The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Sources of Garlic and Its Active Substances
3. Prevention and Treatment of I/R in Different Tissues by Garlic Derivatives
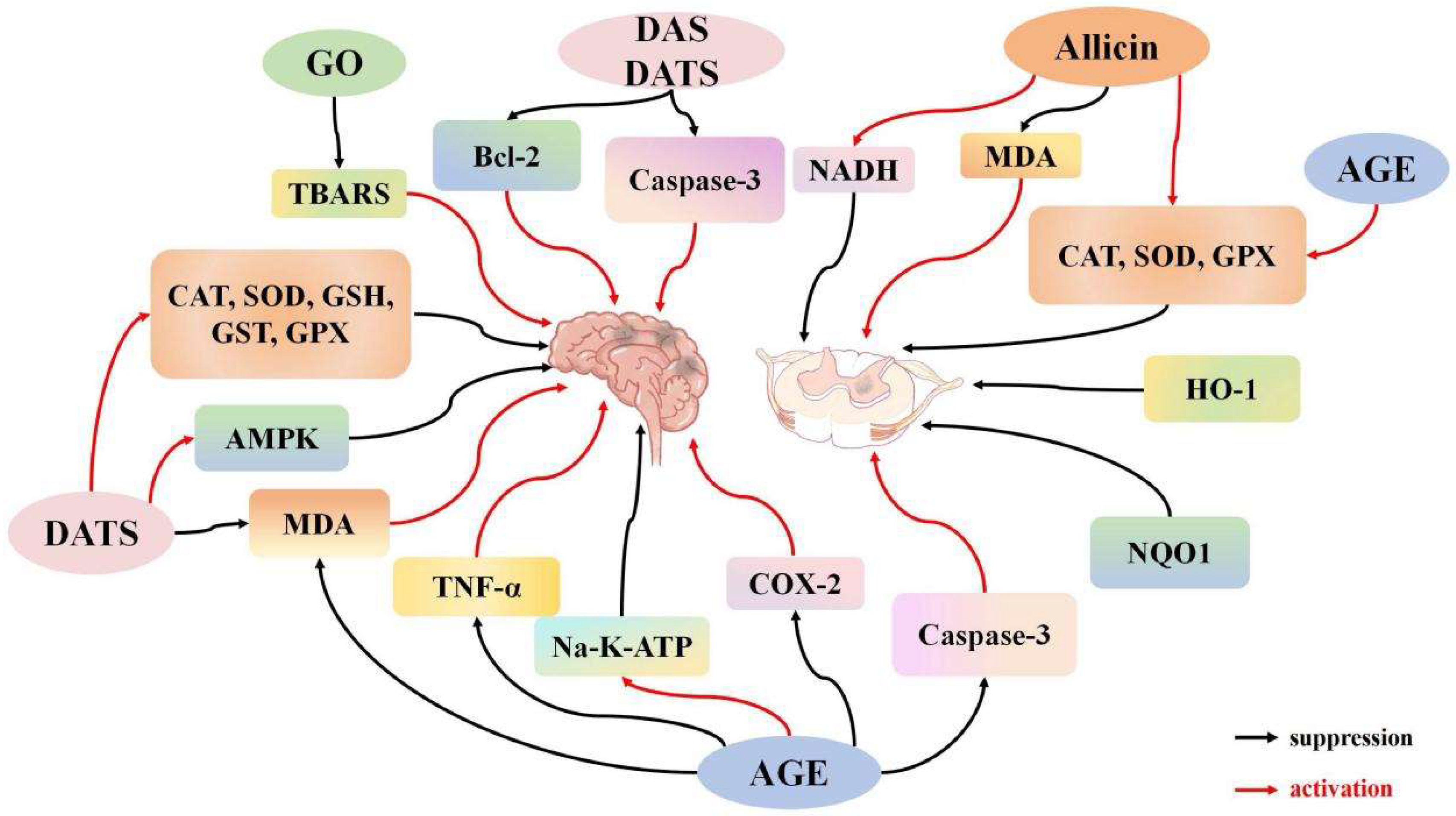
3.1. Impact of Garlic Derivatives on Central Nervous System I/R
3.1.1. Impact of Garlic Derivatives on Cerebral I/R
3.1.2. Effects of Garlic Derivatives on Spinal Cord I/R
3.1.3. Effects of Garlic Derivatives on Eye I/R
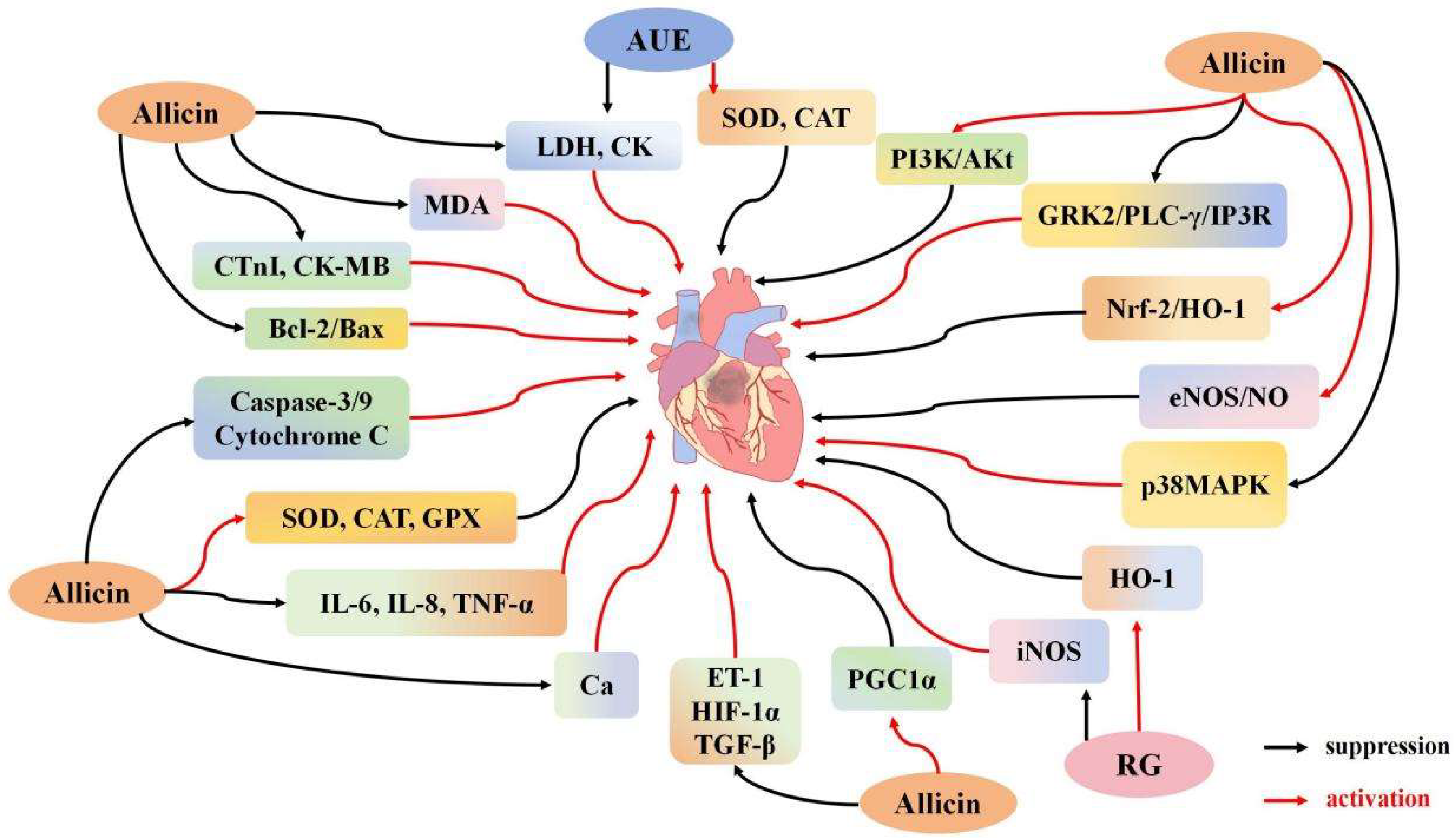
3.2. Effects of Garlic Derivatives on Myocardial I/R
3.2.1. Effect of Garlic Derivatives on Cardiomyocyte Apoptosis
3.2.2. Effects of Garlic Derivatives on Cardiomyocyte Inflammation and Oxidative Stress
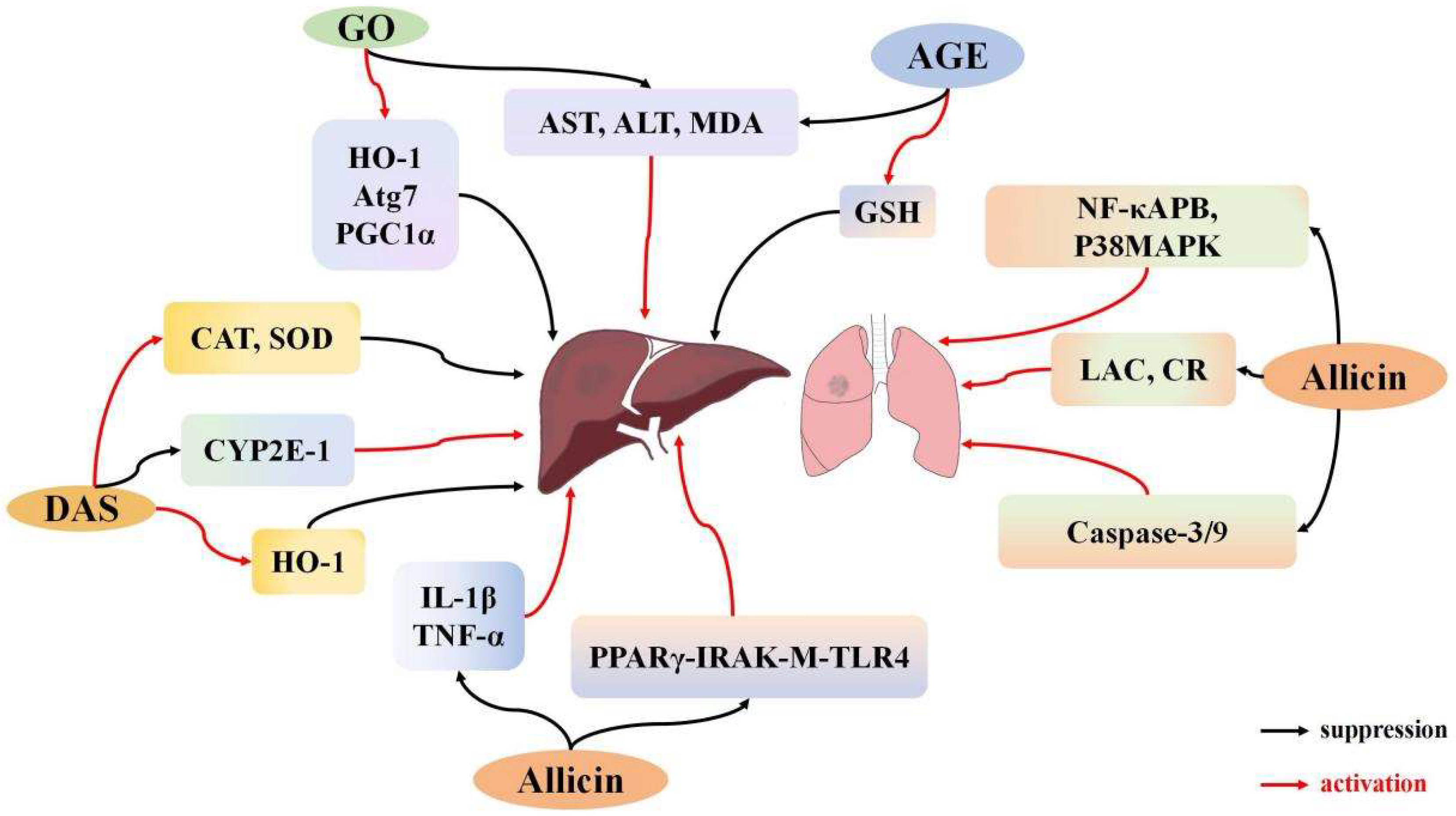
3.3. Effects of Garlic Derivatives on Lung I/R
3.4. Effects of Garlic Derivatives on Liver I/R
3.5. Impact of Garlic Derivatives on Urinary System I/R
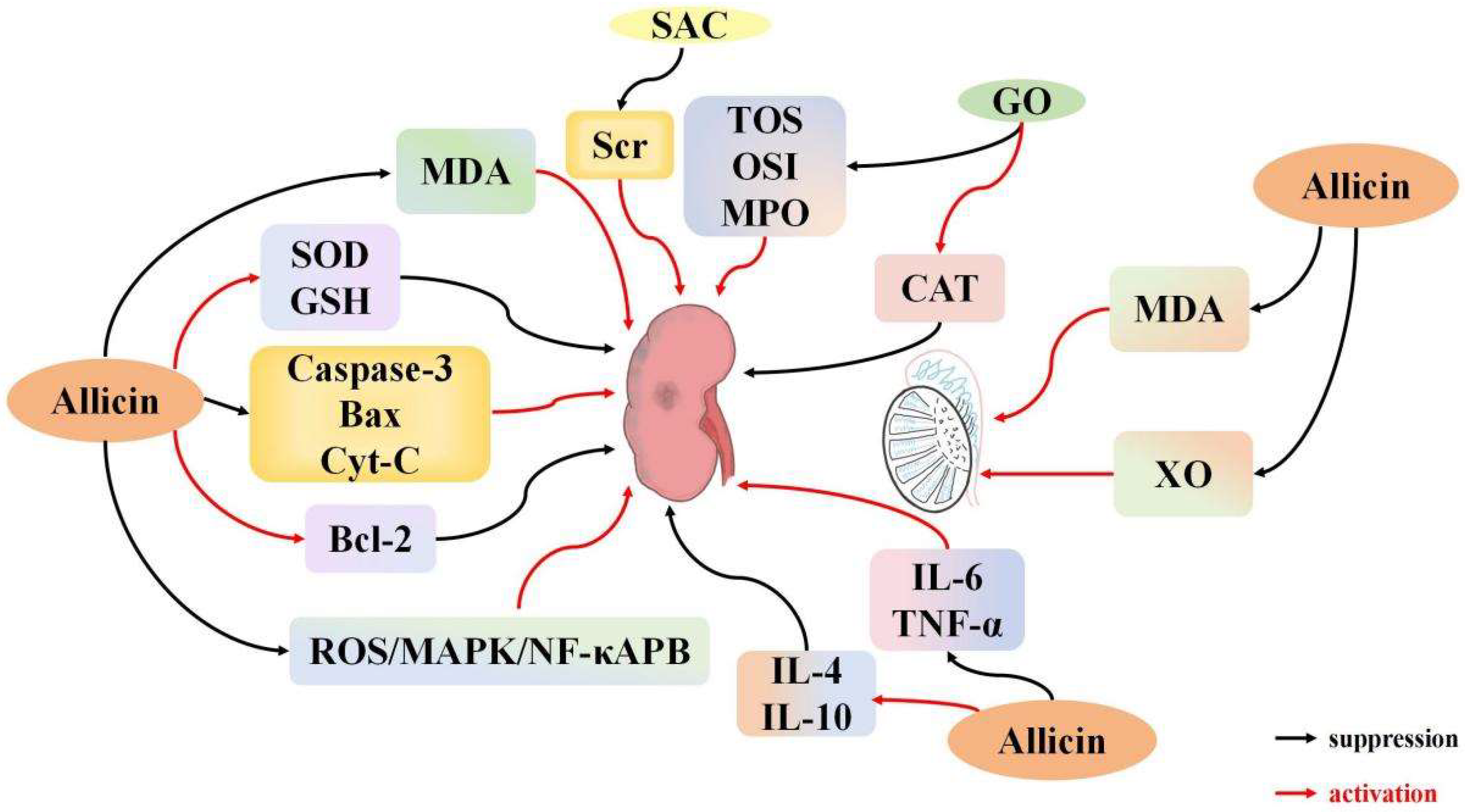
3.5.1. Effects of Garlic Derivatives on Renal I/R
3.5.2. Effects of Garlic Derivatives on Testicular I/R
3.6. Effects of Garlic Derivatives on Skeletal Muscle I/R
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Goncharov, R.G.; Sharapov, M.G. Ischemia-Reperfusion Injury: Molecular Mechanisms of Pathogenesis and Methods of Their Correction. Mol. Biol. 2023, 57, 1150–1174. [Google Scholar] [CrossRef]
- Kishi, M.; Tanaka, H.; Seiyama, A.; Takaoka, M.; Matsuoka, T.; Yoshioka, T.; Sugimoto, H. Pentoxifylline attenuates reperfusion injury in skeletal muscle after partial ischemia. Am. J. Physiol. 1998, 274, H1435–H1442. [Google Scholar] [CrossRef]
- Krause, G.S.; Kumar, K.; White, B.C.; Aust, S.D.; Wiegenstein, J.G. Ischemia, resuscitation, and reperfusion: Mechanisms of tissue injury and prospects for protection. Am. Heart J. 1986, 111, 768–780. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Connern, C.P.; Halestrap, A.P. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem. J. 1994, 302, 321–324. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Gülçin, İ.; Karagöz, B.; Soslu, R.; Alwasel, S.H. A comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, Y.; Wang, P.; Shen, H.; Ling, X.; Xue, X.; Yang, Q.; Zhang, Y.; Xiao, J.; Wang, Z. Role of Hydrogen Sulfide in Myocardial Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. 2021, 77, 30–141. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Seitz, A.P.; Jernigan, P.L.; Gulbins, E.; Edwards, M.J. Ischemia/Reperfusion Injury Alters Sphingolipid Metabolism in the Gut. Cell Physiol. Biochem. 2016, 39, 1262–1270. [Google Scholar] [CrossRef]
- Ornellas, F.M.; Ornellas, D.S.; Martini, S.V.; Castiglione, R.C.; Ventura, G.M.; Rocco, P.R.; Gutfilen, B.; de Souza, S.A.; Takiya, C.M.; Morales, M.M. Bone Marrow-Derived Mononuclear Cell Therapy Accelerates Renal Ischemia-Reperfusion Injury Recovery by Modulating Inflammatory, Antioxidant and Apoptotic Related Molecules. Cell Physiol. Biochem. 2017, 41, 1736–1752. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Majewski, M. Allium sativum: Facts and myths regarding human health. Rocz. Panstw. Zakl. Hig. 2014, 65, 1–8. [Google Scholar]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Bindu Jacob Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Pârvu, M.; Moţ, C.A.; Pârvu, A.E.; Mircea, C.; Stoeber, L.; Roşca-Casian, O.; Ţigu, A.B. Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules 2019, 24, 3958. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gong, S.; Su, L.; Li, C.; Kong, Y. Neuroprotective effects of allicin on ischemia-reperfusion brain injury. Oncotarget 2017, 8, 104492–104507. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Zhao, W.; Li, J.; Li, Q.; Wang, W. Protective effects of allicin against ischemic stroke in a rat model of middle cerebral artery occlusion. Mol. Med. Rep. 2015, 12, 3734–3738. [Google Scholar] [CrossRef]
