Epicardial Adipose Tissue—A Novel Therapeutic Target in Obesity Cardiomyopathy
Abstract
1. Introduction
2. EAT Function Under Physiological Conditions
3. How Does EAT Contribute to the Development of Heart Failure in Obesity?
3.1. Cytokines Secretion and Immune Cells Activation
3.2. Impaired Ventricular Compliance Due to Abnormal Intracardiac Mechanical Interplay
3.3. EAT and Arrhythmogenesis
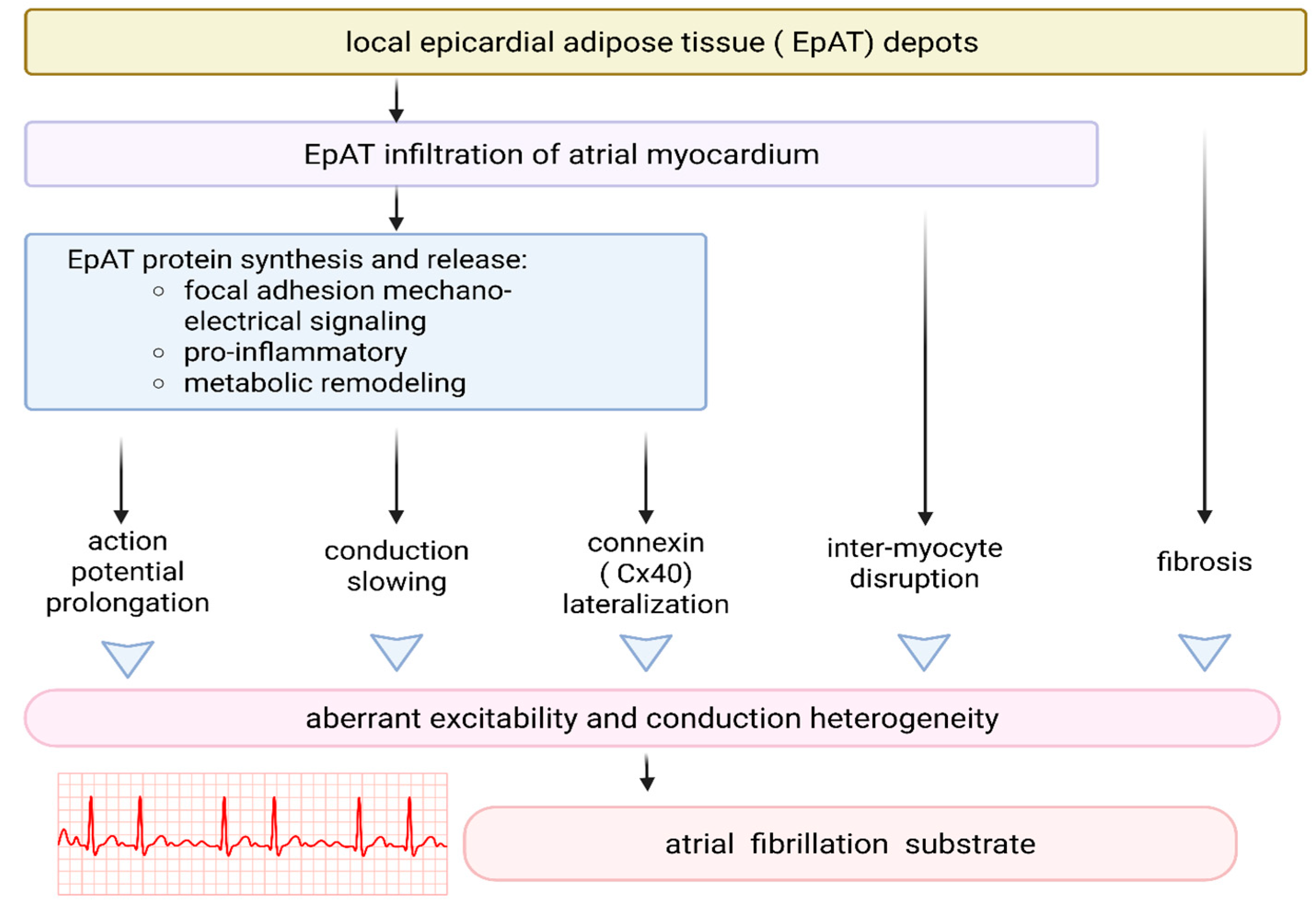
4. Pharmacological Modulation of EAT Function—Potential Implications for the Pathogenesis of Obesity-Related Cardiomyopathy
4.1. Molecular and Functional Pathways Mediating the Effects of GLP-1 and GLP-1/GIP Receptor Agonists on EAT
4.2. Effects of Therapy with GLP-1 vs. Dual GLP-1/GIP Receptor Agonists
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Benn, M.; Marott, S.C.W.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Obesity increases heart failure incidence and mortality: Observational and Mendelian randomization studies totalling over 1 million individuals. Cardiovasc. Res. 2022, 118, 3576–3585. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11. [Google Scholar] [CrossRef]
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; Van Belle, G.; Kessler, R.C. Association between obesity and psychiatric disorders in the US adult population. Arch. Gen. Psychiatry 2006, 63, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, H.; Chen, C.; Pickard, A.S. Cost-effectiveness of pharmacologic treatments for obesity. J. Med. Econ. 2015, 18, 886–897. [Google Scholar] [CrossRef]
- López-Velázquez, J.A.; Silva-Vidal, K.V.; Ponciano-Rodríguez, G.; Chávez-Tapia, N.C.; Arrese, M.; Uribe, M.; Méndez-Sánchez, N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann. Hepatol. 2014, 13, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Knochenhauer, E.S.; Azziz, R. Impact of obesity on the risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 162–168. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Am. J. Gastroenterol. 2005, 100, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk of osteoarthritis in patients with type 2 diabetes mellitus: A population-based cohort study. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef]
- Modena, D.A.O.; Salles, G.F.; Figueiredo Neto, J.A.; Brandão, A.A. Hypertension and menopause: Cardiovascular risk and therapeutic approaches. Rev. Assoc. Med. Bras. 1992, 63, 852–858. [Google Scholar]
- Alpert, M.A.; Alexander, J.K. (Eds.) The Heart and Lung in Obesity, 1st ed.; Futura Publishing Company: Armonk, NY, USA, 1998; pp. 45–56. ISBN 978-0879936770. [Google Scholar]
- Li, C.; Qin, D.; Hu, J.; Yang, Y.; Hu, D.; Yu, B. Inflamed adipose tissue: A culprit underlying obesity and heart failure with preserved ejection fraction. Front. Immunol. 2022, 13, 947147. [Google Scholar] [CrossRef]
- Rana, M.N.; Neeland, I.J. Adipose tissue inflammation and cardiovascular disease: An update. Curr. Diab. Rep. 2022, 22, 27–37. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- González, N.; Moreno-Villegas, Z.; González-Bris, A.; Egido, J.; Lorenzo, Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef]
- Kahn, D.; Macias, E.; Zarini, S.; Garfield, A.; Zemski Berry, K.; MacLean, P.; Gerszten, R.E.; Libby, A.; Solt, C.; Schoen, J.; et al. Exploring visceral and subcutaneous adipose tissue secretomes in human obesity: Implications for metabolic disease. Endocrinology 2022, 163, bqac140. [Google Scholar] [CrossRef]
- Emamat, H.; Jamshidi, A.; Farhadi, A.; Ghalandari, H.; Ghasemi, M.; Tangestani, H. The association between the visceral to subcutaneous abdominal fat ratio and the risk of cardiovascular diseases: A systematic review. BMC Public Health 2024, 24, 1. [Google Scholar] [CrossRef]
- Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; O’Donnel, C.J.; Fox, C.S. Abdominal subcutaneous adipose tissue: A protective fat depot? Diabetes Care 2009, 32, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castell, M.; Samouda, H.; Bocquet, V.; Fagherazzi, G.; Stranges, S.; Huiart, L. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population-based study. Sci. Rep. 2021, 11, 9121. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Karlsson, T.; Rask-Andersen, M.; Pan, G.; Höglund, J.; Wadelius, C.; Ek, W.E.; Johansson, Å. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat. Med. 2019, 25, 1390–1395. [Google Scholar] [CrossRef]
