Uracil–DNA Glycosylase from Beta vulgaris: Properties and Response to Abiotic Stress
Abstract
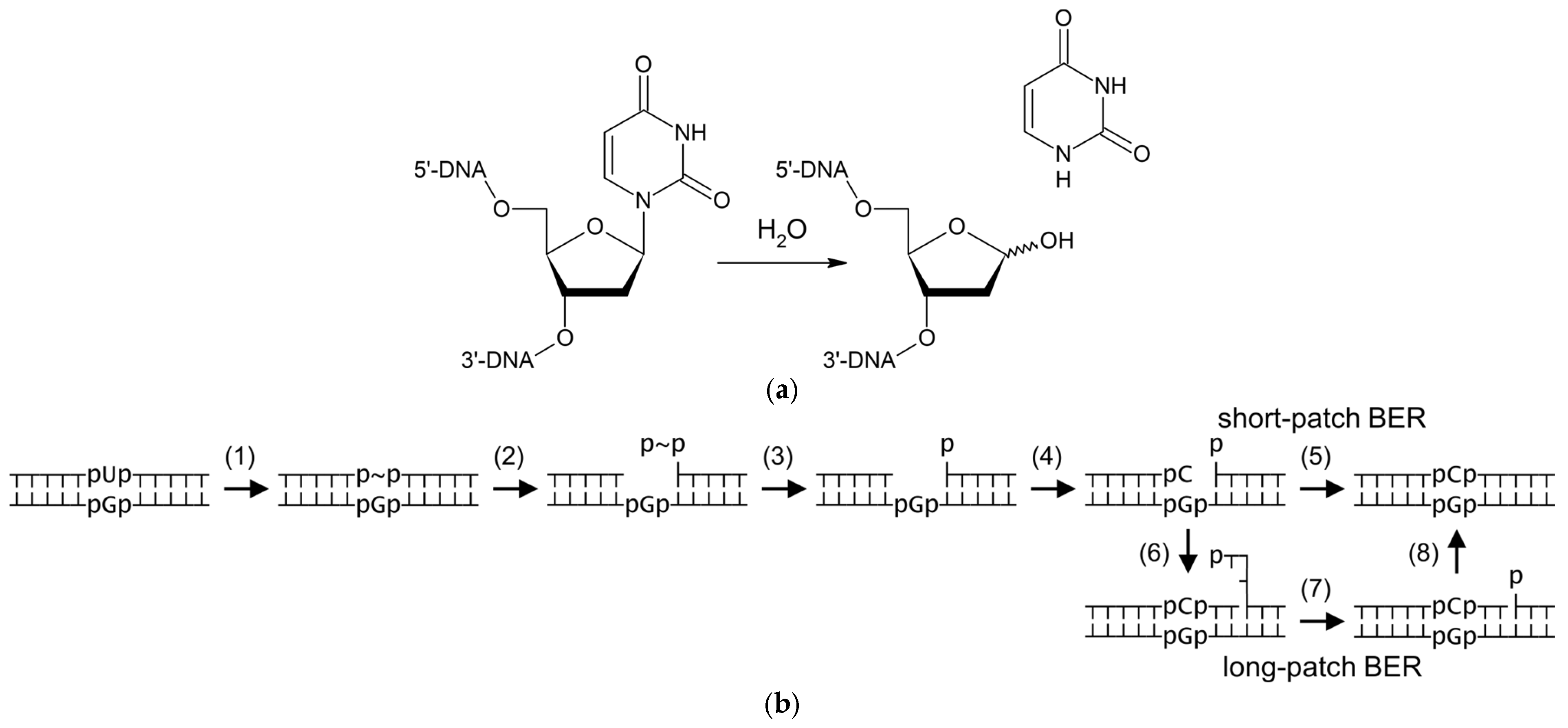
1. Introduction
2. Results
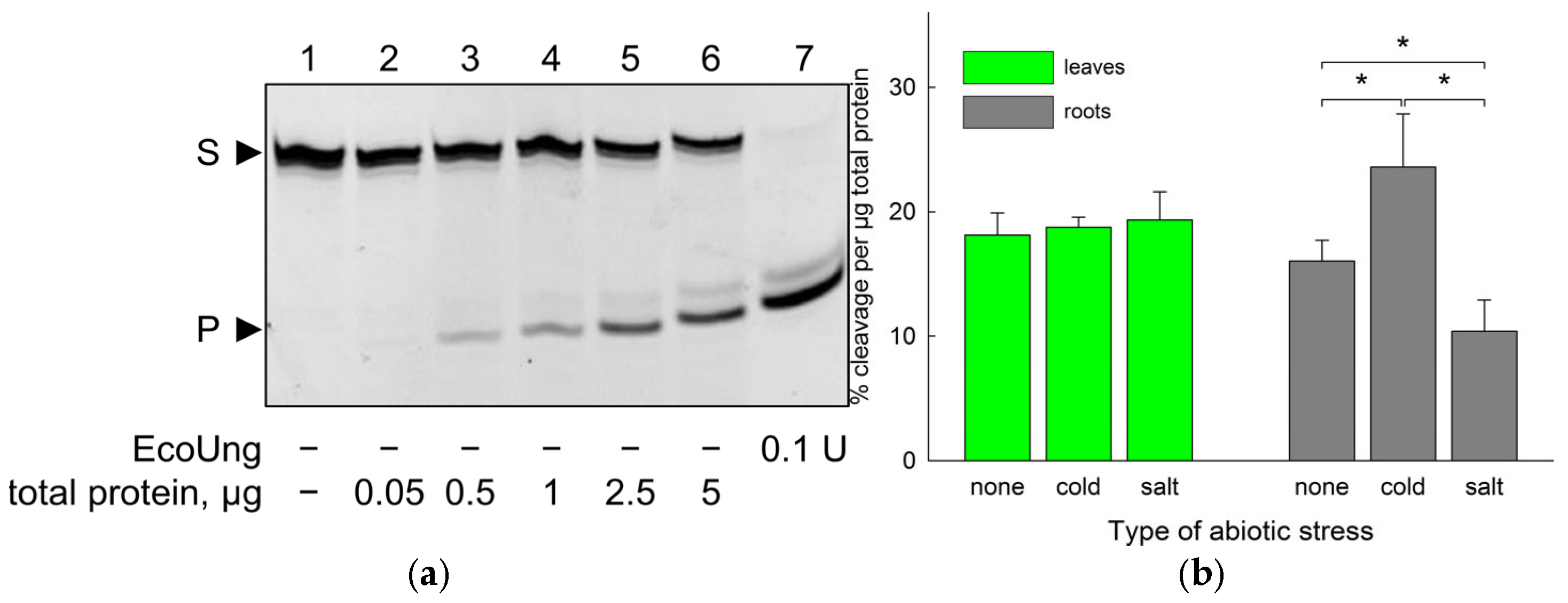
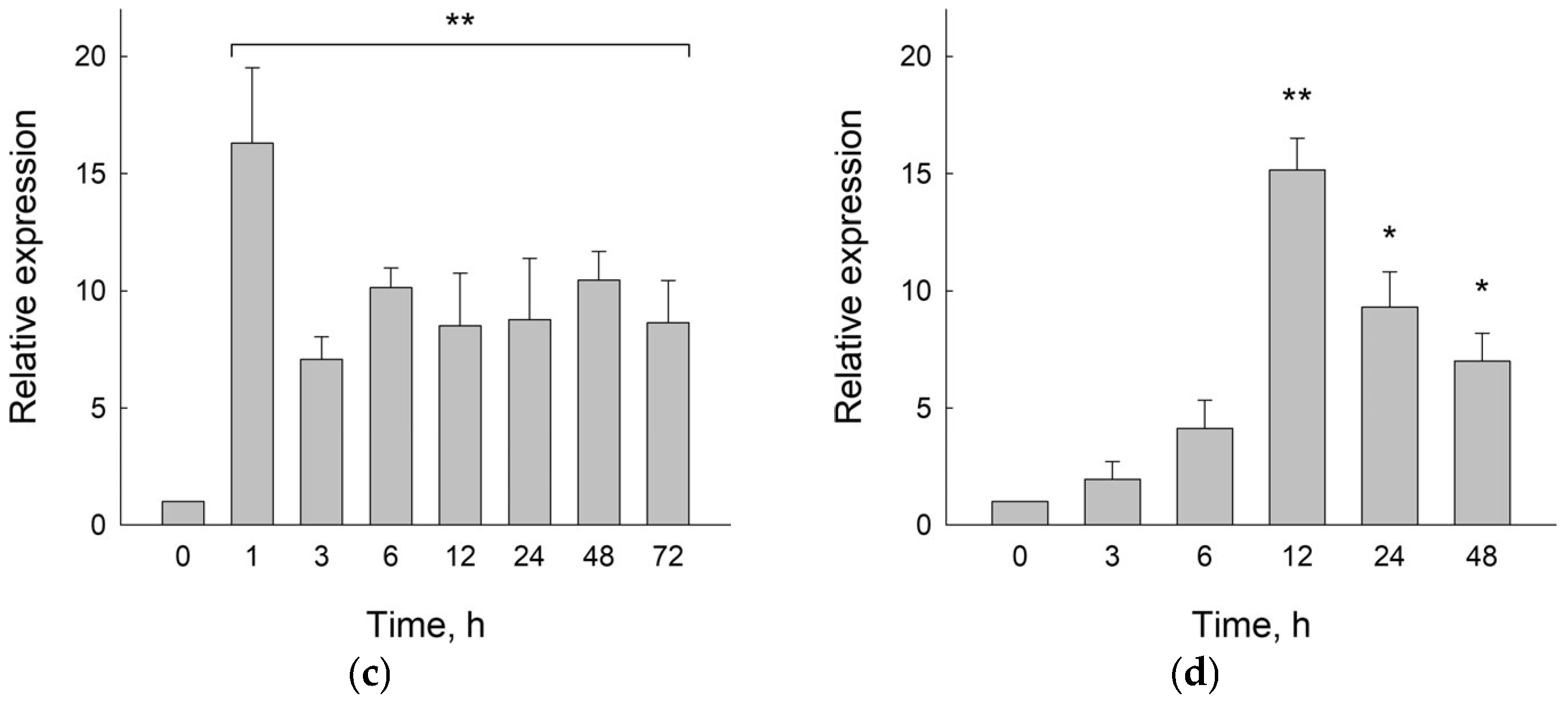
2.1. Uracil–DNA Glycosylase Activity in B. vulgaris
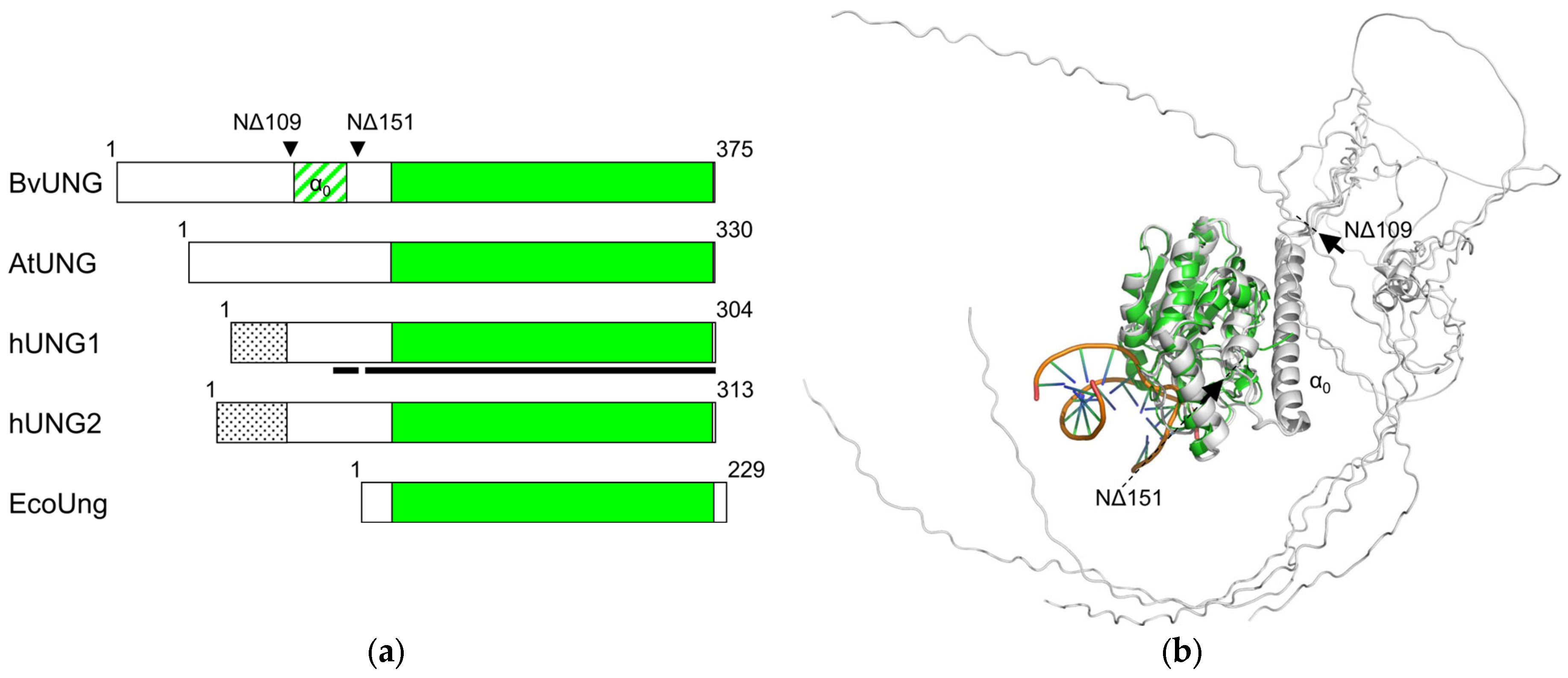
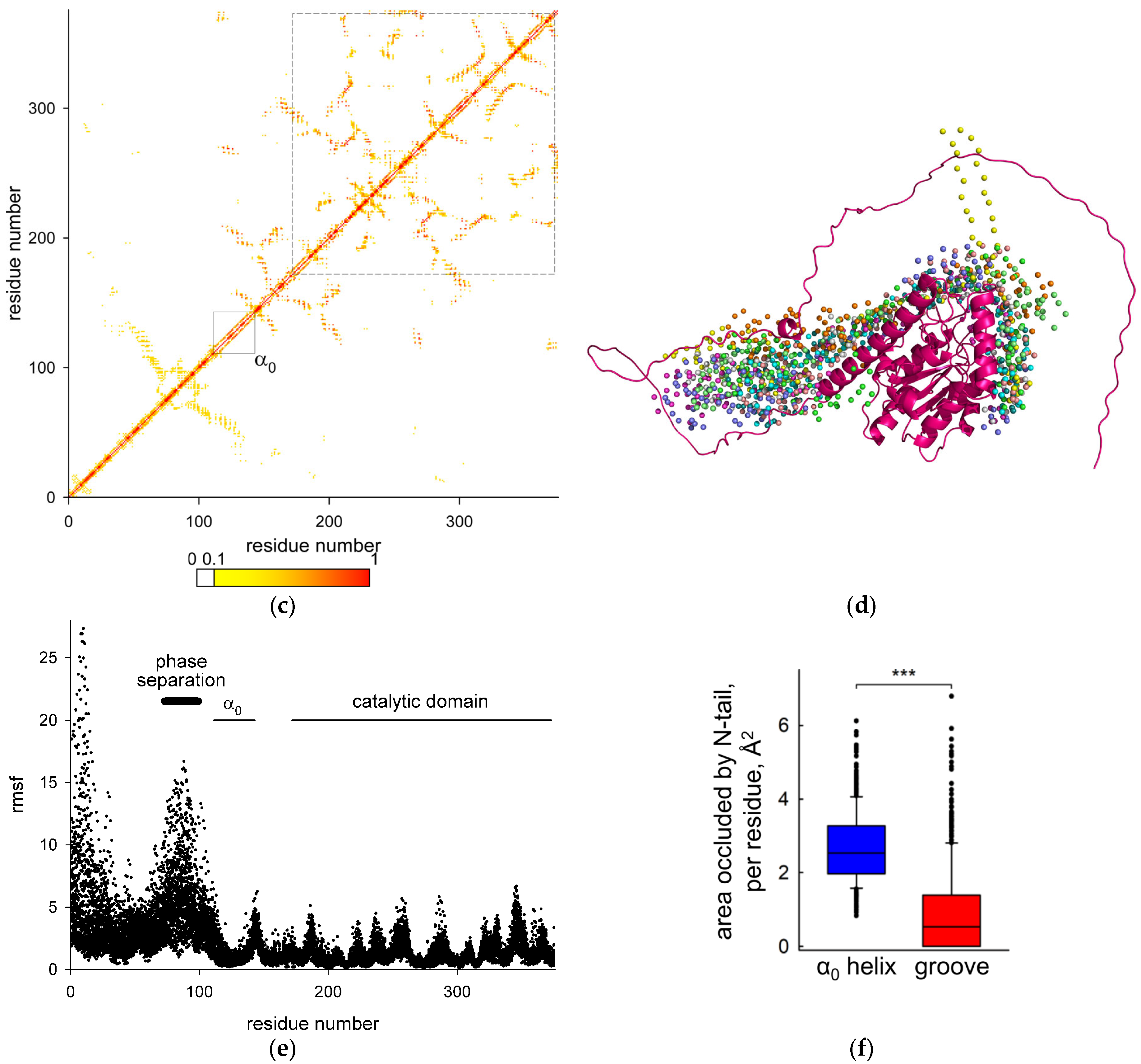
2.2. Predicted Structure of BvUNG
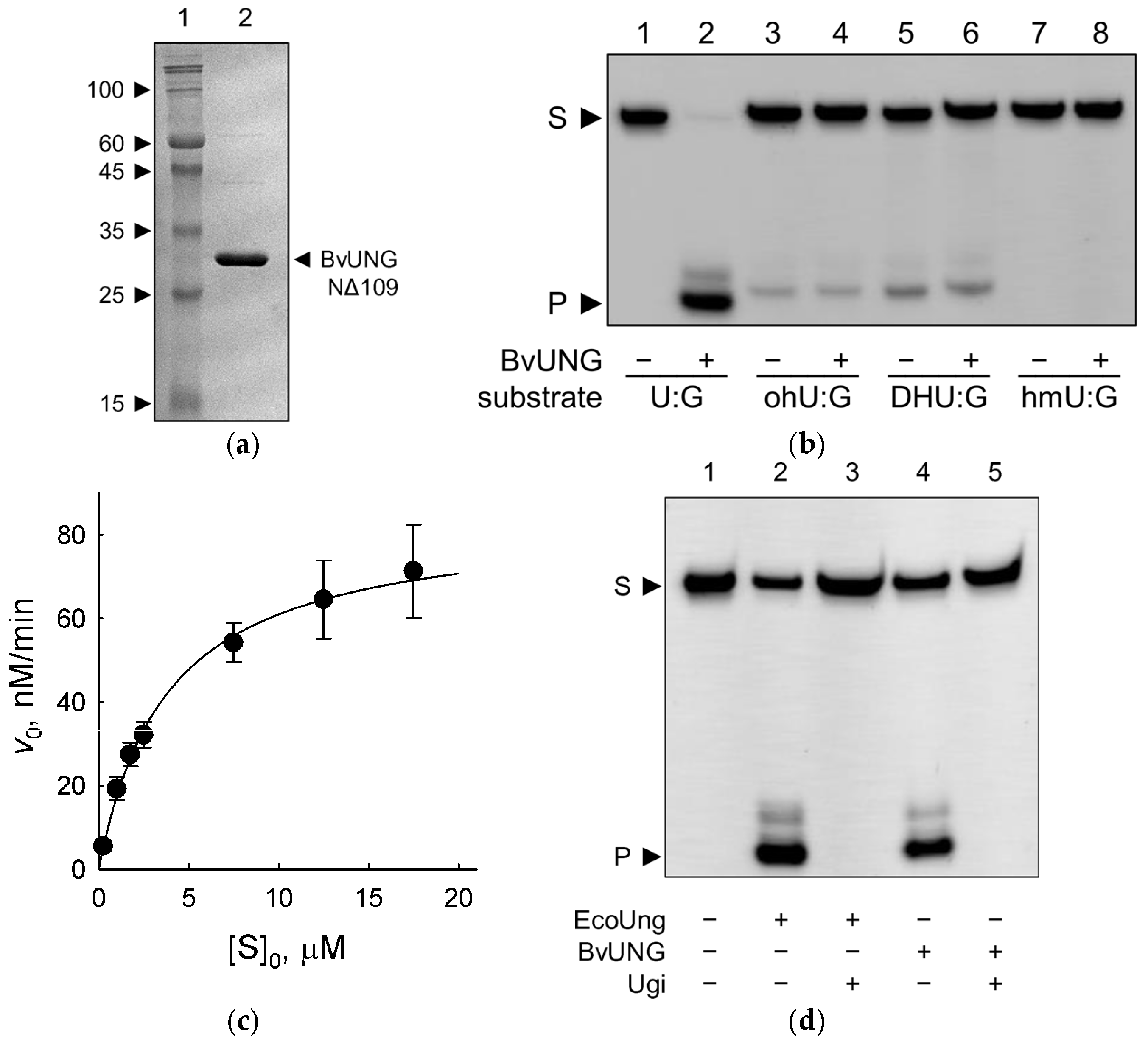
2.3. Activity of Recombinant BvUNG
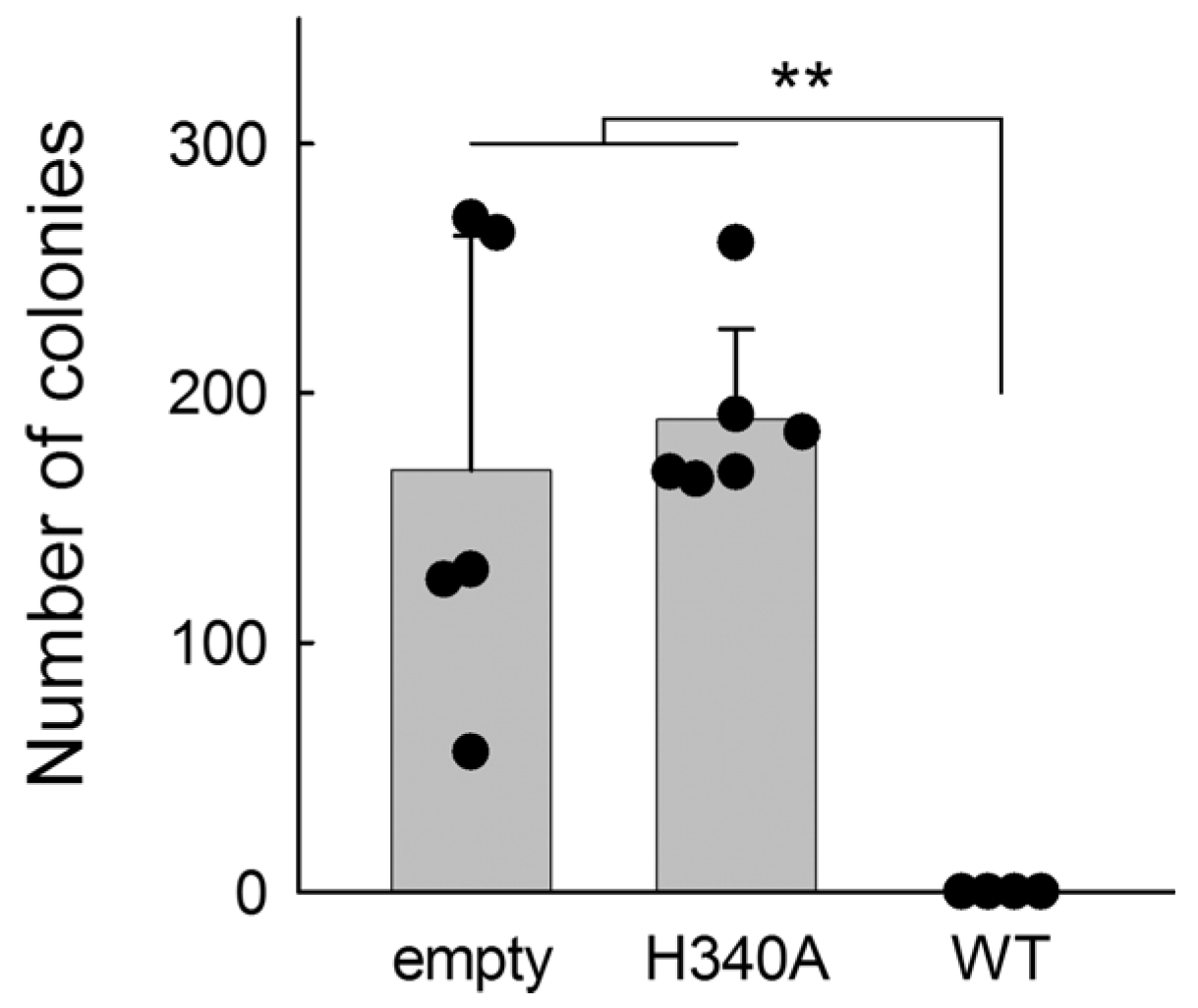
2.4. BvUNG Is Functional in an E. coli Reporter Strain
3. Discussion
4. Materials and Methods
4.1. Enzymes and Oligonucleotides
4.2. Plant Treatment and Extract Preparation
4.3. Uracil Excision Assay in Plant Extracts
4.4. RT-qPCR
4.5. BvUNG Cloning, Mutagenesis, and Purification
4.6. Steady-State Kinetics
4.7. Ugi Inhibition Experiments
4.8. Screening for UNG Activity in E. coli CJ236 Reporter Strain
4.9. Molecular Modeling and Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stivers, J.T.; Drohat, A.C. Uracil DNA glycosylase: Insights from a master catalyst. Arch. Biochem. Biophys. 2001, 396, 1–9. [Google Scholar] [CrossRef]
- Visnes, T.; Doseth, B.; Pettersen, H.S.; Hagen, L.; Sousa, M.M.L.; Akbari, M.; Otterlei, M.; Kavli, B.; Slupphaug, G.; Krokan, H.E. Uracil in DNA and its processing by different DNA glycosylases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 563–568. [Google Scholar] [CrossRef]
- Schormann, N.; Ricciardi, R.; Chattopadhyay, D. Uracil-DNA glycosylases—Structural and functional perspectives on an essential family of DNA repair enzymes. Protein Sci. 2014, 23, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Kavli, B.; Slupphaug, G.; Krokan, H.E. Genomic uracil in biology, immunity and cancer. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 1, pp. 220–248. [Google Scholar]
- Lucas-Lledó, J.I.; Maddamsetti, R.; Lynch, M. Phylogenomic analysis of the uracil-DNA glycosylase superfamily. Mol. Biol. Evol. 2011, 28, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Beard, W.A.; Horton, J.K.; Prasad, R.; Wilson, S.H. Eukaryotic base excision repair: New approaches shine light on mechanism. Annu. Rev. Biochem. 2019, 88, 137–162. [Google Scholar] [CrossRef]
- Hindi, N.N.; Elsakrmy, N.; Ramotar, D. The base excision repair process: Comparison between higher and lower eukaryotes. Cell. Mol. Life Sci. 2021, 78, 7943–7965. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.J.; Simmons, L.A. Bacterial DNA excision repair pathways. Nat. Rev. Microbiol. 2022, 20, 465–477. [Google Scholar] [CrossRef]
- Grin, I.R.; Petrova, D.V.; Endutkin, A.V.; Ma, C.; Yu, B.; Li, H.; Zharkov, D.O. Base excision DNA repair in plants: Arabidopsis and beyond. Int. J. Mol. Sci. 2023, 24, 14746. [Google Scholar] [CrossRef]
- Halliwell, B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 37–52. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 905. [Google Scholar]
- Krokan, H.E.; Sætrom, P.; Aas, P.A.; Pettersen, H.S.; Kavli, B.; Slupphaug, G. Error-free versus mutagenic processing of genomic uracil—Relevance to cancer. DNA Repair 2014, 19, 38–47. [Google Scholar] [CrossRef]
- Pecori, R.; Di Giorgio, S.; Lorenzo, J.P.; Papavasiliou, F.N. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat. Rev. Genet. 2022, 23, 505–518. [Google Scholar] [CrossRef]
- Procházková, D.; Wilhelmová, N.a. Nitric oxide, reactive nitrogen species and associated enzymes during plant senescence. Nitric Oxide 2011, 24, 61–65. [Google Scholar] [CrossRef]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Aranda-Caño, L.; Valderrama, R.; Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Reactive nitrogen species in plant metabolism. In Progress in Botany; Springer: Cham, Switzerland, 2023; pp. 103–152. [Google Scholar] [CrossRef]
- Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130126. [Google Scholar] [CrossRef]
- Ni, G.; Leung, P.M.; Daebeler, A.; Guo, J.; Hu, S.; Cook, P.; Nicol, G.W.; Daims, H.; Greening, C. Nitrification in acidic and alkaline environments. Essays Biochem. 2023, 67, 753–768. [Google Scholar] [CrossRef]
- Boesch, P.; Ibrahim, N.; Paulus, F.; Cosset, A.; Tarasenko, V.; Dietrich, A. Plant mitochondria possess a short-patch base excision DNA repair pathway. Nucleic Acids Res. 2009, 37, 5690–5700. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Dubois, E.; Ariza, R.R.; Doutriaux, M.-P.; Roldán-Arjona, T. Arabidopsis uracil DNA glycosylase (UNG) is required for base excision repair of uracil and increases plant sensitivity to 5-fluorouracil. J. Biol. Chem. 2010, 285, 7475–7483. [Google Scholar] [CrossRef] [PubMed]