- Zhao, R.; Xie, E.; Yang, X.; Gong, B. Alliin alleviates myocardial ischemia-reperfusion injury by promoting autophagy. Biochem. Biophys. Res. Commun. 2019, 512, 236–243. [Google Scholar] [CrossRef]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gómez-Gómez, L.; Ahrazem, O. Tissue-Specific Accumulation of Sulfur Compounds and Saponins in Different Parts of Garlic Cloves from Purple and White Ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Nandagopal Ms, G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing Knowledge on the Bioavailability and Biological Activities of Sulphur Compounds from Alliums: A Potential Drug Candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Seebeck, E. Ueber Alliin, die genuine Muttersubstanz des Knoblauchöls. [About Alliin, the genuine mother substance of garlic oil]. Helv. Chim. Acta 1948, 31, 189–210. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bioavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Rahmani, A.H. Garlic and its Active Compounds: A Potential Candidate in The Prevention of Cancer by Modulating Various Cell Signalling Pathways. Anticancer Agents Med. Chem. 2019, 19, 1314–1324. [Google Scholar] [CrossRef]
- Bhatti, R.; Singh, K.; Ishar, M.P.; Singh, J. The effect of Allium sativum on ischemic preconditioning and ischemia reperfusion induced cardiac injury. Indian J. Pharmacol. 2008, 40, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Czompa, A.; Szoke, K.; Prokisch, J.; Gyongyosi, A.; Bak, I.; Balla, G.; Tosaki, A.; Lekli, I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. Int. J. Mol. Sci. 2018, 19, 1017. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Dinda, A.K.; Manchanda, S.C.; Maulik, S.K. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Savas, M.; Yeni, E.; Ciftci, H.; Yildiz, F.; Gulum, M.; Keser, B.S.; Verit, A.; Utangac, M.; Kocyigit, A.; Celik, H.; et al. The antioxidant role of oral administration of garlic oil on renal ischemia-reperfusion injury. Ren. Fail. 2010, 32, 362–367. [Google Scholar] [CrossRef]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Jeremic, N.; Bolevich, S.; Kartashova, M.; Jeremic, J.; et al. New Insight Into the Cardioprotective Effects of Allium ursinum L. Extract Against Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef]
- Lasheen, N.N.; Elayat, W.M.; Elrefai, M.F.M.; Zaki, W.S.; Ahmed, E.H.; El Sheikh, R.M.N.; Abo Rayas, D.S.A.; Gad, F.R.S. Possible role of garlic oil in ameliorating renal injury after liver ischemia/reperfusion in rats. J. Physiol. Pharmacol. 2019, 70, 765–778. [Google Scholar]
- Wei, S.M.; Huang, Y.M. Protective effect of allicin on ischemia-reperfusion injury caused by testicular torsion-detorsion in rats. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2817–2826. [Google Scholar]
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Shaik, I.H.; George, J.M.; Thekkumkara, T.J.; Mehvar, R. Protective effects of diallyl sulfide, a garlic constituent, on the warm hepatic ischemia-reperfusion injury in a rat model. Pharm. Res. 2008, 25, 2231–2242. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmad, M.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Islam, F. Behavioral and histologic neuroprotection of aqueous garlic extract after reversible focal cerebral ischemia. J. Med. Food. 2006, 9, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, D.; Yang, W.; Zhang, H.; Su, Y.; Gao, B.; Zou, X.; Zhong, Y.; Sun, H.; Xiang, L. Diallyl trisulfide improves spinal cord ischemia-reperfusion injury damage by activating AMPK to stabilize mitochondrial function. J. Orthop. Surg. Res. 2023, 18, 838. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Sehirli, O.; Ipçi, Y.; Ercan, F.; Sirvanci, S.; Gedik, N.; Yeğen, B.C. Aqueous garlic extract alleviates ischaemia-reperfusion-induced oxidative hepatic injury in rats. J. Pharm. Pharmacol. 2005, 57, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Han, W.; Yun, G.; Shi, L. Allicin Protects Renal Function, Improves Oxidative Stress and Lipid Peroxidation in Rats with Chronic Renal Failure. Iran. J. Kidney Dis. 2023, 17, 135–140. [Google Scholar]