- Neeland, I.J.; Gupta, S.; Ayers, C.R.; Turer, A.T.; Rame, J.E.; Das, S.R.; Berry, J.D.; Khera, A.; McGuire, D.K.; Vega, G.L.; et al. Relation of regional fat distribution to left ventricular structure and function. Circ. Cardiovasc. Imaging 2013, 6, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef] [PubMed]
- McAninch, E.A.; Fonseca, T.L.; Poggioli, R.; Panos, A.L.; Salerno, T.A.; Deng, Y.; Li, Y.; Bianco, A.C.; Iacobellis, G. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity 2015, 23, 1267–1278. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Song, Y.; Tan, Y.; Deng, M.; Shan, W.; Zheng, W.; Zhang, B.; Cui, J.; Feng, L.; Shi, L.; Zhang, M.; et al. Epicardial adipose tissue, metabolic disorders, and cardiovascular diseases: Recent advances classified by research methodologies. MedComm 2023, 4, e413. [Google Scholar] [CrossRef]
- Koepp, K.E.; Obokata, M.; Reddy, Y.N.V.; Olson, T.P.; Borlaug, B.A. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. Heart Fail. 2020, 8, 657–666. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ. Heart Fail. 2021, 15, e009238. [Google Scholar] [CrossRef]
- van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Requena-Ibanez, J.; Devesa, A.; Santos-Gallego, C.G.; Badimon, J.J.; Fuster, V. Uncovering the role of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Adv. 2023, 2, 100657. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Iacobellis, G.; Dozio, E.; Basilico, S.; Di Vincenzo, A.; Dubini, C.; Menicanti, L.; Vianello, E.; Meregalli, C.; Ruocco, C.; et al. Human epicardial adipose tissue expresses glucose-dependent insulinotropic polypeptide, glucagon, and glucagon-like peptide-1 receptors as potential targets of pleiotropic therapies. Eur. J. Prev. Cardiol. 2023, 30, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Camarena, V.; Sant, D.W.; Wang, G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm. Metab. Res. 2017, 49, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Vianello, E.; Malavazos, A.E.; Tacchini, L.; Schmitz, G.; Iacobellis, G.; Genoni, G.; De Lorenzo, R.; Di Gregorio, J.; Corsi Romanelli, M.M.; et al. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int. J. Cardiol. 2019, 292, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Wu, C.-K.; Lee, J.-K.; Hsu, J.-C.; Su, M.-Y.M.; Wu, Y.-F.; Lin, T.-T.; Lan, C.-W.; Hwang, J.-J.; Lin, L.-Y. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 445–454. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; DallegrI, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Arbustini, E.; Labellarte, A.; Sommariva, L.; Pawlowski, T.; Manzoli, A.; Pagano, A.; Motolese, M.; Boccanelli, A. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: A permissive role of epicardial fat? Eur. Heart J. 2003, 24, 329–336. [Google Scholar] [CrossRef]
- Bambace, C.; Telesca, M.; Zoico, E.; Sepe, A.; Olioso, D.; Rossi, A.; Corzato, F.; Di Francesco, V.; Mazzucco, A.; Santini, F.; et al. Adiponectin gene expression and adipocyte diameter: A comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc. Pathol. 2011, 20, e153–e156. [Google Scholar] [CrossRef]
- Barchuk, M.; Dutour, A.; Ancel, P.; Svilar, L.; Miksztowicz, V.; Lopez, G.; Rubio, M.; Schreier, L.; Nogueira, J.P.; Valéro, R.; et al. Untargeted lipidomics reveals a specific enrichment in plasmalogens in epicardial adipose tissue and a specific signature in coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 986–1000. [Google Scholar] [CrossRef]
- Park, H.; He, A.; Lodhi, I.J. Lipid regulators of thermogenic fat activation. Trends Endocrinol. Metab. 2019, 30, 710–723. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M.E. Adult epicardial fat exhibits beige features. J. Clin. Endocrinol. Metab. 2013, 98, E1448–E1455. [Google Scholar] [CrossRef]