- Wynn, E.; Purfeerst, E.; Christensen, A. Mitochondrial DNA repair in an Arabidopsis thaliana uracil N-glycosylase mutant. Plants 2020, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.R.; Lescano López, I.; Ayala, A.M.; Alvarez, M.E. The Arabidopsis DNA glycosylase MBD4L repairs the nuclear genome in vivo. Plant J. 2023, 115, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Shi, T.; Sui, R.; Wang, J.; Kang, H.; Yu, Y.; Zhu, Y. The chromatin remodeler ERCC6 and the histone chaperone NAP1 are involved in apurinic/apyrimidinic endonuclease-mediated DNA repair. Plant Cell 2024, 36, 2238–2252. [Google Scholar] [CrossRef]
- Branco-Price, C.; Kawaguchi, R.; Ferreira, R.B.; Bailey-Serres, J. Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann. Bot. 2005, 96, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-h.; Henderson, D.A.; Zhu, J.-K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175. [Google Scholar] [CrossRef] [PubMed]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilon-Smits, E.A.H. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant. 2008, 132, 236–253. [Google Scholar] [CrossRef]
- Wilkins, O.; Bräutigam, K.; Campbell, M.M. Time of day shapes Arabidopsis drought transcriptomes. Plant J. 2010, 63, 715–727. [Google Scholar] [CrossRef]
- Bourbousse, C.; Vegesna, N.; Law, J.A. SOG1 activator and MYB3R repressors regulate a complex DNA damage network in Arabidopsis. Proc. Natl Acad. Sci. USA 2018, 115, E12453–E12462. [Google Scholar] [CrossRef]
- Ge, Y.; He, G.; Wang, Z.; Guo, D.; Qin, R.; Li, R. GISH and BAC-FISH study of apomictic Beta M14. Sci. China C Life Sci. 2007, 50, 242–250. [Google Scholar] [CrossRef]
- Li, H.; Cao, H.; Wang, Y.; Pang, Q.; Ma, C.; Chen, S. Proteomic analysis of sugar beet apomictic monosomic addition line M14. J. Proteom. 2009, 73, 297–308. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, Y.-D.; Yu, L.-J.; Guo, D.-D.; Wang, B.-C. Comparative proteomic analysis of apomictic monosomic addition line of Beta corolliflora and Beta vulgaris L. in sugar beet. Mol. Biol. Rep. 2009, 36, 2093–2098. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhu, N.; Koh, J.; Ma, C.; Pan, Y.; Yu, B.; Chen, S.; Li, H. Proteomic analysis of salt tolerance in sugar beet monosomic addition line M14. J. Proteome Res. 2013, 12, 4931–4950. [Google Scholar] [CrossRef]
- Yu, B.; Li, J.; Koh, J.; Dufresne, C.; Yang, N.; Qi, S.; Zhang, Y.; Ma, C.; Duong, B.V.; Chen, S.; et al. Quantitative proteomics and phosphoproteomics of sugar beet monosomic addition line M14 in response to salt stress. J. Proteom. 2016, 143, 286–297. [Google Scholar] [CrossRef]
- Lv, X.; Jin, Y.; Wang, Y. De novo transcriptome assembly and identification of salt-responsive genes in sugar beet M14. Comput. Biol. Chem. 2018, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, B. Cloning and bioinformatics analysis of BvM14-UNG gene in sugarbeet M14 line. Chin. Agric. Sci. Bull. 2022, 38, 16–22. (In Chinese) [Google Scholar] [CrossRef]
- Talpaert-Borlé, M.; Liuzzi, M. Base-excision repair in carrot cells: Partial purification and characterization of uracil-DNA glycosylase and apurinic/apyrimidinic endodeoxyribonuclease. Eur. J. Biochem. 1982, 124, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, P.; Warner, H. Partial purification and characterization of a uracil-DNA glycosylase from wheat germ. J. Biol. Chem. 1983, 258, 1603–1609. [Google Scholar] [CrossRef]
- Maldonado, A.; Hernández, P.; Gutiérrez, C. Inhibition of uracil-DNA glycosylase increases SCEs in BrdU-treated and visible light-irradiated cells. Exp. Cell Res. 1985, 161, 172–180. [Google Scholar] [CrossRef]
- Bensen, R.J.; Warner, H.R. The partial purification and characterization of nuclear and mitochondrial uracil-DNA glycosylase activities from Zea mays seedlings. Plant Physiol. 1987, 83, 149–154. [Google Scholar] [CrossRef]
- Bensen, R.J.; Warner, H.R. Partial purification and characterization of uracil-DNA glycosylase activity from chloroplasts of Zea mays seedlings. Plant Physiol. 1987, 84, 1102–1106. [Google Scholar] [CrossRef]
- Inamdar, N.M.; Zhang, X.-Y.; Brough, C.L.; Gardiner, W.E.; Bisaro, D.M.; Ehrlich, M. Transfection of heteroduplexes containing uracil·guanine or thymine·guanine mispairs into plant cells. Plant Mol. Biol. 1992, 20, 123–131. [Google Scholar] [CrossRef]
