Role of Plant-Derived Smoke Solution on Plants Under Stress
Abstract
1. Introduction
2. Chemical Nature of PDS
2.1. Karrikins
2.2. Other Components
3. Effects of PDS on Seed Germination Parameters
3.1. Effect of PDS Solutions on Germination in Arabidopsis
3.2. Effects of PDS Solutions on Germination in Other Plants
4. Effects of PDS on Plant Growth Parameters
4.1. Effects of PDS Solution on Morphological Growth Attributes of Plant Growth
4.2. Effects of PDS Solution on Physiological and Biochemical Growth Attributes of Plants
5. Effects of PDS Solution on Plant Stress Tolerance
5.1. Biotic Stress Resistance
5.2. Flooding Stress Tolerance
5.3. Salt Stress Tolerance
5.4. Heavy Metal Stress Tolerance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDS | Plant-derived smoke |
| Karrikins | KARs |
| ROS | ROS |
| TMB | 3,4,5-trimethyl-2(5H)-furanone or Trimethyle butenolides |
| ABA | Abscisic acid |
| GA | Gibberellic acid |
References
- Keeley, J.E.; Pausas, J.G. Evolutionary ecology of fire. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 203–225. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Hiers, J.K.; Varner, J.M.; Hoffman, C.M.; Dickinson, M.B.; Michaletz, S.T.; Loudermilk, E.L.; Butler, B.W. Advances in mechanistic approaches to quantifying biophysical fire effects. Curr. For. Rep. 2018, 4, 161–177. [Google Scholar] [CrossRef]
- Armenteras, D.; González, T.M.; Vargas, J.O.; Meza, M.C.; Oliveras, I. Fire in the ecosystems of northern South America: Advances in the ecology of tropical fires in Colombia, Ecuador and Peru. Caldasia 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B. Fire-released seed dormancy—A global synthesis. Biol. Rev. 2022, 97, 1612–1639. [Google Scholar] [CrossRef] [PubMed]
- Abram, N.J.; Henley, B.J.; Gupta, A.S.; Lippmann, T.J.R.; Clarke, H.; Dowdy, A.J.; Sharples, J.J.; Nolan, R.H.; Zhang, T.; Wooster, M.J.; et al. Connections of climate change and variability to large and extreme forest fires in southeast Australia. Commun. Earth. Environ. 2021, 2, 1–17. [Google Scholar] [CrossRef]
- Zubieta, R.; Ccanchi, Y.; Martínez, A.; Saavedra, M.; Norabuena, E.; Alvarez, S.; Ilbay, M. The role of drought conditions on the recent increase in wildfire occurrence in the high Andean regions of Peru. Int. J. Wildland Fire 2023, 32, 531–544. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Ann. Rev. Plant Biol. 2012, 63, 107–130. [Google Scholar] [CrossRef]
- Kamran, M.; Khan, A.L.; Ali, L.; Hussain, J.; Waqas, M.; Al-Harrasi, A.; Lee, I.J. Hydroquinone; a novel bioactive compound from plant-derived smoke can cue seed germination of lettuce. Front. Chem. 2017, 5, 30. [Google Scholar] [CrossRef]
- van Staden, J.; Sparg, S.G.; Kulkarni, M.G.; Light, M.E. Post-germination effects of the smoke-derived compound 3-methyl-2H-furo [2, 3-c] pyran-2-one, and its potential as a preconditioning agent. Field Crops Res. 2006, 98, 98–105. [Google Scholar] [CrossRef]
- Aslam, M.M.; Khatoon, A.; Jamil, M.; Rehman, S.U.; Komatsu, S. Plant-derived smoke solution: A stress alleviator in crop. J. Plant Growth Regul. 2024, 43, 1707–1724. [Google Scholar] [CrossRef]
- Todorovic, S.; Giba, Z.; Zivkovic, S.; Grubisic, D.; Konjevic, R. Stimulation of empress tree seed germination by liquid smoke. J. Plant Growth Regul. 2005, 47, 141–148. [Google Scholar] [CrossRef]
- Khatoon, A.; Rehman, S.U.; Aslam, M.M.; Jamil, M.; Komatsu, S. Plant-derived smoke affects biochemical mechanism on plant growth and seed germination. Int. J. Mol. Sci. 2020, 21, 7760. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.C.; van Staden, J.; Daws, M.I.; Johnson, T. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. S. Afr. J. Bot. 2003, 69, 514–525. [Google Scholar] [CrossRef]
- Gardner, M.J.; Dalling, K.J.; Light, M.E.; Jäger, A.K.; van Staden, J. Does smoke substitute for red light in the germination of light-sensitive lettuce seeds by affecting gibberellin metabolism? S. Afr. J. Bot. 2001, 67, 636–640. [Google Scholar] [CrossRef]
- Rehman, S.U.; Khatoon, A.; Aslam, M.M.; Jamil, M.; Komatsu, S. Proteomic and biochemical research for exploring the role of plant-derived smoke in food crops. In Advanced Crop Improvement, Volume 2: Case Studies of Economically Important Crops; Raina, A., Wani, M.R., Laskar, R.A., Tomlekova, N., Khan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 457–476. [Google Scholar]
- Malook, I.; Shah, G.; Jan, M.; Shinwari, K.I.; Aslam, M.M.; Rehman, S.U.; Jamil, M. Smoke priming regulates growth and the expression of myeloblastosis and zinc-finger genes in rice under salt stress. Arab. J. Sci. Eng. 2017, 42, 2207–2215. [Google Scholar] [CrossRef]
