Dysregulation of the FGF21–Adiponectin Axis in a Large Cohort of Patients with Severe Obesity and Liver Disease
Abstract
1. Introduction
2. Results
2.1. Participants
2.2. Fibroblast Growth Factor 19 and Choline Metabolism
2.3. Fibroblast Growth Factor 21
2.4. Galectin-3
2.5. Irisin
2.6. Leptin and Adiponectin: The Relevance of Sex Dimorphism
2.7. Network Modeling and Insights into Patterns: The Functional Overlap Between FGF21 and Adiponectin
3. Discussion
4. Strenghts and Limitations of the Study
5. Materials and Methods
5.1. Study Design
5.2. Sampling and Data Collection
5.3. Biochemical and Histological Assessments
5.4. Mass Spectrometry for Selected Metabolites
5.5. Statistical Analyses
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ELISA | Enzyme-linked immunosorbent assay |
| FGF | Fibroblast growth factor |
| FXR | Farnesoid X receptor |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| NAS | Nonalcoholic fatty liver disease activity score |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Ellison-Barnes, A.; Johnson, S.; Gudzune, K. Trends in obesity prevalence among adults aged 18 through 25 years, 1976–2018. JAMA 2021, 326, 2073–2074. [Google Scholar] [CrossRef]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and over: United States, 1960–1962 Through 2017–2018. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (accessed on 18 July 2025).
- Cabré, N.; Luciano-Mateo, F.; Baiges-Gayà, G.; Fernández-Arroyo, S.; Rodríguez-Tomàs, E.; Hernández-Aguilera, A.; París, M.; Sabench, F.; Del Castillo, D.; López-Miranda, J.; et al. Plasma metabolic alterations in patients with severe obesity and non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2020, 51, 374–387. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Cypess, A.M. Reassessing human adipose tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Franco, A.; Castañé, H.; Martínez-Navidad, C.; Placed-Gallego, C.; Hernández-Aguilera, A.; Fernández-Arroyo, S.; Samarra, I.; Canela-Capdevila, M.; Arenas, M.; Zorzano, A.; et al. Metabolic adaptations in severe obesity: Insights from circulating oxylipins before and after weight loss. Clin. Nutr. 2024, 43, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Jangam, T.C.; Desai, S.A.; Patel, V.P.; Pagare, N.B.; Raut, N.D. Exosomes as therapeutic and diagnostic tools: Advances, challenges, and future directions. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Jadhav, K.; Cohen, T.S. Can you trust your gut? Implicating a disrupted intestinal microbiome in the progression of NAFLD/NASH. Front. Endocrinol. 2020, 11, 592157. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Dilts, M.; Pokhrel, S.; Stahl, Z.; Boehme, S.; Wang, X.; Chiang, J.Y.L. Fibroblast growth factor 19 alters bile acids to induce dysbiosis in mice with alcohol-induced liver disease. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 71–87. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, L.; Lin, X.; Cheng, P.; He, L.; Li, X.; Lu, X.; Tan, Y.; Yang, H.; Cai, L.; et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol. Endocrinol. 2015, 29, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adiponectin: Friend or foe in obesity and inflammation. Med. Rev. (2021) 2022, 2, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Asgari, R.; Caceres-Valdiviezo, M.; Wu, S.; Hamel, L.; Humber, B.E.; Agarwal, S.M.; Fletcher, P.J.; Fulton, S.; Hahn, M.K.; Pereira, S. Regulation of energy balance by leptin as an adiposity signal and modulator of the reward system. Mol. Metab. 2025, 91, 102078. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Unraveling the pathophysiologic role of galectin-3 in chronically injured liver. J. Cell. Physiol. 2023, 238, 673–686. [Google Scholar] [CrossRef]
- Camps, J.; Castañé, H.; Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Arenas, M.; Iftimie, S.; Joven, J. On the role of paraoxonase-1 and chemokine ligand 2 (C-C motif) in metabolic alterations linked to inflammation and disease. A 2021 update. Biomolecules 2021, 11, 971. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Olaniyi, P.; Isarinade, T.; Yusuf, I.A. Recent advances in bariatric surgery: A narrative review of weight loss procedures. Ann. Med. Surg. 2023, 85, 6091–6104. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef]