- Zhu, J.W.; Chen, T.; Guan, J.; Liu, W.B.; Liu, J. Neuroprotective effects of allicin on spinal cord ischemia-reperfusion injury via improvement of mitochondrial function in rabbits. Neurochem. Int. 2012, 61, 640–648. [Google Scholar] [CrossRef]
- Ma, L.N.; Li, L.D.; Li, S.C.; Hao, X.M.; Zhang, J.Y.; He, P.; Li, Y.K. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin. J. Integr. Med. 2017, 23, 589–597. [Google Scholar] [CrossRef]
- Gao, T.; Yang, P.; Fu, D.; Liu, M.; Deng, X.; Shao, M.; Liao, J.; Jiang, H.; Li, X. The protective effect of allicin on myocardial ischemia-reperfusion by inhibition of Ca2+ overload-induced cardiomyocyte apoptosis via the PI3K/GRK2/PLC-γ/IP3R signaling pathway. Aging 2021, 13, 19643–19656. [Google Scholar] [CrossRef]
- Sharma, A.K.; Munajjam, A.; Vaishnav, B.; Sharma, R.; Sharma, A.; Kishore, K.; Sharma, A.; Sharma, D.; Kumari, R.; Tiwari, A.; et al. Involvement of adenosine and standardization of aqueous extract of garlic (Allium sativum Linn.) on cardioprotective and cardiodepressant properties in ischemic preconditioning and myocardial ischemia-reperfusion induced cardiac injury. J. Biomed. Res. 2012, 26, 24–36. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, S.; Deng, L.; Li, Y.; Li, H. Effect of Allicin against Ischemia/Hypoxia-Induced H9c2 Myoblast Apoptosis via eNOS/NO Pathway-Mediated Antioxidant Activity. Evid. Based Complement. Alternat Med. 2018, 2018, 3207973. [Google Scholar] [CrossRef]
- Lin, X.; Yu, S.; Chen, Y.; Wu, J.; Zhao, J.; Zhao, Y. Neuroprotective effects of diallyl sulfide against transient focal cerebral ischemia via anti-apoptosis in rats. Neurol. Res. 2012, 34, 32–37. [Google Scholar] [CrossRef]
- Cemil, B.; Gokce, E.C.; Kahveci, R.; Gokce, A.; Aksoy, N.; Sargon, M.F.; Erdogan, B.; Kosem, B. Aged Garlic Extract Attenuates Neuronal Injury in a Rat Model of Spinal Cord Ischemia/Reperfusion Injury. J. Med. Food. 2016, 19, 601–606. [Google Scholar] [CrossRef]
- Deng, X.; Yang, P.; Gao, T.; Liu, M.; Li, X. Allicin attenuates myocardial apoptosis, inflammation and mitochondrial injury during hypoxia-reoxygenation: An in vitro study. BMC Cardiovasc. Disord. 2021, 21, 200. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, H.P.; Huang, F.F.; Wu, W.; Gao, Y.; Chen, Z.B.; Liang, Z.Y.; Liang, T.B. Allicin, a major component of garlic, inhibits apoptosis in vital organs in rats with trauma/hemorrhagic shock. Crit. Care Med. 2008, 36, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ning, J.; Huang, H.; Jiang, S.; Zhuo, D. Allicin protects against renal ischemia-reperfusion injury by attenuating oxidative stress and apoptosis. Int. Urol. Nephrol. 2022, 54, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, J.; Li, A.; Wang, Y.; Shi, Z.; Ke, Y.; Li, X. Garlicin attenuates reperfusion no-reflow in a catheter-based porcine model of acute myocardial infarction. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 285–289. [Google Scholar]
- Colín-González, A.L.; Ortiz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Pedraza-Chaverrí, J.; Maldonado, P.D. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum. Nutr. 2011, 66, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mottaleb, N.A.; Mahmoud, G.S.; Negm, E.A.; Abdel Maksoud, F.M. Garlic antagonizes skeletal muscle ischemia reperfusion injury through regulating inflammation, apoptosis and desmin expression in adult male rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 126–137. [Google Scholar]
