Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis
Abstract
1. Introduction
2. The Acute-Phase Response Mediates Tissue Restoration upon Injury
3. The Central Inflammasome Adapter ASC Couples Pyrin (PYD) and Caspase Recruitment Domain (CARD)-Containing Inflammasome Sensors with Interleukin-1 Bioactivating Effector Machinery
4. Dual Functions of IL-1 Family Members in Balancing Inflammation and Repair Contribute to Epithelial and Mucosal Homeostasis
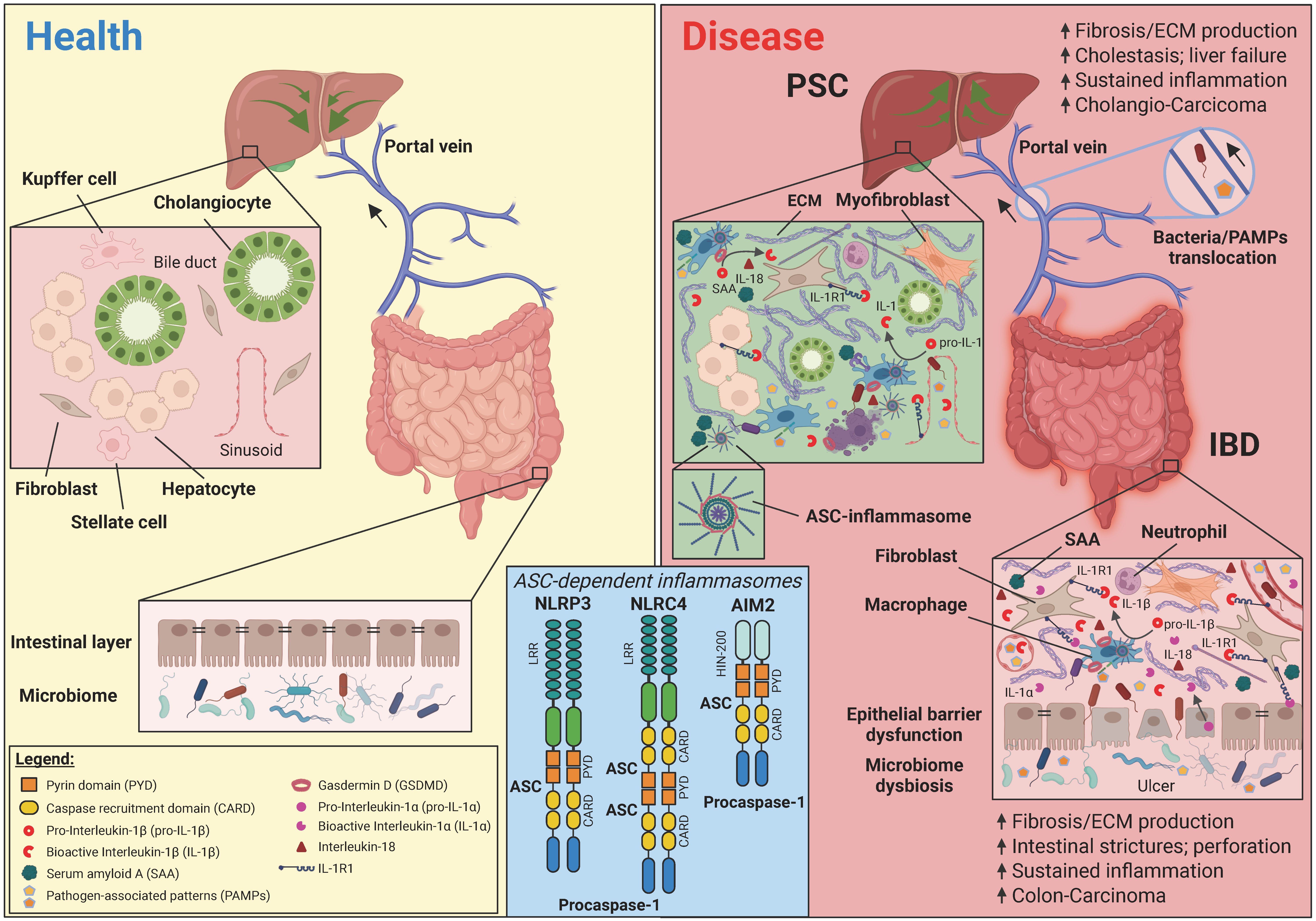
5. ASC-Dependent Inflammasomes and Interleukin-1 Exert Analogous Roles in Inflammation and Fibrosis in PSC and IBD Pathology
6. Serum Amyloid A Proteins Represent Damage-Associated Molecular Patterns and Perpetuate Chronic Inflammation
7. Microbiota–Inflammasome Crosstalk Sustains Chronic Inflammation and Fibrosis by Pathogen-Associated Molecular Patterns
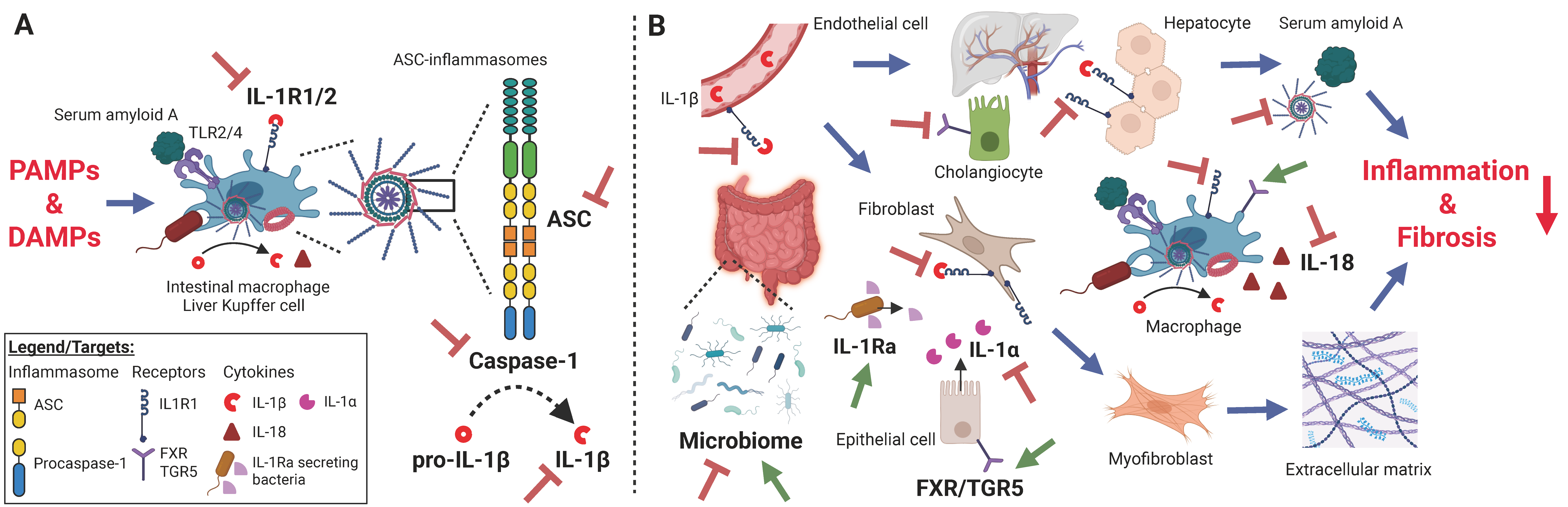
8. Emerging Therapeutic Strategies Targeting ASC-Inflammasomes, Interleukin-1 Family Members, Serum Amyloid A, and the Gut Microbiome in PSC and IBD
8.1. ASC-Targeting Agents
8.2. Caspase-1 Inhibitors
8.3. Interleukin-1α- and Interleukin-1β-Blocking Agents
8.4. IL-1R1/2 Signaling Interference
8.5. Interleukin-18 Inhibitors
8.6. Interleukin-33/ST2 Signaling Blockade
8.7. Serum Amyloid A Targeting Therapeutics
8.8. Microbiome Alterations and Fecal Microbiota Transplantation
8.9. Probiotics
8.10. Farnesoid X Receptor (FXR) and G Protein-Coupled Bile Acid Receptor 1 (TGR5) Bile Receptor Modulation
9. Future Research Directions
10. Conclusions and Clinical Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornillet, M.; Geanon, D.; Bergquist, A.; Bjorkstrom, N.K. Immunobiology of primary sclerosing cholangitis. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Markovinovic, A.; Coward, S.; Herauf, M.; Shaheen, A.A.; Swain, M.; Panaccione, R.; Ma, C.; Lu, C.; Novak, K.; et al. Incidence and Prevalence of Primary Sclerosing Cholangitis: A Meta-analysis of Population-based Studies. Inflamm. Bowel Dis. 2024, 30, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef]
- Bedke, T.; Stumme, F.; Tomczak, M.; Steglich, B.; Jia, R.; Bohmann, S.; Wittek, A.; Kempski, J.; Goke, E.; Bottcher, M.; et al. Protective function of sclerosing cholangitis on IBD. Gut 2024, 73, 1292–1301. [Google Scholar] [CrossRef]
- Bergquist, A.; Montgomery, S.M.; Bahmanyar, S.; Olsson, R.; Danielsson, A.; Lindgren, S.; Prytz, H.; Hultcrantz, R.; Loof, L.A.; Sandberg-Gertzen, H.; et al. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 2008, 6, 939–943. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.I.B.D. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- van Munster, K.N.; Bergquist, A.; Ponsioen, C.Y. Inflammatory bowel disease and primary sclerosing cholangitis: One disease or two? J. Hepatol. 2024, 80, 155–168. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Fricker, Z.P.; Lichtenstein, D.R. Primary Sclerosing Cholangitis: A Concise Review of Diagnosis and Management. Dig. Dis. Sci. 2019, 64, 632–642. [Google Scholar] [CrossRef]
- Assis, D.N.; Bowlus, C.L. Recent Advances in the Management of Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2023, 21, 2065–2075. [Google Scholar] [CrossRef]
- Fung, B.M.; Lindor, K.D.; Tabibian, J.H. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J. Gastroenterol. 2019, 25, 659–671. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Peerani, F.; Castaneda, D.; Torres, J.; Itzkowitz, S.H. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Aust, D.E.; Baretton, G.B.; Sommer, U. Ulcerative colitis-associated carcinogenesis: An update. Pathologie 2023, 44, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Nugent, F.W.; Zelig, M.P.; Munson, J.L.; Schoetz, D.J., Jr. Cholangiocarcinoma and Crohn’s disease. Dig. Dis. Sci. 1994, 39, 667–670. [Google Scholar] [CrossRef]
- Hnatyszyn, A.; Hryhorowicz, S.; Kaczmarek-Rys, M.; Lis, E.; Slomski, R.; Scott, R.J.; Plawski, A. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered. Cancer Clin. Pract. 2019, 17, 18. [Google Scholar] [CrossRef]
- Fung, B.M.; Tabibian, J.H. Cholangiocarcinoma in patients with primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2020, 36, 77–84. [Google Scholar] [CrossRef]
- Yashiro, M. Ulcerative colitis-associated colorectal cancer. World J. Gastroenterol. 2014, 20, 16389–16397. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Nayeri, S.; Borowski, K.; Hernandez-Rocha, C.; Lee, S.H.; Turpin, W.; Stempak, J.M.; Sandhu, I.; Milgrom, R.; Smith, M.I.; et al. Inflammatory bowel disease associated with primary sclerosing cholangitis is associated with an altered gut microbiome and bile acid profile. J. Crohn’s Colitis 2024, 18, 1957–1966. [Google Scholar] [CrossRef]
- Lin, S.N.; Wang, J.; Mukherjee, P.K.; Veisman, I.; Massey, W.J.; Mao, R.; Chandra, J.; Fiocchi, C.; Rieder, F. The functional role of the extracellular matrix in inflammatory bowel disease associated gut fibrosis. Matrix Biol. 2025, 139, 29–48. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef]
- Rieder, F.; Mukherjee, P.K.; Massey, W.J.; Wang, Y.; Fiocchi, C. Fibrosis in IBD: From pathogenesis to therapeutic targets. Gut 2024, 73, 854–866. [Google Scholar] [CrossRef]
- D’Haens, G.; Rieder, F.; Feagan, B.G.; Higgins, P.D.R.; Panes, J.; Maaser, C.; Rogler, G.; Lowenberg, M.; van der Voort, R.; Pinzani, M.; et al. Challenges in the Pathophysiology, Diagnosis, and Management of Intestinal Fibrosis in Inflammatory Bowel Disease. Gastroenterology 2022, 162, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.D.; Chapman, R.W.; Keshav, S.; Montano-Loza, A.J.; Mason, A.L.; Kremer, A.E.; Vetter, M.; de Krijger, M.; Ponsioen, C.Y.; Trivedi, P.; et al. Effects of Vedolizumab in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 179–187.e6. [Google Scholar] [CrossRef] [PubMed]