- Aslam, M.M.; Jamil, M.; Khatoon, A.; El-Hendawy, S.E.; Al-Suhaibani, N.A.; Shakir, K.S.; Malook, I.; Rehman, S.U. Does weeds-derived smoke improve plant growth of wheat? J. Bio Mol. Sci. 2015, 3, 86–96. [Google Scholar]
- Aslam, M.M.; Jamil, M.; Khatoon, A.; El-Hendawy, S.E.; Al-Suhaibani, N.A.; Malook, I.; Rehman, S.U. Physiological and biochemical responses of maize (Zea mays L.) to plant derived smoke solution. Pak. J. Bot. 2017, 49, 435–443. [Google Scholar]
- Kulkarni, M.G.; Ascough, G.D.; van Staden, J. Effects of foliar applications of smoke-water and a smoke-isolated butenolide on seedling growth of okra and tomato. Hort. Sci. 2007, 42, 179–182. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Sparg, S.G.; van Staden, J. Germination and post germination response of Acacia seeds to smoke-water and butenolide, a smoke derived compound. J. Arid Environ. 2007, 69, 177–187. [Google Scholar] [CrossRef]
- Aslam, M.M.; Rehman, S.; Khatoon, A.; Jamil, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Li, X.; Sunohara, Y.; Matsumoto, H.; et al. Molecular responses of maize shoot to a plant derived smoke solution. Int. J. Mol. Sci. 2019, 20, 1319. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Akhter, A.; Jamil, M.; Khatoon, A.; Malook, I.; Rehman, S.U. Effect of plant-derived smoke solution on root of Ipomoea marguerite cuttings under cobalt stress. J. Bio Mol Sci. 2014, 2, 6–11. [Google Scholar]
- Kumari, A.; Papenfus, H.B.; Kulkarni, M.G.; Pošta, M.; van Staden, J. Effect of smoke derivatives on in vitro pollen germination and pollen tube elongation of species from different plant families. Plant Biol. 2015, 17, 825–830. [Google Scholar] [CrossRef]
- Jamil, M.; Kanwal, M.; Aslam, M.M.; Khan, S.U.; Malook, I.; Tu, J.; Rehman, S.U. Effect of plant-derived smoke priming on physiological and biochemical characteristics of rice under salt stress condition. Aust. J. Crop Sci. 2014, 8, 159–170. [Google Scholar]
- Rehman, A.; Rehman, S.U.; Khatoon, A.; Qasim, M.; Itoh, T.; Iwasaki, Y.; Wang, X.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the promotive effect of plant-derived smoke on plant growth of chickpea. J. Proteom. 2018, 176, 56–70. [Google Scholar] [CrossRef]
- Li, X.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Yamaguchi, T.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on soybean during recovery from flooding stress. J. Proteom. 2018, 181, 238–248. [Google Scholar] [CrossRef]
- Zhong, Z.; Kobayashi, T.; Zhu, W.; Imai, H.; Zhao, R.; Ohno, T.; Komatsu, S. Plant-derived smoke enhances plant growth through ornithine-synthesis pathway and ubiquitin-proteasome pathway in soybean. J. Proteom. 2020, 221, 103781. [Google Scholar] [CrossRef] [PubMed]
- Otori, M.; Murashita, Y.; Rehman, S.; Komatsu, S. Proteomic study to understand promotive effects of plant-derived smoke on soybean root growth under flooding stress. Plant Mol. Biol. Rep. 2021, 39, 24–33. [Google Scholar] [CrossRef]
- Komatsu, S.; Kimura, T.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K. Proteomic analysis reveals salt-tolerant mechanism in soybean applied with plant-derived smoke solution. Int. J. Mol. Sci. 2023, 24, 13734. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Rehman, S.U.; Ohno, T. Morphological, biochemical, and proteomic analyses to understand the promotive effects of plant-derived smoke solution on wheat growth under flooding stress. Plants 2022, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Diniyah, A.; Zhu, W.; Nakano, M.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K. Metabolomic and proteomic analyses to reveal the role of plant-derived smoke solution on wheat under salt stress. Int. J. Mol. Sci. 2024, 25, 8216. [Google Scholar] [CrossRef]
- Gupta, S.; Plackova, L.; Kulkarni, M.G.; Dolezal, K.; van Staden, J. Role of smoke stimulatory and inhibitory biomolecules in phytochrome-regulated seed germination of Lactuca sativa. J. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef]
- Aremu, A.O.; Kulkarni, M.G.; Bair, M.O.; Finnie, J.F.; van Staden, J. Growth stimulation effects of smoke-water and vermin-compost leachate on greenhouse grown-tissue cultured ‘Williams’ bananas. J. Plant Growth Regul. 2012, 66, 111–118. [Google Scholar] [CrossRef]
