Sulfur-Containing Heterocyclic Aromatic Hydrocarbons Alter Estrogen Metabolism and Cause DNA Damage and Apoptosis in Granulosa Cells
Abstract
1. Introduction
2. Results
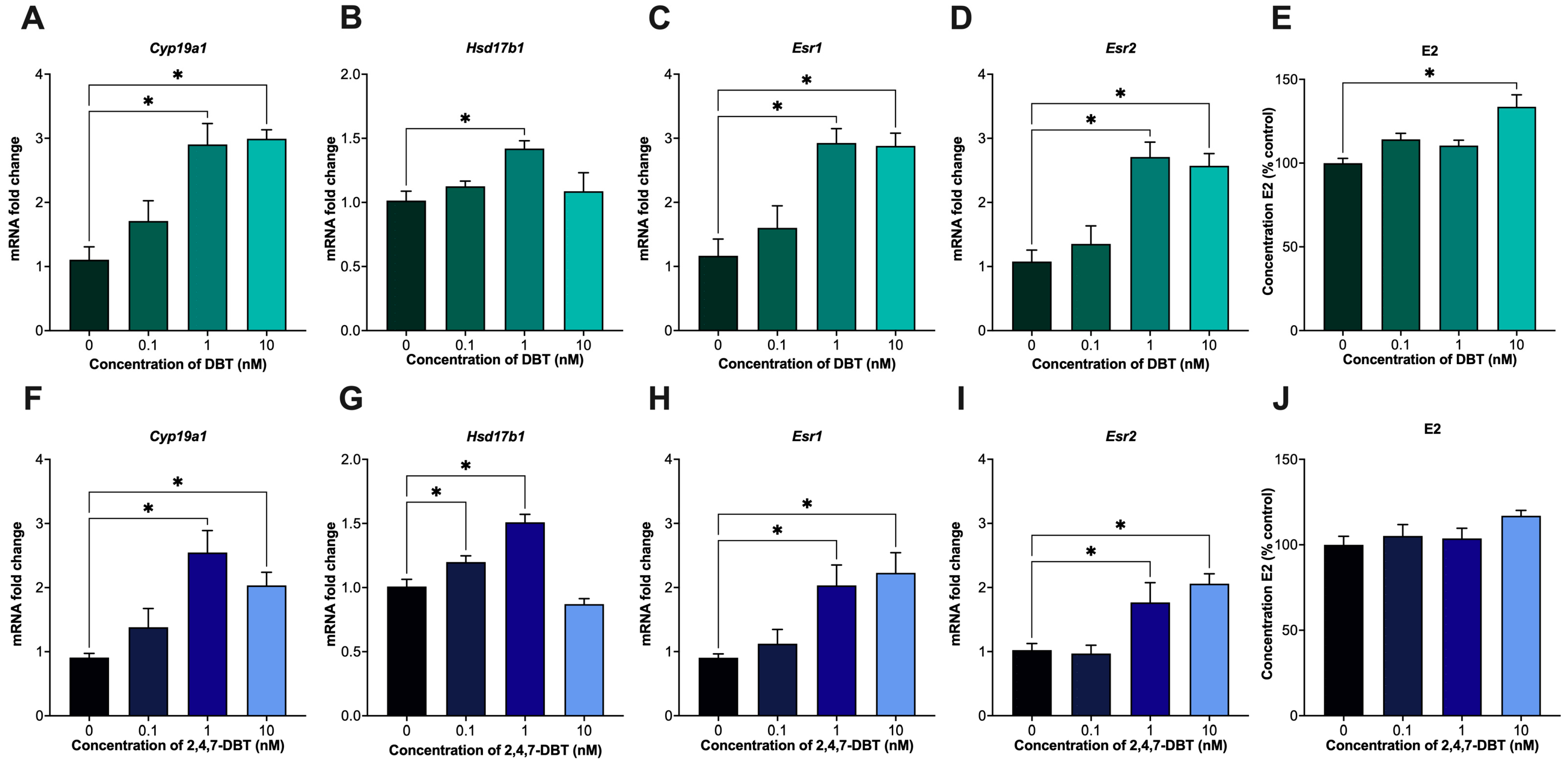
2.1. Exposure to DBT and Alkylated DBT Alters Estrogen Signaling and Synthesis
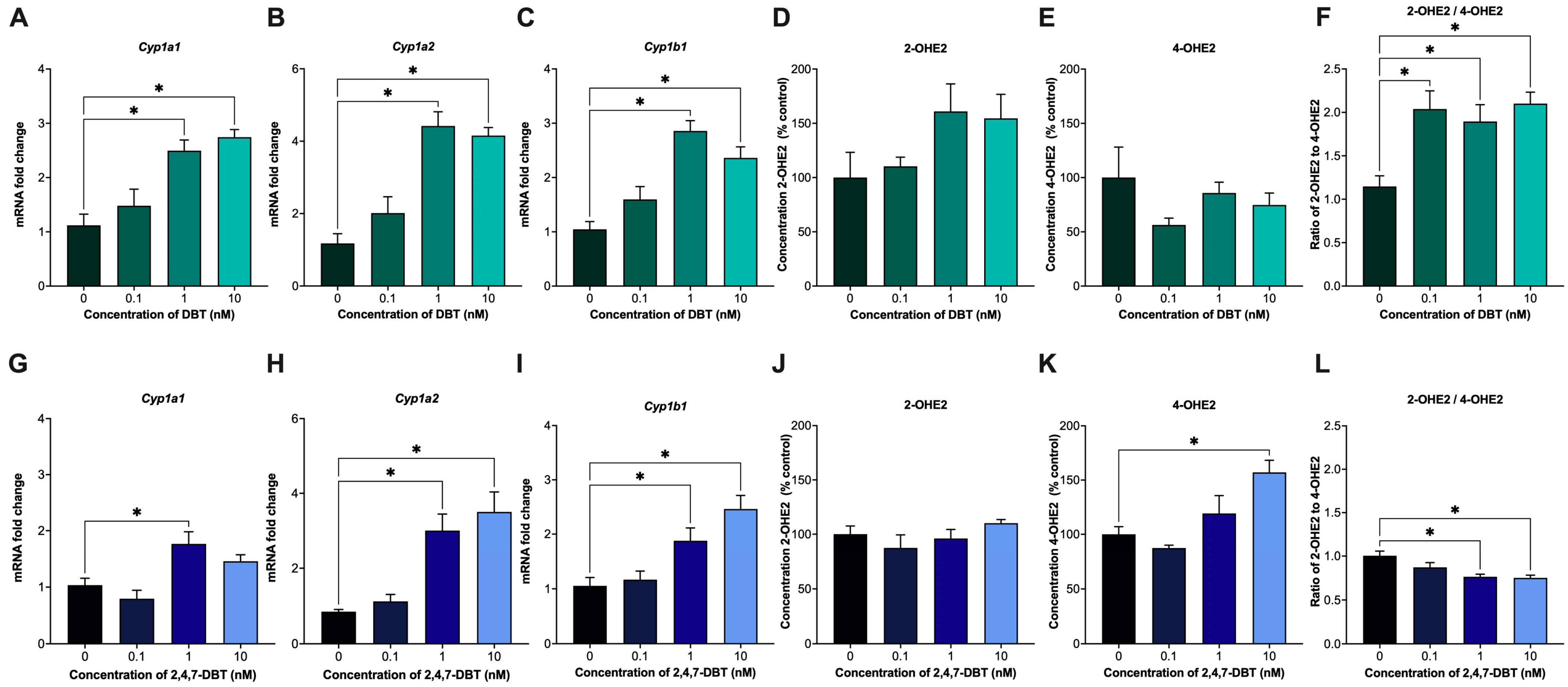
2.2. Exposure to 2,4,7-DBT Increases the Production of Estradiol Metabolite, 4-OHE2
2.3. Both DBT and 2,4,7-DBT Cause DNA Damage in Granulosa Cells
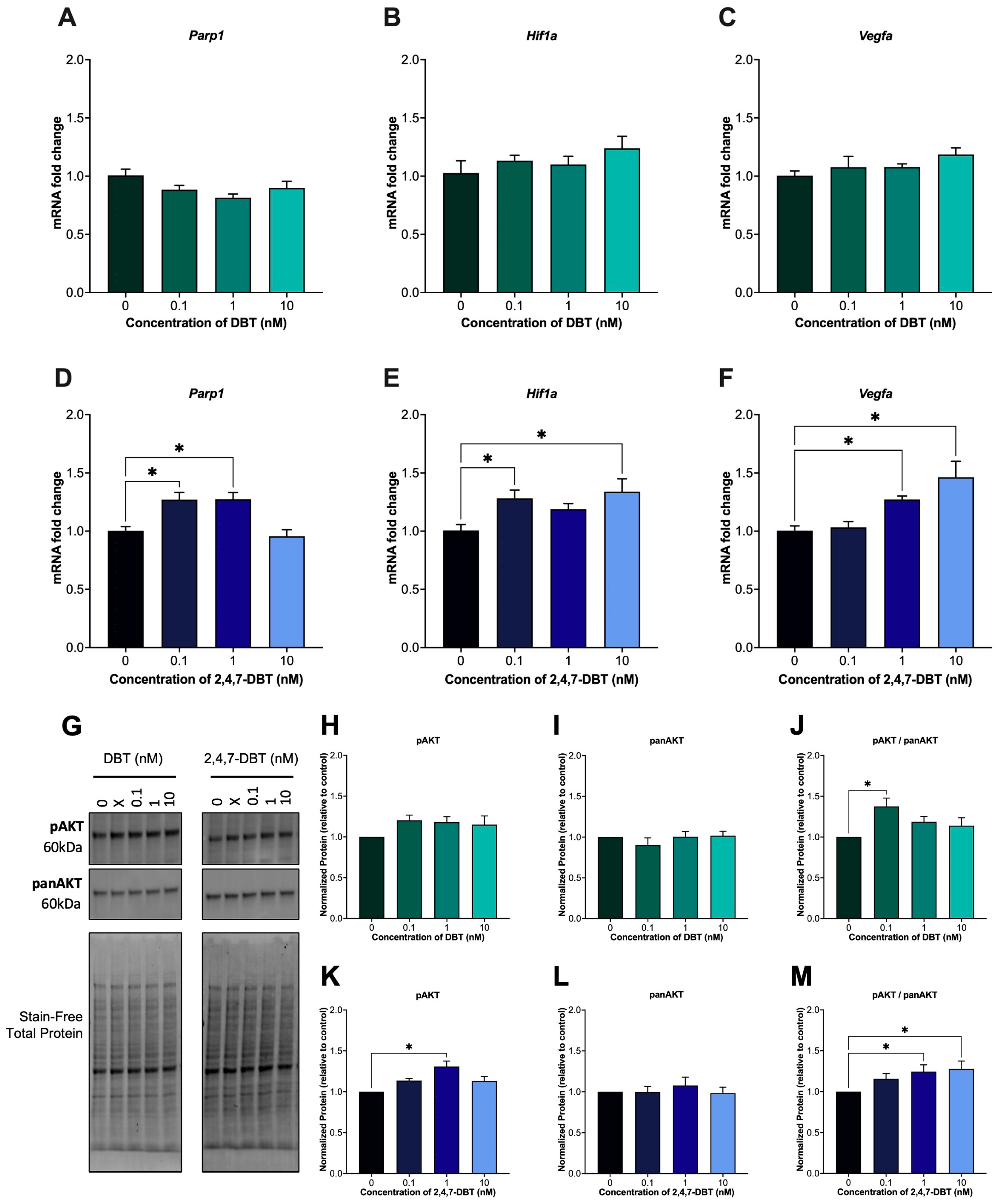
2.4. DBT and 2,4,7-DBT Elicit Distinct Responses to DNA Damage, with 2,4,7-DBT Inducing Parp1 Expression and Activation of the AKT Signaling Pathway
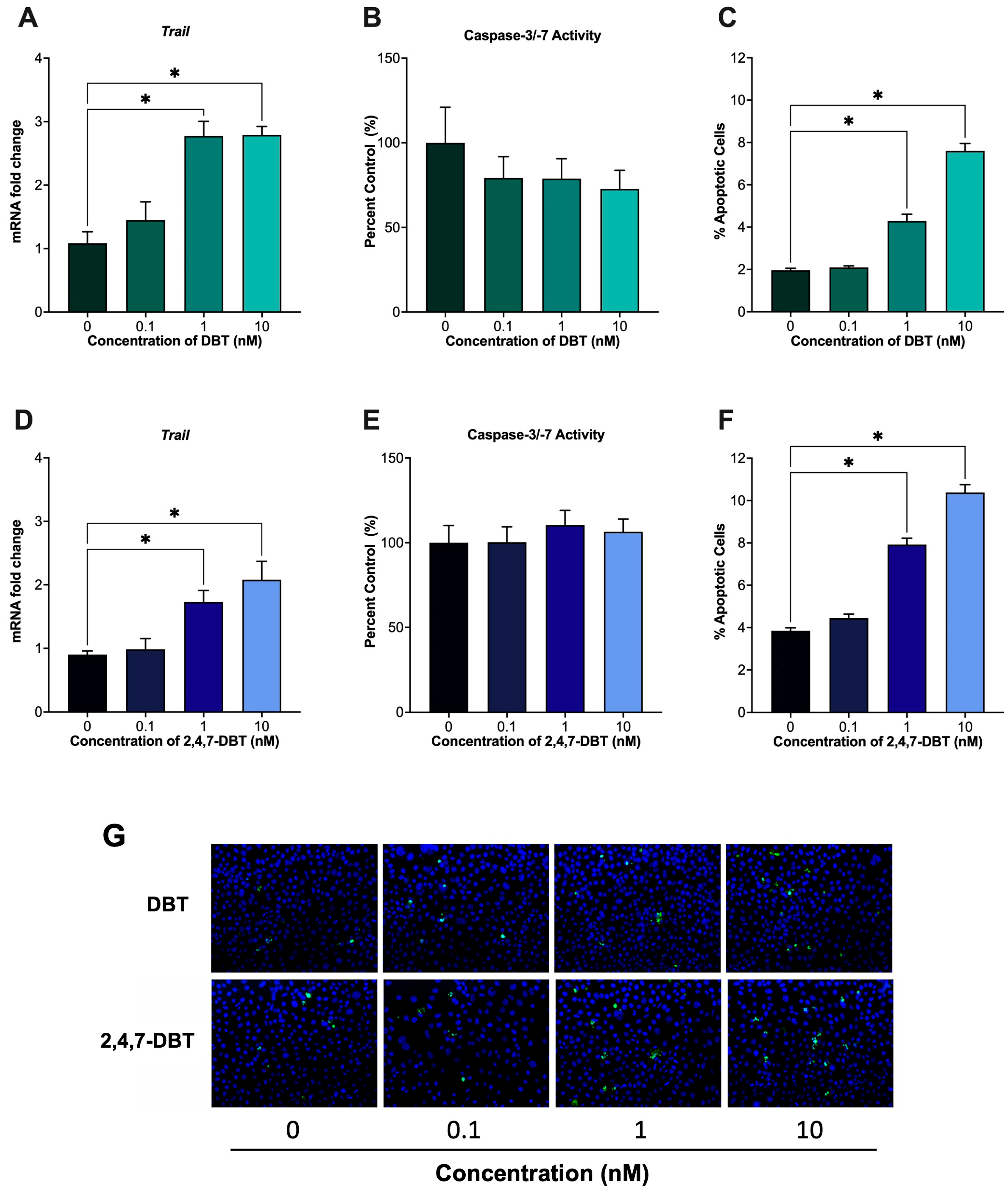
