Disordered Protein Tail Is Wagging Poly(ADP-ribosyl)ation
Abstract
1. Introduction
2. Results
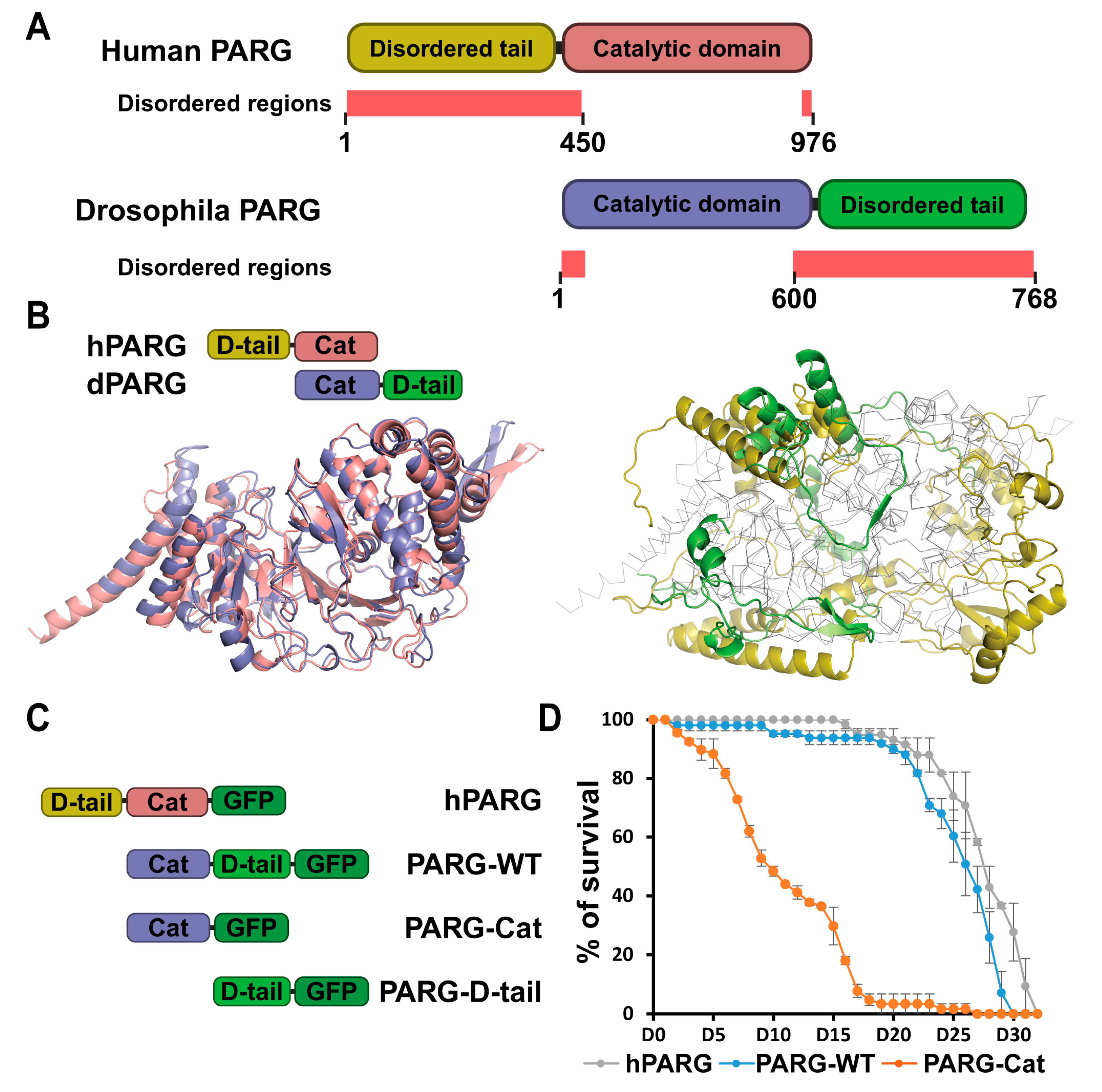
2.1. Despite Structural Differences, Human PARG Is Fully Functional in Drosophila