- Krishnan, A.; Sharma, H.; Yuan, D.; Trollope, A.F.; Chilton, L. The role of epicardial adipose tissue in the development of atrial fibrillation, coronary artery disease and chronic heart failure in the context of obesity and type 2 diabetes mellitus: A narrative review. J. Cardiovasc. Dev. Dis. 2022, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.A.; Manocha, K.; Cheung, J.W.; Lo, J.C. Linking arrhythmias and adipocytes: Insights, mechanisms, and future directions. Front. Physiol. 2018, 9, 1752. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Adhikari, B.K.; Chen, L.; Liu, W.; Wang, Y.; Zhang, H. The role of epicardial adipose tissue dysfunction in cardiovascular diseases: An overview of pathophysiology, evaluation, and management. Front. Endocrinol. 2023, 14, 1167952. [Google Scholar] [CrossRef]
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.P.; et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation 2021, 143, 2475–2493. [Google Scholar] [CrossRef]
- Michailidou, Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr. Opin. Physiol. 2019, 12, 39–43. [Google Scholar] [CrossRef]
- Mirabelli, M.; Misiti, R.; Sicilia, L.; Brunetti, F.S.; Chiefari, E.; Brunetti, A.; Foti, D.P. Hypoxia in human obesity: New insights from inflammation towards insulin resistance—A narrative review. Int. J. Mol. Sci. 2024, 25, 9802. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Dainty, S.; Strudwick, N.; Mihai, A.D.; Watson, J.N.; Dendooven, R.; Paton, A.W.; Paton, J.C.; Schröder, M. Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface. Mol. Biol. Cell 2020, 31, 2597–2629. [Google Scholar] [CrossRef] [PubMed]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef]
- Kang, G.S.; Jo, H.J.; Lee, Y.R.; Oh, T.; Park, H.-J.; Ahn, G.-O. Sensing the oxygen and temperature in the adipose tissues—who’s sensing what? Exp. Mol. Med. 2023, 55, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Yfantis, A.; Mylonis, I.; Chachami, G.; Nikolaidis, M.; Amoutzias, G.D.; Paraskeva, E.; Simos, G. Transcriptional response to hypoxia: The role of HIF-1-associated co-regulators. Cells 2023, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight 2021, 6, e145495. [Google Scholar] [CrossRef]
- Gruzdeva, O.V.; Akbasheva, O.E.; Dyleva, Y.A.; Antonova, L.V.; Matveeva, V.G.; Uchasova, E.G.; Fanaskova, E.V.; Karetnikova, V.N.; Ivanov, S.V.; Barbarash, O.L.; et al. Adipokine and cytokine profiles of epicardial and subcutaneous adipose tissue in patients with coronary heart disease. Bull. Exp. Biol. Med. 2017, 163, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Vaca, L.; Rossen, R.D.; Durante, W.; Hazarika, P.; Mann, D.L. Cellular basis for the negative inotropic effects of tumor necrosis factor-α in the adult mammalian heart. J. Clin. Invest. 1993, 92, 2303–2312. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Li, Y.Y.; McTiernan, C.F.; Feldman, A.M. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc. Res. 1999, 42, 162–172. [Google Scholar] [CrossRef]
- Harding, D.; Fanti, S.; Marelli-Berg, F. Tumour necrosis factor-α in cardiac inflammation: Friend or foe? Cardiovasc. Res. 2024, 120, 1–2. [Google Scholar] [CrossRef]
- Jacobs, M.; Staufenberger, S.; Gergs, U.; Meuter, K.; Brandstätter, K.; Hafner, M.; Ertl, G.; Schorb, W. Tumor necrosis factor-α at acute myocardial infarction in rats and effects on cardiac fibroblasts. J. Mol. Cell. Cardiol. 1999, 31, 1949–1959. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A.; O’Regan, D.J.; Ball, S.G. Tumor necrosis factor α induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: Inhibition by simvastatin. Cardiovasc. Res. 2004, 64, 507–515. [Google Scholar] [CrossRef]
- Awad, A.E.; Kandalam, V.; Chakrabarti, S.; Wang, X.; Penninger, J.M.; Davidge, S.T.; Oudit, G.Y.; Kassiri, Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am. J. Physiol. Cell Physiol. 2010, 298, C679–C692. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. 4Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Guo, X.; Yin, H.; Li, L.; Chen, Y.; Li, J.; Doan, J.; Steinmetz, R.; Liu, Q. Cardioprotective role of tumor necrosis factor receptor-associated factor 2 by suppressing apoptosis and necroptosis. Circulation 2017, 136, 729–742. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α signaling in heart disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Li, W.; Kang, Y.; Xu, Z.; Li, X.; Gao, Y.; Qi, Y. MAPKs/AP-1, not NF-κB, is responsible for MCP-1 production in TNF-α-activated adipocytes. Adipocyte 2022, 11, 477–486. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Tank, J.; Sossalla, S.; Fluschnik, N.; Hinrichs, S.; Maier, L.; Poller, W.; Blankenberg, S.; Schultheiss, H.P.; et al. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic. Res. Cardiol. 2014, 109, 1–16. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar] [CrossRef]