- Naón, D.; Hernández-Alvarez, M.I.; Shinjo, S.; Wieczor, M.; Ivanova, S.; Martins de Brito, O.; Quintana, A.; Hidalgo, J.; Palacín, M.; Aparicio, P.; et al. Splice variants of mitofusin 2 shape the endoplasmic reticulum and tether it to mitochondria. Science 2023, 380, eadh9351. [Google Scholar] [CrossRef]
- Priest, C.; Tontonoz, P. Inter-organ cross-talk in metabolic syndrome. Nat. Metab. 2019, 1, 1177–1188. [Google Scholar] [CrossRef]
- Santos, J.P.M.D.; Maio, M.C.; Lemes, M.A.; Laurindo, L.F.; Haber, J.F.D.S.; Bechara, M.D.; Prado, P.S.D., Jr.; Rauen, E.C.; Costa, F.; Pereira, B.C.A.; et al. Non-alcoholic steatohepatitis (NASH) and organokines: What is now and what will be in the future. Int. J. Mol. Sci. 2022, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Longo, M.; Meroni, M.; Bhattacharya, D.; Paolini, E.; Mughal, S.; Hussain, S.; Anand, S.K.; Gupta, N.; Zhu, Y.; et al. EHBP1 suppresses liver fibrosis in metabolic dysfunction-associated steatohepatitis. Cell Metab. 2025, 37, 1152–1170.e7. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Schnabl, B.; Knight, R.; Loomba, R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef]
- van de Wiel, S.M.W.; Bijsmans, I.T.G.W.; van Mil, S.W.C.; van de Graaf, S.F.J. Identification of FDA-approved drugs targeting the farnesoid X receptor. Sci. Rep. 2019, 9, 2193. [Google Scholar] [CrossRef]
- Wahlström, A.; Aydin, Ö.; Olsson, L.M.; Sjöland, W.; Henricsson, M.; Lundqvist, A.; Marschall, H.U.; Franken, R.; van de Laar, A.; Gerdes, V.; et al. Alterations in bile acid kinetics after bariatric surgery in patients with obesity with or without type 2 diabetes. eBioMedicine 2024, 106, 105265. [Google Scholar] [CrossRef]
- Barb, D.; Kalavalapalli, S.; Godinez Leiva, E.; Bril, F.; Huot-Marchand, P.; Dzen, L.; Rosenberg, J.T.; Junien, J.L.; Broqua, P.; Rocha, A.O.; et al. Pan-PPAR agonist lanifibranor improves insulin resistance and hepatic steatosis in patients with T2D and MASLD. J. Hepatol. 2025, 82, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, W.; Ma, X.; Ren, L.; Feng, M.; Hu, S.; Xue, C.; Chen, R. Roles and mechanisms of choline metabolism in nonalcoholic fatty liver disease and cancers. Front. Biosci. 2024, 29, 182. [Google Scholar] [CrossRef]
