Zinc Transporters of the LIV-1 Subfamily in Various Cancers: Molecular Insights and Research Priorities for Saudi Arabia
Abstract
1. Introduction
2. Zinc Homeostasis
3. Zinc Dysregulation in Disease
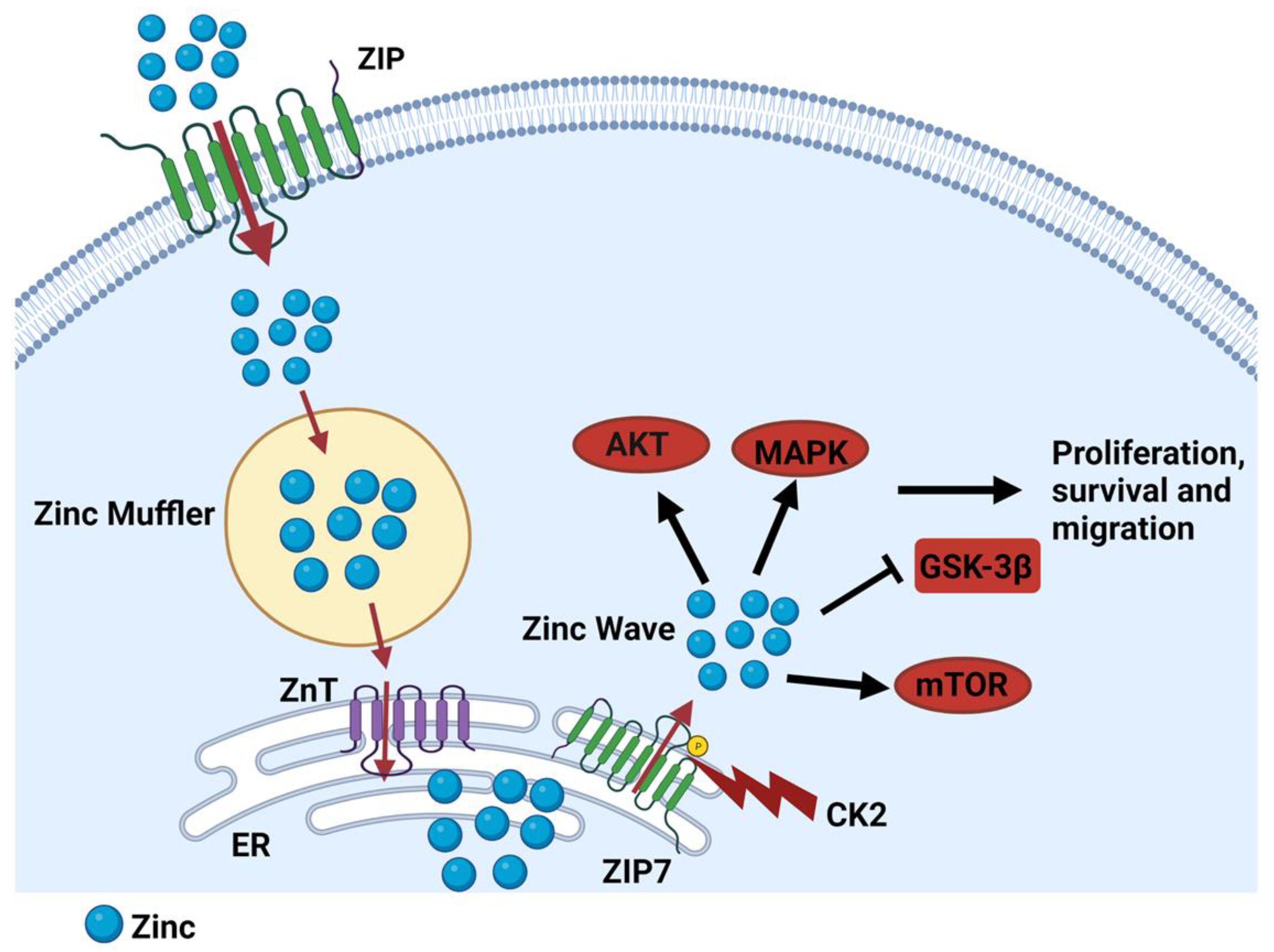
4. The Role of LIV-1 Subfamily Zinc Transporters in Cancer Progression
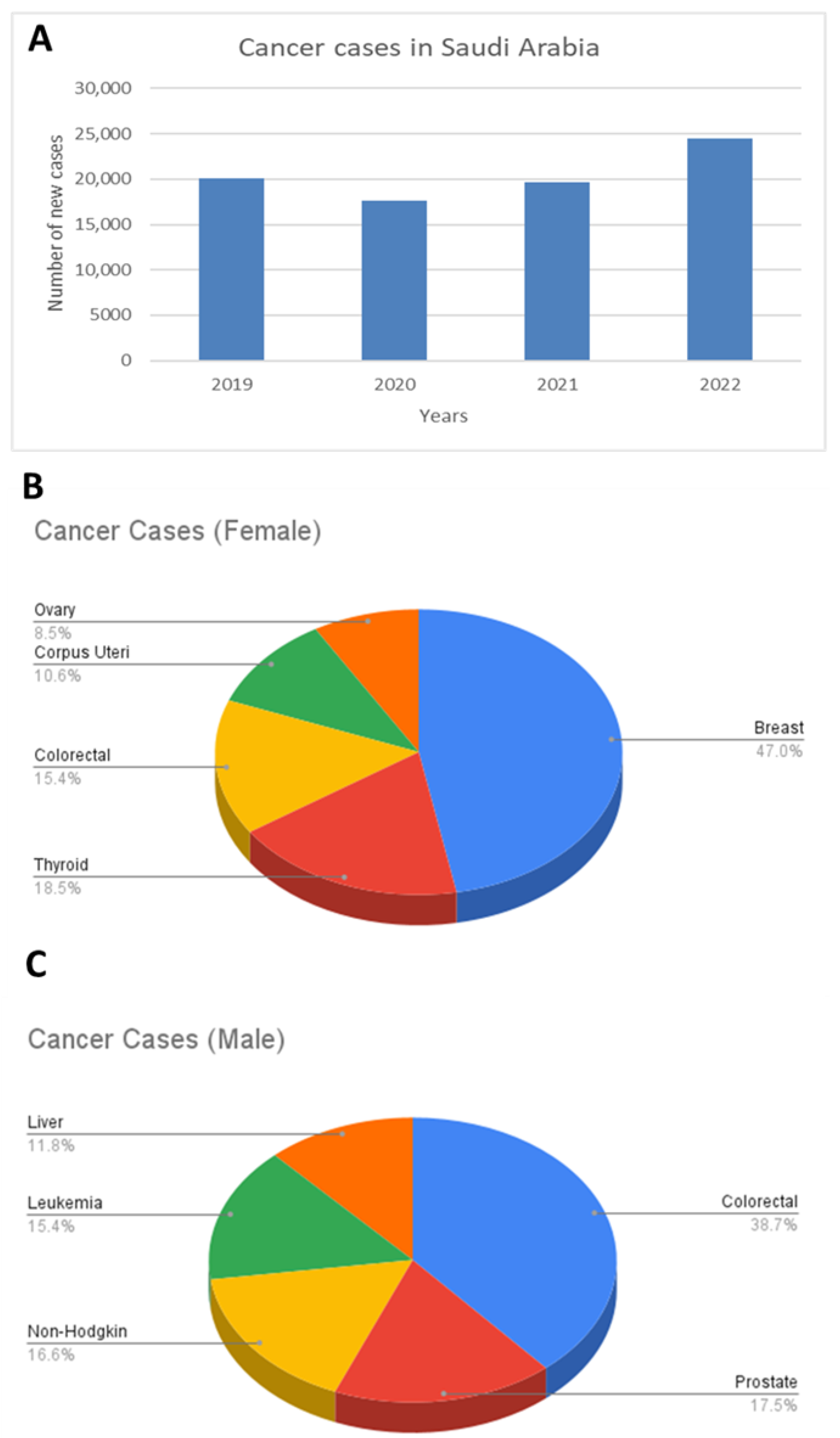
5. Cancer Incidence in Saudi Arabia
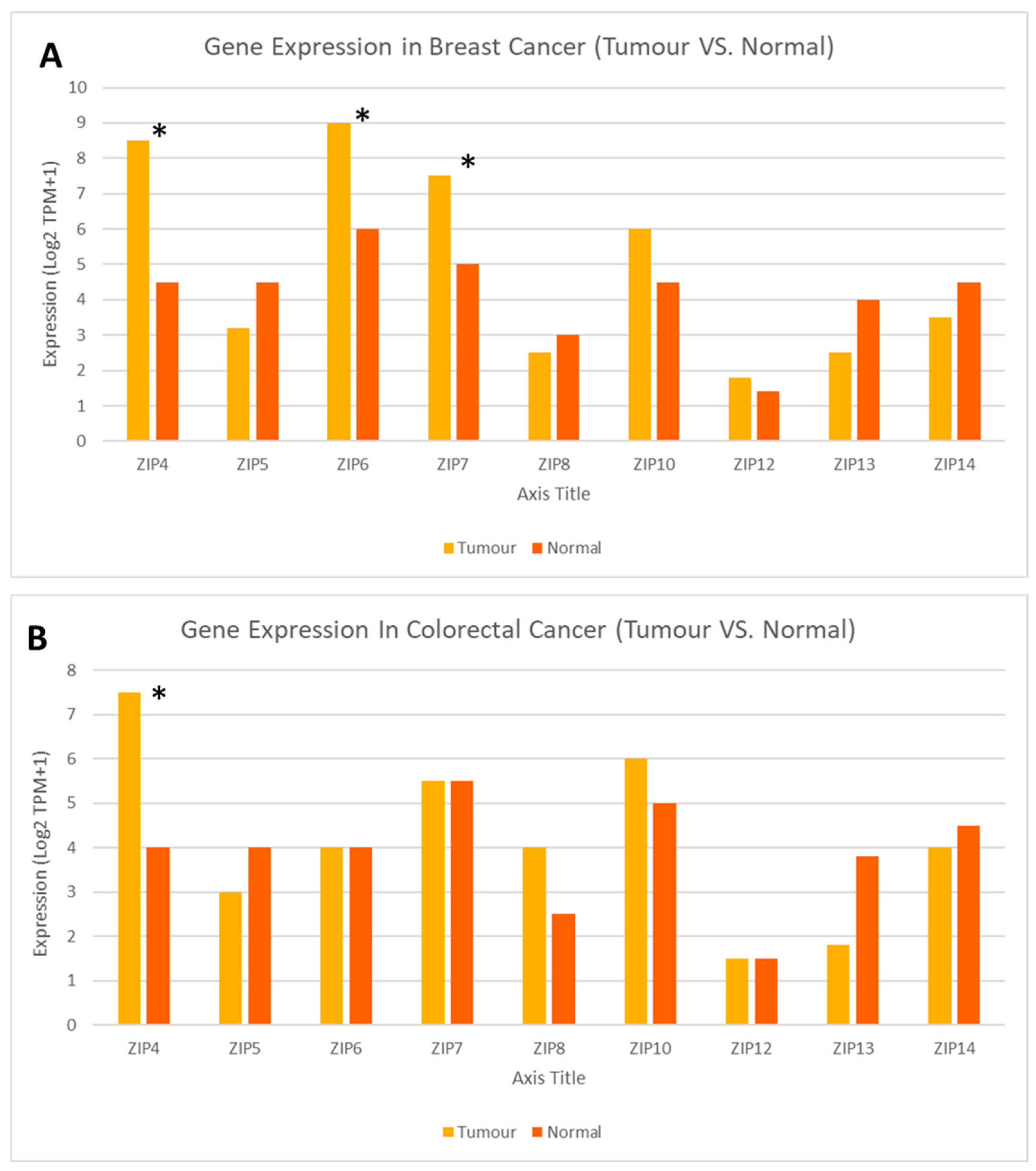
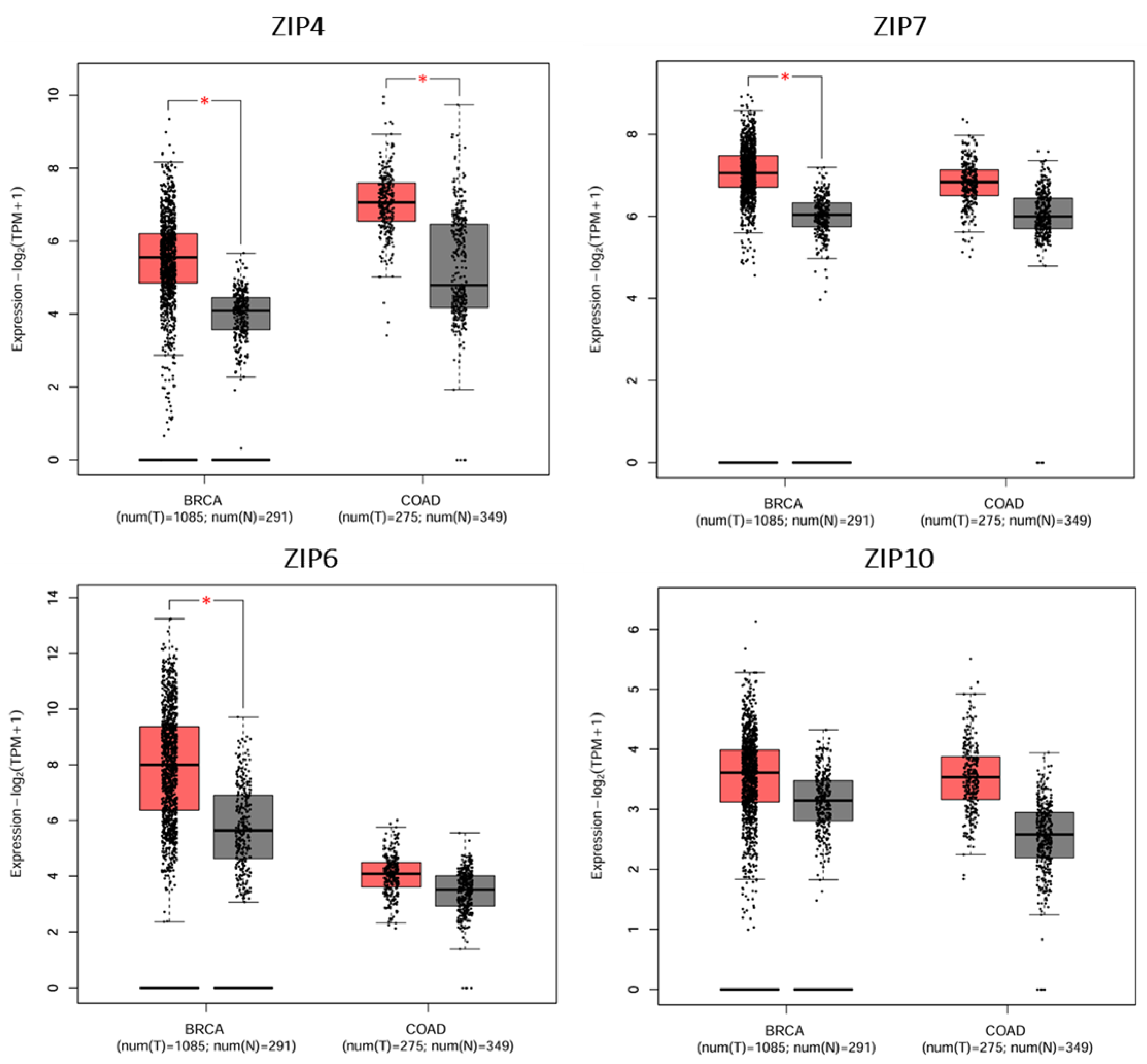
6. Gene Expression of LIV-1 Subfamily ZIP Transporters in Breast and Colorectal Cancers, the Most Common Forms of Cancer in Saudi Arabia
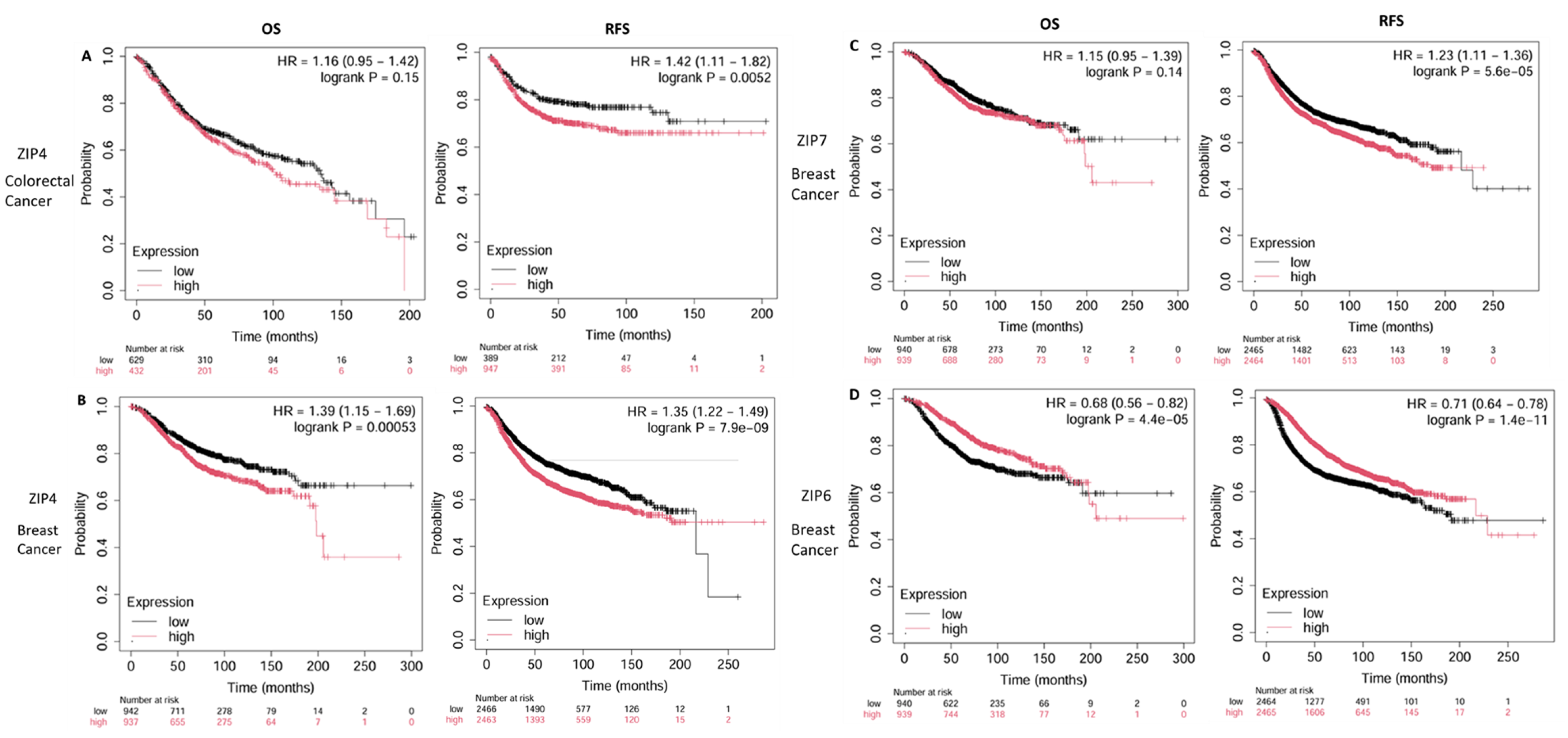
7. Prognostic Relevance of LIV-1 Subfamily ZIP Transporters in Breast and Colorectal Cancers, the Most Common Forms of Cancer in Saudi Arabia
8. Discussion
9. Limitations and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The role of zinc in bone tissue health and regeneration—A review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Cruz, K.J.; de Oliveira, A.R.; Morais, J.B.; Severo, J.S.; Mendes, P.M.; de Sousa Melo, S.R.; de Sousa, G.S.; Marreiro, D.D. Zinc and insulin resistance: Biochemical and molecular aspects. Biol. Trace Elem. Res. 2018, 186, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Te, L.; Liu, J.; Ma, J.; Wang, S. Correlation between serum zinc and testosterone: A systematic review. J. Trace Elem. Med. Biol. 2023, 76, 127124. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.; de Freitas, T.E.; Andrade, A.L.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.; Cruz, K.J.; do Nascimento Marreiro, D. The role of zinc in thyroid hormones metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Maxwell, C.; Volpe, S.L. Effect of zinc supplementation on thyroid hormone function: A case study of two college females. Ann. Nutr. Metab. 2007, 51, 188–194. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef]