- Jamil, M.; Cowart, L.A. Sphingolipids in mitochondria—From function to disease. Front. Cell Dev. Biol. 2023, 11, 1302472. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Oh, E.-T.; Park, H.J. A strategy to prevent atherosclerosis via TNF receptor regulation. FASEB J. 2021, 35, e21391. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.H.; Bui, L.; Le, H.D.; Tran, M.T.; Nguyen, T.H.; Le, K.; Pham, T.N. Associations of different inflammatory factors with atherosclerosis among patients with psoriasis vulgaris. Front. Med. 2024, 11, 1396680. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J. Mol. Cell. Cardiol. 2014, 72, 85–94. [Google Scholar] [CrossRef]
- Nicolaou, A.; Zhao, Z.; Northoff, B.H.; Sass, K.; Herbst, A.; Kohlmaier, A.; Chalaris, A.; Wolfrum, C.; Weber, C.; Steffens, S.; et al. ADAM17 deficiency promotes atherosclerosis by enhanced TNFR2 signaling in mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 247–257. [Google Scholar] [CrossRef]
- Jafree, E.; Del Buono, M.G.; Canada, J.M.; Carbone, S.; Kron, J.; Arena, R.; Van Tassell, B.; Abbate, A.; Trankle, C.R. Interleukin-1 inhibition for the prevention and treatment of heart failure. J. Cardiovasc. Pharmacol. 2024, 83, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Geck, P.; Werdan, K.; Boekstegers, P. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: Evidence for primary impairment of mitochondrial function. Mol. Cell. Biochem. 1997, 177, 61–67. [Google Scholar] [CrossRef]
- Crackower, M.A.; Oudit, G.Y.; Kozieradzki, I.; Sarao, R.; Sun, H.; Sasaki, T.; Hirsch, E.; Suzuki, A.; Shioi, T.; Irie-Sasaki, J.; et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 2002, 110, 737–749. [Google Scholar] [CrossRef]
- Moroni, F.; Golino, M.; Carbone, S.; Trankle, C.; Del Buono, M.G.; Talasaz, A.; Arena, R.; Canada, J.M.; Biondi-Zoccai, G.; Van Tassell, B.; et al. Interleukin-1 blockade in heart failure: An on-treatment and off-treatment cardiorespiratory fitness analysis. ESC Heart Fail. 2023, 10, 3199–3202. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The role of interleukin-6 family members in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef]
- Jing, R.; Long, T.Y.; Pan, W.; Li, F.; Xie, Q.Y. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6283–6291. [Google Scholar] [PubMed]
- Alogna, A.; Koepp, K.E.; Sabbah, M.; Espindola Netto, J.M.; Jensen, M.D.; Kirkland, J.L.; Lam, C.S.P.; Obokata, M.; Petrie, M.C.; Ridker, P.M.; et al. Interleukin-6 in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2023, 11, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; März, W.; Niessner, A.; Delgado, G.; Kleber, M.; Scharnagl, H.; Marx, N.; Schuett, K. IL-6 and hsCRP predict cardiovascular mortality in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Schuett, H.; Luchtefeld, M.; Grothusen, C.; Grote, K.; Schieffer, B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 2009, 102, 215–222. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic review on the role of IL-6 and IL-1β in cardiovascular diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Abubakar, M.; Rasool, H.; Javed, I.; Raza, S.; Abang, L.; Hashim, M.M.A.; Saleem, Z.; Abdullah, R.M.; Faraz, M.A.; Hassan, K.M.; et al. Comparative roles of IL-1, IL-6, IL-10, IL-17, IL-18, IL-22, IL-33, and IL-37 in various cardiovascular diseases with potential insights for targeted immunotherapy. Cureus 2023, 15, e42494. [Google Scholar] [CrossRef]
- Aromolaran, K.A.; Corbin, A.; Aromolaran, A.S. Obesity arrhythmias: Role of IL-6 trans-signaling. Int. J. Mol. Sci. 2024, 25, 8407. [Google Scholar] [CrossRef]
- Li, Y.H.; Rozanski, G.J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc. Res. 1993, 27, 525–530. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhang, Y.; Gao, H.; Nattel, S.; Wang, Z. Impairment of hERG K+ channel function by tumor necrosis factor-alpha: Role of reactive oxygen species as a mediator. J. Biol. Chem. 2004, 279, 13289–13292. [Google Scholar] [CrossRef]