- Schugar, R.C.; Gliniak, C.M.; Osborn, L.J.; Massey, W.; Sangwan, N.; Horak, A.; Banerjee, R.; Orabi, D.; Helsley, R.N.; Brown, A.L.; et al. Gut microbe-targeted choline trimethylamine lyase inhibition improves obesity via rewiring of host circadian rhythms. eLife 2022, 11, e63998. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, J.; Gong, Y.; Gupte, J.; Ye, J.; Weiszmann, J.; Samayoa, K.; Coberly, S.; Gardner, J.; Wang, H.; et al. Fibroblast growth factor receptor 4 (FGFR4) deficiency improves insulin resistance and glucose metabolism under diet-induced obesity conditions. J. Biol. Chem. 2014, 289, 30470–30480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, J.; Xiao, C.; Mo, C.; Ding, B.S. Trimethylamine-N-oxide (TMAO) mediates the crosstalk between the gut microbiota and hepatic vascular niche to alleviate liver fibrosis in nonalcoholic steatohepatitis. Front. Immunol. 2022, 13, 964477. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. A dozen years of discovery: Insights into the physiology and pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef]
- Koh, B.; Xiao, J.; Ng, C.H.; Law, M.; Gunalan, S.Z.; Danpanichkul, P.; Ramadoss, V.; Sim, B.K.L.; Tan, E.Y.; Teo, C.B.; et al. Comparative efficacy of pharmacologic therapies for MASH in reducing liver fat content: Systematic review and network meta-analysis. Hepatology, 2024; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Rolph, T.; Knott, M.; Dubourg, J. FGF21 agonists: An emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J. Hepatol. 2024, 81, 562–576. [Google Scholar] [CrossRef]
- Tang, S.; Borlak, J. A comparative genomic study across 396 liver biopsies provides deep insight into FGF21 mode of action as a therapeutic agent in metabolic dysfunction-associated steatotic liver disease. Clin. Transl. Med. 2025, 15, e70218. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Cabré, N.; Luciano-Mateo, F.; Fernández-Arroyo, S.; Baiges-Gayà, G.; Hernández-Aguilera, A.; Fibla, M.; Fernández-Julià, R.; París, M.; Sabench, F.; Castillo, D.D.; et al. Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism 2019, 99, 81–89. [Google Scholar] [CrossRef]