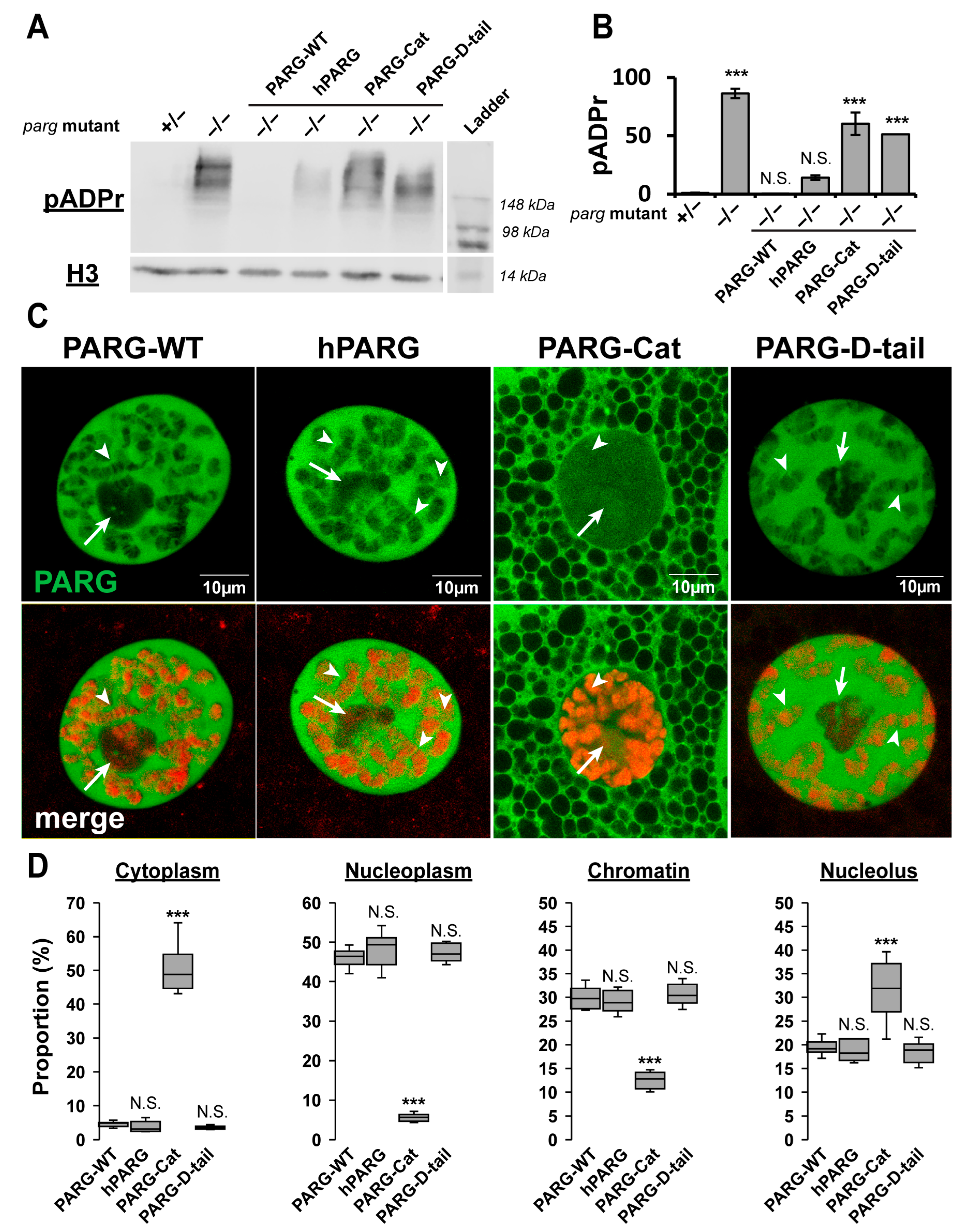
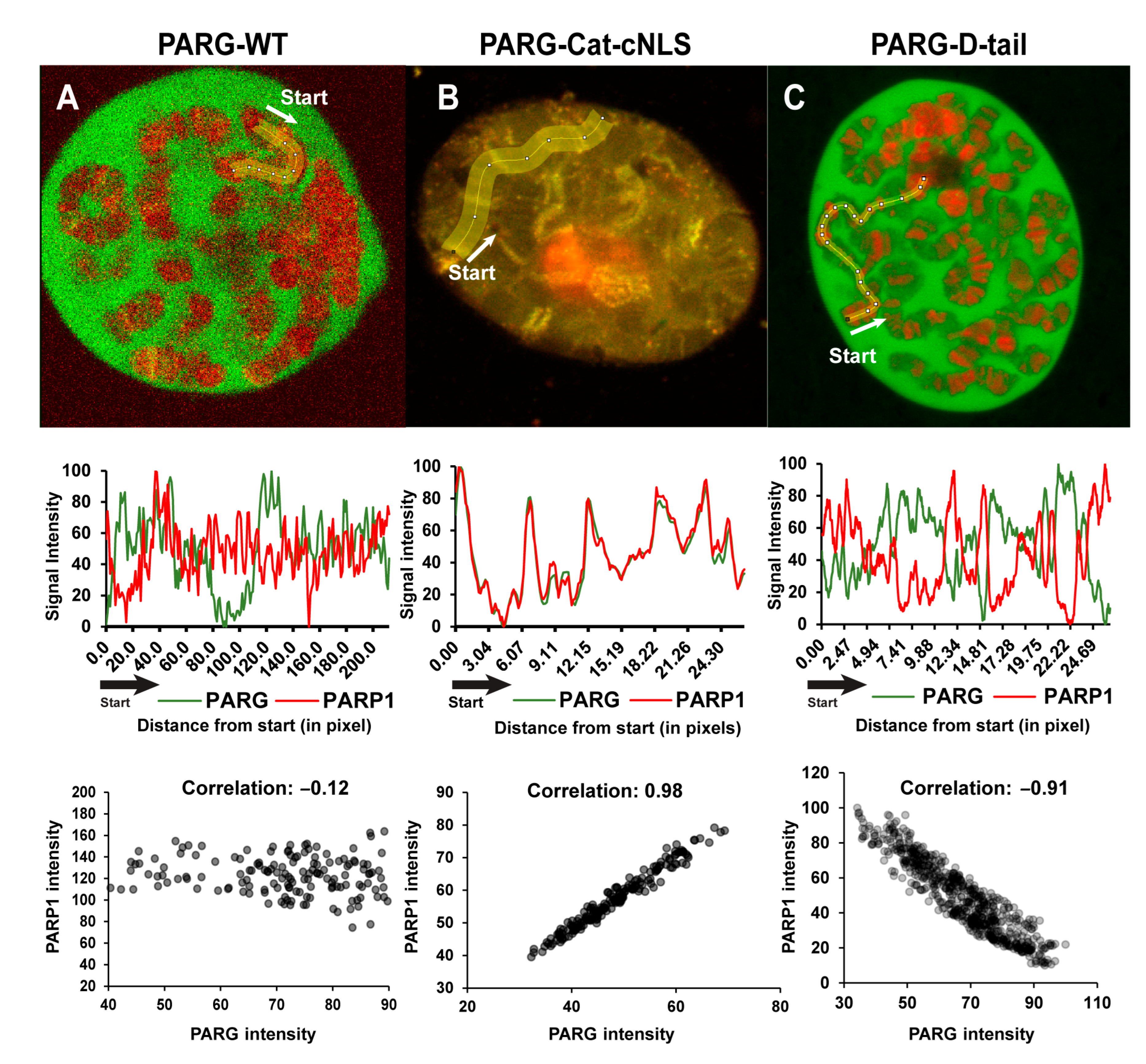
2.2. The Disordered Tail Controls PARG Activity and Subcellular Localization
2.3. The Disordered Tail Is Sufficient to Control PARG Subcellular Localization