- Zundler, S.; Gunther, C.; Kremer, A.E.; Zaiss, M.M.; Rothhammer, V.; Neurath, M.F. Gut immune cell trafficking: Inter-organ communication and immune-mediated inflammation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, E.; Yablecovitch, D.; Baram, L.; Dotan, I.; Sagi, I. ECM remodelling in IBD: Innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2015, 64, 367–372. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biology of interleukin 1. FASEB J. 1988, 2, 108–115. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef]
- Dorner, H.; Stolzer, I.; Mattner, J.; Kaminski, S.; Leistl, S.; Edrich, L.M.; Schwendner, R.; Hobauer, J.; Sebald, A.; Leikam, S.; et al. Gut Pathobiont-Derived Outer Membrane Vesicles Drive Liver Inflammation and Fibrosis in Primary Sclerosing Cholangitis-Associated Inflammatory Bowel Disease. Gastroenterology 2024, 167, 1183–1197.e16. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Moron, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Gutte, P.M.; Grutter, M.G.; Beer, H.D.; et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Investig. 2023, 133, e169304. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Lang, S.; Gottier, C.; Dai, X.; Rawlings, D.J.; Chan, A.C.; Rogler, G.; Scharl, M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017, 13, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454, Erratum in N. Engl. J. Med. 1999, 340, 1376. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Dick, M.S.; Sborgi, L.; Ruhl, S.; Hiller, S.; Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 2016, 7, 11929, Erratum in Nat. Commun. 2017, 8, 15030. https://doi.org/10.1038/ncomms15030. [Google Scholar] [CrossRef] [PubMed]
- Hoss, F.; Rodriguez-Alcazar, J.F.; Latz, E. Assembly and regulation of ASC specks. Cell Mol. Life Sci. 2017, 74, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Leal, V.N.C.; Pontillo, A. Canonical Inflammasomes. In NLR Proteins: Methods and Protocols; Springer: New York, NY, USA, 2023; Volume 2696, pp. 1–27. [Google Scholar] [CrossRef]
- de Alba, E. Structure, interactions and self-assembly of ASC-dependent inflammasomes. Arch. Biochem. Biophys. 2019, 670, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef]
- Franklin, B.S.; Bossaller, L.; De Nardo, D.; Ratter, J.M.; Stutz, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al-Amoudi, A.; et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef]
- Losa, M.; Emmenegger, M.; De Rossi, P.; Schurch, P.M.; Serdiuk, T.; Pengo, N.; Capron, D.; Bieli, D.; Bargenda, N.; Rupp, N.J.; et al. The ASC inflammasome adapter governs SAA-derived protein aggregation in inflammatory amyloidosis. EMBO Mol. Med. 2024, 16, 2024–2042. [Google Scholar] [CrossRef] [PubMed]
- Sahillioglu, A.C.; Sumbul, F.; Ozoren, N.; Haliloglu, T. Structural and dynamics aspects of ASC speck assembly. Structure 2014, 22, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Tang, Y.; Yang, J.; Fu, S.; Li, Y.; Ren, X.; Zhou, N.; Zhao, W.; Gao, J.; Ruan, Z.; et al. Signal-induced NLRP3 phase separation initiates inflammasome activation. Cell Res. 2025, 35, 437–452. [Google Scholar] [CrossRef]
- Masumoto, J.; Taniguchi, S.; Ayukawa, K.; Sarvotham, H.; Kishino, T.; Niikawa, N.; Hidaka, E.; Katsuyama, T.; Higuchi, T.; Sagara, J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 1999, 274, 33835–33838. [Google Scholar] [CrossRef]
- de Alba, E. Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC). J. Biol. Chem. 2009, 284, 32932–32941. [Google Scholar] [CrossRef]
- Masumoto, J.; Taniguchi, S.; Nakayama, J.; Shiohara, M.; Hidaka, E.; Katsuyama, T.; Murase, S.; Sagara, J. Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J. Histochem. Cytochem. 2001, 49, 1269–1275. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Poyet, J.L.; Razmara, M.; Datta, P.; Zhang, Z.; Alnemri, E.S. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 2002, 277, 21119–21122. [Google Scholar] [CrossRef]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schroder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Dig. Dis. Sci. 1988, 33 (Suppl. S3), 25S–35S. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor. Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Savage, N. Interleukin-1 and its receptor. Crit. Rev. Immunol. 1989, 9, 1–20. [Google Scholar] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, I.; Jha, S. Comprehensive review of ASC structure and function in immune homeostasis and disease. Mol. Biol. Rep. 2020, 47, 3077–3096. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Duncan, J.A.; Canna, S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018, 281, 115–123. [Google Scholar] [CrossRef]
- Wang, B.; Tian, Y.; Yin, Q. AIM2 Inflammasome Assembly and Signaling. Adv. Exp. Med. Biol. 2019, 1172, 143–155. [Google Scholar] [CrossRef]
- McEntee, C.P.; Finlay, C.M.; Lavelle, E.C. Divergent Roles for the IL-1 Family in Gastrointestinal Homeostasis and Inflammation. Front. Immunol. 2019, 10, 1266. [Google Scholar] [CrossRef]
- Pinar, A.A.; Samuel, C.S. Immune Mechanisms and Related Targets for the Treatment of Fibrosis in Various Organs. Curr. Mol. Med. 2022, 22, 240–249. [Google Scholar] [CrossRef]
- Song, Z.; Gong, Q.; Guo, J. Pyroptosis: Mechanisms and Links with Fibrosis. Cells 2021, 10, 3509. [Google Scholar] [CrossRef]
- Artlett, C.M. The Mechanism and Regulation of the NLRP3 Inflammasome during Fibrosis. Biomolecules 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Nagy, L.E.; Maher, T.M.; Distler, J.H.W.; Kramann, R.; Hinz, B.; Prunotto, M. Fibrosis: Cross-organ biology and pathways to development of innovative drugs. Nat. Rev. Drug Discov. 2025, 24, 543–569, Erratum in Nat. Rev. Drug Discov. 2025, 24, 399. https://doi.org/10.1038/s41573-025-01190-9. [Google Scholar] [CrossRef]
- Song, A.; Zhu, L.; Gorantla, G.; Berdysz, O.; Amici, S.A.; Guerau-de-Arellano, M.; Madalena, K.M.; Lerch, J.K.; Liu, X.; Quan, N. Salient type 1 interleukin 1 receptor expression in peripheral non-immune cells. Sci. Rep. 2018, 8, 723. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Jackson, M.A.; Korsunsky, I.; Bullers, S.J.; Rue-Albrecht, K.; Christoforidou, Z.; Sathananthan, D.; Thomas, T.; Ravindran, R.; et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 2021, 27, 1970–1981. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, S.J.; Zhou, S.C.; Wu, S.Z.; Wang, H. Inflammasomes and Fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- Cominelli, F.; Nast, C.C.; Clark, B.D.; Schindler, R.; Lierena, R.; Eysselein, V.E.; Thompson, R.C.; Dinarello, C.A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J. Clin. Investig. 1990, 86, 972–980. [Google Scholar] [CrossRef]