- Chow, W.S.; Xu, A.; Woo, Y.C.; Tso, A.W.; Cheung, S.C.; Fong, C.H.; Tse, H.F.; Chau, M.T.; Cheung, B.M.; Lam, K.S. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2454–2459. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Q.; Li, P. Galectin-3 in metabolic disorders: Mechanisms and therapeutic potential. Trends Mol. Med. 2015, 31, 424–437. [Google Scholar] [CrossRef]
- Chamseddine, S.; Yavuz, B.G.; Mohamed, Y.I.; Lee, S.S.; Yao, J.C.; Hu, Z.I.; LaPelusa, M.; Xiao, L.; Sun, R.; Morris, J.S.; et al. Circulating galectin-3: A prognostic biomarker in hepatocellular carcinoma. J. Immunother. Precis. Oncol. 2024, 7, 255–262. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Humphries, D.C.; Herman, K.; Roper, J.A.; Holyer, I.; Mabbitt, J.; Mills, R.; Nilsson, U.J.; Leffler, H.; Pedersen, A.; et al. Effect of GB1107, a novel galectin-3 inhibitor on pro-fibrotic signalling in the liver. Eur. J. Pharmacol. 2024, 985, 177077. [Google Scholar] [CrossRef] [PubMed]
- Comeglio, P.; Guarnieri, G.; Filippi, S.; Cellai, I.; Acciai, G.; Holyer, I.; Zetterberg, F.; Leffler, H.; Kahl-Knutson, B.; Sarchielli, E.; et al. The galectin-3 inhibitor selvigaltin reduces liver inflammation and fibrosis in a high fat diet rabbit model of metabolic-associated steatohepatitis. Front. Pharmacol. 2024, 15, 1430109. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Wei, W.P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.-Q. Relationship between circulating irisin levels and overweight/obesity: A meta-analysis. World J. Clin. Cases 2019, 7, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamada, T.; Katagiri, H. Inter-organ communication involved in brown adipose tissue thermogenesis. Adv. Exp. Med. Biol. 2024, 1461, 161–175. [Google Scholar] [CrossRef]
- Shen, C.; Wu, K.; Ke, Y.; Zhang, Q.; Chen, S.; Li, Q.; Ruan, Y.; Yang, X.; Liu, S.; Hu, J. Circulating irisin levels in patients with MAFLD: An updated systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1464951. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Friedman, J.; Halaas, J. Leptin and the regulation of body weight in Mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Maxim, M.; Soroceanu, R.P.; Vlăsceanu, V.I.; Platon, R.L.; Toader, M.; Miler, A.A.; Onofriescu, A.; Abdulan, I.M.; Ciuntu, B.M.; Balan, G.; et al. Dietary habits, obesity, and bariatric surgery: A review of impact and interventions. Nutrients 2025, 17, 474. [Google Scholar] [CrossRef]