- Bones, A.M. Expression and occurrence of uracil-DNA glycosylase in higher plants. Physiol. Plant. 1993, 88, 682–688. [Google Scholar] [CrossRef]
- Ferrando, B.; Furlanetto, A.L.D.M.; Gredilla, R.; Havelund, J.F.; Hebelstrup, K.H.; Møller, I.M.; Stevnsner, T. DNA repair in plant mitochondria—A complete base excision repair pathway in potato tuber mitochondria. Physiol. Plant. 2019, 166, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Merina, Á.; Ariza, R.R.; Roldán-Arjona, T. Molecular characterization of a putative plant homolog of MBD4 DNA glycosylase. DNA Repair 2013, 12, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Nota, F.; Cambiagno, D.A.; Ribone, P.; Alvarez, M.E. Expression and function of AtMBD4L, the single gene encoding the nuclear DNA glycosylase MBD4L in Arabidopsis. Plant Sci. 2015, 235, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, N.M.; Torres, J.R.; Lescano López, I.; Cobo, S.; Nota, F.; Alvarez, M.E. Alternative splicing of an exitron determines the subnuclear localization of the Arabidopsis DNA glycosylase MBD4L under heat stress. Plant J. 2022, 110, 377–388. [Google Scholar] [CrossRef]
- McGrath, J.M.; Funk, A.; Galewski, P.; Ou, S.; Townsend, B.; Davenport, K.; Daligault, H.; Johnson, S.; Lee, J.; Hastie, A.; et al. A contiguous de novo genome assembly of sugar beet EL10 (Beta vulgaris L.). DNA Res. 2023, 30, dsac033. [Google Scholar] [CrossRef]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef]
- Parikh, S.S.; Mol, C.D.; Slupphaug, G.; Bharati, S.; Krokan, H.E.; Tainer, J.A. Base excision repair initiation revealed by crystal structures and binding kinetics of human uracil-DNA glycosylase with DNA. EMBO J. 1998, 17, 5214–5226. [Google Scholar] [CrossRef]
- Parikh, S.S.; Putnam, C.D.; Tainer, J.A. Lessons learned from structural results on uracil-DNA glycosylase. Mutat. Res. DNA Repair 2000, 460, 183–199. [Google Scholar] [CrossRef]
- Mer, G.; Bochkarev, A.; Gupta, R.; Bochkareva, E.; Frappier, L.; Ingles, C.J.; Edwards, A.M.; Chazin, W.J. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell 2000, 103, 449–456. [Google Scholar] [CrossRef]
- Slupphaug, G.; Markussen, F.-H.; Olsen, L.C.; Aasland, R.; Aarsæther, N.; Bakke, O.; Krokan, H.E.; Helland, D.E. Nuclear and mitochondrial forms of human uracil-DNA glycosylase are encoded by the same gene. Nucleic Acids Res. 1993, 21, 2579–2584. [Google Scholar] [CrossRef]
- Nilsen, H.; Otterlei, M.; Haug, T.; Solum, K.; Nagelhus, T.A.; Skorpen, F.; Krokan, H.E. Nuclear and mitochondrial uracil-DNA glycosylases are generated by alternative splicing and transcription from different positions in the UNG gene. Nucleic Acids Res. 1997, 25, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Rives, A.; Meier, J.; Sercu, T.; Goyal, S.; Lin, Z.; Liu, J.; Guo, D.; Ott, M.; Zitnick, L.; Ma, J.; et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl Acad. Sci. USA 2021, 118, e2016239118. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins Struct. Funct. Bioinform. 2012, 80, 1715–1735. [Google Scholar] [CrossRef]
- Jamroz, M.; Orozco, M.; Kolinski, A.; Kmiecik, S. Consistent view of protein fluctuations from all-atom molecular dynamics and coarse-grained dynamics with knowledge-based force-field. J. Chem. Theory Comput. 2013, 9, 119–125. [Google Scholar] [CrossRef]
- Kurcinski, M.; Oleniecki, T.; Ciemny, M.P.; Kuriata, A.; Kolinski, A.; Kmiecik, S. CABS-flex standalone: A simulation environment for fast modeling of protein flexibility. Bioinformatics 2019, 35, 694–695. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; Khaodeuanepheng, N.P.; Amarasekara, D.L.; Correia, J.J.; Lewis, K.A.; Fitzkee, N.C.; Hough, L.E.; Whitten, S.T. Intrinsically disordered regions that drive phase separation form a robustly distinct protein class. J. Biol. Chem. 2023, 299, 102801. [Google Scholar] [CrossRef]
- Muller-Weeks, S.; Mastran, B.; Caradonna, S. The nuclear isoform of the highly conserved human uracil-DNA glycosylase is an Mr 36,000 phosphoprotein. J. Biol. Chem. 1998, 273, 21909–21917. [Google Scholar] [CrossRef]
- Fischer, J.A.; Muller-Weeks, S.; Caradonna, S. Proteolytic degradation of the nuclear isoform of uracil-DNA glycosylase occurs during the S phase of the cell cycle. DNA Repair 2004, 3, 505–513. [Google Scholar] [CrossRef]
- Lu, X.; Bocangel, D.; Nannenga, B.; Yamaguchi, H.; Appella, E.; Donehower, L.A. The p53-induced oncogenic phosphatase PPM1D interacts with uracil DNA glycosylase and suppresses base excision repair. Mol. Cell 2004, 15, 621–634. [Google Scholar] [CrossRef]