- Theodorakis, N.; Kreouzi, M.; Hitas, C.; Anagnostou, D.; Nikolaou, M. Adipokines and cardiometabolic heart failure with preserved ejection fraction: A state-of-the-art review. Diagnostics 2024, 14, 2677. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of leptin in cardiovascular diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.C.; Lord, R.A.; Anderson, G.M. Multiple leptin signaling pathways in the control of metabolism and fertility: A means to different ends? Int. J. Mol. Sci. 2021, 22, 9210. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L. Selective leptin resistance revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R566–R581. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Jurado-López, R.; Valero-Muñoz, M.; Bartolomé, V.M.; Ballesteros, S.; Luaces, M.; Briones, A.M.; López-Andrés, N.; Miana, M.; Cachofeiro, V.; et al. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114. [Google Scholar] [CrossRef]
- Martelli, D.; Brooks, V.L. Leptin increases: Physiological roles in the control of sympathetic nerve activity, energy balance, and the hypothalamic–pituitary–thyroid axis. Int. J. Mol. Sci. 2023, 24, 2684. [Google Scholar] [CrossRef]
- Na, T.; Dai, D.Z.; Tang, X.Y.; Dai, Y. Upregulation of leptin pathway correlates with abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure and reversal by CPU86017. Naunyn-Schmiedebergs Arch. Pharmacol. 2007, 375, 39–49. [Google Scholar] [CrossRef]
- Mellott, E.; Faulkner, J.L. Mechanisms of leptin-induced endothelial dysfunction. Curr. Opin. Nephrol. Hypertens. 2023, 32, 118–123. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The role of adipokines in inflammatory mechanisms of obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Emerging functions of adipokines in linking the development of obesity and cardiovascular diseases. Mol. Biol. Rep. 2020, 47, 7991–8006. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Ortega-Romero, M.; Jiménez-Corona, A.E. Osteopontin’s relationship with malnutrition and oxidative stress in adolescents. A pilot study. PLoS ONE 2021, 16, e0249057. [Google Scholar] [CrossRef]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a link between inflammation and cancer: The thorax in the spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef]
- Cheong, K.I.; Leu, H.B.; Wu, C.C.; Yin, W.H.; Wang, J.H.; Lin, T.H.; Tseng, W.K.; Chang, K.C.; Chu, S.H.; Yeh, H.I.; et al. The clinical significance of osteopontin on the cardiovascular outcomes in patients with stable coronary artery disease. J. Formos. Med. Assoc. 2023, 122, 328–337. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Criales-Vera, S.; Medina-Leyte, D.; Díaz-Zamudio, M.; Flores-Zapata, A.; Cruz-Robles, D.; López-Meneses, M.; Olvera-Cruz, S.; Ramírez-Marroquín, S.; Flores-Castillo, C.; et al. Bone morphogenetic protein-2 and osteopontin gene expression in epicardial adipose tissue from patients with coronary artery disease is associated with the presence of calcified atherosclerotic plaques. Diabetes Metab. Syndr. Obes. 2020, 13, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Cicekli, I.; Saglam, D.; Takar, N. A new perspective on metabolic syndrome with osteopontin: A comprehensive review. Life 2023, 13, 1608. [Google Scholar] [CrossRef]
- Abdalrhim, A.D.; Marroush, T.S.; Austin, E.E.; Gersh, B.J.; Solak, N.; Rizvi, S.A.; Bailey, K.R.; Kullo, I.J. Plasma osteopontin levels and adverse cardiovascular outcomes in the PEACE trial. PLoS ONE 2016, 11, e0156965. [Google Scholar] [CrossRef]
- Li, S.; Gou, T.; Wang, Q.; Chen, M.; Chen, Z.; Xu, M.; Wang, Y.; Han, D.; Cao, R.; Liu, J.; et al. Ultrasound/optical dual-modality imaging for evaluation of vulnerable atherosclerotic plaques with osteopontin targeted nanoparticles. Macromol. Biosci. 2020, 20, e1900279. [Google Scholar] [CrossRef]
- Strobescu-Ciobanu, C.; Giuşcă, S.E.; Căruntu, I.D.; Amălinei, C.; Rusu, A.; Cojocaru, E.; Popa, R.F.; Lupaşcu, C.D. Osteopontin and osteoprotegerin in atherosclerotic plaque—Are they significant markers of plaque vulnerability? Rom. J. Morphol. Embryol. 2020, 61, 793–801. [Google Scholar] [CrossRef]