- Wang, Z.; Scherer, P. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, Z.; Huang, B.; Cheng, X.; Wang, D.; la Gahu, Z.; Xue, Z.; Da, Y.; Li, D.; et al. Adiponectin-derived active peptide ADP355 exerts anti-inflammatory and anti-fibrotic activities in thioacetamide-induced liver injury. Sci. Rep. 2016, 6, 19445. [Google Scholar] [CrossRef]
- Hui, X.; Feng, T.; Liu, Q.; Gao, Y.; Xu, A. The FGF21-adiponectin axis in controlling energy and vascular homeostasis. J. Mol. Cell Biol. 2016, 8, 110–119. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Xiao, T.M.; Wu, C.X.; Cheng, J.Y.; Li, L.Y. Correlation of adiponectin gene polymorphisms rs266729 and rs3774261 with risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 798417. [Google Scholar] [CrossRef]
- Patt, M.; Karkossa, I.; Krieg, L.; Massier, L.; Makki, K.; Tabei, S.; Karlas, T.; Dietrich, A.; Gericke, M.; Stumvoll, M.; et al. FGF21 and its underlying adipose tissue-liver axis inform cardiometabolic burden and improvement in obesity after metabolic surgery. EBioMedicine 2024, 110, 105458. [Google Scholar] [CrossRef] [PubMed]
- Jirapinyo, P.; McCarty, T.R.; Dolan, R.D.; Shah, R.; Thompson, C.C. Effect of endoscopic bariatric and metabolic therapies on nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 511–524.e1. [Google Scholar] [CrossRef]
- Pais, R.; Aron-Wisnewsky, J.; Bedossa, P.; Ponnaiah, M.; Oppert, J.M.; Siksik, J.M.; Genser, L.; Charlotte, F.; Thabut, D.; Clement, K.; et al. Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology 2022, 76, 456–468. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Wong, V.W.S.; Rinella, M.E.; Boursier, J.; Lazarus, J.V.; Yki-Järvinen, H.; Loomba, R. Metabolic dysfunction-associated steatotic liver disease in adults. Nat. Rev. Dis. Primers 2025, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef]