- Chumpookam, J.; Lin, H.L.; Shiesh, C.C. Effect of smoke-water on seed germination and seedling growth of papaya (Carica papaya cv. Tainung No. 2). Hort. Sci. 2012, 47, 741–744. [Google Scholar] [CrossRef]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.G.; Yun, B.W. Abiotic stress in plants; stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy 2021, 11, 1579. [Google Scholar] [CrossRef]
- Fich, E.A.; Segerson, N.A.; Rose, J.K. The plant polyester cutin: Biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.A.; Jamil, M.; Khan, M.D.; Shakir, S.K.; Rehman, S.U. Effect of plant-derived smoke solutions on physiological and biochemical attributes of maize (Zea mays L.) under salt stress. Pak. J. Bot. 2016, 48, 1763–1774. [Google Scholar]
- Hayat, N.; Afroz, N.; Rehman, S.; Bukhari, S.H.; Iqbal, K.; Khatoon, A.; Nawaz, G. Plant-derived smoke ameliorates salt stress in wheat by enhancing expressions of stress-responsive genes and antioxidant enzymatic activity. Agronomy 2021, 12, 28. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Malook, I.; Rehman, S.U.; Jamil, M. Pb-induced changes in roots of two cultivated rice cultivars grown in lead-contaminated soil mediated by smoke. J. Environ. Sci. Pollut. Res. 2017, 24, 21298–21310. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 97. [Google Scholar] [CrossRef]
- Nelson, D.C.; Riseborough, J.-A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef]
- Nelson, D.C.; Scaffidi, A.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 8897–8902. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef]
- Waters, M.T.; Smith, S.M. KAI2- and MAX2-mediated responses to karrikins and strigolactones are largely independent of HY5 in Arabidopsis seedlings. Mol. Plant 2013, 6, 63–75. [Google Scholar] [CrossRef]
- Stanga, J.P.; Smith, S.M.; Briggs, W.R.; Nelson, D.C. Suppressor of more axillary growth2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 2013, 163, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ruduś, I.; Cembrowska-Lech, D.; Jaworska, A.; Kepczynski, J. Involvement of ethylene biosynthesis and perception during germination of dormant Avena fatua L. caryopses induced by KAR1 or GA3. Planta 2019, 249, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.; Riaz, M.W.; Zhou, X.; Zhu, Z.; Zhou, K. Alleviating dormancy in Brassica oleracea seeds using NO and KAR1 with ethylene biosynthetic pathway, ROS and antioxidant enzymes modifications. BMC Plant Biol. 2019, 19, 577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Z.-X.; Sun, H.; Guo, L.-P. Smoke-isolated karrikins stimulated Tanshinones biosynthesis in Salvia miltiorrhiza through endogenous Nitric Oxide and Jasmonic Acid. Molecules 2019, 24, 1229. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; van Staden, J. Seed priming with smoke water and karrikin improves germination and seedling vigor of Brassica napus under varying environmental conditions. Plant Growth Regul. 2022, 97, 315–326. [Google Scholar] [CrossRef]
- Posta, M.; Light, M.E.; Papenfus, H.B.; van Staden, J.; Kohout, L. Structure–activity relationships of analogs of 3,4,5-trimethylfuran-2(5H)-one with germination inhibitory activities. J Plant Physiol. 2013, 170, 1235–1242. [Google Scholar] [CrossRef]
- Light, M.E.; Burger, B.V.; Staerk, D.; Kohout, L.; van Staden, J. Butenolides from plant-derived smoke: Natural plant-growth regulators with antagonistic actions on seed germination. J. Nat. Prod. 2010, 73, 267–269. [Google Scholar] [CrossRef]
- Nair, J.J.; Pošta, M.; Papenfus, H.B.; Munro, O.Q.; Beier, P.; van Staden, J. Synthesis, X-ray structure determination and germination studies on some smoke-derived karrikins. S. Afr. J Bot. 2014, 91, 53–57. [Google Scholar] [CrossRef]
- Burger, B.V.; Postac, M.; Light, M.E.; Kulkarni, M.G.; Viviersa, M.Z.; van Staden, J. More butenolides from plant-derived smoke with germination inhibitory activity against karrikinolide. S. Afr. J. Bot. 2018, 115, 256–263. [Google Scholar] [CrossRef]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and promote seed germination. Nat. Commun. 2011, 2, 360. [Google Scholar] [CrossRef]
- Wang, M.; Schoettner, M.; Xu, S.; Paetz, C.; Wilde, J.; Baldwin, I.T.; Groten, K. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. New Phytol. 2017, 213, 1755–1770. [Google Scholar] [CrossRef]
- Muzaffar, S.; Ali, B.; Wani, N.A. Effect of catechol, gallic acid and pyrogallic acid on the germination, seedling growth and the level of endogenous phenolics in cucumber (Cucumis sativus L.). Int. J. Life Sci. Biotech. Pharm. Res. 2012, 1, 1–6. [Google Scholar]