- Davidson, H.W.; Wenzlau, J.M.; O’Brien, R.M. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol. Metab. 2014, 25, 415–424. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, H.; Zhao, J. Multifunctional roles of zinc in Alzheimer’s disease. Neurotoxicology 2020, 80, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69. [Google Scholar]
- Allouche-Fitoussi, D.; Breitbart, H. The role of zinc in male fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Alqurashi, R.M.; Abdalla, S.M.; Bin Ammar, A.; Shatwan, I.M.; Alsayegh, A.A.; Alnasser, A.N.; Alfadhliah, J.T.; Alnoubi, A.A.; Fallata, G.A.; Alhumaidan, O.A.; et al. The most popular local and traditional food dishes in different regions of the Kingdom of Saudi Arabia and their cultural significance. Front. Nutr. 2025, 12, 1590522. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.O. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, O.; Almubark, R.; Almarshad, A.; Alqahtani, A.S. The prevalence of vitamin and mineral deficiencies and high levels of non-essential heavy metals in Saudi Arabian adults. Healthcare 2022, 10, 2415. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny. fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc metabolism and metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Bendellaa, M.; Lelièvre, P.; Coll, J.L.; Sancey, L.; Deniaud, A.; Busser, B. Roles of zinc in cancers: From altered metabolism to therapeutic applications. Int. J. Cancer 2024, 154, 7–20. [Google Scholar] [CrossRef]