- Yousefi, K.; Irion, C.I.; Takeuchi, L.M.; Ding, W.; Lambert, G.; Eisenberg, T.; Sukkar, S.; Granzier, H.L.; Methawasin, M.; Lee, D.I.; et al. Osteopontin promotes left ventricular diastolic dysfunction through a mitochondrial pathway. J. Am. Coll. Cardiol. 2019, 73, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz Mohamed, I.; Gadeau, A.P.; Hasan, A.; Abdulrahman, N.; Mraiche, F. Osteopontin: A promising therapeutic target in cardiac fibrosis. Cells 2019, 8, 1558. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and molecular implications of osteopontin in heart failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef]
- López, B.; Ravassa, S.; Moreno, M.U.; González, A.; Beaumont, J.; San José, G.; Querejeta, R.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef]
- Herum, K.M.; Lunde, I.G.; Skrbic, B.; Louch, W.E.; Hasic, A.; Boye, S.; Unger, A.; Brorson, S.H.; Sjaastad, I.; Tønnessen, T.; et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Sano, M. Osteopontin in cardiovascular diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Künzel, S.R.; Hoffmann, M.; Weber, S.; Künzel, K.; Kämmerer, S.; Günscht, M.; Klapproth, E.; Rausch, J.S.E.; Sadek, M.S.; Kolanowski, T.; et al. Diminished PLK2 induces cardiac fibrosis and promotes atrial fibrillation. Circ. Res. 2021, 129, 804–820. [Google Scholar] [CrossRef]
- Lin, R.; Wu, S.; Zhu, D.; Qin, M.; Liu, X. Osteopontin induces atrial fibrosis by activating Akt/GSK-3β/β-catenin pathway and suppressing autophagy. Life Sci. 2020, 245, 117328. [Google Scholar] [CrossRef]
- Soejima, H.; Irie, A.; Fukunaga, T.; Oe, Y.; Kojima, S.; Kaikita, K.; Kawano, H.; Sugiyama, S.; Yoshimura, M.; Kishikawa, H.; et al. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ. J. Off. Jpn. Circ. Soc. 2007, 71, 1879–1884. [Google Scholar] [CrossRef]
- Tardelli, M.; Zeyda, K.; Moreno-Viedma, V.; Wanko, B.; Grün, N.G.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol. Metab. 2016, 5, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (interleukin)-10-STAT3-galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Anzai, A.; Moriyama, H.; Kitakata, H.; Hiraide, T.; Ko, S.; Goto, S.; et al. MerTK expression and ERK activation are essential for the functional maturation of osteopontin-producing reparative macrophages after myocardial infarction. J. Am. Heart Assoc. 2020, 9, e017071. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Zha, Q.; Daniels, C.R.; Steagall, R.J.; Joyner, W.L.; Gadeau, A.P.; Singh, M.; Singh, K. Osteopontin stimulates apoptosis in adult cardiac myocytes via the involvement of CD44 receptors, mitochondrial death pathway, and endoplasmic reticulum stress. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1182–H1191. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Reddy, Y.N.V. The role of the pericardium in heart failure: Implications for pathophysiology and treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Putt, M.E.; Yang, W.; Bertoni, A.G.; Ding, J.; Lima, J.A.C.; Allison, M.A.; Barr, R.G.; Al-Naamani, N.; Patel, R.B.; et al. Association of pericardial fat with cardiac structure, function, and mechanics: The Multi-Ethnic Study of Atherosclerosis. J. Am. Soc. Echocardiogr. 2022, 35, 579–587.e5. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, H.M. Myocardial fat infiltration. Am. Heart J. 1962, 63, 491–496. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Trégouët, D.A.; Pucéat, M.; et al. Reactivation of the epicardium at the origin of myocardial fibro-fatty infiltration during the atrial cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef]
- Krahn, A.D.; Wilde, A.A.M.; Calkins, H.; La Gerche, A.; Cadrin-Tourigny, J.; Roberts, J.D.; Han, H.-C. Arrhythmogenic right ventricular cardiomyopathy. JACC Clin. Electrophysiol. 2022, 8, 533–553. [Google Scholar] [CrossRef]
- Kyriakopoulou, E.; van Kampen, S.J.; Wehrens, M.; Han, S.J.; de Ruiter, H.; Monshouwer-Kloots, J.; Marshall, E.; Brodehl, A.; van der Kraak, P.; te Riele, A.S.J.M.; et al. EPAS1 induction drives myocardial degeneration in desmoplakin-cardiomyopathy. iScience 2025, 28, 111895. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Lacraz, G.P.A.; Vértesy, Á.; van Kampen, S.J.; Perini, I.; de Ruiter, H.; Versteeg, D.; Brodehl, A.; van der Kraak, P.; Giacca, M.; et al. Spatial transcriptomics unveils ZBTB11 as a regulator of cardiomyocyte degeneration in arrhythmogenic cardiomyopathy. Cardiovasc. Res. 2023, 119, 477–491. [Google Scholar] [CrossRef]