- Khan, M.M.A.; Afreen, R.; Quasar, N.; Khanam, N.; Uddin, M. Steam-mediated foliar application of catechol and plant growth regulators enhances the growth attributes, photosynthesis, and essential oil production of lemongrass (Cymbopogon flexuosus Steud.). Biocatal. Agric. Biotechnol. 2023, 48, 102638. [Google Scholar] [CrossRef]
- Flematti, G.R.; Waters, M.T.; Scaffidi, A.; Merritt, D.J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Mol. Plant 2013, 6, 29–37. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D.; Skelton, B.W.; White, A.H. Structural analysis of a potent seed germination stimulant. Aust. J. Chem. 2005, 58, 505–506. [Google Scholar] [CrossRef]
- Meng, Y.; Shuai, H.; Luo, X.; Chen, F.; Zhou, W.; Yang, W.; Shu, K. Karrikins: Regulators involved in phytohormone signaling networks during seed germination and seedling development. Front. Plant Sci. 2017, 7, 2021. [Google Scholar] [CrossRef] [PubMed]
- Kepczynski, J.; Kepczynska, E. Plant-derived smoke and Karrikin 1 in seed priming and seed biotechnology. Plants 2023, 12, 2378. [Google Scholar] [CrossRef]
- Xu, P.; Hu, J.; Chen, H.; Cai, W. SMAX1 interacts with DELLA protein to inhibit seed germination under weak light conditions via gibberellin biosynthesis in Arabidopsis. Cell Rep. 2023, 42, 112740. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Rehman, N.U.; Yu, S.; Zhou, Y.; Haq, B.U.; Wang, J.; Li, P.; Zeng, Z.; Zhao, J. GmMAX2–D14 and –KAI interaction-mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. 2020, 101, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wang, H.; Qiu, Y.; Wang, D.; Xiu, Y.; Zhang, Y.; Pei, M.; Zhao, Y.; Xu, X.; Hang, H. The multifaceted impact of Karrikin signaling in plants. Int. J. Mol. Sci. 2025, 26, 2775. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, S.; Das, D.; Varshney, K.; Kolodziej, M.C.; Villaécija-Aguilar, J.A.; Gutjahr, C. The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis. Proc. Natl. Acad Sci. USA 2020, 117, 21757–21765. [Google Scholar] [CrossRef] [PubMed]
- Villaécija-Aguilar, J.A.; Körösy, C.; Maisch, L.; Hamon-Josse, M.; Petrich, A.; Magosch, S.; Chapman, P.; Bennett, T.; Gutjahr, C. KAI2 promotes Arabidopsis root hair elongation at low external phosphate by controlling local accumulation of AUX1 and PIN2. Curr. Biol. 2022, 32, 228–236. [Google Scholar] [CrossRef]
- Villaécija-Aguilar, J.A.; Hamon-Josse, M.; Carbonnel, S.; Kretschmar, A.; Schmidt, C.; Dawid, C.; Bennett, T.; Gutjahr, C. SMAX1/SMXL2 regulate root and root hair development downstream of KAI2-mediated signalling in Arabidopsis. PLoS Genet. 2019, 15, e1008327. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. J. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Salisbury, F.B., Ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014; pp. 1–761. [Google Scholar]
- Pratyusha, S. Phenolic compounds in the plant development and defense. In Plant Stress Physiology: Plant Stress Physiology—Perspectives in Agriculture; IntechOpen: London, UK, 2022; pp. 125–139. [Google Scholar]
- Charrouf, Z.; Guillaume, D. Phenols and polyphenols from Argania spinosa. Am. J. Food Technol. 2007, 2, 679–683. [Google Scholar] [CrossRef]
- Yam, K.C.; D’Angelo, I.; Kalscheuer, R.; Zhu, H.; Wang, J.X.; Snieckus, V.; Eltis, L.D. Studies of a ring-cleaving dioxygenase illuminate the role of cholesterol metabolism in the pathogenesis of Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000344. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, C.; Shu, C.; Huang, Y.; Yang, M.; Zhou, E. Effects of catechol on growth, antioxidant enzyme activities and melanin biosynthesis gene expression of Rhizoctonia solani AG-1 IA. Can. J. Plant Pathol. 2018, 40, 220–228. [Google Scholar] [CrossRef]
- Lee, I.; Kim, E.; Choi, S.; Kim, D.; Hong, W.; Choi, J.; Choi, H.; Kim, J.; Sable, J.A.; Markkandan, K.; et al. A Raf-like kinase is required for smoke-induced seed dormancy in Arabidopsis thaliana. Plant Biol. 2021, 118, e2020636118. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.W.; Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L. Karrikinolide–a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Tavsanoǧlu, C.; Ergan, G.; Çatav, S.S.; Zare, G.; Küçükakyüz, K.; Özüdoǧru, B. Multiple fire-related cues stimulate germination in Chaenorhinum rubrifolium (Plantaginaceae), a rare annual in the Mediterranean Basin. Seed Sci. Res. 2017, 27, 26–38. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Elgin, E.S.; Dağ, Ç.; Stark, J.L.; Küçükakyüz, K. NMR-based metabolomics reveals that plant-derived smoke stimulates root growth via affecting carbohydrate and energy metabolism in maize. Metabolomics 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Mathnoom, S.N.; Al-Timmen, W.M.A. The effect of smoke water extract on endogenous phytohormones of Cucumis sativus L. seeds exposed to salt stress. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 1–11. [Google Scholar]