- Fischer, J.A.; Muller-Weeks, S.; Caradonna, S.J. Fluorodeoxyuridine modulates cellular expression of the DNA base excision repair enzyme uracil-DNA glycosylase. Cancer Res. 2006, 66, 8829–8837. [Google Scholar] [CrossRef] [PubMed]
- Baehr, C.A.; Huntoon, C.J.; Hoang, S.-M.; Jerde, C.R.; Karnitz, L.M. Glycogen synthase kinase 3 (GSK-3)-mediated phosphorylation of uracil N-glycosylase 2 (UNG2) facilitates the repair of floxuridine-induced DNA lesions and promotes cell survival. J. Biol. Chem. 2016, 291, 26875–26885. [Google Scholar] [CrossRef] [PubMed]
- Weiser, B.P.; Stivers, J.T.; Cole, P.A. Investigation of N-terminal phospho-regulation of uracil DNA glycosylase using protein semisynthesis. Biophys. J. 2017, 113, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Iveland, T.S.; Hagen, L.; Sharma, A.; Sousa, M.M.L.; Sarno, A.; Wollen, K.L.; Liabakk, N.B.; Slupphaug, G. HDACi mediate UNG2 depletion, dysregulated genomic uracil and altered expression of oncoproteins and tumor suppressors in B- and T-cell lines. J. Transl. Med. 2020, 18, 159. [Google Scholar] [CrossRef]
- Sugiyama, N.; Nakagami, H.; Mochida, K.; Daudi, A.; Tomita, M.; Shirasu, K.; Ishihama, Y. Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 2008, 4, 193. [Google Scholar] [CrossRef]
- Shrestha, P.; Kandel, J.; Tayara, H.; Chong, K.T. Post-translational modification prediction via prompt-based fine-tuning of a GPT-2 model. Nat. Commun. 2024, 15, 6699. [Google Scholar] [CrossRef]
- Mol, C.D.; Arvai, A.S.; Sanderson, R.J.; Slupphaug, G.; Kavli, B.; Krokan, H.E.; Mosbaugh, D.W.; Tainer, J.A. Crystal structure of human uracil-DNA glycosylase in complex with a protein inhibitor: Protein mimicry of DNA. Cell 1995, 82, 701–708. [Google Scholar] [CrossRef]
- Slupphaug, G.; Mol, C.D.; Kavli, B.; Arvai, A.S.; Krokan, H.E.; Tainer, J.A. A nucleotide-flipping mechanism from the structure of human uracil–DNA glycosylase bound to DNA. Nature 1996, 384, 87–92. [Google Scholar] [CrossRef]
- Parikh, S.S.; Walcher, G.; Jones, G.D.; Slupphaug, G.; Krokan, H.E.; Blackburn, G.M.; Tainer, J.A. Uracil-DNA glycosylase–DNA substrate and product structures: Conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc. Natl Acad. Sci. USA 2000, 97, 5083–5088. [Google Scholar] [CrossRef]
- Bianchet, M.A.; Seiple, L.A.; Jiang, Y.L.; Ichikawa, Y.; Amzel, L.M.; Stivers, J.T. Electrostatic guidance of glycosyl cation migration along the reaction coordinate of uracil DNA glycosylase. Biochemistry 2003, 42, 12455–12460. [Google Scholar] [CrossRef]
- Moe, E.; Leiros, I.; Riise, E.K.; Olufsen, M.; Lanes, O.; Smalås, A.O.; Willassen, N.P. Optimisation of the surface electrostatics as a strategy for cold adaptation of uracil-DNA N-glycosylase (UNG) from atlantic cod (Gadus morhua). J. Mol. Biol. 2004, 343, 1221–1230. [Google Scholar] [CrossRef]
- Krosky, D.J.; Bianchet, M.A.; Seiple, L.; Chung, S.; Amzel, L.M.; Stivers, J.T. Mimicking damaged DNA with a small molecule inhibitor of human UNG2. Nucleic Acids Res. 2006, 34, 5872–5879. [Google Scholar] [CrossRef]
- Parker, J.B.; Bianchet, M.A.; Krosky, D.J.; Friedman, J.I.; Amzel, L.M.; Stivers, J.T. Enzymatic capture of an extrahelical thymine in the search for uracil in DNA. Nature 2007, 449, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Parker, J.B.; Bianchet, M.; Amzel, L.M.; Stivers, J.T. Impact of linker strain and flexibility in the design of a fragment-based inhibitor. Nat. Chem. Biol. 2009, 5, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Assefa, N.G.; Niiranen, L.; Willassen, N.P.; Smalås, A.; Moe, E. Thermal unfolding studies of cold adapted uracil-DNA N-glycosylase (UNG) from Atlantic cod (Gadus morhua). A comparative study with human UNG. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Ho, C.-H.; Chou, C.-C.; Ko, T.-P.; Huang, M.-F.; Hsu, K.-C.; Wang, A.H.-J. Using structural-based protein engineering to modulate the differential inhibition effects of SAUGI on human and HSV uracil DNA glycosylase. Nucleic Acids Res. 2016, 44, 4440–4449. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, X.; Barnes, C.O.; DeLucia, M.; Cohen, A.E.; Gronenborn, A.M.; Ahn, J.; Calero, G. The DDB1–DCAF1–Vpr–UNG2 crystal structure reveals how HIV-1 Vpr steers human UNG2 toward destruction. Nat. Struct. Mol. Biol. 2016, 23, 933–940. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Moiani, D.; Ahmed, Z.; Arvai, A.S.; Namjoshi, S.; Shin, D.S.; Fedorov, Y.; Selvik, E.J.; Jones, D.E.; Pink, J.; et al. An effective human uracil-DNA glycosylase inhibitor targets the open pre-catalytic active site conformation. Prog. Biophys. Mol. Biol. 2021, 163, 143–159. [Google Scholar] [CrossRef]
