Geniposide Mitigates Insulin Resistance and Hepatic Fibrosis via Insulin Signaling Pathway
Abstract
1. Introduction
2. Results
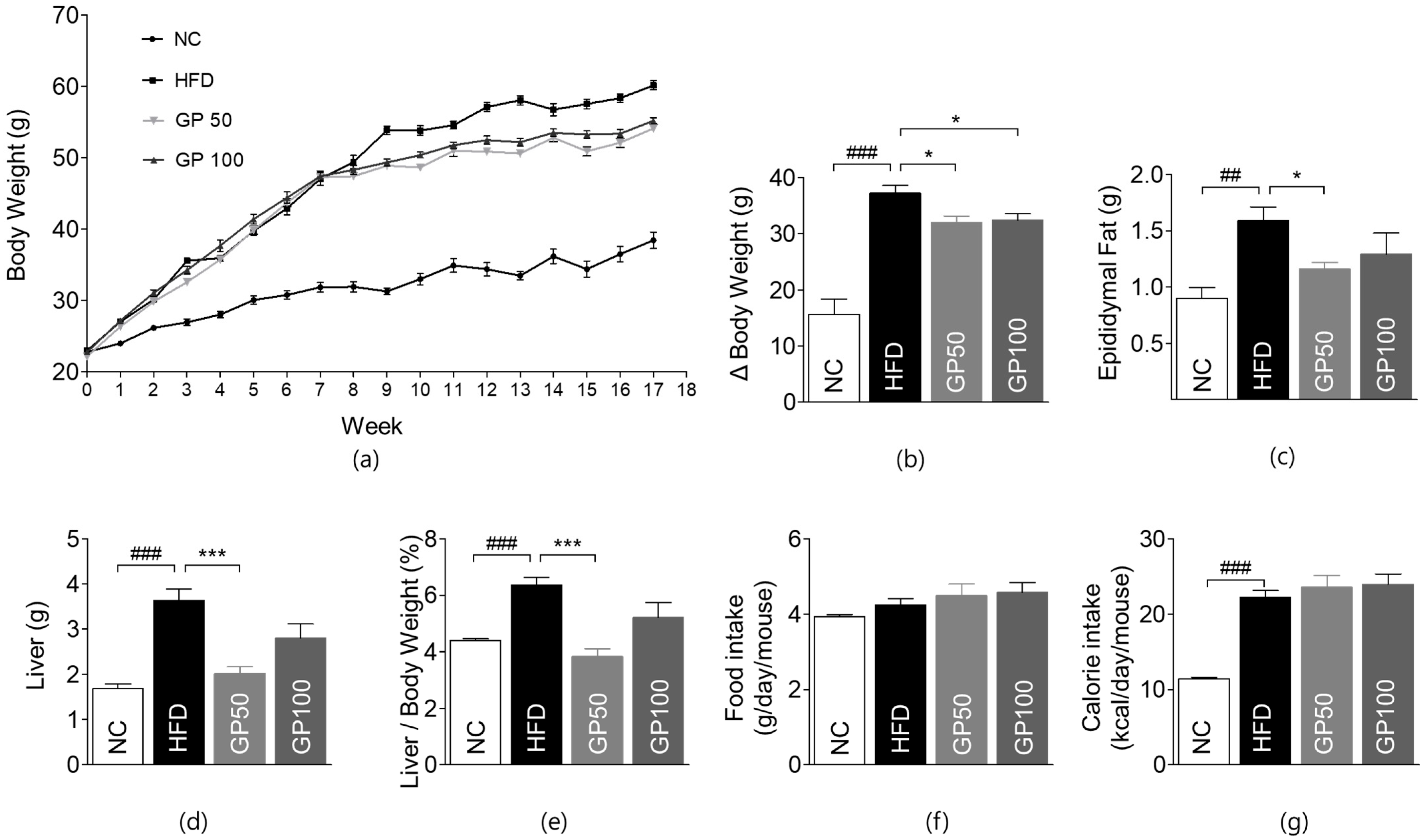
2.1. Effects of GP on Weight Gain
2.2. Effect of GP on Glucose Metabolism
2.3. Effect of GP on Blood Lipid Profile
2.4. Effect of GP on Hepatic and Renal Function Tests
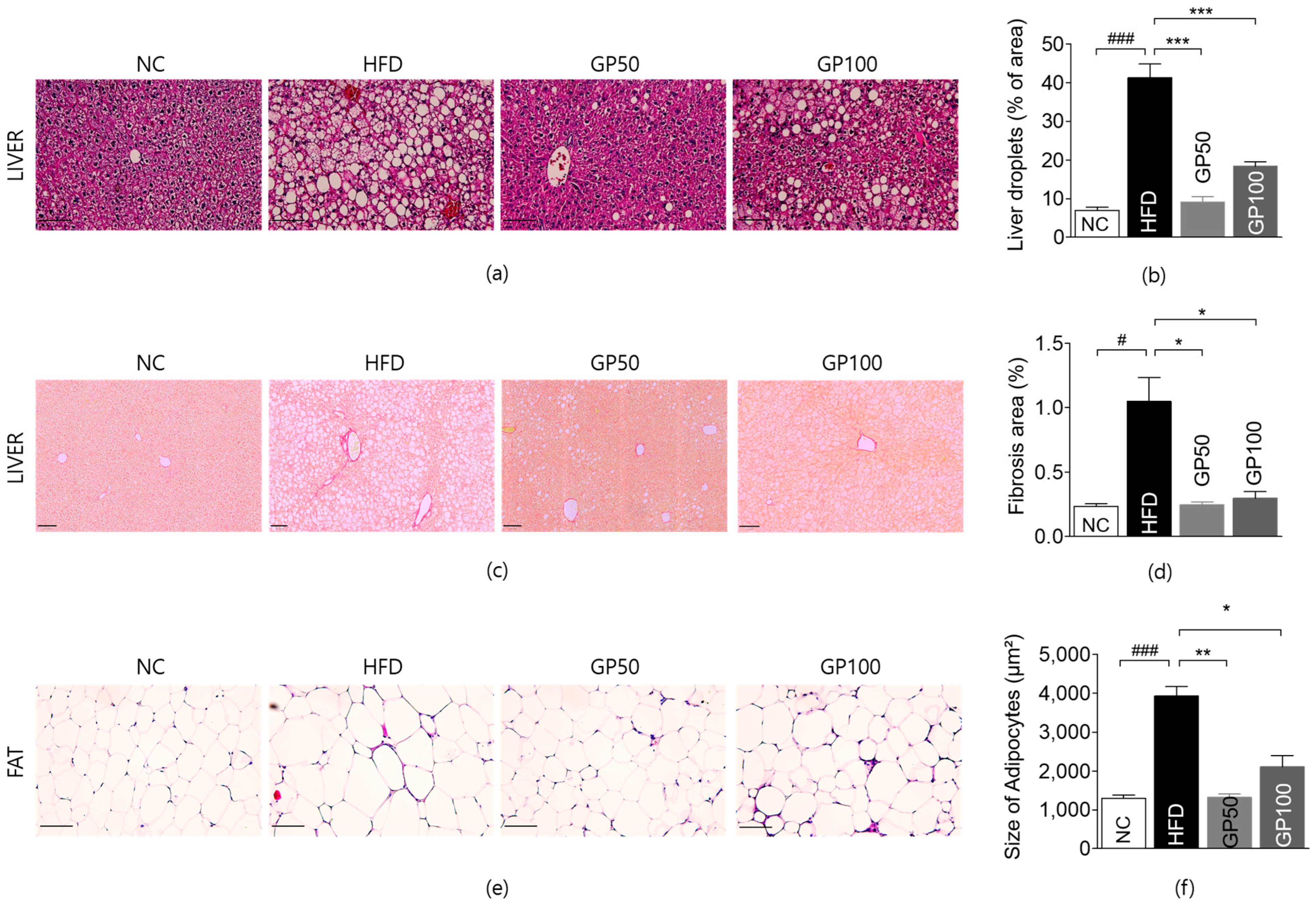
2.5. Effect of GP on Histological Analysis of Liver and Adipose Tissue
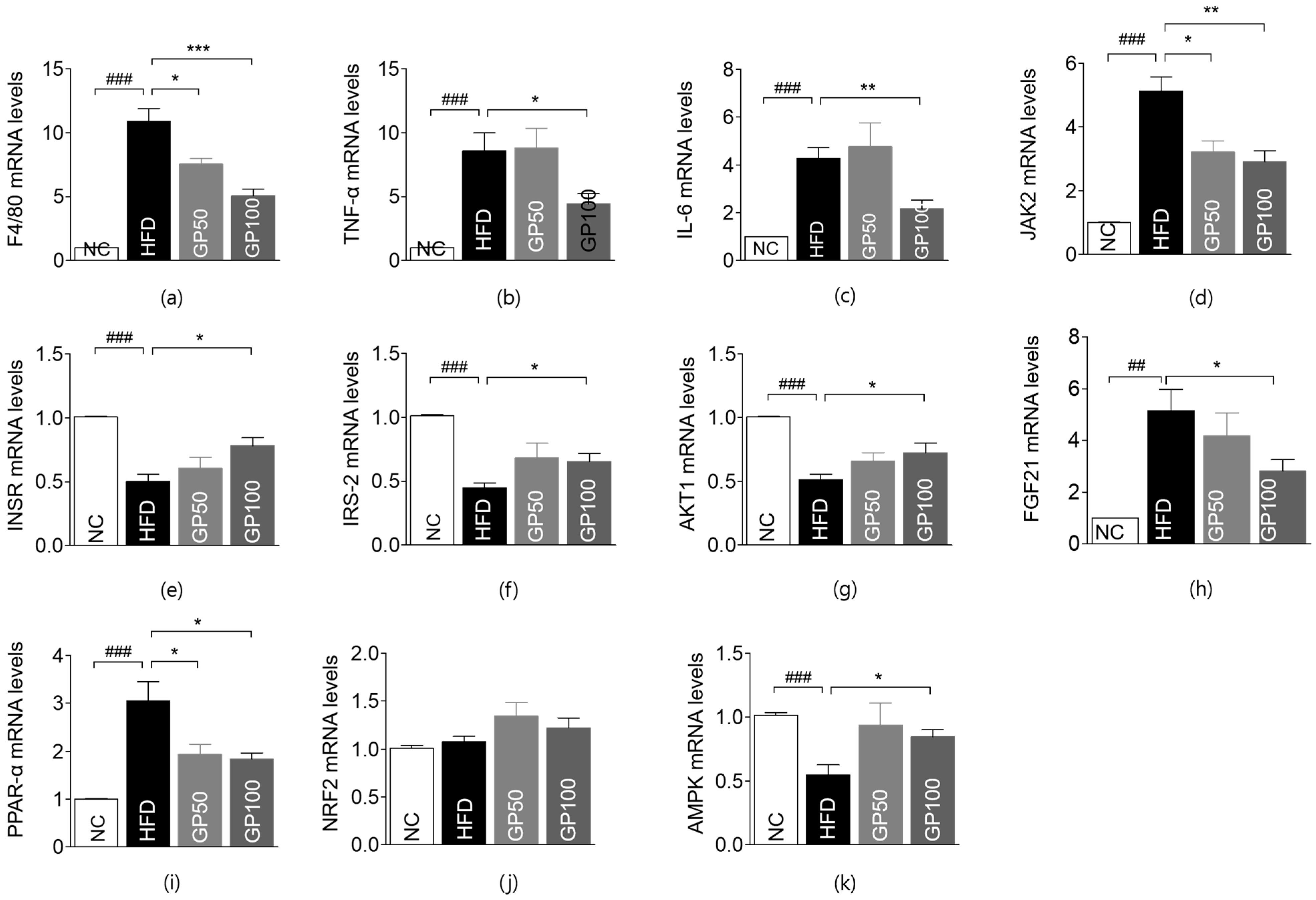
2.6. Effect of GP on Gene Expression in Liver Tissue
2.7. Effect of GP on Gene Expression in Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Animals and Drug
4.2. Experimental Group Allocation and Study Design
4.3. Weight Measurement of Body, Liver, and Fat Tissue
4.4. Assessment on Glucose Tolerance
4.5. Blood Lipid Profile Test
4.6. Hepatic and Renal Function Tests
4.7. Histological Analysis
4.8. RNA Isolation from Tissue
4.9. Real-Time Quantitative RT-PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Murtaza, G.; Riaz, S.; Zafar, M.; Ahsan Raza, M.; Kaleem, I.; Imran, H.; Al-Harbi, A.T.; Sabouri, A.; Asim Niaz, T.; Bashir, S. Examining the growing challenge: Prevalence of diabetes in young adults (Review). Med. Int. 2025, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, H.; Chen, J.; Li, S.P.; Dai, L.; Zhang, Z.R.; Wang, W.Y. Antiinflammation Effects and Mechanisms Study of Geniposide on Rats with Collagen-Induced Arthritis. Phytother. Res. 2017, 31, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wang, G.F.; Liu, Z.Q.; Rao, J.J.; Lu, L.; Xu, W.; Wu, S.G.; Zhang, J.J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bi, L.; Jin, L.; Wang, Y.; Li, Y.; Li, Z.; He, W.; Cui, H.; Miao, J.; Wang, L. Geniposide