- Aschenbrenner, D.; Quaranta, M.; Banerjee, S.; Ilott, N.; Jansen, J.; Steere, B.; Chen, Y.H.; Ho, S.; Cox, K.; Arancibia-Carcamo, C.V.; et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut 2021, 70, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, M.; Kessler, S.; Sadler, T.; West, G.; Homer, C.; McDonald, C.; de la Motte, C.; Fiocchi, C.; Stylianou, E. The epithelial danger signal IL-1alpha is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am. J. Pathol. 2015, 185, 1624–1637. [Google Scholar] [CrossRef]
- Salmela, M.T.; Pender, S.L.; Karjalainen-Lindsberg, M.L.; Puolakkainen, P.; Macdonald, T.T.; Saarialho-Kere, U. Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand. J. Gastroenterol. 2004, 39, 1095–1104. [Google Scholar] [CrossRef]
- Mia, M.M.; Boersema, M.; Bank, R.A. Interleukin-1beta attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-beta1. PLoS ONE 2014, 9, e91559. [Google Scholar] [CrossRef]
- Kamari, Y.; Shaish, A.; Vax, E.; Shemesh, S.; Kandel-Kfir, M.; Arbel, Y.; Olteanu, S.; Barshack, I.; Dotan, S.; Voronov, E.; et al. Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 2011, 55, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, M.; Przytycki, P.F.; Micheletti, R.; Padmanabhan, A.; Ye, L.; Travers, J.G.; Gonzalez-Teran, B.; Silva, A.C.; Duan, Q.; Ranade, S.S.; et al. A transcriptional switch governs fibroblast activation in heart disease. Nature 2021, 595, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Chowdhry, S.; Pizarro, T.T. Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease. Front. Immunol. 2013, 4, 181. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, F.; Nast, C.C.; Llerena, R.; Dinarello, C.A.; Zipser, R.D. Interleukin 1 suppresses inflammation in rabbit colitis. Mediation by endogenous prostaglandins. J. Clin. Investig. 1990, 85, 582–586. [Google Scholar] [CrossRef]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef]
- Takagi, H.; Kanai, T.; Okazawa, A.; Kishi, Y.; Sato, T.; Takaishi, H.; Inoue, N.; Ogata, H.; Iwao, Y.; Hoshino, K.; et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 2003, 38, 837–844. [Google Scholar] [CrossRef]
- Kojouharoff, G.; Hans, W.; Obermeier, F.; Mannel, D.N.; Andus, T.; Scholmerich, J.; Gross, V.; Falk, W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin. Exp. Immunol. 1997, 107, 353–358. [Google Scholar] [CrossRef]
- Kunzmann, L.K.; Schoknecht, T.; Poch, T.; Henze, L.; Stein, S.; Kriz, M.; Grewe, I.; Preti, M.; Hartl, J.; Pannicke, N.; et al. Monocytes as Potential Mediators of Pathogen-Induced T-Helper 17 Differentiation in Patients with Primary Sclerosing Cholangitis (PSC). Hepatology 2020, 72, 1310–1326. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Powrie, F. Translating Immunology into Therapeutic Concepts for Inflammatory Bowel Disease. Annu. Rev. Immunol. 2018, 36, 755–781. [Google Scholar] [CrossRef]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef]
- Hausmann, M.; Bataille, F.; Spoettl, T.; Schreiter, K.; Falk, W.; Schoelmerich, J.; Herfarth, H.; Rogler, G. Physiological role of macrophage inflammatory protein-3 alpha induction during maturation of intestinal macrophages. J. Immunol. 2005, 175, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Kiessling, S.; Mestermann, S.; Webb, G.; Spottl, T.; Andus, T.; Scholmerich, J.; Herfarth, H.; Ray, K.; Falk, W.; et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 2002, 122, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kikuta, J.; Matsui, T.; Hasegawa, T.; Fujii, K.; Okuzaki, D.; Liu, Y.C.; Yoshioka, T.; Seno, S.; Motooka, D.; et al. Periportal macrophages protect against commensal-driven liver inflammation. Nature 2024, 629, 901–909. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- El Hadad, J.; Schreiner, P.; Vavricka, S.R.; Greuter, T. The Genetics of Inflammatory Bowel Disease. Mol. Diagn. Ther. 2024, 28, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Barrett, J.C.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Magyari, L.; Kovesdi, E.; Sarlos, P.; Javorhazy, A.; Sumegi, K.; Melegh, B. Interleukin and interleukin receptor gene polymorphisms in inflammatory bowel diseases susceptibility. World J. Gastroenterol. 2014, 20, 3208–3222. [Google Scholar] [CrossRef]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef]
- Henriksen, E.K.; Melum, E.; Karlsen, T.H. Update on primary sclerosing cholangitis genetics. Curr. Opin. Gastroenterol. 2014, 30, 310–319. [Google Scholar] [CrossRef]
- Jiang, X.; Karlsen, T.H. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 279–295. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Folseraas, T.; Holm, K.; Ellinghaus, E.; Melum, E.; Balschun, T.; Laerdahl, J.K.; Shiryaev, A.; Gotthardt, D.N.; Weismuller, T.J.; et al. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology 2013, 58, 1074–1083. [Google Scholar] [CrossRef]
- Folseraas, T.; Melum, E.; Rausch, P.; Juran, B.D.; Ellinghaus, E.; Shiryaev, A.; Laerdahl, J.K.; Ellinghaus, D.; Schramm, C.; Weismuller, T.J.; et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 2012, 57, 366–375. [Google Scholar] [CrossRef]
- Janse, M.; Lamberts, L.E.; Franke, L.; Raychaudhuri, S.; Ellinghaus, E.; Muri Boberg, K.; Melum, E.; Folseraas, T.; Schrumpf, E.; Bergquist, A.; et al. Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL, and CARD9. Hepatology 2011, 53, 1977–1985. [Google Scholar] [CrossRef]