- Liu, S.; He, Y.; Shi, J.; Liu, L.; Ma, H.; He, L.; Guo, Y. Allicin Attenuates Myocardial Ischemia Reperfusion Injury in Rats by Inhibition of Inflammation and Oxidative Stress. Transplant. Proc. 2019, 51, 2060–2065. [Google Scholar] [CrossRef]
- Li, W.; Huang, R.; Gong, X.; Zhao, Z.; Zhang, L.; Zhou, Q.; Jiang, X.; Tie, H.; Wan, J.; Wang, B. Allicin attenuated hepatic ischemia/reperfusion injury in mice by regulating PPARγ-IRAK-M-TLR4 signal pathway. Food Funct. 2022, 13, 7361–7376. [Google Scholar] [CrossRef]
- Shan, Y.; Chen, D.; Hu, B.; Xu, G.; Li, W.; Jin, Y.; Jin, X.; Jin, X.; Jin, L. Allicin ameliorates renal ischemia/reperfusion injury via inhibition of oxidative stress and inflammation in rats. Biomed. Pharmacother. 2021, 142, 112077. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, L.; Qin, J.J.; Wang, L.; Wang, H.; Zou, Y.; Zhu, X.; Hong, Y.; Zhang, Y.; Liu, Y.; et al. CARD3 Promotes Cerebral Ischemia-Reperfusion Injury Via Activation of TAK1. J. Am. Heart Assoc. 2020, 9, e014920. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.; Chao, X.; Qu, Y.; Zhang, L.; Luo, P.; Xie, K.; Huo, J.; Fei, Z. Neuroprotective effect of osthole against oxygen and glucose deprivation in rat cortical neurons: Involvement of mitogen-activated protein kinase pathway. Neuroscience 2011, 183, 203–211. [Google Scholar] [CrossRef]
- Bautista-Perez, S.M.; Silva-Islas, C.A.; Sandoval-Marquez, O.U.; Toledo-Toledo, J.; Bello-Martínez, J.M.; Barrera-Oviedo, D.; Maldonado, P.D. Antioxidant and Anti-Inflammatory Effects of Garlic in Ischemic Stroke: Proposal of a New Mechanism of Protection through Regulation of Neuroplasticity. Antioxidants 2023, 12, 2126. [Google Scholar] [CrossRef]
- Liu, K.; Yan, L.; Jiang, X.; Yu, Y.; Liu, H.; Gu, T.; Shi, E. Acquired inhibition of microRNA-124 protects against spinal cord ischemia-reperfusion injury partially through a mitophagy-dependent pathway. J. Thorac. Cardiovasc. Surg. 2017, 154, 1498–1508. [Google Scholar] [CrossRef]
- Ege, E.; Ilhan, A.; Gurel, A.; Akyol, O.; Ozen, S. Erdosteine ameliorates neurological outcome and oxidative stress due to ischemia/reperfusion injury in rabbit spinal cord. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 379–386. [Google Scholar] [CrossRef]
- Jiang, M.; Lai, X.; Zhang, Y.; Shen, M.; Ma, H.; Liu, A.; Wu, J.; Yan, J. Artemisinin Alleviates Cerebral Ischemia/Reperfusion-Induced Oxidative Damage via Regulating PHB2-Mediated Autophagy in the Human Neuroblastoma SH-SY5Y Cell Line. Oxid. Med. Cell Longev. 2022, 2022, 6568748. [Google Scholar] [CrossRef]
- Yue, Y.; Zhao, H.; Yue, Y.; Zhang, Y.; Wei, W. Downregulation of Microrna-421 Relieves Cerebral Ischemia/Reperfusion Injuries: Involvement of Anti-apoptotic and Antioxidant Activities. Neuromol. Med. 2020, 22, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Rovcanin, B.; Medic, B.; Kocic, G.; Cebovic, T.; Ristic, M.; Prostran, M. Molecular Dissection of Renal Ischemia-Reperfusion: Oxidative Stress and Cellular Events. Curr. Med. Chem. 2016, 23, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A. Aqueous garlic extract attenuates hepatitis and oxidative stress induced by galactosamine/lipoploysaccharide in rats. Phytother. Res. 2008, 22, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Chipuk, J.E. Death upon a kiss: Mitochondrial outer membrane composition and organelle communication govern sensitivity to BAK/BAX-dependent apoptosis. Chem. Biol. 2014, 21, 114–123. [Google Scholar] [CrossRef]
- Mikhailov, V.; Mikhailova, M.; Pulkrabek, D.J.; Dong, Z.; Venkatachalam, M.A.; Saikumar, P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001, 21, 18361–18374. [Google Scholar] [CrossRef]