- Shah, F.A.; Wei, X.; Wang, Q.; Liu, W.; Wang, D.; Yao, Y.; Wu, L. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. J. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Demir, I.; Light, M.E.; van Staden, J.; Kenanoglu, B.B.; Celikkol, T. Improving seedling growth of unaged and aged aubergine seeds with smoke-derived butenolides. Seed Sci. Technol. 2009, 37, 255–260. [Google Scholar] [CrossRef]
- Tieu, A.; Dixon, K.A.; Sivasithamparam, K.; Plummer, J.A. Germination of four species of native Western Australian plant using plant-derived smoke. Aust. J. Bot. 1999, 47, 207–219. [Google Scholar] [CrossRef]
- Mavi, K.; Light, M.E.; Demir, I.; van Staden, J.; Yasar, F. Positive effect of smoke-derived butenolide priming on melon seedling emergence and growth. N. Z. J. Crop Hortic. Sci. 2010, 38, 147–155. [Google Scholar] [CrossRef]
- Sparg, S.G.; Kulkarni, M.G.; Light, M.E.; van Staden, J. Improving seedling vigour of indigenous medicinal plants with smoke. Bioresour. Technol. 2005, 96, 1323–1330. [Google Scholar] [CrossRef]
- Merritt, D.J.; Kristiansen, M.; Flematti, G.R.; Turner, S.R.; Ghisalberti, E.L.; Trengove, R.D.; Dixon, K.W. Effects of a butenolide present in smoke on light-mediated germination of Australian Asteraceae. Seed Sci. Res. 2006, 16, 29–35. [Google Scholar] [CrossRef]
- Daws, M.I.; Pritchard, H.W.; van Staden, J. Butenolide from plant-derived smoke functions as a strigolactone analogue: Evidence from parasitic weed seed germination. S. Afr. J. Bot. 2008, 74, 116–120. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Mershad, M.R.; Asl, A.M.; Sepehri, A. Plant-derived smoke solution and potassium nitrate affect seed germination and seed vigour in four medicinal plant species. J. Bodenkultur. 2010, 61, 5–12. [Google Scholar]
- Clarke, S.; French, K. Germination response to heat and smoke of 22 Poaceae species from grassy woodlands. Aust. J. Bot. 2005, 53, 445–454. [Google Scholar] [CrossRef]
- El-Nour, H.H.B. Effects of smoke water on seeds germination, seedling growth of some vegetables and green yield productivity of Phaseolus vulgaris. Al-Azhar J. Agric. Res. 2021, 46, 124–138. [Google Scholar] [CrossRef]
- Jiang, Z.; Verhoeven, A.; Li, Y.; Geertsma, R.; Sasidharan, R.; van Zanten, M. Deciphering acclimation to sublethal combined and sequential abiotic stresses in Arabidopsis thaliana. Plant Physiol. 2024, kiae581. [Google Scholar] [CrossRef] [PubMed]
- Antala, M.; Sytar, O.; Rastogi, A.; Brestic, M. Potential of Karrikins as novel plant growth regulators in agriculture. Plants 2019, 9, 43. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Abdelrahman, M.; Rahman, M.M.; Tran, C.D.; Nguyen, K.H.; Watanabe, Y.; Itouga, M.; Li, W.; Wang, Z.; Mochida, K.; et al. Karrikin receptor KAI2 coordinates salt tolerance mechanisms in Arabidopsis thaliana. Plant Cell Physiol. 2022, 63, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Hurkan, K.Y.; Aki, C. Preparation of plant-derived smoke for stimulating seed germination and quantification of karrikins using high performance liquid chromatography. J. Agric. Sci. 2023, 29, 800–810. [Google Scholar]
- Wang, L.; Xu, Q.; Yu, H.; Ma, H.; Li, X.; Yang, J.; Wang, B. Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis. Plant Cell 2020, 32, 2251–2270. [Google Scholar] [CrossRef]
- Sparg, S.G.; Kulkarni, M.G.; van Staden, J. Aerosol smoke and smoke-water stimulation of seedling vigor of a commercial maize cultivar. J. Crop Sci. 2006, 46, 1336–1340. [Google Scholar] [CrossRef]
- Doherty, L.C.; Cohn, M.A. Seed dormancy in red rice (Oryza sativa L.). XI. Commercial liquid smoke elicits germination. Seed Sci. Res. 2000, 10, 415–421. [Google Scholar] [CrossRef]
- Momin, A.; Naheed, R.; Jamil, M.; Rehman, S.U.; Khatoon, A.; Rha, E.S. Combined effect of silicon dioxide nanoparticles and plant-derived smoke solution on physiological and biochemical parameters of pea plant (Pisum sativum L.). Int. J. Appl. Exp. Biol. 2024, 3, 227–237. [Google Scholar] [CrossRef]