- Kanazawa, H.; Yamabe, H.; Enomoto, K.; Koyama, J.; Morihisa, K.; Hoshiyama, T.; Matsui, K.; Ogawa, H. Importance of pericardial fat in the formation of complex fractionated atrial electrogram region in atrial fibrillation. Int. J. Cardiol. 2014, 174, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of cardiac arrhythmogenesis by epicardial adipose tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages facilitate electrical conduction in the heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef]
- Rook, M.B.; Jongsma, H.J.; de Jonge, B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflug. Arch. 1989, 414, 95–98. [Google Scholar] [CrossRef]
- Bentley, D.C.; Pulbutr, P.; Chan, S.; Smith, P.A. Etiology of the membrane potential of rat white fat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E161–E175. [Google Scholar] [CrossRef]
- MacCannell, K.A.; Bazzazi, H.; Chilton, L.; Shibukawa, Y.; Clark, R.B.; Giles, W.R. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys. J. 2007, 92, 4121–4132. [Google Scholar] [CrossRef]
- Kostecki, G.M.; Shi, Y.; Chen, C.S.; Piekarski, B.L.; Zhu, R.; Jiang, Y.; Pu, K.; Majesky, M.W.; Trayanova, N.A.; Tung, L.; et al. Optogenetic current in myofibroblasts acutely alters electrophysiology and conduction of co-cultured cardiomyocytes. Sci. Rep. 2021, 11, 4430. [Google Scholar] [CrossRef]
- Ernault, A.C.; Verkerk, A.O.; Bayer, J.D.; Aras, K.; Montañés-Agudo, P.; Mohan, R.A.; Veldkamp, M.; Rivaud, M.R.; de Winter, R.; Kawasaki, M.; et al. Secretome of atrial epicardial adipose tissue facilitates reentrant arrhythmias by myocardial remodeling. Heart Rhythm. 2022, 19, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; de Winter, R.; Fabrizi, B.; Bracht, J.W.P.; Hau, C.; van Amersfoorth, S.C.M.; Meulendijks, E.R.; Tijsen, A.J.; Cócera Ortega, L.; van der Made, I.; et al. MicroRNAs in extracellular vesicles released from epicardial adipose tissue promote arrhythmogenic conduction slowing. Heart Rhythm O2 2023, 4, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; Rampoldi, A.; Chao, M.; Li, D.; Armand, L.; Hwang, H.; Liu, R.; Jha, R.; Fu, H.; Maxwell, J.T.; et al. Functional and molecular effects of TNF-α on human iPSC-derived cardiomyocytes. Stem Cell Res. 2021, 52, 102218. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef]
- Cornier, M.A. A Review of Current Guidelines for the Treatment of Obesity. Am. J. Manag. Care 2022, 28, 288–296. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2183–2204. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Janssen, L.G.M.; Nahon, K.J.; Bracké, K.F.M.; van den Broek, D.; Smit, R.; Sardjoe Mishre, A.S.D.; Koorneef, L.L.; Martinez-Tellez, B.; Burakiewicz, J.; Kan, H.E.; et al. Twelve weeks of exenatide treatment increases [18F]fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metabolism 2020, 106, 154167. [Google Scholar] [CrossRef]
- Corbin, K.D.; Carnero, E.A.; Allerton, T.D.; Tillner, J.; Bock, C.P.; Luyet, P.P.; Göbel, B.; Hall, K.D.; Parsons, S.A.; Ravussin, E.; et al. Glucagon-like peptide-1/glucagon receptor agonism associates with reduced metabolic adaptation and higher fat oxidation: A randomized trial. Obesity 2023, 31, 350–362. [Google Scholar] [CrossRef]
- von Scholten, B.J.; Persson, F.; Rosenlund, S.; Eugen-Olsen, J.; Pielak, T.; Faber, J.; Hansen, T.W.; Rossing, P. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: A sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes. Metab. 2017, 19, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Daousi, C.; Pinkney, J.H.; Cleator, J.; Wilding, J.P.; Ranganath, L.R. Acute peripheral administration of synthetic human GLP-1 (7–36 amide) decreases circulating IL-6 in obese patients with type 2 diabetes mellitus: A potential role for GLP-1 in modulation of the diabetic pro-inflammatory state? Regul. Pept. 2013, 183, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- Zobel, E.H.; Ripa, R.S.; von Scholten, B.J.; Rotbain Curovic, V.; Kjaer, A.; Hansen, T.W.; Rossing, P.; Størling, J. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 2021, 11, 18522. [Google Scholar] [CrossRef]
- Sudo, M.; Li, Y.; Hiro, T.; Takayama, T.; Mitsumata, M.; Shiomi, M.; Sugitani, M.; Matsumoto, T.; Hao, H.; Hirayama, A.; et al. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: Serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2017, 265, 283–291. [Google Scholar] [CrossRef]