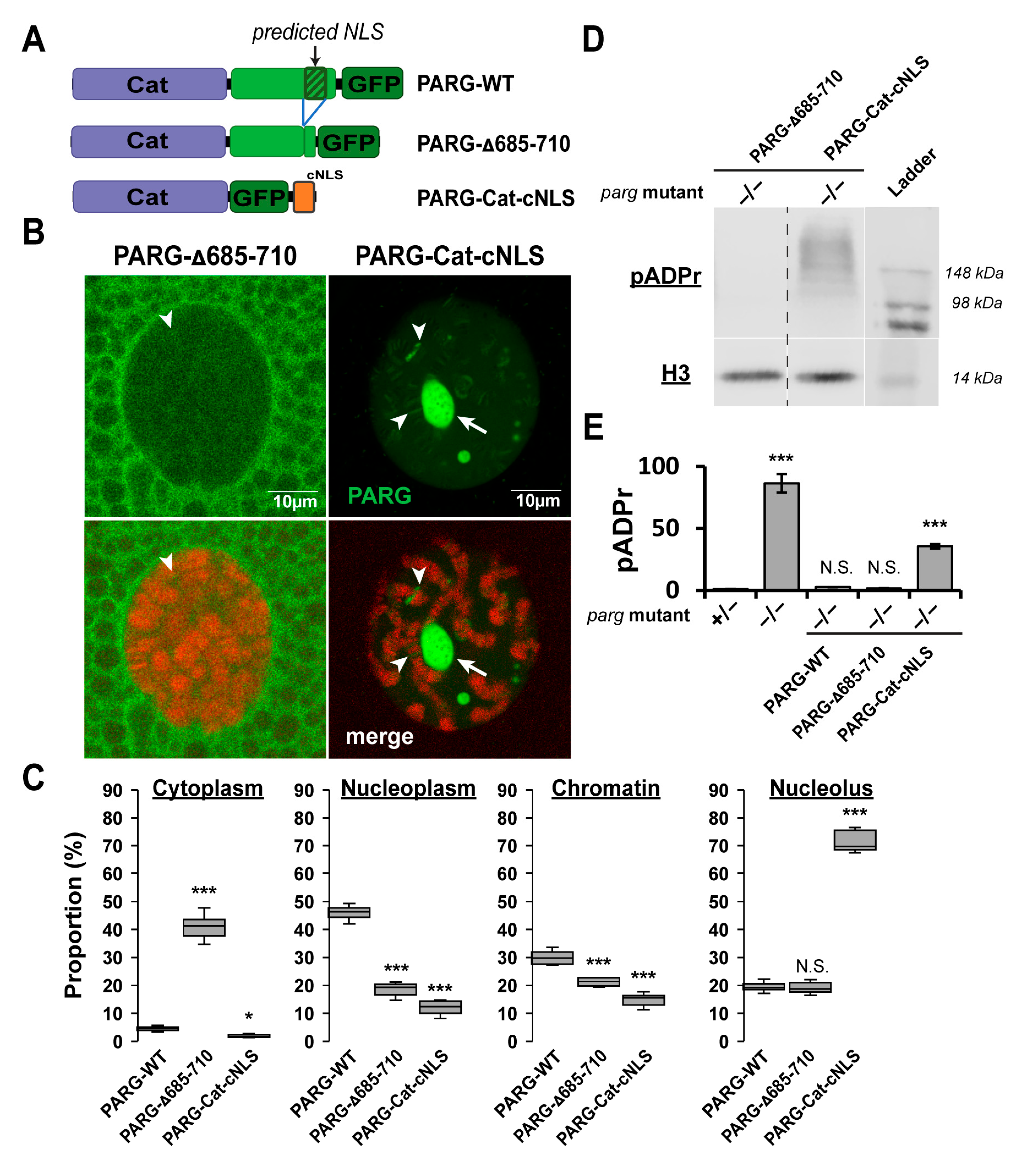
2.4. The Disordered Tail Controls PARG Nuclear–Cytoplasmic Trafficking and Ensures Functional Nuclear Localization
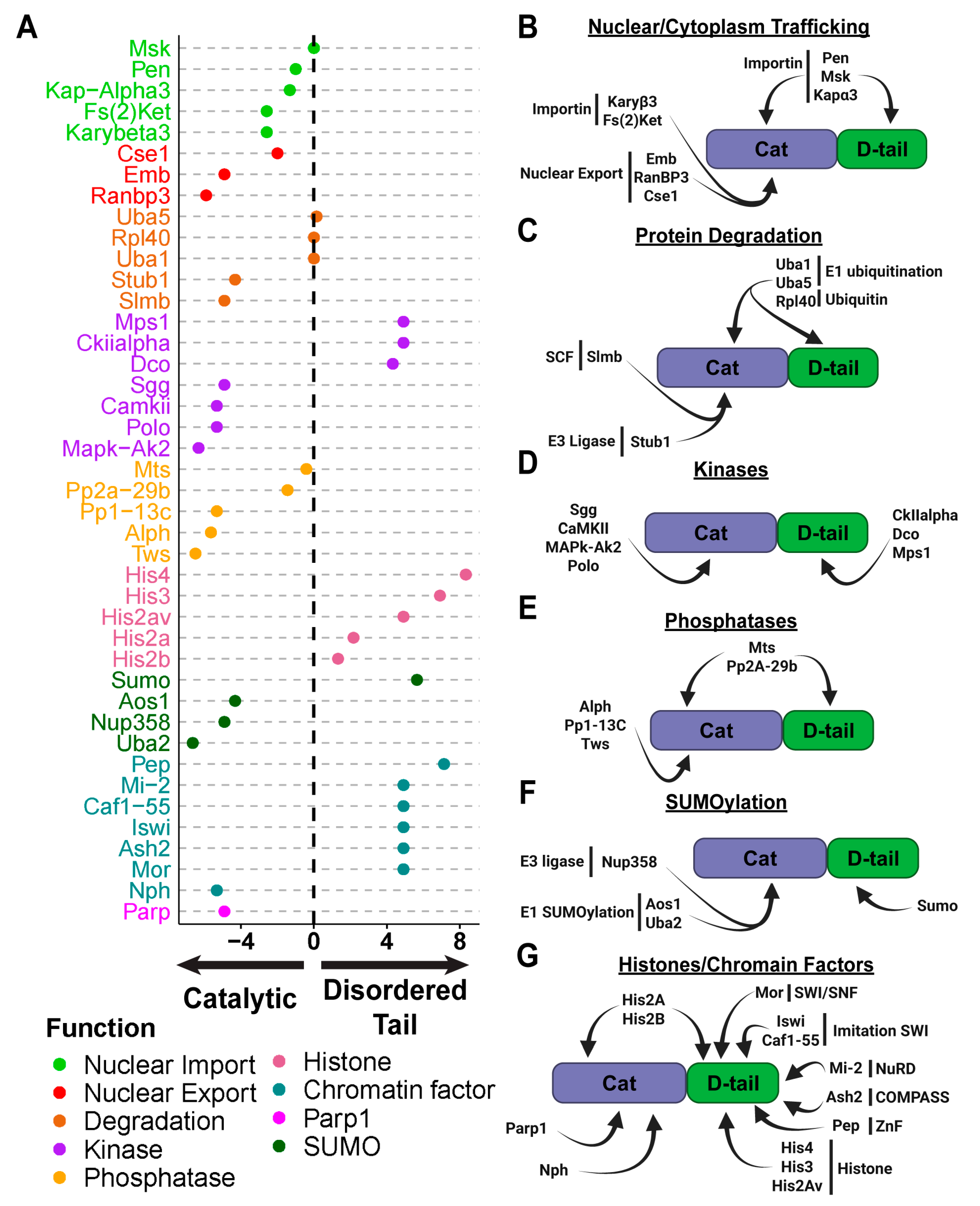
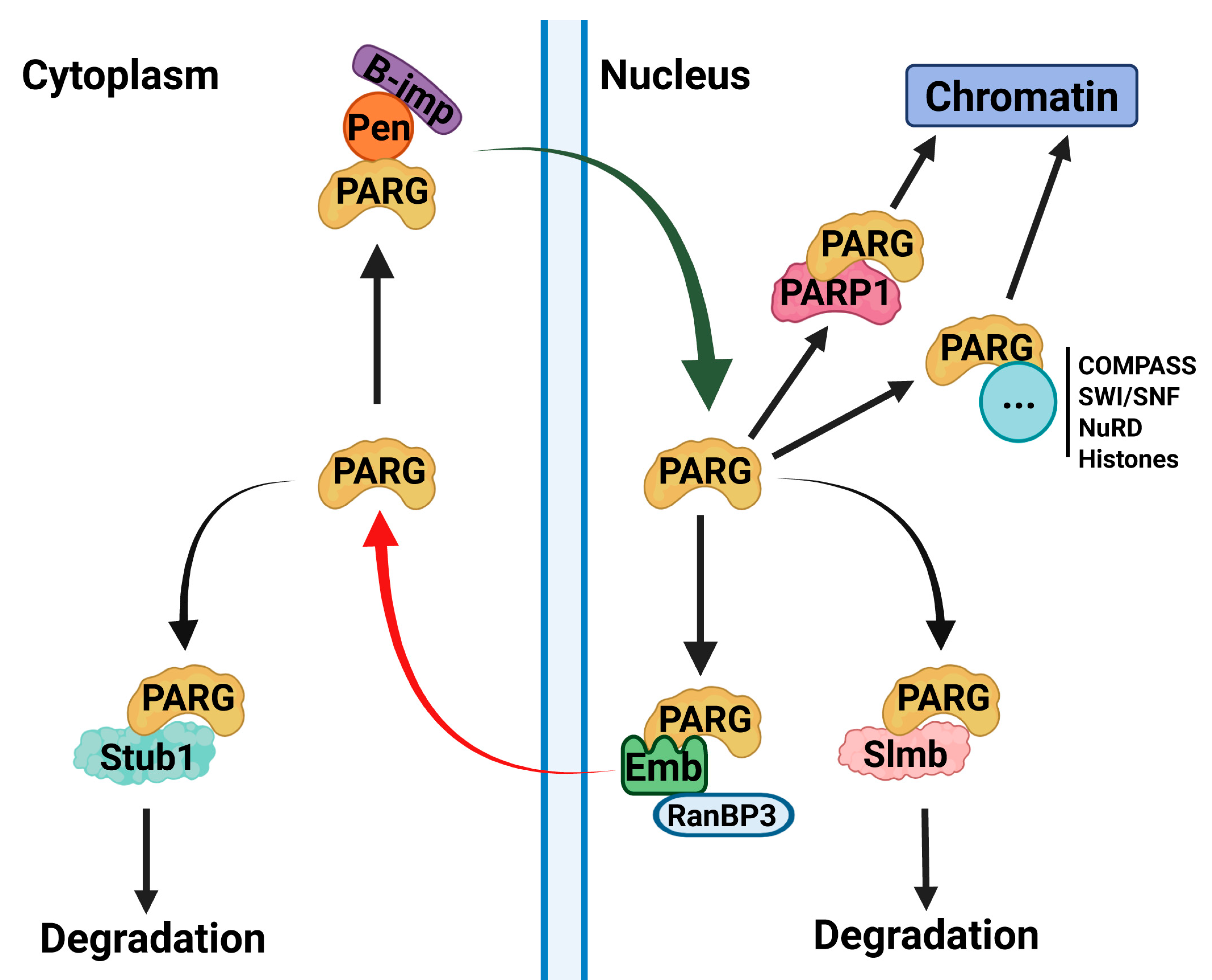
2.5. Interaction with the Protein Degradation Machinery Is Primarily Mediated Through the PARG Catalytic Domain
2.6. Distinct Kinases Regulate PARG Phosphorylation at Catalytic and Disordered Tail Regions
2.7. SUMOylation as a Potential Mechanism of PARG Regulation
2.8. The Disordered Tail Controls PARG Chromatin Binding
3. Discussion
4. Materials and Methods
- PARGWT: parg27.1/parg27.1; P{w+, UASt::PARG-WT-EGFP}, P{w+, UASt::PARP1-DsRed}, P{w+, Gawb(69b)-Gal4}/TM2.