- Bertran, N.; Camps, J.; Fernandez-Ballart, J.; Arija, V.; Ferre, N.; Tous, M.; Simo, D.; Murphy, M.M.; Vilella, E.; Joven, J. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J. Lab. Clin. Med. 2005, 145, 41–46. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Cabré, N.; Luciano-Mateo, F.; Chapski, D.J.; Baiges-Gaya, G.; Fernández-Arroyo, S.; Hernández-Aguilera, A.; Castañé, H.; Rodríguez-Tomàs, E.; París, M.; Sabench, F.; et al. Laparoscopic sleeve gastrectomy in patients with severe obesity restores adaptive responses leading to nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2022, 23, 7830. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Ruiz-Canela, M.; Bulló, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gómez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites From Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J. Am. Heart Assoc. 2017, 6, e006524. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Bulló, M.; Hernández-Alonso, P.; Ruiz-Canela, M.; Li, J.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Choline Metabolism and Risk of Atrial Fibrillation and Heart Failure in the PREDIMED Study. Clin Chem. 2021, 67, 288–297. [Google Scholar] [CrossRef] [PubMed]
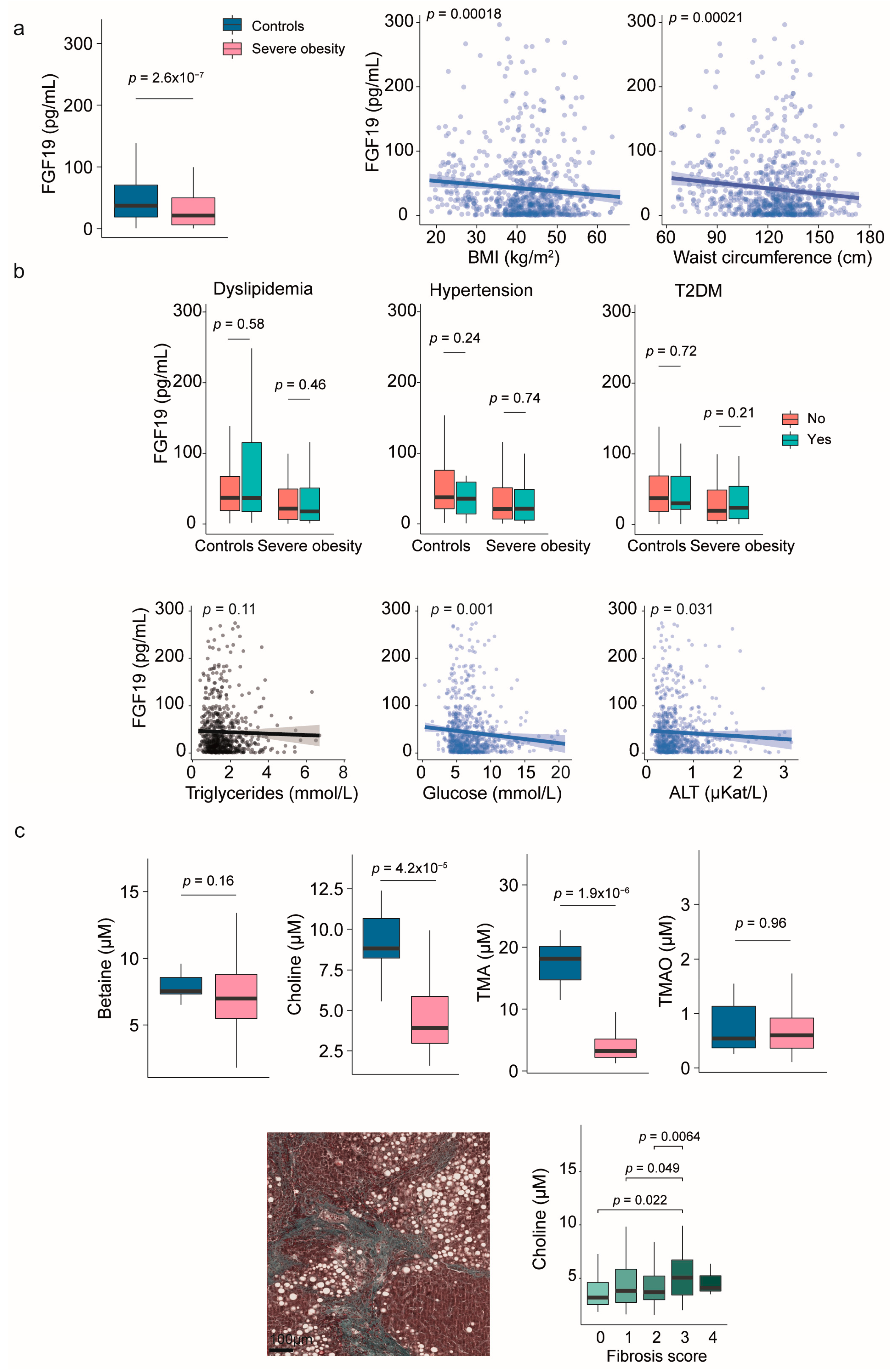


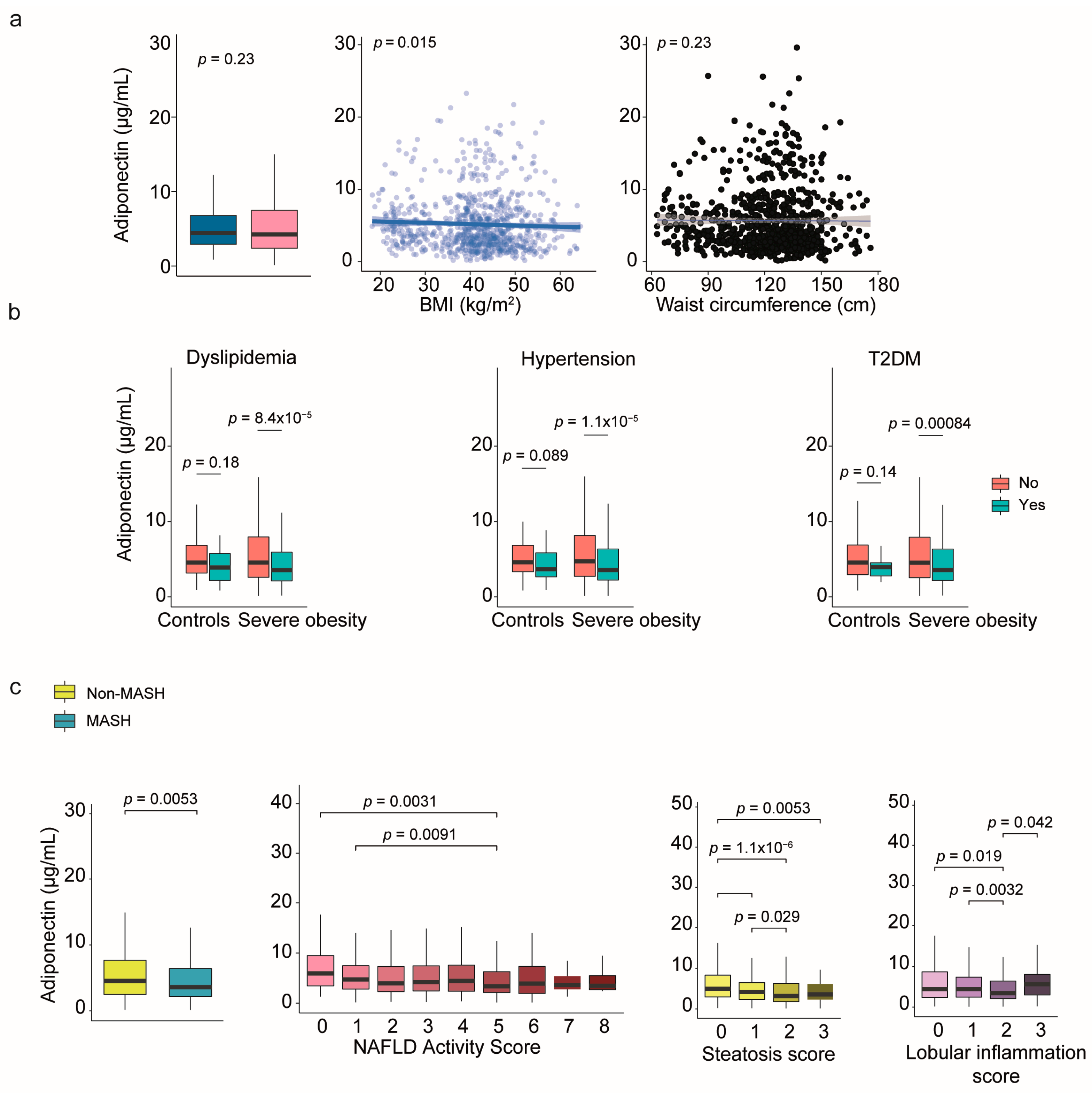
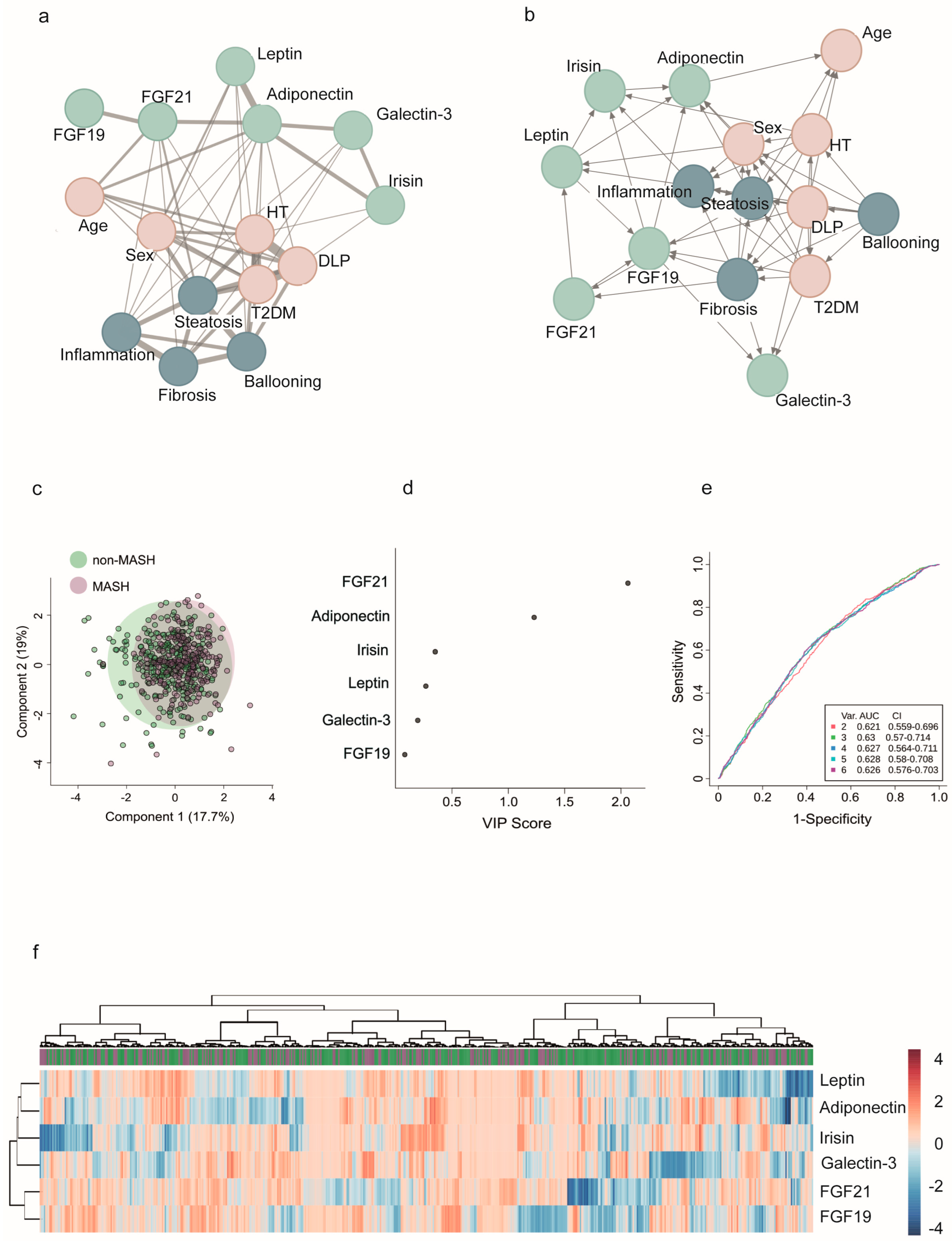

| Variable | Controls (n = 258) | Severe Obesity (n = 923) | p-Value |
|---|---|---|---|
| Women, n (%) | 121 (47.1) | 677 (73.4) | <0.001 |
| Age (years) | 45 (35–62) | 49 (41–56) | 0.347 |
| BMI (kg/m2) | 26.7 (23.3–29.8) | 43.9 (40.3–48.4) | <0.001 |
| Waist circumference (cm) | 89 (78–98) | 130 (120–139) | <0.001 |
| T2DM, n (%) | 18 (7.0) | 253 (27.4) | <0.001 |
| Hypertension, n (%) | 40 (15.5) | 406 (44.0) | <0.001 |
| Dyslipidemia, n (%) | 25 (9.7) | 234 (25.4) | <0.001 |
| Conventional biochemical variables | |||
| Glucose (mmol/L) | 4.7 (4.3–5.2) | 6.7 (5.5–8.5) | <0.001 |
| Insulin (pmol/L) | 47.0 (29.3–65.8) | 67.8 (37.7–109.5) | <0.001 |
| HOMA-IR | 1.4 (0.9–2.2) | 3.2 (1.7–5.7) | <0.001 |
| Triglycerides (mmol/L) | 1.0 (0.7–1.5) | 1.5 (1.2–2.0) | <0.001 |
| Cholesterol (mmol/L) | 5.2 (4.7–5.9) | 4.0 (3.5–4.7) | <0.001 |
| LDL (mmol/L) | 3.1 (2.6–3.8) | 2.4 (1.9–3.0) | <0.001 |
| HDL (mmol/L) | 1.4 (1.2–1.8) | 1.0 (0.8–1.2) | <0.001 |
| ALT (μKat/L) | 0.3 (0.2–0.4) | 0.6 (0.4–0.9) | <0.001 |
| AST (μKat/L) | 0.3 (0.3–0.4) | 0.6 (0.4–0.8) | <0.001 |
