Journal of Molecular Sciences. 2025; 26(17):8264. https://doi.org/10.3390/ijms26178264
Chicago/Turabian StylePérez-Aguirre, Sonia Guadalupe, Rosa María Vigueras-Villaseñor, Herlinda Bonilla-Jaime, Sergio Montes, Sonia Galván-Arzate, Joel Hernández-Rodríguez, Sergio Marín de Jesús, Leticia Carrizales-Yañez, Julio Cesar Rojas-Castañeda, and Marcela Arteaga-Silva. 2025. "Epigallocatechin-3-Gallate Enhances Antioxidant Activity and Improves Testicular and Epididymal Histology in Cadmium-Exposed Prepubertal Rats" International Journal of Molecular Sciences 26, no. 17: 8264. https://doi.org/10.3390/ijms26178264
APA StylePérez-Aguirre, S. G., Vigueras-Villaseñor, R. M., Bonilla-Jaime, H., Montes, S., Galván-Arzate, S., Hernández-Rodríguez, J., Marín de Jesús, S., Carrizales-Yañez, L., Rojas-Castañeda, J. C., & Arteaga-Silva, M. (2025). Epigallocatechin-3-Gallate Enhances Antioxidant Activity and Improves Testicular and Epididymal Histology in Cadmium-Exposed Prepubertal Rats. International Journal of Molecular Sciences, 26(17), 8264. https://doi.org/10.3390/ijms26178264