- Ullah, G.; Ibrahim, M.; Nawaz, G.; Khatoon, A.; Jamil, M.; Rehman, S.U.; Tariq, A. Plant-derived smoke mitigates the inhibitory effects of the auxin inhibitor 2, 3, 5-triiodo benzoic acid (TIBA) by enhancing root architecture and biochemical parameters in maize. Plants 2023, 12, 2604. [Google Scholar] [CrossRef]
- Shah, G.; Tu, J.; Fayyaz, M.; Masood, S.; Ullah, H.; Jamil, M. Moringa oleifera smoke induced positive changes in biochemical, metabolic, and antioxidant profile of rice seedling under cadmium stress. Int. J. Phytoremediat. 2023, 25, 1337–1347. [Google Scholar] [CrossRef]
- Singh, G.M.; Zhang, F.; Schaefer, D.A.; Goldberg, S.; Xu, J. Smoke induced seed germination in maize in response to self and other plants biomass-derived smoke. Ecol. Environ. Conserv. 2023, 28, 288–293. [Google Scholar]
- Taylor, J.L.S.; van Staden, J. Root initiation in Vigna radiates (L.) Wilczek hypocotyls cuttings are stimulated by smoke-derived extracts. J. Plant Growth Regul. 1996, 18, 165–168. [Google Scholar] [CrossRef]
- Jain, N.; van Staden, J. A plant-derived smoke improves early growth of tomato seedlings. J. Plant Growth Regul. 2006, 50, 139–148. [Google Scholar] [CrossRef]
- Kulkurni, M.G.; Ascough, G.D.; van Staden, J. Smoke-water and smoke-isolated butenolide improve growth and yield of tomatoes under greenhouse conditions. Hortic. Technol. 2008, 18, 449–454. [Google Scholar] [CrossRef]
- Jamil, M.; Malook, I.; Parveen, S.; Naz, T.; Ali, A.; Rehman, S.U. Smoke priming, a potent protective agent against salinity: Effect on proline accumulation, elemental uptake, pigmental attributes and protein banding patterns of rice (Oryza sativa). J. Stress. Physiol. Biochem. 2013, 169–183. [Google Scholar]
- Pinit, S.; Ariyakulkiat, L.; Chaiwanon, J. Rice straw-derived smoke water promotes rice root growth under phosphorus deficiency by modulating oxidative stress and photosynthetic gene expression. Sci. Rep. 2014, 13, 14802. [Google Scholar]
- Aremu, A.O.; Masondo, N.A.; van Staden, J. Smoke–water stimulates secondary metabolites during in vitro seedling development in Tulbaghia species. S. Afr. J. Bot. 2014, 91, 49–52. [Google Scholar] [CrossRef]
- Soos, V.; Sebestyen, E.; Juhasz, A.; Pinter, J.; Light, M.E.; van Staden, J.; Balazs, E. Stress-related genes define essential steps in the response of maize seedlings to smoke-water. Funct. Integr. Genomics. 2009, 9, 231–242. [Google Scholar] [CrossRef]
- Tan, Z.Z.; Wang, Y.T.; Zhang, X.X.; Jiang, H.Y.; Li, Y.; Zhuang, L.L.; Yang, Z.M. Karrikin1 enhances drought tolerance in creeping bentgrass in association with antioxidative protection and regulation of stress-responsive gene expression. Agronomy 2023, 13, 675. [Google Scholar] [CrossRef]
- Elsadek, M.A.; Yousef, E.A. Smoke-water enhances germination and seedling growth of four horticultural crops. Plants 2019, 8, 104. [Google Scholar] [CrossRef]
- Basaran, U.; Dogrusoz, M.C.; Gulumser, E.; Mut, H. Using smoke solutions in grass pea (Lathyrus sativus L.) to improve germination and seedling growth and reduce toxic compound ODAP. Turk. J. Agric. For. 2019, 43, 518–526. [Google Scholar] [CrossRef]
- Asaf, S.; Imran, Q.M.; Jan, R.; Lubna, L.; Khatoon, A.; Young, J.H.; Rehman, S. Plant derived smoke promotes seed germination and alleviates auxin stress in carrot. J. Agric. Biol. Sci. 2014, 9, 308–314. [Google Scholar]
- Shah, A.A.; Khan, W.U.; Yasin, N.A.; Akram, W.; Ahmad, A.; Abbas, M.; Safdar, M.N. Butanolide alleviated cadmium stress by improving plant growth, photosynthetic parameters and antioxidant defense system of Brassica oleracea. Chemosphere 2020, 261, 127728. [Google Scholar] [CrossRef] [PubMed]
- Shabir, S.; Ilyas, N.; Asif, S.; Iqbal, M.; Kanwal, S.; Ali, Z. Deciphering the role of plant-derived smoke solution in ameliorating saline stress and improving physiological, biochemical, and growth responses of wheat. J. Plant Growth Regul. 2022, 41, 2769–2786. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Q.; Zhou, J.; Guo, L. Metabolomics reveals the influences of smoke-water and karrikinolide on the biosynthesis of flavonoids and terpenoids in Salvia miltiorrhiza. Funct. Plant Biol. 2021, 48, 321–332. [Google Scholar] [CrossRef]
- van Staden, J.; Jager, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Sreekissoon, A.; Finnie, J.F.; van Staden, J. Effects of smoke water on germination, seedling vigour and growth of Sceletium tortupsum. S Afr. J. Bot. 2021, 139, 427–431. [Google Scholar] [CrossRef]
- Demir, I.; Ozuaydin, F.; Yasar, J.; van Staden, J. Effect of smoke-derived butenolide priming treatment on pepper and salvia seeds in relation to transplant quality and catalase activity. S. Afr. J. Bot. 2012, 78, 83–87. [Google Scholar] [CrossRef]