- Kavli, B.; Sundheim, O.; Akbari, M.; Otterlei, M.; Nilsen, H.; Skorpen, F.; Aas, P.A.; Hagen, L.; Krokan, H.E.; Slupphaug, G. hUNG2 is the major repair enzyme for removal of uracil from U:A matches, U:G mismatches, and U in single-stranded DNA, with hSMUG1 as a broad specificity backup. J. Biol. Chem. 2002, 277, 39926–39936. [Google Scholar] [CrossRef]
- Putnam, C.D.; Tainer, J.A. Protein mimicry of DNA and pathway regulation. DNA Repair 2005, 4, 1410–1420. [Google Scholar] [CrossRef]
- Gommers-Ampt, J.H.; Borst, P. Hypermodified bases in DNA. FASEB J. 1995, 9, 1034–1042. [Google Scholar] [CrossRef]
- Weigele, P.; Raleigh, E.A. Biosynthesis and function of modified bases in bacteria and their viruses. Chem. Rev. 2016, 116, 12655–12687. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Cedergren-Zeppezauer, E.S.; Wilson, K.S. Homotrimeric dUTPases: Structural solutions for specific recognition and hydrolysis of dUTP. Curr. Protein Pept. Sci. 2001, 2, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Vértessy, B.G.; Tóth, J. Keeping uracil out of DNA: Physiological role, structure and catalytic mechanism of dUTPases. Acc. Chem. Res. 2009, 42, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kouzminova, E.A.; Kuzminov, A. Chromosomal fragmentation in dUTPase-deficient mutants of Escherichia coli and its recombinational repair. Mol. Microbiol. 2004, 51, 1279–1295. [Google Scholar] [CrossRef]
- Ting, H.; Kouzminova, E.A.; Kuzminov, A. Synthetic lethality with the dut defect in Escherichia coli reveals layers of DNA damage of increasing complexity due to uracil incorporation. J. Bacteriol. 2008, 190, 5841–5854. [Google Scholar] [CrossRef]
- Drohat, A.C.; Jagadeesh, J.; Ferguson, E.; Stivers, J.T. Role of electrophilic and general base catalysis in the mechanism of Escherichia coli uracil DNA glycosylase. Biochemistry 1999, 38, 11866–11875. [Google Scholar] [CrossRef]
- Mol, C.D.; Arvai, A.S.; Slupphaug, G.; Kavli, B.; Alseth, I.; Krokan, H.E.; Tainer, J.A. Crystal structure and mutational analysis of human uracil-DNA glycosylase: Structural basis for specificity and catalysis. Cell 1995, 80, 869–878. [Google Scholar] [CrossRef]
- Grossman, T.H.; Kawasaki, E.S.; Punreddy, S.R.; Osburne, M.S. Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 1998, 209, 95–103. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Endutkin, A.V.; Panferova, E.P.; Barmatov, A.E.; Zharkov, D.O. DNA glycosylases for 8-oxoguanine repair in Staphylococcus aureus. DNA Repair 2021, 105, 103160. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.K.; Weiss, B. Specific mutator effects of ung (uracil-DNA glycosylase) mutations in Escherichia coli. J. Bacteriol. 1982, 151, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.J.; Sen, S.; Choo, Y.J.; Beltrao, P.; Zietek, M.; Chaba, R.; Lee, S.; Kazmierczak, K.M.; Lee, K.J.; Wong, A.; et al. Phenotypic landscape of a bacterial cell. Cell 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Impellizzeri, K.J.; Anderson, B.; Burgers, P.M.J. The spectrum of spontaneous mutations in a Saccharomyces cerevisiae uracil-DNA-glycosylase mutant limits the function of this enzyme to cytosine deamination repair. J. Bacteriol. 1991, 173, 6807–6810. [Google Scholar] [CrossRef]
- Burgers, P.M.J.; Klein, M.B. Selection by genetic transformation of a Saccharomyces cerevisiae mutant defective for the nuclear uracil-DNA-glycosylase. J. Bacteriol. 1986, 166, 905–913. [Google Scholar] [CrossRef]
- Jarolim, S.; Ayer, A.; Pillay, B.; Gee, A.C.; Phrakaysone, A.; Perrone, G.G.; Breitenbach, M.; Dawes, I.W. Saccharomyces cerevisiae genes involved in survival of heat shock. G3 Genes Genomes Genet. 2013, 3, 2321–2333. [Google Scholar] [CrossRef]
- Mülleder, M.; Calvani, E.; Alam, M.T.; Wang, R.K.; Eckerstorfer, F.; Zelezniak, A.; Ralser, M. Functional metabolomics describes the yeast biosynthetic regulome. Cell 2016, 167, 553–565.e512. [Google Scholar] [CrossRef]
- Imai, K.; Slupphaug, G.; Lee, W.-I.; Revy, P.; Nonoyama, S.; Catalan, N.; Yel, L.; Forveille, M.; Kavli, B.; Krokan, H.E.; et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat. Immunol. 2003, 4, 1023–1028. [Google Scholar] [CrossRef]