- Maret, W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Zupo, R.; Sila, A.; Castellana, F.; Bringiotti, R.; Curlo, M.; De Pergola, G.; De Nucci, S.; Giannelli, G.; Mastronardi, M.; Sardone, R. Prevalence of zinc deficiency in inflammatory bowel disease: A systematic review and meta-analysis. Nutrients 2022, 14, 4052. [Google Scholar] [CrossRef] [PubMed]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Farr, G.; Nimmanon, T.; Ziliotto, S.; Gee, J.M.; Taylor, K.M. The importance of targeting signalling mechanisms of the SLC39A family of zinc transporters to inhibit endocrine resistant breast cancer. Explor. Target. Anti-Tumor Ther. 2022, 3, 224. [Google Scholar]
- Xu, X.; Guo, H.J.; Xie, H.Y.; Li, J.; Zhuang, R.Z.; Ling, Q.; Zhou, L.; Wei, X.Y.; Liu, Z.K.; Ding, S.M.; et al. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int. J. Biol. Sci. 2014, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dong, J.; Li, F.; Wei, Z.; Tian, Y. Knockdown of SLC39A7 suppresses cell proliferation, migration and invasion in cervical cancer. EXCLI J. 2017, 16, 1165. [Google Scholar] [PubMed]
- Saleh, S.A.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef]
- Seiki, Y.; Ikezawa, K.; Kai, Y.; Takada, R.; Kawabata, M.; Kishimoto, H.; Hosokawa, K.; Watsuji, K.; Kozumi, K.; Urabe, M.; et al. Impact of zinc deficiency on the prognosis of unresectable pancreatic cancer. Pancreatology 2025, 25, 552–557. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Li, A.; Zhang, Y. Association between serum zinc levels and lung cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 78. [Google Scholar] [CrossRef]