Ameliorates Liver Fibrosis Through Reducing Oxidative Stress and Inflammatory Respose, Inhibiting Apoptosis and Modulating Overall Metabolism. Front. Pharmacol. 2021, 12, 772635. [Google Scholar] [CrossRef]
- Ma, T.; Huang, C.; Zong, G.; Zha, D.; Meng, X.; Li, J.; Tang, W. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J. Pharm. Pharmacol. 2011, 63, 587–593. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, Y.; Yan, J.; Liu, J.; Li, L. Geniposide promotes autophagy to inhibit insulin resistance in HepG2 cells via P62/NF-kappaB/GLUT-4. Mol. Med. Rep. 2017, 16, 7237–7244. [Google Scholar] [CrossRef]
- Zhao, W.; Pu, M.; Shen, S.; Yin, F. Geniposide improves insulin resistance through AMPK-mediated Txnip protein degradation in 3T3-L1 adipocytes. Acta Biochim. Biophys. Sin. 2021, 53, 160–169. [Google Scholar] [CrossRef]
- Liu, J.; Song, C.; Nie, C.; Sun, Y.; Wang, Y.; Xue, L.; Fan, M.; Qian, H.; Wang, L.; Li, Y. A novel regulatory mechanism of geniposide for improving glucose homeostasis mediated by circulating RBP4. Phytomedicine 2022, 95, 153862. [Google Scholar] [CrossRef]
- Chen, P.; Chen, Y.; Wang, Y.; Cai, S.; Deng, L.; Liu, J.; Zhang, H. Comparative Evaluation of Hepatoprotective Activities of Geniposide, Crocins and Crocetin by CCl4-Induced liver Injury in Mice. Biomol. Ther. 2016, 24, 156–162. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, J.; Liu, S.; Hu, Q.; Hu, S.; Zeng, J.; Ma, X.; Zhang, X. Bidirectional effects of geniposide in liver injury: Preclinical evidence construction based on meta-analysis. J. Ethnopharmacol. 