- Jacob, C.O. Cytokines and anti-cytokines. Curr. Opin. Immunol. 1989, 2, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Urieli-Shoval, S.; Cohen, P.; Eisenberg, S.; Matzner, Y. Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J. Histochem. Cytochem. 1998, 46, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, K.; Palming, J.; Olofsson, L.E.; Gummesson, A.; Svensson, P.A.; Lystig, T.C.; Jennische, E.; Brandberg, J.; Torgerson, J.S.; Carlsson, B.; et al. A microarray search for genes predominantly expressed in human omental adipocytes: Adipose tissue as a major production site of serum amyloid A. J. Clin. Endocrinol. Metab. 2005, 90, 2233–2239. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hall, J.A.; Kroehling, L.; Wu, L.; Najar, T.; Nguyen, H.H.; Lin, W.Y.; Yeung, S.T.; Silva, H.M.; Li, D.; et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 2020, 180, 79–91.e16. [Google Scholar] [CrossRef]
- Sack, G.H. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef]
- Sack, G.H. Serum Amyloid A (SAA) Proteins. Subcell. Biochem. 2020, 94, 421–436. [Google Scholar] [CrossRef]
- Benditt, E.P.; Hoffman, J.S.; Eriksen, N.; Parmelee, D.C.; Walsh, K.A. SAA, an apoprotein of HDL: Its structure and function. Ann. N. Y. Acad. Sci. 1982, 389, 183–189. [Google Scholar] [CrossRef]
- Sellar, G.C.; Jordan, S.A.; Bickmore, W.A.; Fantes, J.A.; van Heyningen, V.; Whitehead, A.S. The human serum amyloid A protein (SAA) superfamily gene cluster: Mapping to chromosome 11p15.1 by physical and genetic linkage analysis. Genomics 1994, 19, 221–227. [Google Scholar] [CrossRef]
- Papareddy, P.; Herwald, H. From immune activation to disease progression: Unraveling the complex role of Serum Amyloid A proteins. Cytokine Growth Factor. Rev. 2025, 83, 77–84. [Google Scholar] [CrossRef]
- Davis, T.A.; Conradie, D.; Shridas, P.; de Beer, F.C.; Engelbrecht, A.M.; de Villiers, W.J.S. Serum Amyloid A Promotes Inflammation-Associated Damage and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1329–1341. [Google Scholar] [CrossRef]
- Ye, R.D.; Sun, L. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef]
- Niemi, K.; Teirila, L.; Lappalainen, J.; Rajamaki, K.; Baumann, M.H.; Oorni, K.; Wolff, H.; Kovanen, P.T.; Matikainen, S.; Eklund, K.K. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J. Immunol. 2011, 186, 6119–6128. [Google Scholar] [CrossRef]
- Sun, L.; Ye, R.D. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef]
- Westermark, G.T.; Westermark, P. Serum amyloid A and protein AA: Molecular mechanisms of a transmissible amyloidosis. FEBS Lett. 2009, 583, 2685–2690. [Google Scholar] [CrossRef]
- Van Steenbergen, W.; Braeye, L.; Harlet, R.; Nevens, F.; Fevery, J.; Desmet, V.; Roskams, T.; Pirenne, J. Primary sclerosing cholangitis complicated by amyloid A amyloidosis: Complete regression of the nephrotic syndrome by liver transplantation. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1265–1270. [Google Scholar] [CrossRef]
- Shridas, P.; Tannock, L.R. Role of serum amyloid A in atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 320–325. [Google Scholar] [CrossRef]
- Yassine, H.N.; Trenchevska, O.; He, H.; Borges, C.R.; Nedelkov, D.; Mack, W.; Kono, N.; Koska, J.; Reaven, P.D.; Nelson, R.W. Serum amyloid a truncations in type 2 diabetes mellitus. PLoS ONE 2015, 10, e0115320. [Google Scholar] [CrossRef]
- Connolly, M.; Veale, D.J.; Fearon, U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1296–1303. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Tie, Y.; Zhang, J.; Huang, P.; Xie, Y.; Zhang, L.; Tang, X.; Zeng, Z.; Li, L.; et al. Serum amyloid A for predicting prognosis in patients with newly diagnosed Crohn’s disease. BMJ Open Gastroenterol. 2024, 11, e001497. [Google Scholar] [CrossRef]
- Yarur, A.J.; Quintero, M.A.; Jain, A.; Czul, F.; Barkin, J.S.; Abreu, M.T. Serum Amyloid A as a Surrogate Marker for Mucosal and Histologic Inflammation in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 158–164. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Z.; Wang, Y.; Li, G.; Bai, X. Serum amyloid A and interleukin -1beta facilitate LDL transcytosis across endothelial cells and atherosclerosis via NF-kappaB/caveolin-1/cavin-1 pathway. Atherosclerosis 2023, 375, 87–97. [Google Scholar] [CrossRef]
- Connolly, M.; Rooney, P.R.; McGarry, T.; Maratha, A.X.; McCormick, J.; Miggin, S.M.; Veale, D.J.; Fearon, U. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann. Rheum. Dis. 2016, 75, 1392–1398. [Google Scholar] [CrossRef]
- Hatanaka, E.; Dermargos, A.; Armelin, H.A.; Curi, R.; Campa, A. Serum amyloid A induces reactive oxygen species (ROS) production and proliferation of fibroblast. Clin. Exp. Immunol. 2011, 163, 362–367. [Google Scholar] [CrossRef]
- Emmenegger, M.; Zografou, C.; Dai, Y.; Hoyt, L.R.; Gudneppanavar, R.; Chincisan, A.; Rehrauer, H.; Noé, F.J.; Zajac, N.; Meisl, G.; et al. The Cystic Fibrosis Transmembrane Regulator Controls Tolerogenic Responses to Food Allergens in Mice and Humans. medRxiv 2024. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Yu, S.; Sun, Y.; Shao, X.; Zhou, Y.; Yu, Y.; Kuai, X.; Zhou, C. Leaky Gut in IBD: Intestinal Barrier-Gut Microbiota Interaction. J. Microbiol. Biotechnol. 2022, 32, 825–834. [Google Scholar] [CrossRef]
- Dhillon, A.K.; Kummen, M.; Troseid, M.; Akra, S.; Liaskou, E.; Moum, B.; Vesterhus, M.; Karlsen, T.H.; Seljeflot, I.; Hov, J.R. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019, 39, 371–381. [Google Scholar] [CrossRef]
- Navaneethan, U. Hepatobiliary manifestations of ulcerative colitis: An example of gut-liver crosstalk. Gastroenterol. Rep. 2014, 2, 193–200. [Google Scholar] [CrossRef]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2023, 16, 92. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut microbiome in primary sclerosing cholangitis: A review. World J. Gastroenterol. 2020, 26, 2768–2780. [Google Scholar] [CrossRef]
- Bogatic, D.; Bryant, R.V.; Lynch, K.D.; Costello, S.P. Systematic review: Microbial manipulation as therapy for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2023, 57, 23–36. [Google Scholar] [CrossRef]
- Kummen, M.; Thingholm, L.B.; Ruhlemann, M.C.; Holm, K.; Hansen, S.H.; Moitinho-Silva, L.; Liwinski, T.; Zenouzi, R.; Storm-Larsen, C.; Midttun, O.; et al. Altered Gut Microbial Metabolism of Essential Nutrients in Primary Sclerosing Cholangitis. Gastroenterology 2021, 160, 1784–1798.e0. [Google Scholar] [CrossRef]