- Rada, C.; Williams, G.T.; Nilsen, H.; Barnes, D.E.; Lindahl, T.; Neuberger, M.S. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 2002, 12, 1748–1755. [Google Scholar] [CrossRef]
- Kavli, B.; Andersen, S.; Otterlei, M.; Liabakk, N.B.; Imai, K.; Fischer, A.; Durandy, A.; Krokan, H.E.; Slupphaug, G. B cells from hyper-IgM patients carrying UNG mutations lack ability to remove uracil from ssDNA and have elevated genomic uracil. J. Exp. Med. 2005, 201, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Harms, C.; Sobol, R.W.; Cardozo-Pelaez, F.; Linhart, H.; Winter, B.; Balkaya, M.; Gertz, K.; Gay, S.B.; Cox, D.; et al. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J. Neurosci. 2008, 28, 7219–7230. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Gertz, K.; Overall, R.W.; Harms, C.; Klein, J.; Page, M.M.; Stuart, J.A.; Endres, M. Folate deficiency increases mtDNA and D-1 mtDNA deletion in aged brain of mice lacking uracil-DNA glycosylase. Exp. Neurol. 2011, 228, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D.; Inada, N.; Hather, G.; Kleindt, C.K.; Wildermuth, M.C. Laser microdissection of Arabidopsis cells at the powdery mildew infection site reveals site-specific processes and regulators. Proc. Natl Acad. Sci. USA 2010, 107, 460–465. [Google Scholar] [CrossRef]
- Lister, R.; Chew, O.; Lee, M.-N.; Heazlewood, J.L.; Clifton, R.; Parker, K.L.; Millar, A.H.; Whelan, J. A transcriptomic and proteomic characterization of the Arabidopsis mitochondrial protein import apparatus and its response to mitochondrial dysfunction. Plant Physiol. 2004, 134, 777–789. [Google Scholar] [CrossRef]
- Clifton, R.; Lister, R.; Parker, K.L.; Sappl, P.G.; Elhafez, D.; Millar, A.H.; Day, D.A.; Whelan, J. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol. Biol. 2005, 58, 193–212. [Google Scholar] [CrossRef]
- Lin, W.-D.; Liao, Y.-Y.; Yang, T.J.W.; Pan, C.-Y.; Buckhout, T.J.; Schmidt, W. Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol. 2004, 155, 1383–1402. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Long, T.A.; Wang, J.Y.; Jung, J.W.; Mace, D.; Pointer, S.; Barron, C.; Brady, S.M.; Schiefelbein, J.; Benfey, P.N. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 2008, 320, 942–945. [Google Scholar] [CrossRef]
- Chan, Z.; Grumet, R.; Loescher, W. Global gene expression analysis of transgenic, mannitol-producing, and salt-tolerant Arabidopsis thaliana indicates widespread changes in abiotic and biotic stress-related genes. J. Exp. Bot. 2011, 62, 4787–4803. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Ihnatowicz, A.; Pesaresi, P.; Varotto, C.; Richly, E.; Schneider, A.; Jahns, P.; Salamini, F.; Leister, D. Mutants for photosystem I subunit D of Arabidopsis thaliana: Effects on photosynthesis, photosystem I stability and expression of nuclear genes for chloroplast functions. Plant J. 2004, 37, 839–852. [Google Scholar] [CrossRef]
- Biehl, A.; Richly, E.; Noutsos, C.; Salamini, F.; Leister, D. Analysis of 101 nuclear transcriptomes reveals 23 distinct regulons and their relationship to metabolism, chromosomal gene distribution and co-ordination of nuclear and plastid gene expression. Gene 2005, 344, 33–41. [Google Scholar] [CrossRef]
- Bonardi, V.; Pesaresi, P.; Becker, T.; Schleiff, E.; Wagner, R.; Pfannschmidt, T.; Jahns, P.; Leister, D. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 2005, 437, 1179–1182. [Google Scholar] [CrossRef]
- Mazzella, M.a.A.; Arana, M.a.V.n.; Staneloni, R.J.; Perelman, S.; Rodriguez Batiller, M.a.J.; Muschietti, J.; Cerdán, P.D.; Chen, K.; Sánchez, R.A.; Zhu, T.; et al. Phytochrome control of the Arabidopsis transcriptome anticipates seedling exposure to light. Plant Cell 2005, 17, 2507–2516. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef]
- González-Pérez, S.; Gutiérrez, J.; García-García, F.; Osuna, D.; Dopazo, J.; Lorenzo, Ó.; Revuelta, J.L.; Arellano, J.B. Early transcriptional defense responses in Arabidopsis cell suspension culture under high-light conditions. Plant Physiol. 2011, 156, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Horiuchi, Y.; Ueda, Y.; Mizuta, Y.; Kubo, T.; Yano, K.; Yamaki, S.; Tsuda, K.; Nagata, T.; Niihama, M.; et al. Rice expression atlas in reproductive development. Plant Cell Physiol. 2010, 51, 2060–2081. [Google Scholar] [CrossRef] [PubMed]