2.5. Exposure to 1 and 10 nM DBT or 2,4,7-DBT Increased Granulosa Cell Apoptosis via Caspase-Independent Mechanisms
3. Discussion
4. Materials and Methods
4.1. Cell Culture Maintenance and Treatment
4.2. RNA Isolation and Quantitative Real-Time PCR
4.3. Protein Isolation and Western Blot
4.4. Estradiol Quantification via ELISA
4.5. Hydroxyestradiol Quantification via HPLC-MS/MS
4.6. Immunofluorescence Microscopy for γH2Ax
4.7. Caspase-3/-7 Activity
4.8. TUNEL Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AOSR | Alberta oil sands region |
| PACs | Polycyclic aromatic compounds |
| PAHs | Polycyclic aromatic hydrocarbons |
| DBT | Dibenzothiophene |
| 2,4,7-DBT | 2,4,7-Trimethyldibenzothiophene |
| SIGCs | Spontaneously immortalized granulosa cell |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| AhR | Aryl hydrocarbon receptor |
| CYP | Cytochrome P450 |
| EROD | Ethoxyresorufin-O-deethylase |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| COMT | Catechol-O-methyltransferase |
| DDR | DNA damage response |
| PARP1 | Poly(ADP-ribose) polymerase-1 |
| AKT | Protein kinase B |
| pAKT | Phosphorylated protein kinase B |
References
- Dubé, M.G.; Dunlop, J.M.; Davidson, C.; Beausoleil, D.L.; Hazewinkel, R.R.O.; Wyatt, F. History, Overview, and Governance of Environmental Monitoring in the Oil Sands Region of Alberta, Canada. Integr. Environ. Assess. Manag. 2022, 18, 319–332. [Google Scholar] [CrossRef]
- Alberta Energy Outlook (ST98); Alberta Energy Regulator: Edmonton, AB, Canada, 2024.