- Ostadmohammadi, S.; Azimirad, M.; Houri, H.; Naseri, K.; Javanmard, E.; Mirjalali, H.; Yadegar, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Zali, M.R. Characterization of the gut microbiota in patients with primary sclerosing cholangitis compared to inflammatory bowel disease and healthy controls. Mol. Biol. Rep. 2021, 48, 5519–5529. [Google Scholar] [CrossRef]
- Folseraas, T.; Liaskou, E.; Anderson, C.A.; Karlsen, T.H. Genetics in PSC: What do the “risk genes” teach us? Clin. Rev. Allergy Immunol. 2015, 48, 154–164. [Google Scholar] [CrossRef]
- Chen, S.N.; Tan, Y.; Xiao, X.C.; Li, Q.; Wu, Q.; Peng, Y.Y.; Ren, J.; Dong, M.L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef]
- Katt, J.; Schwinge, D.; Schoknecht, T.; Quaas, A.; Sobottka, I.; Burandt, E.; Becker, C.; Neurath, M.F.; Lohse, A.W.; Herkel, J.; et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013, 58, 1084–1093. [Google Scholar] [CrossRef]
- Hao, H.; Cao, L.; Jiang, C.; Che, Y.; Zhang, S.; Takahashi, S.; Wang, G.; Gonzalez, F.J. Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metab. 2017, 25, 856–867.e5. [Google Scholar] [CrossRef]
- Shi, T.; Malik, A.; Yang Vom Hofe, A.; Matuschek, L.; Mullen, M.; Lages, C.S.; Kudira, R.; Singh, R.; Zhang, W.; Setchell, K.D.R.; et al. Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci. Transl. Med. 2022, 14, eabi4354. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef]
- Huynh, D.; Rubtsov, D.; Khaing, M.M. Efficacy of Biologics in the Treatment of Primary Sclerosing Cholangitis Associated With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e56182. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Khatri, V.; Kalyanasundaram, R. Therapeutic implications of inflammasome in inflammatory bowel disease. FASEB J. 2021, 35, e21439. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, W.; Zhang, J.; Mei, Y.; Bao, J.; Hou, S.; Zhou, X.; Mao, L. Inflammasome-targeting natural compounds in inflammatory bowel disease: Mechanisms and therapeutic potential. Front. Immunol. 2022, 13, 963291. [Google Scholar] [CrossRef]
- Perera, A.P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S.S.; Robertson, A.A.B.; Schroder, K.; Kunde, D.; Eri, R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. [Google Scholar] [CrossRef]
- Umiker, B.; Lee, H.H.; Cope, J.; Ajami, N.J.; Laine, J.P.; Fregeau, C.; Ferguson, H.; Alves, S.E.; Sciammetta, N.; Kleinschek, M.; et al. The NLRP3 inflammasome mediates DSS-induced intestinal inflammation in Nod2 knockout mice. Innate Immun. 2019, 25, 132–143. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chen, Y.; Li, X.; Jiang, Z. Decursinol angelate relieves inflammatory bowel disease by inhibiting the ROS/TXNIP/NLRP3 pathway and pyroptosis. Front. Pharmacol. 2024, 15, 1520040. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lin, X.; Huang, G.G.; Zhou, R.; Lei, S.Y.; Ren, J.; Zhang, K.R.; Feng, C.L.; Wu, Y.W.; Tang, W. Atranorin inhibits NLRP3 inflammasome activation by targeting ASC and protects NLRP3 inflammasome-driven diseases. Acta Pharmacol. Sin. 2023, 44, 1687–1700. [Google Scholar] [CrossRef]
- Cai, H.; Liu, Z.; Sun, P.; Zhou, Y.; Yan, Y.; Luo, Y.; Zhang, X.; Wu, R.; Liang, X.; Wu, D.; et al. Discovery of a dual-acting inhibitor of interleukin-1beta and STATs for the treatment of inflammatory bowel disease. RSC Med. Chem. 2024, 15, 193–206. [Google Scholar] [CrossRef]
- Wang, L.; Dong, X.; Feng, S.; Pan, H.; Jang, X.; Chen, L.; Zhao, Y.; Chen, W.; Huang, Z. VX765 alleviates dextran sulfate sodium-induced colitis in mice by suppressing caspase-1-mediated pyroptosis. Int. Immunopharmacol. 2022, 102, 108405. [Google Scholar] [CrossRef]
- Yin, Q.; Pi, X.; Jiang, Y.; Ren, G.; Liu, Z.; Liu, H.; Wang, M.; Sun, W.; Li, S.; Gao, Z.; et al. An immuno-blocking agent targeting IL-1beta and IL-17A reduces the lesion of DSS-induced ulcerative colitis in mice. Inflammation 2021, 44, 1724–1736. [Google Scholar] [CrossRef]
- Liso, M.; Verna, G.; Cavalcanti, E.; De Santis, S.; Armentano, R.; Tafaro, A.; Lippolis, A.; Campiglia, P.; Gasbarrini, A.; Mastronardi, M.; et al. Interleukin 1beta Blockade Reduces Intestinal Inflammation in a Murine Model of Tumor Necrosis Factor-Independent Ulcerative Colitis. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 151–171. [Google Scholar] [CrossRef]
- Namai, F.; Shigemori, S.; Ogita, T.; Sato, T.; Shimosato, T. Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Exp. Mol. Med. 2020, 52, 1627–1636. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, J.; Xi, S.; Qi, X.; Shen, S.; Ge, Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur. J. Pharm. Biopharm. 2019, 137, 112–121. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.; Renooij, W.; Murzilli, S.; Klomp, L.W.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, B.; Yu, J.; Pan, Q.; Yu, Y.; Feng, X.; Chen, W.; Yang, J.; Gao, C.; Cao, H. ROS-responsive nanoparticle delivery of obeticholic acid mitigate primary sclerosing cholangitis. J. Control Release 2024, 374, 112–126. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, S.; Yu, X.; Zhang, L.; Zhang, Z.; Fu, Y.; Liu, W.; Mu, Y.; Zhang, H.; Liu, P.; et al. Total astragalus saponins attenuate primary sclerosing cholangitis in mice by upregulation of TGR5. Phytother. Res. 2024, 38, 4502–4518. [Google Scholar] [CrossRef]
- Shaul, E.; Conrad, M.A.; Dawany, N.; Patel, T.; Canavan, M.C.; Baccarella, A.; Weinbrom, S.; Aleynick, D.; Sullivan, K.E.; Kelsen, J.R. Canakinumab for the treatment of autoinflammatory very early onset-inflammatory bowel disease. Front. Immunol. 2022, 13, 972114. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Kang, Y.H.; Griffith, A.E.; Konnikova, L.; Mascanfroni, I.D.; Redhu, N.S.; Frei, S.M.; Field, M.; Doty, A.L.; et al. Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016, 151, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.; Bayliss, C.; Bond, S.; Dowling, F.; Galea, J.; Jairath, V.; Lamb, C.; Probert, C.; Timperley-Preece, E.; Watson, A.; et al. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: The IASO trial. BMJ Open 2019, 9, e023765. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Vuppalanchi, R.; Levy, C.; Floreani, A.; Andreone, P.; LaRusso, N.F.; Shrestha, R.; Trotter, J.; Goldberg, D.; Rushbrook, S.; et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 2020, 73, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Tevethia, H.V.; Arab, J.P.; Candia, R.; Premkumar, M.; Kumar, P.; Sharma, M.; Reddy, D.N.; Padaki, N.R. Efficacy and safety of obeticholic acid in liver disease-A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101675. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Liang, B.; Ding, P.; Hou, X.; Wei, W.; Ma, Y. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 2022, 928, 175091. [Google Scholar] [CrossRef]