- Human PARG (hPARG): parg27.1/parg27.1; P{w+, UASt::hPARG-EGFP}, P{w+, UASt::PARP1-DsRed}, P{w+, Gawb(69b)-Gal4}/TM2.
- PARGcat: parg27.1/parg27.1; P{w+, UASt::PARG-Cat-EGFP}, P{w+, UASt::PARP1-DsRed}, P{w+, Gawb(69b)-Gal4}/TM2.
- PARGCter: parg27.1/FM7i-GFP; P{w+, UASt::PARG-D-tail-EGFP}, P{w+, UASt::PARP1-DsRed}, P{w+, Gawb(69b)3Gal4}/Tm2.
- Forward: AAAGCGGCCGCATGCAAGAATTCAGGTCACACTTG
- Reverse: AAAGGTACCGTCAACAATATCGTCCTTTTCGTC
- Forward: AAGGTACCATGCAAGAATTCAGGTCACACTTG
- Reverse: TTGGATCCGGCGGATGCTCCCTC
- Forward: AAGAATTCATGGAAGCTGGAAGCTCTAGAG
- Reverse: AAGGTACCGTCAACAATATCGTCCTTTTCGTC
- Forward primer: AAAGCGGCCGCATGCAAGAATTCAGGTCACACTTG
- Reverse primer: AAAGGTACCGTCAACAATATCGTCCTTTTCGTCCTTGTCGGTCACATCCTTTTCATAATGG
- Forward primer: AAGCGGCCGCATGCAAGAATTCAGGTCACACTTG
- Reverse primer: TTGGTACCGGCGGATGCTCCCTCTC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.; Ren, C.; Wang, W.; Li, J.; Gong, X. Computational Prediction of Protein Intrinsically Disordered Region Related Interactions and Functions. Genes 2023, 14, 432. [Google Scholar] [CrossRef]
- Brodsky, S.; Jana, T.; Mittelman, K.; Chapal, M.; Kumar, D.K.; Carmi, M.; Barkai, N. Intrinsically Disordered Regions Direct Transcription Factor In Vivo Binding Specificity. Mol. Cell 2020, 79, 459–471.e454. [Google Scholar] [CrossRef] [PubMed]
- Tulin, A.; Spradling, A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 2003, 299, 560–562. [Google Scholar] [CrossRef]
- Bordet, G.; Lodhi, N.; Guo, D.; Kossenkov, A.; Tulin, A.V. Poly(ADP-ribose) polymerase 1 in genome-wide expression control in Drosophila. Sci. Rep. 2020, 10, 21151. [Google Scholar] [CrossRef]
- Bordet, G.; Karpova, I.; Tulin, A.V. Poly(ADP-ribosyl)ating enzymes cooperate to coordinate development. Sci. Rep. 2022, 12, 22120. [Google Scholar] [CrossRef]
- Bordet, G.; Kotova, E.; Tulin, A.V. Poly(ADP-ribosyl)ating pathway regulates development from stem cell niche to longevity control. Life Sci. Alliance 2022, 5, e202101071. [Google Scholar] [CrossRef]
- Bordet, G.; Bamgbose, G.; Tulin, A.V. Poly(ADP-ribosyl)ating enzymes coordinate changes in the expression of metabolic genes with developmental progression. Sci. Rep. 2023, 13, 20320. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.; Bai, P. PARPs, PAR and NAD Metabolism and Their Inhibitors in Cancer. Cancers 2020, 12, 3494. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Petesch, S.J.; Lis, J.T. Activator-induced spread of poly(ADP-ribose) polymerase promotes nucleosome loss at Hsp70. Mol. Cell 2012, 45, 64–74. [Google Scholar] [CrossRef]
- Thomas, C.; Ji, Y.; Wu, C.; Datz, H.; Boyle, C.; MacLeod, B.; Patel, S.; Ampofo, M.; Currie, M.; Harbin, J.; et al. Hit and run versus long-term activation of PARP-1 by its different domains fine-tunes nuclear processes. Proc. Natl. Acad. Sci. USA 2019, 116, 9941–9946. [Google Scholar] [CrossRef]
- Kotova, E.; Jarnik, M.; Tulin, A.V. Uncoupling of the transactivation and transrepression functions of PARP1 protein. Proc. Natl. Acad. Sci. USA 2010, 107, 6406–6411. [Google Scholar] [CrossRef]
- Koh, D.W.; Lawler, A.M.; Poitras, M.F.; Sasaki, M.; Wattler, S.; Nehls, M.C.; Stoger, T.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc. Natl. Acad. Sci. USA 2004, 101, 17699–17704. [Google Scholar] [CrossRef]