- Westman, C.N.; Joly, T.L. Oil Sands Extraction in Alberta, Canada: A Review of Impacts and Processes Concerning Indigenous Peoples. Hum. Ecol. 2019, 47, 233–243. [Google Scholar] [CrossRef]
- Chibwe, L.; Roberts, S.; Shang, D.; Yang, F.; Manzano, C.A.; Wang, X.; Kirk, J.L.; Muir, D.C.G. A One-Century Sedimentary Record of N- and S-Polycyclic Aromatic Compounds in the Athabasca Oil Sands Region in Canada. Chemosphere 2020, 260, 127641. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Sharma, K.; Brassard, B.W.; Hazewinkel, R. Polycyclic Aromatic Hydrocarbon Deposition in the Snowpack of the Athabasca Oil Sands Region of Alberta, Canada. Water Air Soil. Pollut. 2014, 225, 1910. [Google Scholar] [CrossRef]
- Boutin, C.; Carpenter, D.J. Assessment of Wetland/Upland Vegetation Communities and Evaluation of Soil-Plant Contamination by Polycyclic Aromatic Hydrocarbons and Trace Metals in Regions near Oil Sands Mining in Alberta. Sci. Total Environ. 2017, 576, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; de Solla, S.R.; Head, J.A.; Hodson, P.V.; Parrott, J.L.; Thomas, P.J.; Berthiaume, A.; Langlois, V.S. Polycyclic Aromatic Compounds (PACs) in the Canadian Environment: Exposure and Effects on Wildlife. Environ. Pollut. 2020, 265, 114863. [Google Scholar] [CrossRef]
- Achten, C.; Andersson, J.T. Overview of Polycyclic Aromatic Compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186. [Google Scholar] [CrossRef]
- Kelly, E.N.; Short, J.W.; Schindler, D.W.; Hodson, P.V.; Ma, M.; Kwan, A.K.; Fortin, B.L. Oil Sands Development Contributes Polycyclic Aromatic Compounds to the Athabasca River and Its Tributaries. Proc. Natl. Acad. Sci. USA 2009, 106, 22346–22351. [Google Scholar] [CrossRef]
- Cooke, C.A.; Drevnick, P.E. Transboundary Atmospheric Pollution from Mountaintop Coal Mining. Environ. Sci. Technol. Lett. 2022, 9, 943–948. [Google Scholar] [CrossRef]
- Cooke, C.A.; Holland, K.M.; Emmerton, C.A.; Drevnick, P.E.; Criscitiello, A.S.; Newton, B. Mountaintop Removal Coal Mining Contaminates Snowpack across a Broad Region. Environ. Sci. Technol. 2024, 58, 11718–11726. [Google Scholar] [CrossRef]
- Masto, R.E.; Singh, M.K.; Rout, T.K.; Kumar, A.; Kumar, S.; George, J.; Selvi, V.A.; Dutta, P.; Tripathi, R.C.; Srivastava, N.K. Health Risks from PAHs and Potentially Toxic Elements in Street Dust of a Coal Mining Area in India. Environ. Geochem. Health 2019, 41, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Bolden, A.L.; Rochester, J.R.; Schultz, K.; Kwiatkowski, C.F. Polycyclic Aromatic Hydrocarbons and Female Reproductive Health: A Scoping Review. Reprod. Toxicol. 2017, 73, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, S.; Liu, X.; Kim, C.; Jung, D.; Yim, U.H.; Shim, W.J.; Khim, J.S.; Giesy, J.P.; Choi, K. Endocrine Disrupting Potential of PAHs and Their Alkylated Analogues Associated with Oil Spills. Environ. Sci. Process Impacts 2017, 19, 1117–1125. [Google Scholar] [CrossRef]
- Kavanagh, R.J.; Frank, R.A.; Oakes, K.D.; Servos, M.R.; Young, R.F.; Fedorak, P.M.; MacKinnon, M.D.; Solomon, K.R.; Dixon, D.G.; Van Der Kraak, G. Fathead Minnow (Pimephales Promelas) Reproduction Is Impaired in Aged Oil Sands Process-Affected Waters. Aquat. Toxicol. 2011, 101, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Barros, Á.; Álvarez, D.; Velando, A. Long-Term Reproductive Impairment in a Seabird after the Prestige Oil Spill. Biol. Lett. 2014, 10, 20131041. [Google Scholar] [CrossRef]
- Rafiee, A.; Hoseini, M.; Akbari, S.; Mahabee-Gittens, E.M. Exposure to Polycyclic Aromatic Hydrocarbons and Adverse Reproductive Outcomes in Women: Current Status and Future Perspectives. Rev. Environ. Health 2024, 39, 305–311. [Google Scholar] [CrossRef]
- Lim, J.; Lawson, G.W.; Nakamura, B.N.; Ortiz, L.; Hur, J.A.; Kavanagh, T.J.; Luderer, U. Glutathione-Deficient Mice Have Increased Sensitivity to Transplacental Benzo[a]Pyrene-Induced Premature Ovarian Failure and Ovarian Tumorigenesis. Cancer Res. 2013, 73, 908–917. [Google Scholar] [CrossRef]
- Ye, X.; Pan, W.; Li, C.; Ma, X.; Yin, S.; Zhou, J.; Liu, J. Exposure to Polycyclic Aromatic Hydrocarbons and Risk for Premature Ovarian Failure and Reproductive Hormones Imbalance. J. Environ. Sci. 2020, 91, 1–9. [Google Scholar] [CrossRef]