- Bakhshi, S.; Shamsi, S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: Latest evidence and therapeutic outcomes. Int. Immunopharmacol. 2022, 106, 108595. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.A.B.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef]
- Huo, S.; Li, B.; Du, J.; Zhang, X.; Zhang, J.; Wang, Q.; Song, M.; Li, Y. Dibutyl phthalate induces liver fibrosis via p38MAPK/NF-kappaB/NLRP3-mediated pyroptosis. Sci. Total Environ. 2023, 897, 165500. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Ning, X.; Li, S.; Xue, D.; Wei, C.; Zhu, Z.; Sheng, L.; Lu, B.; Li, Y.; et al. Directly targeting ASC by lonidamine alleviates inflammasome-driven diseases. J. Neuroinflamm. 2022, 19, 315. [Google Scholar] [CrossRef]
- Siegmund, B.; Lehr, H.A.; Fantuzzi, G.; Dinarello, C.A. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 2001, 98, 13249–13254. [Google Scholar] [CrossRef] [PubMed]
- Auer, H.; Mobley, J.A.; Ayers, L.W.; Bowen, J.; Chuaqui, R.F.; Johnson, L.A.; Livolsi, V.A.; Lubensky, I.A.; McGarvey, D.; Monovich, L.C.; et al. The effects of frozen tissue storage conditions on the integrity of RNA and protein. Biotech. Histochem. 2014, 89, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Ni, H.; Huang, J.; Yu, G.; Zhang, Z.; Zhang, Q. VX-765 prevents intestinal ischemia-reperfusion injury by inhibiting NLRP3 inflammasome. Tissue Cell 2022, 75, 101718. [Google Scholar] [CrossRef] [PubMed]
- Menghini, P.; Corridoni, D.; Butto, L.F.; Osme, A.; Shivaswamy, S.; Lam, M.; Bamias, G.; Pizarro, T.T.; Rodriguez-Palacios, A.; Dinarello, C.A.; et al. Neutralization of IL-1alpha ameliorates Crohn’s disease-like ileitis by functional alterations of the gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 26717–26726. [Google Scholar] [CrossRef]
- Shah, A.; Jones, M.P.; Callaghan, G.; Fairlie, T.; Ma, X.; Culver, E.L.; Stuart, K.; De Cruz, P.; O’Beirne, J.; Tabibian, J.H.; et al. Efficacy and safety of biologics in primary sclerosing cholangitis with inflammatory bowel disease: A systematic review and meta-analysis. Hepatol. Commun. 2024, 8, e0347. [Google Scholar] [CrossRef]
- Onac, I.A.; Clarke, B.D.; Tacu, C.; Lloyd, M.; Hajela, V.; Batty, T.; Thoroughgood, J.; Smith, S.; Irvine, H.; Hill, D.; et al. Secukinumab as a potential trigger of inflammatory bowel disease in ankylosing spondylitis or psoriatic arthritis patients. Rheumatology 2021, 60, 5233–5238. [Google Scholar] [CrossRef]
- Fobelo Lozano, M.J.; Serrano Gimenez, R.; Castro Fernandez, M. Emergence of Inflammatory Bowel Disease During Treatment With Secukinumab. J. Crohn’s Colitis 2018, 12, 1131–1133. [Google Scholar] [CrossRef]
- Hueber, W.; Sands, B.E.; Lewitzky, S.; Vandemeulebroecke, M.; Reinisch, W.; Higgins, P.D.; Wehkamp, J.; Feagan, B.G.; Yao, M.D.; Karczewski, M.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef]
- Soylu, A.; Yildiz, G.; Torun Bayram, M.; Kavukcu, S. IL-1beta blockade in periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: Case-based review. Rheumatol. Int. 2021, 41, 183–188. [Google Scholar] [CrossRef]
- Schedel, J.; Bach, B.; Kummerle-Deschner, J.B.; Kotter, I. Autoinflammatory syndromes/fever syndromes. Hautarzt 2011, 62, 389–401, quiz 402. [Google Scholar] [CrossRef]
- Arnold, D.D.; Yalamanoglu, A.; Boyman, O. Systematic Review of Safety and Efficacy of IL-1-Targeted Biologics in Treating Immune-Mediated Disorders. Front. Immunol. 2022, 13, 888392. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Fornai, M.; Colucci, R.; Benvenuti, L.; D’Antongiovanni, V.; Natale, G.; Fulceri, F.; Giorgis, M.; Marini, E.; Gastaldi, S.; et al. A Comparative Study on the Efficacy of NLRP3 Inflammasome Signaling Inhibitors in a Pre-clinical Model of Bowel Inflammation. Front. Pharmacol. 2018, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Vaja, S.; Subramanian, S.; Brezina, B.; Probert, C.S.; Steel, A.; Lofthouse, M.; Speight, R.A.; Lamb, C.; Sebastian, S.; et al. OP33 Results of a randomised controlled trial to evaluate Interleukin 1 blockade with anakinra in patients with acute severe ulcerative colitis (IASO). J. Crohn’s Colitis 2023, 17 (Suppl. S1), i43–i46. [Google Scholar] [CrossRef]
- Kaly, L.; Rozenbaum, M.; Rimar, D.; Slobodin, G.; Boulman, N.; Awisat, A.; Ginsberg, S.; Jiries, N.; Rosner, I. Ulcerative Colitis and Familial Mediterranean Fever: Can Anakinra Treat Both? ACG Case Rep. J. 2019, 6, e00143. [Google Scholar] [CrossRef] [PubMed]
- Mora-Buch, R.; Dotti, I.; Planell, N.; Calderon-Gomez, E.; Jung, P.; Masamunt, M.C.; Llach, J.; Ricart, E.; Batlle, E.; Panes, J.; et al. Epithelial IL-1R2 acts as a homeostatic regulator during remission of ulcerative colitis. Mucosal Immunol. 2016, 9, 950–959. [Google Scholar] [CrossRef]
- Molgora, M.; Supino, D.; Mantovani, A.; Garlanda, C. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol. Rev. 2018, 281, 233–247. [Google Scholar] [CrossRef]
- Curcic, I.B.; Kizivat, T.; Petrovic, A.; Smolic, R.; Tabll, A.; Wu, G.Y.; Smolic, M. Therapeutic Perspectives of IL1 Family Members in Liver Diseases: An Update. J. Clin. Transl. Hepatol. 2022, 10, 1186–1193. [Google Scholar] [CrossRef]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; Le Maitre, C. Interleukin 1 is a key driver of inflammatory bowel disease-demonstration in a murine IL-1Ra knockout model. Oncotarget 2019, 10, 3559–3575. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, K.J.; Park, J.; Chi, S.; Han, J.; Bang, Y.; Kim, S.M.; Kang, S.G.; Cha, S.H.; Han, Y.H. Disruption of IL-18 signaling via engineered IL-18BP biologics alleviates experimental cholestatic liver disease. Biomed. Pharmacother. 2023, 167, 115587. [Google Scholar] [CrossRef]