- Cheng, P.; Dai, W.; Wang, F.; Lu, J.; Shen, M.; Chen, K.; Li, J.; Zhang, Y.; Wang, C.; Yang, J.; et al. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem. Biophys. Res. Commun. 2014, 443, 1162–1168. [Google Scholar] [CrossRef]
- Saitoh, Y.; Ouchida, R.; Kayasuga, A.; Miwa, N. Anti-apoptotic defense of bcl-2 gene against hydroperoxide-induced cytotoxicity together with suppressed lipid peroxidation, enhanced ascorbate uptake, and upregulated Bcl-2 protein. J. Cell. Biochem. 2003, 89, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Chen, C.; Guo, Y.; Meng, X.; Kan, C. Allicin attenuates lipopolysaccharide-induced acute lung injury in neonatal rats via the PI3K/Akt pathway. Mol. Med. Rep. 2018, 17, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, X.; Li, X.; Ma, C.; Xu, L.; Guo, X.; Li, J.; Wang, H.; Han, Y. Allicin Protects against Cisplatin-Induced Stria Vascularis Damage: Possible Relation to Inhibition of Caspase-3 and PARP-1-AIF-Mediated Apoptotic Pathways. ORL J. Otorhinolaryngol. Relat. Spec. 2019, 81, 202–214. [Google Scholar] [CrossRef]
- Kiss, L.; Bocsik, A.; Walter, F.R.; Ross, J.; Brown, D.; A Mendenhall, B.; Crews, S.R.; Lowry, J.; Coronado, V.; E Thompson, D.; et al. From the Cover: In Vitro and In Vivo Blood-Brain Barrier Penetration Studies with the Novel Cyanide Antidote Candidate Dimethyl Trisulfide in Mice. Toxicol. Sci. 2017, 2, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Fan, J.; Ji, C.; Wang, Z.; Ge, X.; Wang, J.; Ye, W.; Yin, G.; Cai, W.; Liu, W. USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia-reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ. 2022, 29, 1164–1175. [Google Scholar] [CrossRef]
- Wynn, M.M.; Acher, C.W. A modern theory of spinal cord ischemia/injury in thoracoabdominal aortic surgery and its implications for prevention of paralysis. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1088–1099. [Google Scholar] [CrossRef]
- Bell, M.T.; Puskas, F.; Agoston, V.A.; Cleveland, J.C., Jr.; Freeman, K.A.; Gamboni, F.; Herson, P.S.; Meng, X.; Smith, P.D.; Weyant, M.J.; et al. Toll-like receptor 4-dependent microglial activation mediates spinal cord ischemia-reperfusion injury. Circulation 2013, 128, S152–S156. [Google Scholar] [CrossRef]
- Andreadi, C.K.; Howells, L.M.; Atherfold, P.A.; Manson, M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharmacol. 2006, 69, 1033–1040. [Google Scholar] [CrossRef]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef]
- Agarwal, A.; Bolisetty, S. Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 111–122. [Google Scholar]
- Tu, G.; Zhang, Y.-F.; Wei, W.; Li, L.; Zhang, Y.; Yang, J.; Xing, Y. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol. Med. Rep. 2016, 13, 2320–2326. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.; So, K.F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE 2014, 1, e84800. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, W. Oxypaeoniflorin improves myocardial ischemia/reperfusion injury by activating the Sirt1/Foxo1 signaling pathway. Acta Biochim. Pol. 2020, 67, 239–245. [Google Scholar] [CrossRef]
- Peng, K.; Liu, H.; Yan, B.; Meng, X.W.; Song, S.Y.; Ji, F.H.; Xia, Z. Inhibition of cathepsin S attenuates myocardial ischemia/reperfusion injury by suppressing inflammation and apoptosis. J. Cell Physiol. 2021, 236, 1309–1320. [Google Scholar] [CrossRef]
- Charles, C.J.; Li, R.R.; Yeung, T.; Mazlan, S.M.I.; Lai, R.C.; de Kleijn, D.P.V.; Lim, S.K.; Richards, A.M. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization with MRI in a Porcine Model. Front. Cardiovasc. Med. 2020, 7, 601990. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, J.; Bai, J.; Pu, P.; Liu, J.; Wang, F.; Ruan, B. Suv39h1 protects from myocardial ischemia-reperfusion injury in diabetic rats. Cell Physiol. Biochem. 2014, 33, 1176–1185. [Google Scholar] [CrossRef]