- Sadeu, J.C.; Foster, W.G. Effect of in Vitro Exposure to Benzo[a]Pyrene, a Component of Cigarette Smoke, on Folliculogenesis, Steroidogenesis and Oocyte Nuclear Maturation. Reprod. Toxicol. 2011, 31, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Archibong, A.E.; Ramesh, A.; Inyang, F.; Niaz, M.S.; Hood, D.B.; Kopsombut, P. Endocrine Disruptive Actions of Inhaled Benzo(a)Pyrene on Ovarian Function and Fetal Survival in Fisher F-344 Adult Rats. Reprod. Toxicol. 2012, 34, 635–643. [Google Scholar] [CrossRef]
- Zajda, K.; Rak, A.; Ptak, A.; Gregoraszczuk, E.L. Compounds of PAH Mixtures Dependent Interaction between Multiple Signaling Pathways in Granulosa Tumour Cells. Toxicol. Lett. 2019, 310, 14–22. [Google Scholar] [CrossRef]
- Luderer, U.; Meier, M.J.; Lawson, G.W.; Beal, M.A.; Yauk, C.L.; Marchetti, F. In Utero Exposure to Benzo [a] Pyrene Induces Ovarian Mutations at Doses That Deplete Ovarian Follicles in Mice. Environ. Mol. Mutagen. 2019, 60, 410–420. [Google Scholar] [CrossRef]
- Golzadeh, N.; Barst, B.D.; Baker, J.M.; Auger, J.C.; McKinney, M.A. Alkylated Polycyclic Aromatic Hydrocarbons Are the Largest Contributor to Polycyclic Aromatic Compound Concentrations in Traditional Foods of the Bigstone Cree Nation in Alberta, Canada. Environ. Pollut. 2021, 275, 116625. [Google Scholar] [CrossRef]
- Hellou, J.; Payne, J.F.; Hamilton, C. Polycyclic Aromatic Compounds in Northwest Atlantic COD (Gadus Morhua). Environ. Pollut. 1994, 84, 197–202. [Google Scholar] [CrossRef]
- Provencher, J.F.; Thomas, P.J.; Pauli, B.; Braune, B.M.; Franckowiak, R.P.; Gendron, M.; Savard, G.; Sarma, S.N.; Crump, D.; Zahaby, Y.; et al. Polycyclic Aromatic Compounds (PACs) and Trace Elements in Four Marine Bird Species from Northern Canada in a Region of Natural Marine Oil and Gas Seeps. Sci. Total Environ. 2020, 744, 140959. [Google Scholar] [CrossRef]
- Madill, R.E.A.; Orzechowski, M.T.; Chen, G.; Brownlee, B.G.; Bunce, N.J. Preliminary Risk Assessment of the Wet Landscape Option for Reclamation of Oil Sands Mine Tailings: Bioassays with Mature Fine Tailings Pore Water. Environ. Toxicol. 2001, 16, 197–208. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.-G.; Simoneit, B.R.T.; Shi, S.; Zhang, L.; Yang, F. Qualitative and Quantitative Analysis of Dibenzothiophene, Its Methylated Homologues, and Benzonaphthothiophenes in Crude Oils, Coal, and Sediment Extracts. J. Chromatogr. A 2012, 1233, 126–136. [Google Scholar] [CrossRef]
- Schuster, J.K.; Harner, T.; Su, K.; Mihele, C.; Eng, A. First Results from the Oil Sands Passive Air Monitoring Network for Polycyclic Aromatic Compounds. Environ. Sci. Technol. 2015, 49, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, L.M.J.; Roy, J.; Glozier, N.E.; Dirk, L.; Cooke, C.A. Dissolved Polycyclic Aromatic Compounds in Canada’s Athabasca River in Relation to Oil Sands from 2013 through 2019. Environ. Monit. Assess. 2023, 195, 1354. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.; Farwell, A.; Mark Hewitt, L.; MacKinnon, M.; George Dixon, D. The Effects of Dimethylated and Alkylated Polycyclic Aromatic Hydrocarbons on the Embryonic Development of the Japanese Medaka. Ecotoxicol. Environ. Saf. 2005, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Guzzolino, E.; Milella, M.S.; Forini, F.; Borsò, M.; Rutigliano, G.; Gorini, F.; Zucchi, R.; Saba, A.; Bianchi, F.; Iervasi, G.; et al. Thyroid Disrupting Effects of Low-Dose Dibenzothiophene and Cadmium in Single or Concurrent Exposure: New Evidence from a Translational Zebrafish Model. Sci. Total Environ. 2021, 769, 144703. [Google Scholar] [CrossRef]
- Ruberg, E.J.; Elliott, J.E.; Williams, T.D. Review of Petroleum Toxicity and Identifying Common Endpoints for Future Research on Diluted Bitumen Toxicity in Marine Mammals. Ecotoxicology 2021, 30, 537–551. [Google Scholar] [CrossRef]
- Wayland, M.; Headley, J.V.; Peru, K.M.; Crosley, R.; Brownlee, B.G. Levels of Polycyclic Aromatic Hydrocarbons and Dibenzothiophenes in Wetland Sediments and Aquatic Insects in the Oil Sands Area of Northeastern Alberta, Canada. Environ. Monit. Assess. 2007, 136, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Maletz, S.; Krauss, M.; Bluhm, K.; Schiwy, S.; Kuckelkorn, J.; Tiehm, A.; Brack, W.; Hollert, H. Heterocyclic Aromatic Hydrocarbons Show Estrogenic Activity upon Metabolization in a Recombinant Transactivation Assay. Environ. Sci. Technol. 2014, 48, 5892–5901. [Google Scholar] [CrossRef]