- Jouybari, L.; Kiani, F.; Akbari, A.; Sanagoo, A.; Sayehmiri, F.; Aaseth, J.; Chartrand, M.S.; Sayehmiri, K.; Chirumbolo, S.; Bjørklund, G. A meta-analysis of zinc levels in breast cancer. J. Trace Elem. Med. Biol. 2019, 56, 90–99. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef]
- Rusch, P.; Hirner, A.V.; Schmitz, O.; Kimmig, R.; Hoffmann, O.; Diel, M. Zinc distribution within breast cancer tissue of different intrinsic subtypes. Arch. Gynecol. Obstet. 2021, 303, 195–205. [Google Scholar] [CrossRef]
- Sohrabi, M.; Gholami, A.; Azar, M.H.; Yaghoobi, M.; Shahi, M.M.; Shirmardi, S.; Nikkhah, M.; Kohi, Z.; Salehpour, D.; Khoonsari, M.R.; et al. Trace element and heavy metal levels in colorectal cancer: Comparison between cancerous and non-cancerous tissues. Biol. Trace Elem. Res. 2018, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.C. Increased expression of zinc transporter ZIP4, ZIP11, ZnT1, and ZnT6 predicts poor prognosis in pancreatic cancer. J. Trace Elem. Med. Biol. 2021, 65, 126734. [Google Scholar] [CrossRef] [PubMed]
- Desouki, M.M.; Geradts, J.; Milon, B.; Franklin, R.B.; Costello, L.C. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 2007, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef]
- Althubiti, M.A.; Eldein, M.M. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J. 2018, 39, 1259. [Google Scholar] [CrossRef]
- Alqahtani, W.S.; Almufareh, N.A.; Domiaty, D.M.; Albasher, G.; Alduwish, M.A.; Alkhalaf, H.; Almuzzaini, B.; Al-Marshidy, S.S.; Alfraihi, R.; Elasbali, A.M.; et al. Epidemiology of cancer in Saudi Arabia thru 2010–2019: A systematic review with constrained meta-analysis. AIMS Public Health 2020, 7, 679. [Google Scholar] [CrossRef]