- Maarman, G.; Marais, E.; Lochner, A.; du Toit, E.F. Effect of chronic CPT-1 inhibition on myocardial ischemia-reperfusion injury (I/R) in a model of diet-induced obesity. Cardiovasc. Drugs Ther. 2012, 26, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 12, 773–789. [Google Scholar] [CrossRef]
- Freude, B.; Masters, T.N.; Kostin, S.; Robicsek, F.; Schaper, J. Cardiomyocyte apoptosis in acute and chronic conditions. Basic Res. Cardiol. 1998, 93, 85–89. [Google Scholar] [CrossRef]
- Wang, D.; Chen, T.Y.; Liu, F.J. Che-1 attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by upregulation of Nrf2 signaling. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1084–1093. [Google Scholar]
- Zhang, S.; Wang, Y.; Wang, P.; Xuan, J. miR-708 affords protective efficacy in anoxia/reoxygenation-stimulated cardiomyocytes by blocking the TLR4 signaling via targeting HMGB1. Mol. Cell. Probes 2020, 54, 101653. [Google Scholar] [CrossRef]
- Wu, M.; Lu, S.; Zhong, J.; Huang, K.; Zhang, S. Protective Effects of Pterostilbene Against Myocardial Ischemia/Reperfusion Injury in Rats. Inflammation 2017, 40, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Rietz, B.; Isensee, H.; Strobach, H.; Makdessi, S.; Jacob, R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol Cell Biochem. 1993, 1–2, 143–150. [Google Scholar] [CrossRef]
- Khatua, T.N.; Borkar, R.M.; Mohammed, S.A.; Dinda, A.K.; Srinivas, R.; Banerjee, S.K. Novel Sulfur Metabolites of Garlic Attenuate Cardiac Hypertrophy and Remodeling through Induction of Na+/K+-ATPase Expression. Front. Pharmacol. 2017, 8, 18. [Google Scholar] [CrossRef]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef]
- Abdelall, A.M.A.; Khames, A.; Bekhit, A.A.; Fathy, M. Potential Effect of Etoricoxib in Reducing Inflammation in Methotrexate-Induced Pulmonary Injury in Rats: Role of Oxidative Stress and the TLR4/p38-MAPK/NF-κB Signaling Pathway. Inflammation 2024, 48, 2407–2416. [Google Scholar] [CrossRef]
- Batirel, H.F.; Naka, Y.; Kayano, K.; Okada, K.; Vural, K.; Pinsky, D.J.; Oz, M.C. Intravenous allicin improves pulmonary blood flow after ischemia-reperfusion injury in rats. J. Cardiovasc. Surg. 2002, 43, 175–179. [Google Scholar]
- Teodoro, J.S.; Da Silva, R.T.; Machado, I.F.; Panisello-Roselló, A.; Roselló-Catafau, J.; Rolo, A.P.; Palmeira, C.M. Shaping of Hepatic Ischemia/Reperfusion Events: The Crucial Role of Mitochondria. Cells 2022, 11, 688. [Google Scholar] [CrossRef]
- Aydin, I.; Sehitoglu, I.; Ozer, E.; Kalkan, Y.; Tumkaya, L.; Cure, M.C.; Cure, E. High dose zoledronic acid increases ischemia-reperfusion damage of the liver. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3567–3575. [Google Scholar]
- Zhou, Y.; Tan, Z.; Huang, H.; Zeng, Y.; Chen, S.; Wei, J.; Huang, G.; Qian, C.; Yuan, G.; He, S. Baicalein pre-treatment alleviates hepatic ischemia/reperfusion injury in mice by regulating the Nrf2/ARE pathway. Exp. Ther. Med. 2021, 22, 1380. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yu, K.H.; Zhen, S.Q. MiR-93 blocks STAT3 to alleviate hepatic injury after ischemia-reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5295–5304. [Google Scholar]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Siempis, T.; Theodorakou, E.; Tsoulfas, G. Hepatic ischemia and reperfusion injury and trauma: Current concepts. Arch. Trauma Res. 2013, 2, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Samra, Y.A.; Hamed, M.F.; El-Sheakh, A.R. Hepatoprotective effect of allicin against acetaminophen-induced liver injury: Role of inflammasome pathway, apoptosis, and liver regeneration. J. Biochem. Mol. Toxicol. 2020, 34, e22470. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liao, Y.; Wang, Q.; Liu, L.; Yang, W. Current studies and potential future research directions on biological effects and related mechanisms of allicin. Crit. Rev. Food Sci. Nutr. 2023, 63, 7722–7748. [Google Scholar] [CrossRef]