- Raez-Villanueva, S.; Perono, G.A.; Jamshed, L.; Thomas, P.J.; Holloway, A.C. Effects of Dibenzothiophene, a Sulfur-containing Heterocyclic Aromatic Hydrocarbon, and Its Alkylated Congener, 2,4,7-trimethyldibenzothiophene, on Placental Trophoblast Cell Function. J. Appl. Toxicol. 2021, 41, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Bolden, A.L.; Schultz, K.; Pelch, K.E.; Kwiatkowski, C.F. Exploring the Endocrine Activity of Air Pollutants Associated with Unconventional Oil and Gas Extraction. Environ. Health 2018, 17, 26. [Google Scholar] [CrossRef]
- Pescatori, S.; Berardinelli, F.; Albanesi, J.; Ascenzi, P.; Marino, M.; Antoccia, A.; di Masi, A.; Acconcia, F. A Tale of Ice and Fire: The Dual Role for 17β-Estradiol in Balancing DNA Damage and Genome Integrity. Cancers 2021, 13, 1583. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. A Unifying Mechanism in the Initiation of Cancer and Other Diseases by Catechol Quinones. Ann. N. Y. Acad. Sci. 2004, 1028, 247–257. [Google Scholar] [CrossRef]
- Miao, S.; Yang, F.; Wang, Y.; Shao, C.; Zava, D.T.; Ding, Q.; Shi, Y.E. 4-Hydroxy Estrogen Metabolite, Causing Genomic Instability by Attenuating the Function of Spindle-Assembly Checkpoint, Can Serve as a Biomarker for Breast Cancer. Am. J. Transl. Res. 2019, 11, 4992–5007. [Google Scholar] [PubMed]
- Liehr, J.G.; Ricci, M.J. 4-Hydroxylation of Estrogens as Marker of Human Mammary Tumors. Proc. Natl. Acad. Sci. USA 1996, 93, 3294–3296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H. Resveratrol Inhibits TCDD-Induced Expression of CYP1A1 and CYP1B1 and Catechol Estrogen-Mediated Oxidative DNA Damage in Cultured Human Mammary Epithelial Cells. Carcinogenesis 2004, 25, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX Phosphorylation: A Marker for DNA Damage. In DNA Repair Protocols; Bjergbæk, L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 920, pp. 613–626. ISBN 978-1-61779-997-6. [Google Scholar]
- Kim, Y.; Seol, D.-W. TRAIL, a Mighty Apoptosis Inducer. Mol. Cells 2003, 15, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A Review of Human and Animals Exposure to Polycyclic Aromatic Hydrocarbons: Health Risk and Adverse Effects, Photo-Induced Toxicity and Regulating Effect of Microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef]
- Xia, Z.; Idowu, I.; Marvin, C.; Thomas, P.J.; Johnson, W.; Francisco, O.; Stetefeld, J.; Crimmins, B.; Fry, M.; Tomy, G.T. Identification of Halogenated Polycyclic Aromatic Hydrocarbons in Biological Samples from Alberta Oil-Sands Region. Chemosphere 2019, 215, 206–213. [Google Scholar] [CrossRef]
- Kurek, J.; Kirk, J.L.; Muir, D.C.G.; Wang, X.; Evans, M.S.; Smol, J.P. Legacy of a Half Century of Athabasca Oil Sands Development Recorded by Lake Ecosystems. Proc. Natl. Acad. Sci. USA 2013, 110, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Einaudi, L.; Courbiere, B.; Tassistro, V.; Prevot, C.; Sari-Minodier, I.; Orsiere, T.; Perrin, J. In Vivo Exposure to Benzo(a)Pyrene Induces Significant DNA Damage in Mouse Oocytes and Cumulus Cells. Human Reprod. 2014, 29, 548–554. [Google Scholar] [CrossRef]
- Ganesan, S.; Bhattacharya, P.; Keating, A.F. 7,12-Dimethylbenz[a]Anthracene Exposure Induces the DNA Repair Response in Neonatal Rat Ovaries. Toxicol. Appl. Pharmacol. 2013, 272, 690–696. [Google Scholar] [CrossRef]
- Neal, M.S.; Zhu, J.; Holloway, A.C.; Foster, W.G. Follicle Growth Is Inhibited by Benzo-[a]-Pyrene, at Concentrations Representative of Human Exposure, in an Isolated Rat Follicle Culture Assay. Human Reprod. 2007, 22, 961–967. [Google Scholar] [CrossRef]
- Yazawa, T.; Islam, M.S.; Imamichi, Y.; Watanabe, H.; Yaegashi, K.; Ida, T.; Sato, T.; Kitano, T.; Matsuzaki, S.; Umezawa, A.; et al. Comparison of Placental HSD17B1 Expression and Its Regulation in Various Mammalian Species. Animals 2023, 13, 622. [Google Scholar] [CrossRef]
- Yivgi-Ohana, N.; Sher, N.; Melamed-Book, N.; Eimerl, S.; Koler, M.; Manna, P.R.; Stocco, D.M.; Orly, J. Transcription of Steroidogenic Acute Regulatory Protein in the Rodent Ovary and Placenta: Alternative Modes of Cyclic Adenosine 3′, 5′-Monophosphate Dependent and Independent Regulation. Endocrinology 2009, 150, 977–989. [Google Scholar] [CrossRef]
- Whitlock, J.P. Induction of Cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 103–125. [Google Scholar] [CrossRef]
- Hinger, G.; Brinkmann, M.; Bluhm, K.; Sagner, A.; Takner, H.; Eisenträger, A.; Braunbeck, T.; Engwall, M.; Tiehm, A.; Hollert, H. Some Heterocyclic Aromatic Compounds Are Ah Receptor Agonists in the DR-CALUX Assay and the EROD Assay with RTL-W1 Cells. Environ. Sci. Pollut. Res. 2011, 18, 1297–1304. [Google Scholar] [CrossRef]