- Alessy, S.A.; Al-Zahrani, A.; Alhomoud, S.; Alaskar, A.; Haoudi, A.; Alkheilewi, M.A.; Alhamali, M.; Alsharm, A.A.; Asiri, M.; Alqahtani, S.A. Towards a comprehensive cancer control policy in Saudi Arabia. Lancet Oncol. 2025, 26, e360–e368. [Google Scholar] [CrossRef]
- Alqahtani, B.; Elnaggar, R.K.; Alshehri, M.M.; Khunti, K.; Alenazi, A. National and regional prevalence rates of diabetes in Saudi Arabia: Analysis of national survey data. Int. J. Diabetes Dev. Ctries. 2023, 43, 392–397. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, Y.; Qi, H.; Liang, L.; Wu, H.; Yuan, J.; Hu, Q. Evaluation of the prognostic values of solute carrier (SLC) family 39 genes for patients with lung adenocarcinoma. Aging 2021, 13, 5312. [Google Scholar] [CrossRef]
- Franz, M.C.; Anderle, P.; Bürzle, M.; Suzuki, Y.; Freeman, M.R.; Hediger, M.A.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef]
- Barresi, V.; Valenti, G.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell. Biochem. 2018, 119, 9707–9719. [Google Scholar] [CrossRef]
- Ziliotto, S.; Gee, J.M.; Ellis, I.O.; Green, A.R.; Finlay, P.; Gobbato, A.; Taylor, K.M. Activated zinc transporter ZIP7 as an indicator of anti-hormone resistance in breast cancer. Metallomics 2019, 11, 1579–1592. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Morris, S.; Flanagan, L.; Taylor, K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 2017, 9, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, Y.; Ju, Z.; Zhang, Z. ZIP7 (SLC39A7) expression in colorectal cancer and its correlation with clinical prognosis. Transl. Cancer Res. 2020, 9, 6471. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ren, L.; Yang, J.; Wang, B.; Xing, T.; Chen, H.; Chen, M. miR-15a-3p suppresses prostate cancer cell proliferation and invasion by targeting SLC39A7 via downregulating Wnt/β-catenin signaling pathway. Cancer Biother. Radiopharm. 2019, 34, 472–479. [Google Scholar] [CrossRef]
- Sheng, N.; Yan, L.; You, W.; Tan, G.; Gong, J.; Chen, H.; Yang, Y.; Hu, L.; Wang, Z. Knockdown of SLC39A7 inhibits cell growth and induces apoptosis in human colorectal cancer cells. Acta Biochim. Biophys. Sin. 2017, 49, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Hogstrand, C.; Kille, P.; Ackland, M.L.; Hiscox, S.; Taylor, K.M. A mechanism for epithelial–mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem. J. 2013, 455, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.W.; Yang, X.; Wang, R.; Qian, W.; Xu, R.Z.; Lyles, R.; Osunkoya, A.O.; Zhou, B.P.; Vessella, R.L.; Zayzafoon, M.; et al. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PLoS ONE 2011, 6, e27720. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Z.; Wang, S.; Zhou, Q.; Ma, Z.; Liu, C.; Huang, B.; Zheng, Z.; Yang, L.; Zou, Y.; et al. SLC39A10 upregulation predicts poor prognosis, promotes proliferation and migration, and correlates with immune infiltration in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 8, 899–912. [Google Scholar] [CrossRef]
- Ren, X.; Feng, C.; Wang, Y.; Chen, P.; Wang, S.; Wang, J.; Cao, H.; Li, Y.; Ji, M.; Hou, P. SLC39A10 promotes malignant phenotypes of gastric cancer cells by activating the CK2-mediated MAPK/ERK and PI3K/AKT pathways. Exp. Mol. Med. 2023, 55, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Nakano, H.; Toyomaki, Y.; Hanada, K.; Nomura, K. Novel SLC39A4 mutations in acrodermatitis enteropathica. J. Investig. Dermatol. 