- Gagne, J.P.; Moreel, X.; Gagne, P.; Labelle, Y.; Droit, A.; Chevalier-Pare, M.; Bourassa, S.; McDonald, D.; Hendzel, M.J.; Prigent, C.; et al. Proteomic investigation of phosphorylation sites in poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase. J. Proteome Res. 2009, 8, 1014–1029. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, D.; Del Conte, A.; Mehdiabadi, M.; Aspromonte, M.C.; Blum, M.; Tesei, G.; von Bulow, S.; Lindorff-Larsen, K.; Tosatto, S.C.E. MOBIDB in 2025: Integrating ensemble properties and function annotations for intrinsically disordered proteins. Nucleic Acids Res. 2025, 53, D495–D503. [Google Scholar] [CrossRef] [PubMed]
- Slade, D.; Dunstan, M.S.; Barkauskaite, E.; Weston, R.; Lafite, P.; Dixon, N.; Ahel, M.; Leys, D.; Ahel, I. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature 2011, 477, 616–620. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Chakravarty, D. Intrinsically disordered proteins/regions and insight into their biomolecular interactions. Biophys. Chem. 2022, 283, 106769. [Google Scholar] [CrossRef]
- Macara, I.G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001, 65, 570–594. [Google Scholar] [CrossRef]
- Collier, S.; Chan, H.Y.; Toda, T.; McKimmie, C.; Johnson, G.; Adler, P.N.; O’Kane, C.; Ashburner, M. The Drosophila embargoed gene is required for larval progression and encodes the functional homolog of schizosaccharomyces Crm1. Genetics 2000, 155, 1799–1807. [Google Scholar] [CrossRef]
- Freedman, D.A.; Levine, A.J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 1998, 18, 7288–7293. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.K.; Kotchetkov, R.; Cariou, S.; Resch, A.; Lupetti, R.; Beniston, R.G.; Melchior, F.; Hengst, L.; Slingerland, J.M. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol. Biol. Cell 2003, 14, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, J.; Zhong, K.; Tong, A.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef]
- Wong, J.J.; Li, S.; Lim, E.K.; Wang, Y.; Wang, C.; Zhang, H.; Kirilly, D.; Wu, C.; Liou, Y.C.; Wang, H.; et al. A Cullin1-based SCF E3 ubiquitin ligase targets the InR/PI3K/TOR pathway to regulate neuronal pruning. PLoS Biol. 2013, 11, e1001657. [Google Scholar] [CrossRef]
- Ballinger, C.A.; Connell, P.; Wu, Y.; Hu, Z.; Thompson, L.J.; Yin, L.Y.; Patterson, C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999, 19, 4535–4545. [Google Scholar] [CrossRef]
- Chabu, C.; Doe, C.Q. Twins/PP2A regulates aPKC to control neuroblast cell polarity and self-renewal. Dev. Biol. 2009, 330, 399–405. [Google Scholar] [CrossRef]
- Geiss-Friedlander, R.; Melchior, F. Concepts in sumoylation: A decade on. Nat. Rev. Mol. Cell Biol. 2007, 8, 947–956. [Google Scholar] [CrossRef]
- Werner, A.; Flotho, A.; Melchior, F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol. Cell 2012, 46, 287–298. [Google Scholar] [CrossRef]
- Smith, M.; Turki-Judeh, W.; Courey, A.J. SUMOylation in Drosophila Development. Biomolecules 2012, 2, 331–349. [Google Scholar] [CrossRef]
- LaJeunesse, D.; Shearn, A. Trans-regulation of thoracic homeotic selector genes of the Antennapedia and bithorax complexes by the trithorax group genes: Absent, small, and homeotic discs 1 and 2. Mech. Dev. 1995, 53, 123–139. [Google Scholar] [CrossRef]
- Crosby, M.A.; Miller, C.; Alon, T.; Watson, K.L.; Verrijzer, C.P.; Goldman-Levi, R.; Zak, N.B. The trithorax group gene moira encodes a brahma-associated putative chromatin-remodeling factor in Drosophila melanogaster. Mol. Cell. Biol. 1999, 19, 1159–1170. [Google Scholar] [CrossRef]