- Sivakumar, P.V.; Westrich, G.M.; Kanaly, S.; Garka, K.; Born, T.L.; Derry, J.M.; Viney, J.L. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: Blocking interleukin 18 attenuates intestinal damage. Gut 2002, 50, 812–820. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1beta and IL-18. Biochem. Pharmacol. 2018, 155, 150–161, Erratum in Biochem. Pharmacol. 2024, 222, 116089. [Google Scholar] [CrossRef]
- Mu, J.; Maeda, K.; Ohashi, A.; Urano, T.; Nariai, Y.; Kamino, H.; Nakamura, M.; Yamamura, T.; Sawada, T.; Ishikawa, E.; et al. Monoclonal Antibodies Against Mature Interleukin-18 Ameliorate Colitis and Repair Goblet Cell Function. Dig. Dis. Sci. 2024, 69, 2573–2585. [Google Scholar] [CrossRef]
- Williams, M.A.; O’Callaghan, A.; Corr, S.C. IL-33 and IL-18 in Inflammatory Bowel Disease Etiology and Microbial Interactions. Front. Immunol. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Li, M.; Yu, J.M.; Yang, S.Y.; Zou, L.; Yang, G.J.; Zhang, L.L. IL-33/ST2 axis in diverse diseases: Regulatory mechanisms and therapeutic potential. Front. Immunol. 2025, 16, 1533335. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, M.A.; Pichery, M.; Murdoch, J.R.; Foligne, B.; Ortega, N.; Normand, S.; Mertz, K.; Sanmugalingam, D.; Brault, L.; Grandjean, T.; et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut 2013, 62, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Tsounis, E.P.; Triantos, C. Molecular Mechanisms Underlying IL-33-Mediated Inflammation in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 24, 623. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2017, 2017, 4810258. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zheng, C.; Chen, G.; Shen, Z.; Zheng, S.; Dong, R. Correlation of Interleukin-33/ST2 Receptor and Liver Fibrosis Progression in Biliary Atresia Patients. Front. Pediatr. 2019, 7, 403. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.Y.; Lv, S.; Wang, H.; Gong, M.; Du, N.; Liu, H.; Zhang, N.; Jing, J.; Zhou, C.; et al. Interleukin-33 promotes disease progression in patients with primary biliary cirrhosis. Tohoku J. Exp. Med. 2014, 234, 255–261. [Google Scholar] [CrossRef][Green Version]
- Stute, M.; Kreysing, M.; Zorn, M.; Michl, P.; Gauss, A. Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein. Int. J. Mol. Sci. 2024, 25, 1177. [Google Scholar] [CrossRef]
- Wakai, M.; Hayashi, R.; Tanaka, S.; Naito, T.; Kumada, J.; Nomura, M.; Takigawa, H.; Oka, S.; Ueno, Y.; Ito, M.; et al. Serum amyloid A is a better predictive biomarker of mucosal healing than C-reactive protein in ulcerative colitis in clinical remission. BMC Gastroenterol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Al-Shakhshir, S.; Quraishi, M.N.; Mullish, B.; Patel, A.; Vince, A.; Rowe, A.; Homer, V.; Jackson, N.; Gyimah, D.; Shabir, S.; et al. FAecal micRobiota transplantation in primary sclerosinG chOlangitis (FARGO): Study protocol for a randomised, multicentre, phase IIa, placebo-controlled trial. BMJ Open 2025, 15, e095392. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Caviglia, G.P.; Abdulle, A.; Pellicano, R.; Ditto, M.C.; Morino, M.; Fusaro, E.; Saracco, G.M.; Bugianesi, E.; Astegiano, M. Adalimumab Therapy Improves Intestinal Dysbiosis in Crohn’s Disease. J. Clin. Med. 2019, 8, 1646. [Google Scholar] [CrossRef]
- Li, Z.J.; Gou, H.Z.; Zhang, Y.L.; Song, X.J.; Zhang, L. Role of intestinal flora in primary sclerosing cholangitis and its potential therapeutic value. World J. Gastroenterol. 2022, 28, 6213–6229. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guan, X.X.; Tang, Y.J.; Sun, J.F.; Wang, X.K.; Wang, W.D.; Fan, J.M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Lin, B.; Lei, Y.; Zhang, Y.; Zhang, Y.; Chen, B.; Mao, Q.; Kim, J.J.; Cao, Q. Efficacy of probiotic supplementation and impact on fecal microbiota in patients with inflammatory bowel disease: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2025, 83, e65–e73. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, M.M.; Yuan, Y.; Rodriguez-Lago, I.; Sousa-Pimenta, M.; Dias, C.C.; Barreiro-de Acosta, M.; Jairath, V.; Magro, F. Efficacy and safety of probiotics in IBD: An overview of systematic reviews and updated meta-analysis of randomized controlled trials. United Eur. Gastroenterol. J. 2024, 12, 960–981. [Google Scholar] [CrossRef] [PubMed]
- Vleggaar, F.P.; Monkelbaan, J.F.; van Erpecum, K.J. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study. Eur. J. Gastroenterol. Hepatol. 2008, 20, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 2009, 183, 6251–6261. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, N.; Guo, Y.; Cui, S.; Ge, C.; He, Q.; Pan, X.; Wang, G.; Wang, H.; Hao, H. Combined obeticholic acid and apoptosis inhibitor treatment alleviates liver fibrosis. Acta Pharm. Sin. B 2019, 9, 526–536. [Google Scholar] [CrossRef]
- Fu, T.; Li, Y.; Oh, T.G.; Cayabyab, F.; He, N.; Tang, Q.; Coulter, S.; Truitt, M.; Medina, P.; He, M.; et al. FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc. Natl. Acad. Sci. USA 2022, 119, e2213041119. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Sohal, A.; Kowdley, K.V. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis Therapy Landscape. Am. J. Gastroenterol. 2025, 120, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Spomer, L.; Klindt, C.; Fuchs, K.; Stindt, J.; Deutschmann, K.; Hohne, J.; Liaskou, E.; Hov, J.R.; Karlsen, T.H.; et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. 2021, 75, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Liu, F.; Zhu, X.R.; Suo, F.Y.; Jia, Z.J.; Yao, S.K. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J. Gastroenterol. 2021, 27, 3609–3629. [Google Scholar] [CrossRef]