- Marvanová, S.; Pěnčíková, K.; Pálková, L.; Ciganek, M.; Petráš, J.; Lněničková, A.; Vondráček, J.; Machala, M. Benzo [b] Naphtho [d] Thiophenes and Naphthylbenzo [b] Thiophenes: Their Aryl Hydrocarbon Receptor-Mediated Activities and Environmental Presence. Sci. Total Environ. 2023, 879, 162924. [Google Scholar] [CrossRef]
- Lam, M.M.; Bülow, R.; Engwall, M.; Giesy, J.P.; Larsson, M. Methylated PACs Are More Potent than Their Parent Compounds: A Study of Aryl Hydrocarbon Receptor-Mediated Activity, Degradability, and Mixture Interactions in the H4IIE- Luc Assay: Methylated PACs Are More Potent than Their Parent Compounds. Environ. Toxicol. Chem. 2018, 37, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Yue, W.; Wang, J.-P. Estrogen Metabolites and Breast Cancer. Steroids 2015, 99, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Na, H.-K.; Hurh, Y.-J.; Surh, Y.-J. 4-Hydroxyestradiol Induces Oxidative Stress and Apoptosis in Human Mammary Epithelial Cells: Possible Protection by NF-κB and ERK/MAPK. Toxicol. Appl. Pharmacol. 2005, 208, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Sakakibara, H.; Takemura, H.; Shimoi, K. 4-Hydroxyestradiol Induces γ-H2AX in the Presence of an Inhibitor of Catechol-O-Methyltransferase in Human Breast Cancer MCF-7 Cells. Genes Environ. 2012, 34, 129–135. [Google Scholar] [CrossRef]
- Amat, A.; Pfohl-Leszkowicz, A.; Castegnaro, M. Genotoxic Activity Of Thiophenes On Liver Human Cell Line (HepG2). Polycycl. Aromat. Compd. 2004, 24, 733–742. [Google Scholar] [CrossRef]
- Williamson, L.M.; Lees-Miller, S.P. Estrogen Receptor α-Mediated Transcription Induces Cell Cycle-Dependent DNA Double-Strand Breaks. Carcinogenesis 2011, 32, 279–285. [Google Scholar] [CrossRef]
- Wei, H.; Yu, X. Functions of PARylation in DNA Damage Repair Pathways. Genom. Proteom. Bioinform. 2016, 14, 131–139. [Google Scholar] [CrossRef]
- Conceição, C.J.F.; Moe, E.; Ribeiro, P.A.; Raposo, M. PARP1: A Comprehensive Review of Its Mechanisms, Therapeutic Implications and Emerging Cancer Treatments. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189282. [Google Scholar] [CrossRef]
- Lee, E.-J.; Oh, S.-Y.; Kim, M.-K.; Ahn, S.H.; Son, B.H.; Sung, M.-K. Modulatory Effects of α- and γ-Tocopherols on 4-Hydroxyestradiol Induced Oxidative Stresses in MCF-10A Breast Epithelial Cells. Nutr. Res. Pract. 2009, 3, 185–191. [Google Scholar] [CrossRef]
- Martí, J.M.; Garcia-Diaz, A.; Delgado-Bellido, D.; O’Valle, F.; González-Flores, A.; Carlevaris, O.; Rodríguez-Vargas, J.M.; Amé, J.C.; Dantzer, F.; King, G.L.; et al. Selective Modulation by PARP-1 of HIF-1α-Recruitment to Chromatin during Hypoxia Is Required for Tumor Adaptation to Hypoxic Conditions. Redox Biol. 2021, 41, 101885. [Google Scholar] [CrossRef]
- Gao, N.; Nester, R.A.; Sarkar, M.A. 4-Hydroxy Estradiol but Not 2-Hydroxy Estradiol Induces Expression of Hypoxia-Inducible Factor 1α and Vascular Endothelial Growth Factor A through Phosphatidylinositol 3-Kinase/Akt/FRAP Pathway in OVCAR-3 and A2780-CP70 Human Ovarian Carcinoma Cells. Toxicol. Appl. Pharmacol. 2004, 196, 124–135. [Google Scholar] [CrossRef]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A Nuclease That Mediates Cell Death Induced by DNA Damage and Poly(ADP-Ribose) Polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.N.; Blais, J.M.; Chan, H.M. Neurotoxicity of Alkylated Polycyclic Aromatic Compounds in Human Neuroblastoma Cells. J. Toxicol. Environ. Health A 2017, 80, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Keren-Tal, I.; Suh, B.S.; Dantes, A.; Lindner, S.; Oren, M.; Amsterdam, A. Involvement of P53 Expression in cAMP-Mediated Apoptosis in Immortalized Granulosa Cells. Exp. Cell Res. 1995, 218, 283–295. [Google Scholar] [CrossRef]
- Amsterdam, A.; Sasson, R.; Keren-Tal, I.; Aharoni, D.; Dantes, A.; Rimon, E.; Land, A.; Cohen, T.; Dor, Y.; Hirsh, L. Alternative Pathways of Ovarian Apoptosis: Death for Life. Biochem. Pharmacol. 2003, 66, 1355–1362. [Google Scholar] [CrossRef]
- Ballachey, B.E.; Kloecker, K.A. Hydrocarbons in Hair, Livers and Intestines of Sea Otters (Enhydra Lurris) Found Dead along the Path of the Exxon Valdez Oil Spill; U.S. Geological Survey, Biological Resources Division: Anchorage, AK, USA, 1997.