2003, 120, 963–966. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.M.; Cousins, R.J.; et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef]
- Weaver, B.P.; Zhang, Y.; Hiscox, S.; Guo, G.L.; Apte, U.; Taylor, K.M.; Sheline, C.T.; Wang, L.; Andrews, G.K. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS ONE 2010, 5, e13158. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Liu, T.; Deng, S.H.; Han, R.; Xu, Y. SLC39A4 expression is associated with enhanced cell migration, cisplatin resistance, and poor survival in non-small cell lung cancer. Sci. Rep. 2017, 7, 7211. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Wang, C. Analysis of the prognostic significance of solute carrier (SLC) family 39 genes in breast cancer. Biosci. Rep. 2020, 40, BSR20200764. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L. UKLF/PCBP2 axis governs the colorectal cancer development by transcriptionally activating SLC39A4. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119755. [Google Scholar] [CrossRef]
- Nolin, E.; Gans, S.; Llamas, L.; Bandyopadhyay, S.; Brittain, S.M.; Bernasconi-Elias, P.; Carter, K.P.; Loureiro, J.J.; Thomas, J.R.; Schirle, M.; et al. Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat. Chem. Biol. 2019, 15, 179–188. [Google Scholar] [CrossRef]
- Saudi Health Council; National Cancer Center. Cancer Incidence Report 2022—Saudi Arabia; Saudi Health Council: Riyadh, Saudi Arabia, 2022. Available online: https://shc.gov.sa/en/NCC/Activities/Pages/NewAR.aspx (accessed on 9 May 2025).