- Boavida, L.C.; Borges, F.; Becker, J.D.; Feijó, J.A. Whole genome analysis of gene expression reveals coordinated activation of signaling and metabolic pathways during pollen-pistil interactions in Arabidopsis. Plant Physiol. 2011, 155, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, Z.; Kow, Y.W.; Purmal, A.A.; Cunningham, R.P.; Wallace, S.S. New substrates for old enzymes: 5-hydroxy-2′-deoxycytidine and 5-hydroxy-2′-deoxyuridine are substrates for Escherichia coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2′-deoxyuridine is a substrate for uracil DNA N-glycosylase. J. Biol. Chem. 1994, 269, 18814–18820. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Karakaya, A.; Jaruga, P.; Slupphaug, G.; Krokan, H.E. Novel activities of human uracil DNA N-glycosylase for cytosine-derived products of oxidative DNA damage. Nucleic Acids Res. 1996, 24, 418–422. [Google Scholar] [CrossRef]
- Slupphaug, G.; Eftedal, I.; Kavli, B.; Bharati, S.; Helle, N.M.; Haug, T.; Levine, D.W.; Krokan, H.E. Properties of a recombinant human uracil-DNA glycosylase from the UNG gene and evidence that UNG encodes the major uracil-DNA glycosylase. Biochemistry 1995, 34, 128–138. [Google Scholar] [CrossRef]
- Nagelhus, T.A.; Haug, T.; Singh, K.K.; Keshav, K.F.; Skorpen, F.; Otterlei, M.; Bharati, S.; Lindmo, T.; Benichou, S.; Benarous, R.; et al. A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J. Biol. Chem. 1997, 272, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, M.; Warbrick, E.; Nagelhus, T.A.; Haug, T.; Slupphaug, G.; Akbari, M.; Aas, P.A.; Steinsbekk, K.; Bakke, O.; Krokan, H.E. Post-replicative base excision repair in replication foci. EMBO J. 1999, 18, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Boyko, A.; Golubov, A.; Bilichak, A.; Kovalchuk, I. Chlorine ions but not sodium ions alter genome stability of Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1066–1078. [Google Scholar] [CrossRef]
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Ramakanth, K.K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell 2017, 170, 102–113.E14. [Google Scholar] [CrossRef]
- Nisa, M.-U.; Huang, Y.; Benhamed, M.; Raynaud, C. The plant DNA damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Chen, X.; Tian, D.; Kong, X.; Chen, Q.; Abd Allah, E.F.; Hu, X.; Jia, A. The role of nitric oxide signalling in response to salt stress in Chlamydomonas reinhardtii. Planta 2016, 244, 651–669. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. Interactive role of exogenous 24 Epibrassinolide and endogenous NO in Brassica juncea L. under salinity stress: Evidence for NR-dependent NO biosynthesis. Nitric Oxide 2020, 97, 33–47. [Google Scholar] [CrossRef]
- Han, B.; Gu, J.; Zhao, L.; Guo, H.; Xie, Y.; Zhao, S.; Song, X.; Han, L.; Liu, L. Factors affecting the radiosensitivity of hexaploid wheat to γ-irradiation: Radiosensitivity of hexaploid wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0165187. [Google Scholar] [CrossRef]
- Pirredda, M.; Fañanás-Pueyo, I.; Oñate-Sánchez, L.; Mira, S. Seed longevity and ageing: A review on physiological and genetic factors with an emphasis on hormonal regulation. Plants 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shen, X.; Liang, N.; Xie, Z.; Tian, Z.; Zhang, L.; Guo, J.; Wei, F.; Shi, G.; Wei, X. Integrated cytological and transcriptomic analyses provide new insights into restoration of pollen viability in synthetic allotetraploid Brassica carinata. Plant Cell Rep. 2024, 43, 234. [Google Scholar] [CrossRef] [PubMed]
- Sayed, L.M.; Khaled, K.A.M.; Samaha, G.M.; Saber, A.A. Integrating phenotypic, molecular, and bioinformatics approaches for developing drought-tolerant sesame. Sci. Rep. 2025, 15, 21897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, D.; Grin, I.R.; Zharkov, D.O.; Yu, B. Developing plant-derived DNA repair enzyme resources through studying the involvement of base excision repair DNA glycosylases in stress responses of plants. Physiol. Plant. 2025, 177, e70162. [Google Scholar] [CrossRef]
- Veillet, F.; Perrot, L.; Chauvin, L.; Kermarrec, M.-P.; Guyon-Debast, A.; Chauvin, J.-E.; Nogué, F.; Mazier, M. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 2019, 20, 402. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Wang, F.; Zhao, S.; Feng, F.; Song, J.; Zhang, C.; Yang, J. Increasing cytosine base editing scope and efficiency with engineered Cas9-PmCDA1 fusions and the modified sgRNA in rice. Front. Genet. 2019, 10, 379. [Google Scholar] [CrossRef]