- Garibay, D.; Zaborska, K.E.; Shanahan, M.; Zheng, Q.; Kelly, K.M.; Montrose, D.C.; Dannenberg, A.J.; Miller, A.D.; Sethupathy, P.; Cummings, B.P. TGR5 Protects Against Colitis in Mice, but Vertical Sleeve Gastrectomy Increases Colitis Severity. Obes. Surg. 2019, 29, 1593–1601. [Google Scholar] [CrossRef]
- Nguyen, O.T.P.; Misun, P.M.; Hierlemann, A.; Lohasz, C. A Versatile Intestine-on-Chip System for Deciphering the Immunopathogenesis of Inflammatory Bowel Disease. Adv. Healthc. Mater. 2024, 13, e2302454. [Google Scholar] [CrossRef]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023, 7, 011502. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, eabq4473. [Google Scholar] [CrossRef]
- Bronson, R.; Lyu, J.; Xiong, J. Transcriptome analysis reveals molecular signature and cell-type difference of Homo sapiens endothelial-to-mesenchymal transition. G3 Genes Genomes Genet. 2023, 13, jkad243. [Google Scholar] [CrossRef]
- Jun, Y.K.; Kim, N.; Yoon, H.; Park, J.H.; Kim, H.K.; Choi, Y.; Lee, J.A.; Shin, C.M.; Park, Y.S.; Lee, D.H. Molecular Activity of Inflammation and Epithelial-Mesenchymal Transition in the Microenvironment of Ulcerative Colitis. Gut Liver 2024, 18, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hikiba, Y.; Hirata, Y.; Font-Burgada, J.; Sakamoto, K.; Hayakawa, Y.; Taniguchi, K.; Umemura, A.; Kinoshita, H.; Sakitani, K.; et al. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
| Agent/Drug | Disease and Specimen | Main Read-Outs | Target/Mechanism | Reference(s) |
|---|---|---|---|---|
| Preclinical studies | ||||
| MCC950 | IBD; Murine | Biochemical markers (i.e., cytokine assessment), disease activity score | Inhibits ASC oligomerization | [158,159] |
| Decursinol Angelate (DA) | IBD; Murine | Biochemical markers (i.e., cytokine, enzyme, and metabolite assessment), histology, gene expression, disease activity score | Suggests disruption of NLRP3–Caspase-1 interaction | [160] |
| Atranorin | IBD; Murine | Biochemical markers (i.e., cytokine, enzyme, barrier, and signaling protein assessment), colon length, intestinal barrier permeability, histology, gene expression, disease activity score | Inhibits ASC oligomerization | [161] |
| Compound 10 v | IBD; Murine | Biochemical markers (i.e., cytokine, inflammasome, and signaling protein assessment), colon length, fecal blood index, body weight loss, histology | Blocks NLRP3-ASC and AIM-2-ASC interaction and STAT1/5 signaling pathways | [162] |
| Belnacasan (VX765) | IBD; Murine | Biochemical markers (i.e., Cytokine, inflammasome and signaling protein assessment), colon length, body weight loss, histology | Caspase-1 mediated pyroptosis suppression | [163] |
| FL-BsAb1/17 | IBD; Murine | Biochemical markers (i.e., cytokine, enzyme, apoptosis protein, metabolite assessment), colon length, histology, apoptosis and cytokine gene expression, disease activity score | Bispecific IL-1β and IL-17 neutralizing antibody | [164] |
| Anakinra | IBD; Murine | Biochemical markers (i.e., cytokine, chemokines, signaling protein assessment), cytokine, chemokine and signaling gene expression, cellular IFNγ T and Foxp3+ Treg cells, histology score, colon length, body weight loss, disease activity index | IL-1R1 blockade, including TNF-independent models | [165] |
| Microcapsules or genetically modified Lactococcus lactis | IBD; Murine | Biochemical markers (i.e., cytokine and enzyme assessment), cellular L-17A positive T cells assessment, body weight loss, disease activity index, histology | IL-1Ra-containing capsules or -secreting microbiota | [166,167] |
| INT-747 | IBD; Murine, enterocyte-like cells, patient-derived lamina propria mononuclear cells, monocytes and dendritic cells | Biochemical markers (i.e., cytokine analysis), body weight loss, epithelial permeability, rectal bleeding, colon length, ulceration status, goblet cell loss, histology (i.e., immune cell infiltration) | Modulation of inflammasome activation by FXR agonism | [168] |
| Obeticholic acid (OCA) containing nanoparticles | PSC; Murine, patient-derived organoids | Biochemical markers (i.e., cytokine, chemokine, signaling protein and enzyme analysis), body weight, histology (i.e., fibrosis, macrophages, ROS levels, apoptosis markers), liver injury score | Modulation of inflammasome activation by FXR agonism | [169] |
| Total astragalus saponins (TAS) | PSC; Murine | Biochemical markers (i.e., cytokine assessment), cholestasis parameter, histology (i.e., collagen deposition, ductular rection and fibrosis) | IL-1β and IL-6 expression downregulation by TGR5 upregulation and reduced NF-κB p65 phosphorylation | [170] |
| Clinical studies | ||||
| Canakinumab | IBD; Human Phase II | Clinical activity index, biochemical markers (i.e., CRP) | IL-1β neutralization | [171] |
| Anakinra | IBD; Human Phase I | Clinical symptoms, histology | IL-1R1 blockade | [172,173] |
| IBD; Human Phase II | Clinical symptoms, histology, endoscopy scoring | [174] | ||
| Obeticholic acid (OCA) | PSC; Human Phase II | Biochemical markers (i.e., ALP) | Modulation of inflammasome activation by FXR agonism | [175,176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losa, M.; Schwarzfischer, M.; Emmenegger, M.; Spalinger, M.R.; Rogler, G.; Scharl, M. Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Int. J. Mol. Sci. 2025, 26, 8042. https://doi.org/10.3390/ijms26168042
Losa M, Schwarzfischer M, Emmenegger M, Spalinger MR, Rogler G, Scharl M. Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. International Journal of Molecular Sciences. 2025; 26(16):8042. https://doi.org/10.3390/ijms26168042
Chicago/Turabian StyleLosa, Marco, Marlene Schwarzfischer, Marc Emmenegger, Marianne R. Spalinger, Gerhard Rogler, and Michael Scharl. 2025. "Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis" International Journal of Molecular Sciences 26, no. 16: 8042. https://doi.org/10.3390/ijms26168042
APA StyleLosa, M., Schwarzfischer, M., Emmenegger, M., Spalinger, M. R., Rogler, G., & Scharl, M. (2025). Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. International Journal of Molecular Sciences, 26(16), 8042. https://doi.org/10.3390/ijms26168042