- Hobson, C.; Ozrazgat-Baslanti, T.; Kuxhausen, A.; Thottakkara, P.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and Mortality Associated with Postoperative Acute Kidney Injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 2009, 130, 41–50. [Google Scholar] [CrossRef]
- Chok, M.K.; Ferlicot, S.; Conti, M.; Almolki, A.; Dürrbach, A.; Loric, S.; Benoît, G.; Droupy, S.; Eschwège, P. Renoprotective potency of heme oxygenase-1 induction in rat renal ischemia-reperfusion. Inflamm. Allergy-Drug Targets 2009, 8, 252–259. [Google Scholar] [CrossRef]
- Meng, F.J.; Hou, Z.W.; Li, Y.; Yang, Y.; Yu, B. The protective effect of picroside II against hypoxia/reoxygenation injury in neonatal rat cardiomyocytes. Pharm. Biol. 2012, 50, 1226–1232. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Oh, C.J.; Kim, M.J.; Lee, J.M.; Kim, D.H.; Kim, I.Y.; Park, S.; Kim, Y.; Lee, K.B.; Lee, S.H.; Lim, C.W.; et al. Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion. Kidney Int. 2023, 104, 724–739. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Segoviano-Murillo, S.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Cruz, C.; Maldonado, P.D.; Pedraza-Chaverrí, J. S-allylcysteine ameliorates ischemia and reperfusion induced renal damage. Phytother. Res. 2008, 22, 836–840. [Google Scholar] [CrossRef]
- Ali, S.I.; Alhusseini, N.F.; Atteia, H.H.; Idris, R.A.; Hasan, R.A. Renoprotective effect of a combination of garlic and telmisartan against ischemia/reperfusion-induced kidney injury in obese rats. Free Radic. Res. 2016, 50, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Unsal, A.; Eroglu, M.; Avci, A.; Cimentepe, E.; Guven, C.; Derya Balbay, M.; Durak, I. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand. J. Urol. Nephrol. 2006, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, W.Y.; Mostafa-Hedeab, G.; Sayed AboBakr Ali, A.H.; Fawzy, M.A.; Ahmed, A.F.; Bahaa El-Deen, M.A.; Welson, N.N.; Aly Labib, D.A. Idebenone regulates sirt1/Nrf2/TNF-α pathway with inhibition of oxidative stress, inflammation, and apoptosis in testicular torsion/detorsion in juvenile rats. Hum. Exp. Toxicol. 2022, 41, 9603271221102515. [Google Scholar] [CrossRef]
- Garbaisz, D.; Turoczi, Z.; Aranyi, P.; Fulop, A.; Rosero, O.; Hermesz, E.; Ferencz, A.; Lotz, G.; Harsanyi, L.; Szijarto, A. Attenuation of skeletal muscle and renal injury to the lower limb following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS ONE 2014, 9, e101067. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Ma, J.; Yang, P.; Zhou, Z.; Song, T.; Jia, L.; Ye, X.; Yan, W.; Sun, J.; Ye, T.; Zhu, L. GYY4137-induced p65 sulfhydration protects synovial macrophages against pyroptosis by improving mitochondrial function in osteoarthritis development. J. Adv. Res. 2025, 71, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.P.; Wei, W.; Gauld, J.W.; Auld, J.; Özcan, F.; Aslan, M.; Mutus, B. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is inactivated by S-sulfuration in vitro. Free Radic. Biol. Med. 2015, 89, 512–521. [Google Scholar] [CrossRef] [PubMed]
| Action | Allicin (Concentration) | Models | Mechanisms | Publication Date | Reference |
|---|---|---|---|---|---|
| Antioxidant | Allicin (0.5%) | Myocardial I/R | LDH, CK ↓ | 2008 | Bhatti et al. [27] |
| RG (300 mg/kg), ABG (300 mg/kg) | Myocardial I/R | HO-1, iNOS ↑ | 2018 | Czompa et al. [28] | |
| Allicin (125, 250, 500 mg/kg) | Myocardial I/R | SOD, GSH, and cardiac catalase ↑ | 2002 | Banerjee et al. [29] | |
| GO (200 mg/kg) | Kidney I/R | TOS, OSI, MPO, NO, and PC ↓ | 2010 | Savas et al. [30] | |