- World Health Organization; International Agency for Research on Cancer. Cancer Today; WHO: Geneva, Switzerland, 2016; Available online: https://gco.iarc.fr/today/en/dataviz/maps-heatmap?mode=population&sexes=1 (accessed on 17 July 2025).
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef]
- Nashar, R.M.; Almurshed, K.S. Colorectal cancer: A case control study of dietary factors, king faisal specialist hospital and researh center, riyadh, saudi arabia. J. Fam. Community Med. 2008, 15, 57–64. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chatterjee, N.; Shamim, M.A.; Rani, I.; Dhar, A.; Tondolo, V.; Rongioletti, M.; Rizzo, G.; Goswami, K.; Squitti, R. Serum zinc status of patients with colorectal cancer: A systematic review and meta-analysis. J. Trace Elem. Minerals 2024, 9, 100185. [Google Scholar] [CrossRef]
- Zhang, Y.; Bharadwaj, U.; Logsdon, C.D.; Chen, C.; Yao, Q.; Li, M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 2010, 16, 1423–1430. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, S.; Yu, N.; Li, H. ZIP4: A promising early diagnostic and therapeutic targets for pancreatic cancer. Am. J. Cancer Res. 2024, 14, 4652. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Liu, L.; Qiang, G.; Zhu, J. Serum zinc level and tissue ZIP4 expression are related to the prognosis of patients with zes I–III colon cancer. Transl. Cancer Res. 2020, 9, 5585. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Ogle, O.; Burt, A.; Gee, J.M.; Andrews, G.K.; Kille, P.; Hogstrand, C.; Maret, W.; Taylor, K.M. The ZIP6/ZIP10 heteromer is essential for the zinc-mediated trigger of mitosis. Cell. Mol. Life Sci. 2021, 78, 1781–1798. [Google Scholar] [CrossRef]
- Chen, P.H.; Wu, J.; Xu, Y.; Ding, C.K.; Mestre, A.A.; Lin, C.C.; Yang, W.H.; Chi, J.T. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 2021, 12, 198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.M.; Taylor, K.M. Zinc Transporters of the LIV-1 Subfamily in Various Cancers: Molecular Insights and Research Priorities for Saudi Arabia. Int. J. Mol. Sci. 2025, 26, 8080. https://doi.org/10.3390/ijms26168080
Alzahrani AM, Taylor KM. Zinc Transporters of the LIV-1 Subfamily in Various Cancers: Molecular Insights and Research Priorities for Saudi Arabia. International Journal of Molecular Sciences. 2025; 26(16):8080. https://doi.org/10.3390/ijms26168080
Chicago/Turabian StyleAlzahrani, Ahmed M., and Kathryn M. Taylor. 2025. "Zinc Transporters of the LIV-1 Subfamily in Various Cancers: Molecular Insights and Research Priorities for Saudi Arabia" International Journal of Molecular Sciences 26, no. 16: 8080. https://doi.org/10.3390/ijms26168080
APA StyleAlzahrani, A. M., & Taylor, K. M. (2025). Zinc Transporters of the LIV-1 Subfamily in Various Cancers: Molecular Insights and Research Priorities for Saudi Arabia. International Journal of Molecular Sciences, 26(16), 8080. https://doi.org/10.3390/ijms26168080