- Khan, P.; Rehman, S.; Jamil, M.; Irfan, S.; Waheed, M.A.; Aslam, M.M.; Kanwal, M.; Shakir, S.K. Alleviation of boron stress through plant derived smoke extracts in Sorghum bicolor. J. Stress Physiol. Biochem. 2014, 10, 153–165. [Google Scholar]
- Iqbal, M.; Asif, S.; Ilyas, N.; Raja, N.I.; Hussain, M.; Sabir, S.; Faz, M.N.A.; Rauf, A. Effect of plant derived smoke on germination and post germination expression of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 806–813. [Google Scholar] [CrossRef]
- Kamran, M.; Khan, A.L.; Waqas, M.; Imran, Q.M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Kim, M.J.; Lee, I.J. Effects of plant-derived smoke on the growth dynamics of Barnyard Grass (Echinochloa crus-galli). Acta Agric. Scand.—B Soil Plant Sci. 2014, 64, 121–128. [Google Scholar] [CrossRef]
- Murashita, Y.; Nishiuchi, T.; Rehman, S.U.; Komatsu, S. Subcellular proteomics to understand promotive effect of plant-derived smoke solution on soybean root. J. Proteomes 2021, 9, 39. [Google Scholar] [CrossRef]
- Bell, T.L.; Stephens, S.; Moritz, M.A. Short-term physiological effects of smoke on grapevine leaves. Int. J. Wildland Fire 2013, 22, 933–946. [Google Scholar] [CrossRef]
- Salomon, M.V.; Piccoli, P.; Pinter, I.F.; Stirk, W.A.; Kulkarni, M.; van Staden, J.; Bottini, R. Bacteria and smoke-water extract improve growth and induce the synthesis of volatile defense mechanisms in Vitis vinifera L. J. Plant Physiol. Biochem. 2017, 120, 1–9. [Google Scholar] [CrossRef]
- Khan, M.H.U.; Khattak, J.Z.K.; Jamil, M.; Malook, I.; Khan, S.U.; Jan, M.; Din, I.; Saud, S.; Kamran, M.; Alharby, H.; et al. Bacillus safensis with plant-derived smoke stimulates rice growth under saline conditions. Environ. Sci. Pollut. Res. 2017, 24, 23850–23863. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Huang, J. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Kucukakyuz, K.; Catav, S.S. Physiological effects of smoke-water and Karrikinolide on wheat seedlings grown under boron stress. Russ. J. Plant Physiol. 2021, 68, 552–558. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nawaz, S.; Iqbal, K.; Rehman, S.; Ullah, R.; Nawaz, G.; Peluso, I. Plant-derived smoke solution alleviates cellular oxidative stress caused by arsenic and mercury by modulating the cellular antioxidative defense system in wheat. Plants 2022, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Melville, K.T.; Waters, M.T. Karrikin signalling: Impacts on plant development and abiotic stress tolerance. J. Exp. Bot. 2024, 75, 1174–1186. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Ha, C.V.; Watanabe, Y.; Osakabe, Y.; Leyva-González, M.A.; Sato, M.; Toyooka, K.; Voges, L.; et al. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017, 3, e1007076. [Google Scholar] [CrossRef]
- Li, W.; Gupta, A.; Tian, H.; Nguyen, K.H.; Tran, C.D.; Watanabe, Y.; Tran, L.S.P. Different strategies of strigolactone and karrikin signals in regulating the resistance of Arabidopsis thaliana to water-deficit stress. Plant Signal Behav. 2020, 15, e178932. [Google Scholar] [CrossRef]
- Yagcı, A.; Daler, S.; Kaya, O. An innovative approach: Alleviating cadmium toxicity in grapevine seedlings using smoke solution derived from the burning of vineyard pruning waste. J. Physiol. Plant. 2024, 176, e14624. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Zaman, W. Plant biotic and abiotic stresses. Life 2024, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Biswas, S.; Das, R. Organic forming to mitigate biotic stresses under climate change scenario. Bull. Natl. Res. Cent. 2024, 48, 71. [Google Scholar] [CrossRef]
- Yaman, K.P. Physiological stress in agricultural crops: An overview. Biol. Forum Int. J. 2021, 13, 277–292. [Google Scholar]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; d’Errico, G.; Laudonia, S.; Vinale, F.; Abd-ElGawad, A. Biochar-derived smoke-water exerts biological effects on nematodes, insects, and higher plants but not fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef]
- Saini, N.; Kataria, R.; Bhardwaj, I.; Panchal, P. Importance of flavonoids in agriculture. In Flavonoids as Nutraceuticals; Kesharwani, R.K., Saini, D., Kesarvani, R.K., Sharma, A.K., Eds.; Apple Academic Press: New York, NY, USA, 2024; pp. 37–55. [Google Scholar]
- Ghebrehiwot, H.M.; Kulkarni, M.G.; Kirkman, K.P.; van Staden, J. Smoke-water and a smoke-isolated butenolide improve germination and seedling vigor of Eragorostis tef (Zucc.) Trotter under high temperature and low osmotic potential. J. Agron. Crop Sci. 2008, 194, 270–277. [Google Scholar] [CrossRef]