2024, 319, 117061. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Deyoung, S.M.; Saltiel, A.R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E166–E174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.G.; Ko, H.J.; Cho, Y.R.; Kim, H.J.; Ma, Z.; Yu, T.Y.; Friedline, R.H.; Kurt-Jones, E.; Finberg, R.; Fischer, M.A.; et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009, 58, 2525–2535. [Google Scholar] [CrossRef]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Martin, R.G.; Duncan, R.E.; Choi, D.; Lu, S.Y.; Schroer, S.A.; Cai, E.P.; Luk, C.T.; Hopperton, K.E.; Domenichiello, A.F.; et al. Hepatocyte-specific deletion of Janus kinase 2 (JAK2) protects against diet-induced steatohepatitis and glucose intolerance. J. Biol. Chem. 2012, 287, 10277–10288. [Google Scholar] [CrossRef] [PubMed]
- Thirone, A.C.; JeBailey, L.; Bilan, P.J.; Klip, A. Opposite effect of JAK2 on insulin-dependent activation of mitogen-activated protein kinases and Akt in muscle cells: Possible target to ameliorate insulin resistance. Diabetes 2006, 55, 942–951. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef]
- Handy, R.M.; Holloway, G.P. Insights into the development of insulin resistance: Unraveling the interaction of physical inactivity, lipid metabolism and mitochondrial biology. Front. Physiol. 2023, 14, 1151389. [Google Scholar] [CrossRef]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Jani, R.; Jenkinson, C.P.; Coletta, D.K.; Kaku, K.; DeFronzo, R.A. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E401–E406. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Regnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef]