| GGT (μKat/L) | 0.2 (0.2–0.4) | 0.4 (0.2–0.6) | <0.001 |
| Organokines and metabolites | |||
| FGF19 (pg/mL) | 37.2 (19.0–70.7) | 21.3 (6.2–49.9) | <0.001 |
| Betaine (μM) | 7.5 (7.3–8.6) | 7.0 (5.5–8.8) | 0.163 |
| Choline (μM) | 8.8 (8.2–10.7) | 3.9 (3.0–5.9) | <0.001 |
| TMA (μM) | 18.1 (14.7–20.1) | 3.2 (2.2–5.2) | <0.001 |
| TMAO (μM) | 0.5 (0.4–1.1) | 0.6 (0.4–0.9) | 0.957 |
| FGF21 (pg/mL) | 119.7 (25.6–222.8) | 164.2 (54.5–326.1) | <0.001 |
| Galectin-3 (ng/mL) | 11.3 (6.2–17.3) | 12.7 (6.6–21.2) | 0.080 |
| Irisin (ng/mL) | 1.1 (0.6–1.6) | 1.5 (0.8–2.4) | <0.001 |
| Leptin (ng/mL) | 12.0 (5.4–23.0) | 51.6 (26.7–85.1) | <0.001 |
| Adiponectin (μg/mL) | 4.4 (2.9–6.8) | 4.2 (2.4–7.5) | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañé, H.; Jiménez-Franco, A.; Onoiu, A.-I.; Cambra-Cortés, V.; Hernández-Aguilera, A.; Parada, D.; Riu, F.; Zorzano, A.; Camps, J.; Joven, J. Dysregulation of the FGF21–Adiponectin Axis in a Large Cohort of Patients with Severe Obesity and Liver Disease. Int. J. Mol. Sci. 2025, 26, 8510. https://doi.org/10.3390/ijms26178510
Castañé H, Jiménez-Franco A, Onoiu A-I, Cambra-Cortés V, Hernández-Aguilera A, Parada D, Riu F, Zorzano A, Camps J, Joven J. Dysregulation of the FGF21–Adiponectin Axis in a Large Cohort of Patients with Severe Obesity and Liver Disease. International Journal of Molecular Sciences. 2025; 26(17):8510. https://doi.org/10.3390/ijms26178510
Chicago/Turabian StyleCastañé, Helena, Andrea Jiménez-Franco, Alina-Iuliana Onoiu, Vicente Cambra-Cortés, Anna Hernández-Aguilera, David Parada, Francesc Riu, Antonio Zorzano, Jordi Camps, and Jorge Joven. 2025. "Dysregulation of the FGF21–Adiponectin Axis in a Large Cohort of Patients with Severe Obesity and Liver Disease" International Journal of Molecular Sciences 26, no. 17: 8510. https://doi.org/10.3390/ijms26178510
APA StyleCastañé, H., Jiménez-Franco, A., Onoiu, A.-I., Cambra-Cortés, V., Hernández-Aguilera, A., Parada, D., Riu, F., Zorzano, A., Camps, J., & Joven, J. (2025). Dysregulation of the FGF21–Adiponectin Axis in a Large Cohort of Patients with Severe Obesity and Liver Disease. International Journal of Molecular Sciences, 26(17), 8510. https://doi.org/10.3390/ijms26178510