- Idowu, I.; Johnson, W.; Francisco, O.; Obal, T.; Marvin, C.; Thomas, P.J.; Sandau, C.D.; Stetefeld, J.; Tomy, G.T. Comprehensive Two-Dimensional Gas Chromatography High-Resolution Mass Spectrometry for the Analysis of Substituted and Unsubstituted Polycyclic Aromatic Compounds in Environmental Samples. J. Chromatogr. A 2018, 1579, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Newell, E.E.; Eccles, K.; Holloway, A.C.; Idowu, I.; Xia, Z.; Hassan, E.; Tomy, G.; Quenneville, C. Co-Exposures to Trace Elements and Polycyclic Aromatic Compounds (PACs) Impacts North American River Otter (Lontra canadensis) Baculum. Chemosphere 2021, 265, 128920. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Maloy, A.; Alexander, S.; Andreas, A.; Nyunoya, T.; Chandra, D. Stain-Free Total-Protein Normalization Enhances the Reproducibility of Western Blot Data. Anal. Biochem. 2022, 654, 114840. [Google Scholar] [CrossRef]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The Use of Total Protein Stains as Loading Controls: An Alternative to High-Abundance Single-Protein Controls in Semi-Quantitative Immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A Key Player in DNA Damage Response and a Promising Target for Cancer Therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef] [PubMed]
- Jariyasopit, N.; Tung, P.; Su, K.; Halappanavar, S.; Evans, G.J.; Su, Y.; Khoomrung, S.; Harner, T. Polycyclic Aromatic Compounds in Urban Air and Associated Inhalation Cancer Risks: A Case Study Targeting Distinct Source Sectors. Environ. Pollut. 2019, 252, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Chen, Y.-J.; Chen, Y.; Lu, Y.; Li, R.; Dong, C.; Hu, D.; Cai, Z. Discovery of Emerging Sulfur-Containing PAHs in PM2.5: Contamination Profiles and Potential Health Risks. J. Hazard. Mater. 2021, 416, 125795. [Google Scholar] [CrossRef]
- Mori, Y.; Taneda, S.; Hayashi, H.; Sakushima, A.; Kamata, K.; Suzuki, A.K.; Yoshino, S.; Sakata, M.; Sagai, M.; Seki, K. Estrogenic Activities of Chemicals in Diesel Exhaust Particles. Biol. Pharm. Bull. 2002, 25, 145–146. [Google Scholar] [CrossRef] [PubMed]

| Accession Number | Gene Name | Symbol | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|---|---|
| NM_012512.2 | Beta-2 microglobulin | B2m | AATTCACACCCACCGAGACC | GCTCCTTCAGAGTGACGTGT |
| NM_012583.2 | Hypoxanthine phosphoribosyl transferase 1 | Hprt | GCAGTACAGCCCCAAAATGG | GGTCCTTTTCACCAGCAAGCT |
| NM_012851.2 | Hydroxysteroid 17-Beta Dehydrogenase 1 | Hsd17b1 | AAGGTCTGTGCGAGAGTCTG | TTTCATGGAAGGCTGTGTGC |
| NM_017085.2 | Cytochrome P450 Family 19 Subfamily A Member 1 | Cyp19a1 | CTGTCCA TTCCAGCACCC TT | AGTAGTTTGGCTGTGGCTC C |
| NM_012689.1 | Estrogen Receptor 1 | Esr1 | GCTCCACTTCAGCACATTCC | GGATTCGCAGAACCTTGTGG |
| NM_012754.3 | Estrogen Receptor 2 | Esr2 | TGAGGCGGACAGACTACAGA | GGTAAGGGGTGCGTAACCAA |
| NM_012540.3 | Cytochrome P450 Family 1 Subfamily A Member 1 | Cyp1a1 | TTGGGGAGGTTACTGGTTCTG | GAGTTAGGGAGGTAACGGAGG |
| NM_012541.3 | Cytochrome P450 Family 1 Subfamily A Member 2 | Cyp1a2 | AGGGAATGCTGTGGACTTCTT | AGTGTTCCTGGACTGTTTTCTG |
| NM_012940.2 | Cytochrome P450 Family 1 Subfamily B Member 1 | Cyp1b1 | CAACCCAACTTACCATACGTCA | AAACAAAGGTGTTGGCAG |
| NM_013063.2 | Poly(ADP-Ribose) Polymerase 1 | Parp1 | AAGTGCGAACTACTGCCACA | CTTTGACACTGTGCTTGCCC |
| NM_024359.1 | Hypoxia-Inducible Factor 1 Subunit Alpha | Hif1a | CAGACCCAGTTACAGAAACCTAC | GATTCATCAGTGGTGGCAGTTG |
| NM_031836.3 | Vascular Endothelial Growth Factor A | Vegfa | TCTCCCAGATCGGTGACAGT | AAGGAATGTGTGGTGGGGAC |
| NM_145681.2 | Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand | Trail | GGACGGAGGGAGGATCAAAC | CAGTATGGGATCGGGGTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perono, G.A.; Tomy, T.; Loudon, K.; Jamshed, L.; Garlisi, B.; Lauks, S.; Lockington, C.; Ruan, C.; Tomy, G.T.; Petrik, J.J.; et al. Sulfur-Containing Heterocyclic Aromatic Hydrocarbons Alter Estrogen Metabolism and Cause DNA Damage and Apoptosis in Granulosa Cells. Int. J. Mol. Sci. 2025, 26, 8004. https://doi.org/10.3390/ijms26168004
Perono GA, Tomy T, Loudon K, Jamshed L, Garlisi B, Lauks S, Lockington C, Ruan C, Tomy GT, Petrik JJ, et al. Sulfur-Containing Heterocyclic Aromatic Hydrocarbons Alter Estrogen Metabolism and Cause DNA Damage and Apoptosis in Granulosa Cells. International Journal of Molecular Sciences. 2025; 26(16):8004. https://doi.org/10.3390/ijms26168004
Chicago/Turabian StylePerono, Genevieve A., Thane Tomy, Kara Loudon, Laiba Jamshed, Bianca Garlisi, Sylvia Lauks, Cielle Lockington, Celina Ruan, Gregg T. Tomy, James J. Petrik, and et al. 2025. "Sulfur-Containing Heterocyclic Aromatic Hydrocarbons Alter Estrogen Metabolism and Cause DNA Damage and Apoptosis in Granulosa Cells" International Journal of Molecular Sciences 26, no. 16: 8004. https://doi.org/10.3390/ijms26168004
APA StylePerono, G. A., Tomy, T., Loudon, K., Jamshed, L., Garlisi, B., Lauks, S., Lockington, C., Ruan, C., Tomy, G. T., Petrik, J. J., Thomas, P. J., & Holloway, A. C. (2025). Sulfur-Containing Heterocyclic Aromatic Hydrocarbons Alter Estrogen Metabolism and Cause DNA Damage and Apoptosis in Granulosa Cells. International Journal of Molecular Sciences, 26(16), 8004. https://doi.org/10.3390/ijms26168004