| Allicin (125, 250, 500 mg/kg) | Myocardial I/R | SOD, CAT, and dp/dt max ↑ | 2021 | Rankovic et al. [31] | |
| GO (5 mL/kg) | Liver I/R | HO-1, ATG7, and PGC1α ↑ | 2019 | Lasheen et al. [32] | |
| Allicin (50 mg/kg) | Testicular I/R | xanthine oxidase, ROS ↓ | 2024 | Wei et al. [33] | |
| Garlic oil (23 mg/kg, 46 mg/kg) | Brain I/R | TBARS ↓ | 2003 | Gupta et al. [34] | |
| DAS (1.75 mmol/kg) | Liver I/R | HO-1 ↑, CYP2E1 ↓ | 2008 | Shaik et al. [35] | |
| Allicin (500 mg/kg) | Brain I/R | MDA ↓, GSH, CAT, SOD, Na+-K+ -ATPase ↑ | 2006 | Saleem et al. [36] | |
| DATS (40 mg/kg) | Brain I/R | Bax, cleaved caspase-3, MDA ↓, AMPK, Bcl-2, GSH, SOD ↑ | 2023 | Sun et al. [37] | |
| AGE (1 mL/kg) | Liver I/R | AST, ALT, MDA, and GSH ↓ | 2005 | Sener et al. [38] | |
| Kidney I/R | ROS/MAPK/NF-κARB pathway ↓, SOD, GSH ↑, MDA, ROS ↓ | 2023 | Xu N et al. [39] | ||
| Alleviating mitochondrial dysfunction | Allicin (1, 10, 50 mg/kg) | Spinal cord I/R | ROS, mitochondrial cytochrome C ↓, CAT, SOD, GPX, GST ↑ | 2012 | Zhu et al. [40] |
| Anti-apoptotic | Allicin (1.2, 1.8, 3.6 mg/kg) | Myocardial I/R | Bcl-2/Bax pathway and LDH, CK ↓ | 2017 | Ma et al. [41] |
| Allicin (1.88 mg/kg) | Myocardial I/R | GRK2, PLC-α, IP3R signaling pathways ↓ | 2021 | Gao et al. [42] | |
| Allicin (0.05%) | Myocardial I/R | Bax/Bcl-2 phosphorylation levels of P-38MAPK and JNK ↓ | 2012 | Sharma et al. [43] | |
| Allicin (0.2, 1, 5 μM) | H9C2 cell I/H | Bax, MDA ↓, eNOS/NO pathway, Nrf-2, HO-1, Bcl-2, SOD ↑ | 2018 | Ma et al. [44] | |
| DAS (100, 150, 200 mg/kg) | Brain I/R | caspase-3 ↓, Bcl-2 ↑ | 2012 | Lin et al. [45] | |
| AGE (250 mg/kg) | Spinal cord I/R | MDA, NO, TNF-α, caspase-3 ↓, SOD, GSH-Px, CAT ↑ | 2016 | Cemil B et al. [46] | |
| Hypoxia-reoxygenation model of myocardial cells | Bax, cleaved caspase-3, IL-6, TNF-α, cytochrome C ↓, Bcl-2 ↑ | 2021 | Deng X et al. [47] | ||
| Allicin (30 μg/kg) | Lung I/R | P38 MAPK pathway ↑, nuclear factor NF-κAPB, phosphorylated P38 MAPK, Caspase-3, Caspase-9 ↓ | 2008 | Zhang Y et al. [48] | |
| Allicin (40, 50, 60 mg/kg) | Kidney I/R | Caspase-3, Bax, MDA ↓, Bcl-2 ↑ | 2022 | Li M et al. [49] | |
| Anti-inflammatory | Allicin (1.88 mg/kg) | Myocardial I/R | IL-6, TNF-α, EV-1 ↓ | 2012 | Peng et al. [50] |
| Allicin (1.2 mL/kg) | Brain I/R | TNF-α, COX-2 ↓ | 2011 | Colín-González et al. [51] | |
| Allicin (500 mg/kg) | Skeletal muscle I/R | W/D, IL-1 ↓, IL-10 ↑ | 2019 | Abd El-Mottaleb et al. [52] | |
| Allicin (50 mg/kg) | Myocardial I/R | p38MAPK pathway, p-p38, MDA, TNF-α, IL-6, IL-8 ↓, SOD, CAT, GPx ↑ | 2019 | Liu S et al. [53] | |
| Liver I/R | IL-1β, TNF-α, PPARγ-IRAK-M-TLR4 signaling pathway ↓ | 2022 | Li W et al. [54] | ||
| Kidney I/R | SOD, IL-4, IL-10, Bcl-2 ↑, MDA, IL-6, TNF-α, Bax, Caspase-3, Cyt-C ↓ | 2021 | Shan Y et al. [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Han, N.; Mao, C.; Chen, J.; Cheng, N.; Zhao, J.; Song, Y.; Sun, X. The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2025, 26, 8257. https://doi.org/10.3390/ijms26178257
Wang C, Han N, Mao C, Chen J, Cheng N, Zhao J, Song Y, Sun X. The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury. International Journal of Molecular Sciences. 2025; 26(17):8257. https://doi.org/10.3390/ijms26178257
Chicago/Turabian StyleWang, Chunlei, Ning Han, Caiyun Mao, Jiaxu Chen, Nana Cheng, Jieyou Zhao, Yunjia Song, and Xutao Sun. 2025. "The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury" International Journal of Molecular Sciences 26, no. 17: 8257. https://doi.org/10.3390/ijms26178257
APA StyleWang, C., Han, N., Mao, C., Chen, J., Cheng, N., Zhao, J., Song, Y., & Sun, X. (2025). The Therapeutic Potential of Garlic-Derived Organic Polysulfides for Ischemia-Reperfusion Injury. International Journal of Molecular Sciences, 26(17), 8257. https://doi.org/10.3390/ijms26178257