- Kramer, C.M.; Borlaug, B.A.; Zile, M.R.; Ruff, D.; DiMaria, J.M.; Menon, V.; Ou, Y.; Zarante, A.M.; Hurt, K.C.; Murakami, M.; et al. Tirzepatide Reduces LV Mass and Paracardiac Adipose Tissue in Obesity-Related Heart Failure: A SUMMIT CMR Substudy. J. Am. Coll. Cardiol. 2025, 85, 699–706. [Google Scholar] [CrossRef]
- Berg, G.; Barchuk, M.; Lobo, M.; Nogueira, J.P. Effect of glucagon-like peptide-1 (GLP-1) analogues on epicardial adipose tissue: A meta-analysis. Diabetes Metab. Syndr. 2022, 16, 102562. [Google Scholar] [CrossRef]
- Iacobellis, G.; Villasante Fricke, A.C. Effects of semaglutide versus dulaglutide on epicardial fat thickness in subjects with Type 2 diabetes and obesity. J. Endocr. Soc. 2020, 4, bvaa013. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Alkhezi, O.S.; Alahmed, A.A.; Alfayez, O.M.; Alzuman, O.A.; Almutairi, A.R.; Almohammed, O.A. Comparative effectiveness of glucagon-like peptide-1 receptor agonists for the management of obesity in adults without diabetes: A network meta-analysis of randomized clinical trials. Obes. Rev. 2023, 24, e13589. [Google Scholar] [CrossRef]
- Permana, H.; Yanto, T.A.; Hariyanto, T.I. Efficacy and safety of tirzepatide as novel treatment for type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials: Tirzepatide for T2DM. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102640. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; Theodorou, A.; Safouris, A.; Fischer, U.; Kelly, P.J.; Dawson, J.; Katan, M.; Katsanos, A.H.; Lambadiari, V.; et al. Risk of major adverse cardiovascular events and all-cause mortality under treatment with GLP-1 RAs or the dual GIP/GLP-1 receptor agonist tirzepatide in overweight or obese adults without diabetes: A systematic review and meta-analysis. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241281903. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef] [PubMed]


| Function | Cytokines Related with Particular Function |
|---|---|
| Pro-inflammatory, proatherogenic | TNF-α |
| MCP-1 | |
| IL-1, IL-1β, IL-1Ra, IL6, IL8, IL10 CRP PAI-1 Prostaglandin D(2), haptoglobin, α1-glycoprotein, JNK sPLA2-IIA, fatty-acid-binding protein 4 RANTES ICAM | |
| Proliferative factors | NGF |
| FLT-1 | |
| Anti-inflammatory, anti-atherogenic | Adiponectin Adrenomedullin |
| Insulin mimetic, markers of visceral fat | Resistin |
| Visfatin Omentin | |
| Brown fat differentiation transcription factors | PRDM-16 |
| PGC-1α | |
| Thermogenic | UCP-1 |
| Vascular remodeling, blood pressure control, myocardial hypertrophy, adipogenesis | Angiotensin Angiotensinogen Leptin |
| Receptors | Angiotensinogen type 1 receptor |
| TLRs PPAR-γ GLUT-4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszniewski, K.; Grudniewska, A.; Szabłowska-Gadomska, I.; Pilichowska-Paszkiet, E.; Zaborska, B.; Zgliczyński, W.; Dudek, P.; Bik, W.; Sota, M.; Mrozikiewicz-Rakowska, B. Epicardial Adipose Tissue—A Novel Therapeutic Target in Obesity Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 7963. https://doi.org/10.3390/ijms26167963
Wiszniewski K, Grudniewska A, Szabłowska-Gadomska I, Pilichowska-Paszkiet E, Zaborska B, Zgliczyński W, Dudek P, Bik W, Sota M, Mrozikiewicz-Rakowska B. Epicardial Adipose Tissue—A Novel Therapeutic Target in Obesity Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(16):7963. https://doi.org/10.3390/ijms26167963
Chicago/Turabian StyleWiszniewski, Kacper, Anna Grudniewska, Ilona Szabłowska-Gadomska, Ewa Pilichowska-Paszkiet, Beata Zaborska, Wojciech Zgliczyński, Piotr Dudek, Wojciech Bik, Marcin Sota, and Beata Mrozikiewicz-Rakowska. 2025. "Epicardial Adipose Tissue—A Novel Therapeutic Target in Obesity Cardiomyopathy" International Journal of Molecular Sciences 26, no. 16: 7963. https://doi.org/10.3390/ijms26167963
APA StyleWiszniewski, K., Grudniewska, A., Szabłowska-Gadomska, I., Pilichowska-Paszkiet, E., Zaborska, B., Zgliczyński, W., Dudek, P., Bik, W., Sota, M., & Mrozikiewicz-Rakowska, B. (2025). Epicardial Adipose Tissue—A Novel Therapeutic Target in Obesity Cardiomyopathy. International Journal of Molecular Sciences, 26(16), 7963. https://doi.org/10.3390/ijms26167963