- Shen, B.; Feng, H.; Cheng, J.; Li, Z.; Jin, M.; Zhao, L.; Wang, Q.; Qin, H.; Liu, G. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J. Cell. Mol. Med. 2020, 24, 5097–5108. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Sun, C.; Liu, S.; Yan, Y.; Pan, H.; Fan, M.; Xue, L.; Nie, C.; Zhang, H.; et al. Geniposide reduces cholesterol accumulation and increases its excretion by regulating the FXR-mediated liver-gut crosstalk of bile acids. Pharmacol. Res. 2020, 152, 104631. [Google Scholar] [CrossRef]
- Wang, S.; Ge, S.; Chen, Y.; Zhou, F.; Wang, J.; Chen, L.; Chen, Y.; Yu, R.; Huang, L. Acute and subacute hepatotoxicity of genipin in mice and its potential mechanism. Heliyon 2023, 9, e21834. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M. Towards precision medicine in non-alcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 2023, 24, 885–899. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.Y.; Ren, X.X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef]



| Forward Primers | Reverse Primers | |
|---|---|---|
| F4/80 | 5′-CTCTGTGGTCCCACCTTCAT-3′ | 5′-GATGGCCAAGGATCTGAAAA-3′ |
| TNF-α | 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ | 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′ |
| IL-6 | 5′-AACGATGATGCACTTGCAGA-3′ | 5′-GAGCATTGGAAATTGGGGTA-3′ |
| IL-10 | 5′-CGGGAAGACAATAACTGCACCC-3′ | 5′-CGGTTAGCAGTATGTTGTCCAGC-3′ |
| IFN-γ | 5′-ACTGGCAAAAGGATGGIGAC-3′ | 5′-TGAGCTCATTGAATGCTTGG-3′ |
| MAPK14 p38 | GAGCGTTACCAGAACCTGTCTC | AGTAACCGCAGTTCTCTGTAGGT |