- Deuring, R.; Fanti, L.; Armstrong, J.A.; Sarte, M.; Papoulas, O.; Prestel, M.; Daubresse, G.; Verardo, M.; Moseley, S.L.; Berloco, M.; et al. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol. Cell 2000, 5, 355–365. [Google Scholar] [CrossRef]
- Denslow, S.A.; Wade, P.A. The human Mi-2/NuRD complex and gene regulation. Oncogene 2007, 26, 5433–5438. [Google Scholar] [CrossRef]
- Calvo-Martin, J.M.; Papaceit, M.; Segarra, C. Evidence of neofunctionalization after the duplication of the highly conserved Polycomb group gene Caf1-55 in the obscura group of Drosophila. Sci. Rep. 2017, 7, 40536. [Google Scholar] [CrossRef]
- Amero, S.A.; Elgin, S.C.; Beyer, A.L. A unique zinc finger protein is associated preferentially with active ecdysone-responsive loci in Drosophila. Genes Dev. 1991, 5, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Box, J.K.; Paquet, N.; Adams, M.N.; Boucher, D.; Bolderson, E.; O’Byrne, K.J.; Richard, D.J. Nucleophosmin: From structure and function to disease development. BMC Mol. Biol. 2016, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Hoege, C.; Pyrowolakis, G.; Jentsch, S. SUMO, ubiquitin’s mysterious cousin. Nat. Rev. Mol. Cell Biol. 2001, 2, 202–210. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Henley, J.M. Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 2010, 428, 133–145. [Google Scholar] [CrossRef]
- Hanai, S.; Kanai, M.; Ohashi, S.; Okamoto, K.; Yamada, M.; Takahashi, H.; Miwa, M. Loss of poly(ADP-ribose) glycohydrolase causes progressive neurodegeneration in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Tulin, A.; Stewart, D.; Spradling, A.C. The Drosophila heterochromatic gene encoding poly(ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development. Genes Dev. 2002, 16, 2108–2119. [Google Scholar] [CrossRef]
- Karpova, Y.; Wu, C.; Divan, A.; McDonnell, M.E.; Hewlett, E.; Makhov, P.; Gordon, J.; Ye, M.; Reitz, A.B.; Childers, W.E.; et al. Non-NAD-like PARP-1 inhibitors in prostate cancer treatment. Biochem. Pharmacol. 2019, 167, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Larsen, B.; Lin, Z.Y.; Breitkreutz, A.; Mellacheruvu, D.; Fermin, D.; Qin, Z.S.; Tyers, M.; Gingras, A.C.; Nesvizhskii, A.I. SAINT: Probabilistic scoring of affinity purification-mass spectrometry data. Nat. Methods 2011, 8, 70–73. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordet, G.; Karpova, Y.; Espeseth, S.; Mitzel, G.; Bigelow, Z.; Tulin, A.V. Disordered Protein Tail Is Wagging Poly(ADP-ribosyl)ation. Int. J. Mol. Sci. 2025, 26, 8166. https://doi.org/10.3390/ijms26178166
Bordet G, Karpova Y, Espeseth S, Mitzel G, Bigelow Z, Tulin AV. Disordered Protein Tail Is Wagging Poly(ADP-ribosyl)ation. International Journal of Molecular Sciences. 2025; 26(17):8166. https://doi.org/10.3390/ijms26178166
Chicago/Turabian StyleBordet, Guillaume, Yaroslava Karpova, Saraynia Espeseth, Gavin Mitzel, Zachary Bigelow, and Alexei V. Tulin. 2025. "Disordered Protein Tail Is Wagging Poly(ADP-ribosyl)ation" International Journal of Molecular Sciences 26, no. 17: 8166. https://doi.org/10.3390/ijms26178166
APA StyleBordet, G., Karpova, Y., Espeseth, S., Mitzel, G., Bigelow, Z., & Tulin, A. V. (2025). Disordered Protein Tail Is Wagging Poly(ADP-ribosyl)ation. International Journal of Molecular Sciences, 26(17), 8166. https://doi.org/10.3390/ijms26178166