- Hu, L.; Amoo, O.; Liu, Q.; Cai, S.; Zhu, M.; Shen, X.; Yu, K.; Zhai, Y.; Yang, Y.; Xu, L.; et al. Precision genome engineering through cytidine base editing in rapeseed (Brassica napus L.). Front. Genome Ed. 2020, 2, 605768. [Google Scholar] [CrossRef]
- Shirazi Parsa, H.; Sabet, M.S.; Moieni, A.; Shojaeiyan, A.; Dogimont, C.; Boualem, A.; Bendahmane, A. CRISPR/Cas9-mediated cytosine base editing using an improved transformation procedure in melon (Cucumis melo L.). Int. J. Mol. Sci. 2023, 24, 11189. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Base editing in crops: Current advances, limitations and future implications. Plant Biotechnol. J. 2020, 18, 20–31. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. CRISPR/Cas system-mediated base editing in crops: Recent developments and future prospects. Plant Cell Rep. 2024, 43, 271. [Google Scholar] [CrossRef] [PubMed]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef] [PubMed]
- Gegenheimer, P. Preparation of extracts from plants. Methods Enzymol. 1990, 182, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Fang, H.; Shi, H.; Chen, K.; Zhang, Z.; Tan, X. Selection of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in Brassica napus under various stress conditions. Mol. Genet. Genom. 2014, 289, 1023–1035. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Popov, A.V.; Vorobjev, Y.N.; Zharkov, D.O. MDTRA: A molecular dynamics trajectory analyzer with a graphical user interface. J. Comput. Chem. 2013, 34, 319–325. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]

| ID | Sequence, 5′→3′ |
|---|---|
| Cloning | |
| BvUNG_fwd | CGTCAAAGAAATGAAATCTCTCCGA |
| BvUNG_rev | GCTTCAGGCACAAGTCATTCCAT |
| RT-qPCR | |
| BvUNG_RT_fwd | ATCCTGCCCAAGAATGACGG |
| BvUNG_RT_rev | CGTGGTTGGGAGCATTACCT |
| Site-directed and deletion mutagenesis | |
| BvUNG_H340A_fwd | TAAATCAGCTGCTCCTTCTGGTCTTTC |
| BvUNG_H340A_rev | AGAATATGGTGCTTAGAC |
| BvUNG_NΔ109_fwd | ACTGCTGAGCAGAAGTTCAGAATGGAG |
| BvUNG_NΔ109_rev | ATGGCTGCCGCGCGG |
| BvUNG_NΔ151_fwd | AAGGAGCTCCTGGTGGAGGATTCGTG |
| BvUNG_NΔ151_rev | ATGGCTGCCGCGCGGCAC |
| Enzyme activity and kinetic studies | |
| 23U | Fluo-CTCTCCCTTCUCTCCTTTCCTCT |
| 23ohU | Fluo-CTCTCCCTTCXCTCCTTTCCTCT (X = 5-hydroxyuracil) |
| 23DHU | Fluo-CTCTCCCTTCXCTCCTTTCCTCT (X = 5,6-dihydrouracil) |
| 23hmU | Fluo-CTCTCCCTTCXCTCCTTTCCTCT (X = 5-hydroxymethyluracil) |
| 23Ups | Fluo-CTCTCCCTTCUCTCCTTTCCTpsCpsT |
| 23compA | AGAGGAAAGGAGAGAAGGGAGAG |
| 23compG | AGAGGAAAGGAGGGAAGGGAGAG |
| Modification Type | Site 1 |
|---|---|
| Ser/Thr phosphorylation | S85, S89, S91 |
| N-linked glycosylation | N120, N203 |
| O-linked glycosylation | S52, S53, S54, T56, T64, S66, S357 |
| S-nitrosylation | C133, C256 |
| Acetylation | K127, K136, K174, K251 |
| Malonylation | K127, K251 |
| Glutarylation | K121, K136 |
| Glutathionylation | C256 |
| Succinylation | K291, K292 |
| SUMOylation | K337 |
| Formylation | K251, K332 |
| Substrate | KM, µM | kcat, min−1 | kcat/KM, µM−1·min−1 |
|---|---|---|---|
| U | 27 ± 12 | 540 ± 170 | 20 ± 11 |
| U:A | 15 ± 3 | 230 ± 30 | 16 ± 4 |
| U:G | 3.8 ± 0.8 | 420 ± 30 | 110 ± 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, D.V.; Zateeva, M.V.; Zhang, L.; Zhang, J.; Zhao, Y.; Permyakova, N.V.; Zagorskaya, A.A.; Zharkov, V.D.; Endutkin, A.V.; Yu, B.; et al. Uracil–DNA Glycosylase from Beta vulgaris: Properties and Response to Abiotic Stress. Int. J. Mol. Sci. 2025, 26, 8221. https://doi.org/10.3390/ijms26178221
Petrova DV, Zateeva MV, Zhang L, Zhang J, Zhao Y, Permyakova NV, Zagorskaya AA, Zharkov VD, Endutkin AV, Yu B, et al. Uracil–DNA Glycosylase from Beta vulgaris: Properties and Response to Abiotic Stress. International Journal of Molecular Sciences. 2025; 26(17):8221. https://doi.org/10.3390/ijms26178221
Chicago/Turabian StylePetrova, Daria V., Maria V. Zateeva, Lijun Zhang, Jiajia Zhang, Ying Zhao, Natalya V. Permyakova, Alla A. Zagorskaya, Vasily D. Zharkov, Anton V. Endutkin, Bing Yu, and et al. 2025. "Uracil–DNA Glycosylase from Beta vulgaris: Properties and Response to Abiotic Stress" International Journal of Molecular Sciences 26, no. 17: 8221. https://doi.org/10.3390/ijms26178221
APA StylePetrova, D. V., Zateeva, M. V., Zhang, L., Zhang, J., Zhao, Y., Permyakova, N. V., Zagorskaya, A. A., Zharkov, V. D., Endutkin, A. V., Yu, B., Ma, C., Li, H., Zharkov, D. O., & Grin, I. R. (2025). Uracil–DNA Glycosylase from Beta vulgaris: Properties and Response to Abiotic Stress. International Journal of Molecular Sciences, 26(17), 8221. https://doi.org/10.3390/ijms26178221