| JAK2 | GCTACCAGATGGAAACTGTGCG | GCCTCTGTAATGTTGGTGAGATC |
| INSR | 5′-AGATGAGAGGTGCAGTGTGGCT-3′ | 5′-GGTTCCTTTGGCTCTTGCCACA-3′ |
| IRS-1 | AAGCACCTGGTGGCTCTCTA | TCAGGATAACCTGCCAGACC |
| IRS-2 | ATACCGCCTATGCCTGTCTG | TGGTCTCATGGATGTTCTGC |
| protein kinase B (Akt) | 5′-TGGACTTCCGATCAGGCTCAC-3′ | 5′-GCCCTTGCCCAGTAGCTTCA-3 |
| IGF1R | 5′-CGGGATCTCATCAGCTTCACAG-3′ | 5′-TCCTTGTTCGGAGGCAGGTCTA-3′ |
| FGF21 | 5′-ATCAGGGAGGATGGAACAGTGG-3′ | 5′-AGCTCCATCTGGCTGTTGGCAA-3′ |
| PPAR-α | 5′-ACCACTACGGAGTTCACGCATG-3′ | 5′-GAATCTTGCAGCTCCGATCACAC-3′ |
| NRF2 | 5′-CTGAACTCCTGGACGGGACTA-3′ | 5′-CGGTGGGTCTCCGTAAATGG-3′ |
| AMPK | 5′-GGTGTACGGAAGGCAAAATGGC-3′ | 5′-CAGGATTCTTCCTTCGTACACGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-H.; Lee, M.-S.; Lee, B.-C. Geniposide Mitigates Insulin Resistance and Hepatic Fibrosis via Insulin Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 8079. https://doi.org/10.3390/ijms26168079
Oh S-H, Lee M-S, Lee B-C. Geniposide Mitigates Insulin Resistance and Hepatic Fibrosis via Insulin Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(16):8079. https://doi.org/10.3390/ijms26168079
Chicago/Turabian StyleOh, Seung-Hyun, Min-Seong Lee, and Byung-Cheol Lee. 2025. "Geniposide Mitigates Insulin Resistance and Hepatic Fibrosis via Insulin Signaling Pathway" International Journal of Molecular Sciences 26, no. 16: 8079. https://doi.org/10.3390/ijms26168079
APA StyleOh, S.-H., Lee, M.-S., & Lee, B.-C. (2025). Geniposide Mitigates Insulin Resistance and Hepatic Fibrosis via Insulin Signaling Pathway. International Journal of Molecular Sciences, 26(16), 8079. https://doi.org/10.3390/ijms26168079