- Syman, K.; Turpanova, R.; Nazarova, G.A.; Berdenkulova, A.Z.; Tulindinova, G.K.; Korogod, N.P.; Izbassarova, Z.Z.; Aliyeva, Z.G.; Utegaliyeva, R. Role of oxidative stress enzymes in abiotic and biotic stress. Casp. J. Environ. Sci. 2024, 22, 521–528. [Google Scholar]
- Francis, B.; Aravindakumar, C.T.; Simon, S. Regulatory role of Strigolactones in biotic stress tolerance. In Strigolactones Synthesis, Application and Role in Plants; Bashri, G., Hayat, S., Bajguz, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 189–200. [Google Scholar]
- Yang, T.; Lian, Y.; Wang, C. Comparing and contrasting the multiple roles of butenolide plant growth regulators: Strigolactones and karrikins in plant development and adaptation to abiotic stresses. Int. J. Mol. Sci. 2019, 20, 6270. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.; Verhoeven, J.T.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Change Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Qian, L.; Duan, K. Evaluating the impacts of flooding on crop yields by different meteorological indices: A regional case study in the middle-lower reach of the Yangtze River, China. Ecol. Indic. 2024, 162, 112068. [Google Scholar] [CrossRef]
- Bhoite, R.; Han, Y.; Chaitanya, A.K.; Varshney, R.K.; Sharma, D.L. Genomic approaches to enhance adaptive plasticity to cope with soil constraints amidst climate change in wheat. Plant Genome 2024, 17, e20358. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High salinity inhibits soil bacterial community mediating nitrogen cycling. Appl. Environ. Microbiol. 2021, 87, e0136621. [Google Scholar] [CrossRef]
- Sarri, E.; Termentzi, A.; Abraham, E.M.; Papadopoulos, G.K.; Baira, K.; Machera, K.; Loukas, V.; Komaitis, F.; Tani, E. Salinity stress alters the secondary metabolic profile of M. sativa, M. arborea and their hybrid (Alborea). Int. J. Mol. Sci. 2021, 22, 4882. [Google Scholar] [CrossRef] [PubMed]
- Catav, S.S.; Surgun-Acar, Y.; Zemheri-Navruz, F. Physiological, biochemical, and molecular responses of wheat seedlings to salinity and plant-derived smoke. S. Afr. J. Bot. 2021, 39, 148–157. [Google Scholar] [CrossRef]
- Singh, S.; Uddin, M.; Khan, M.M.A.; Chishti, A.S.; Singh, S.; Bhat, U.H. The role of plant-derived smoke and karrikinolide in abiotic stress mitigation: An Omic approach. Plant Stress 2023, 7, 100147. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Dumat, C. Heavy Metal Stress and Crop Productivity. In Crop Production and Global Environmental Issues; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Tran, L.S.P., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
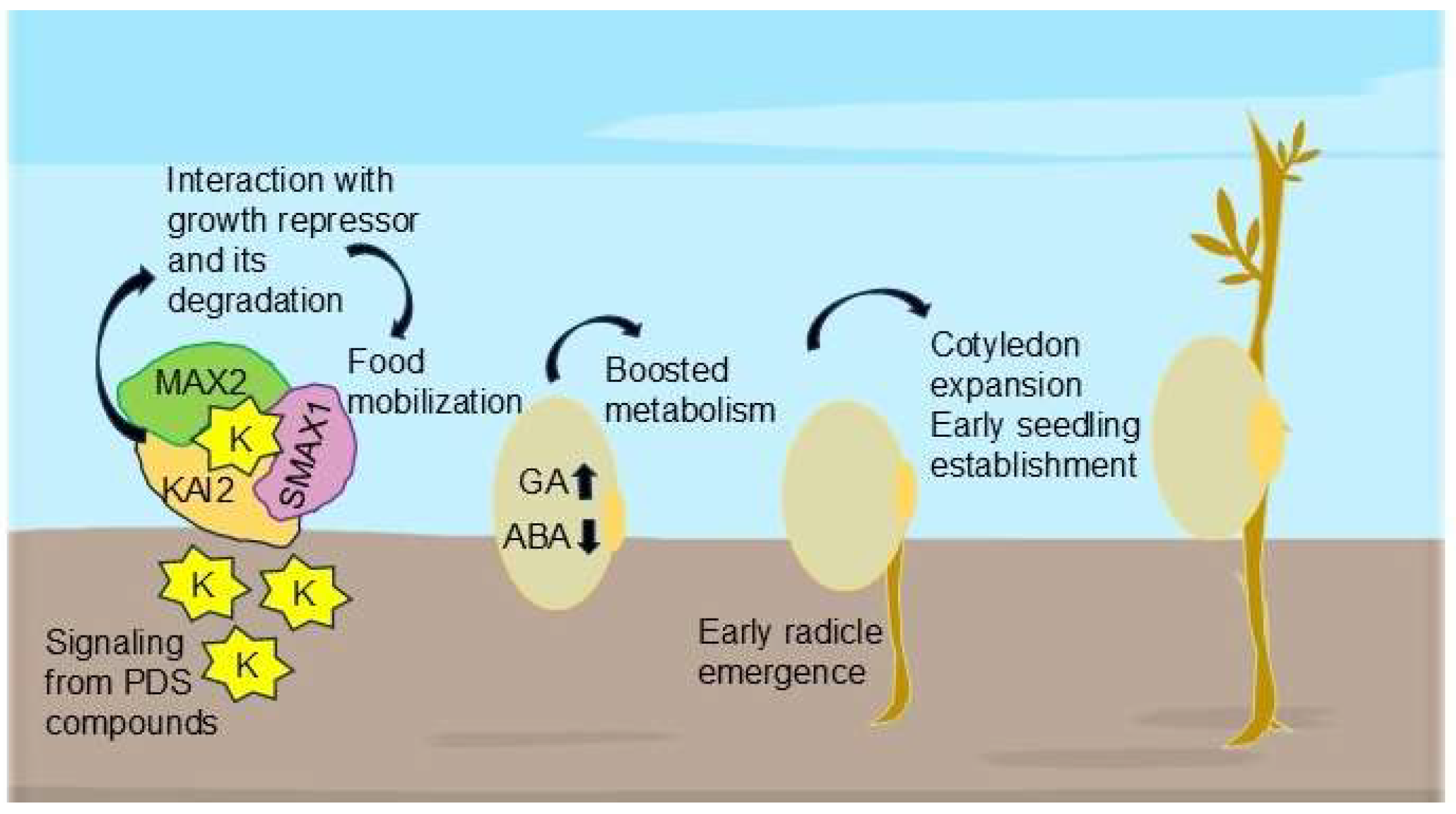
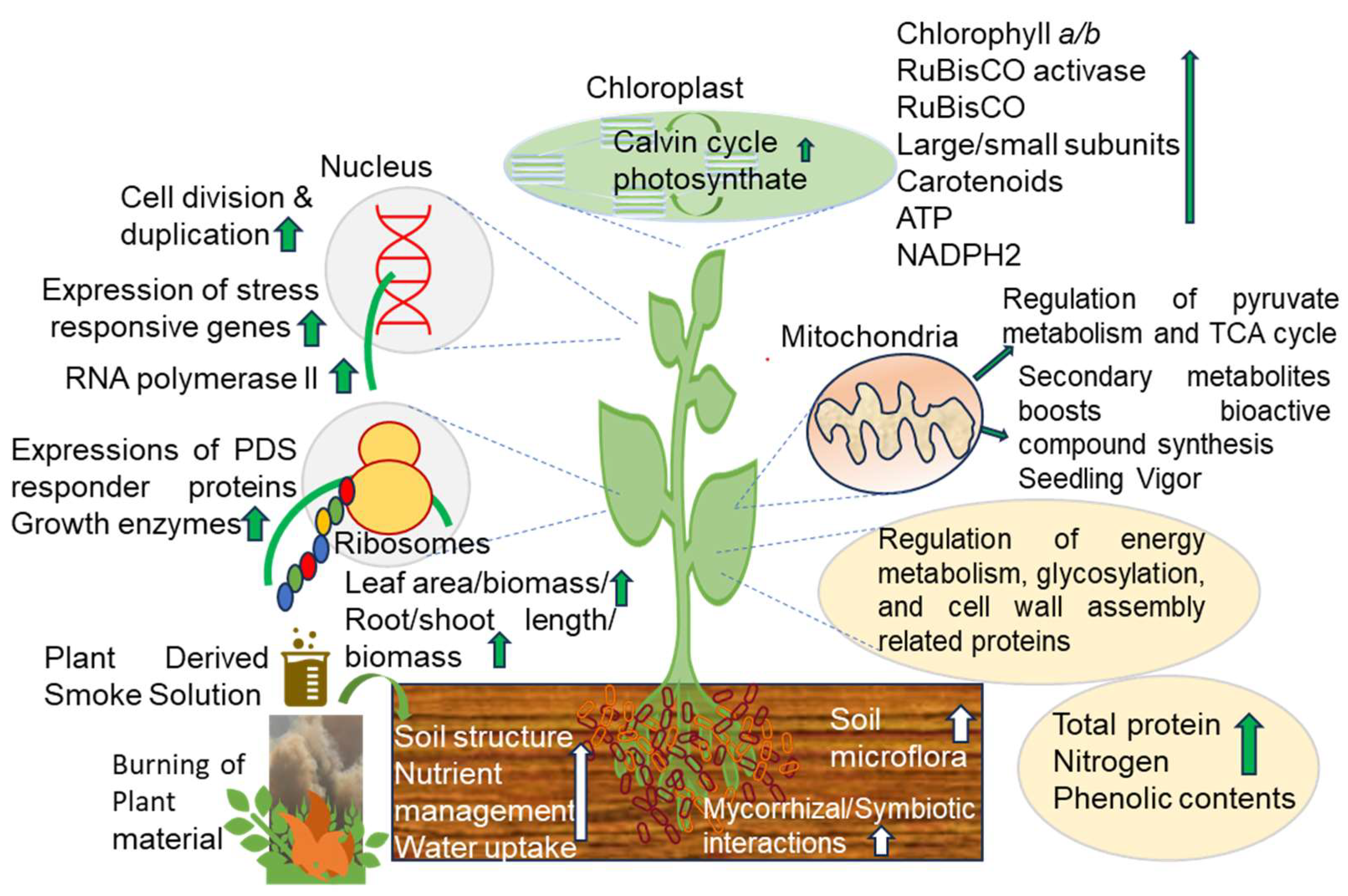

| Solution Applied | Solution Concentrations | Seed Germination and Morphological and Biochemical Parameters Studied | Results | Ref. |
|---|---|---|---|---|
| Arabidopsis | ||||
| KAR | 1 µM | Seed germination | Increased | [41] |
| KAR | 1 µM | Seed germination, hypocotyl length, bud growth | Increased | [42] |
| KAR | 1 µM | Seed germination, hypocotyl length | Increased | [43] |
| KAR | 1 µM, 10 µM | Hypocotyl length | Increased | [44] |
| KAR | 1 µM | Seed germination, hypocotyl length, cotyledon area, cotyledon petiole length | Increased | [45] |
| Lettuce | ||||
| KAR | 10−1 µM | Seed germination | Increased | [32] |
| KAR | 10−1 µM | α-amylase, biomolecules, lipase activity | Increased | [32] |
| Wild oat (Avena sativa L.) | ||||
| KAR | 3000 µM | Seed germination, coat rupture, coleorhiza emergence, root emergence, water contents (%) | Increased | [46] |
| KAR | 3 × 10−3 µM | ACC synthase, ACC oxidase | Increased | [46] |
| Cabbage (Brassica oleracea L.) | ||||
| KAR | 3 × 10−3 µM | Seed germination, relative water contents | Increased | [47] |
| KAR | 3 × 10−3 µM | SOD, CAT | Decreased | [47] |
| KAR | 3 × 10−3 µM | Glutathione reductase | Increased | [47] |
| KAR | 3 × 10−3 µM | BOACS7, BOACS9, BOACS 11, BOACS2 genes | Increased | [47] |
| Redroot sage (Salvia miltiorrhiza Bunge) | ||||
| KAR | 10−3 µM | Tanshinone I contents, NO contents, jasmonic acid contents | Increased | [48] |
| Brassica (Brassica napus L.) | ||||
| KAR | 10−1 µM, 10−2 µM | Seed germination, mean germination index, germination rate | Increased | [49] |
| Lettuce | ||||
| Trimethyle butenolides | 0.1 µM, 1 µM, 10 µM, 100 µM | Seed germination | Decreased | [50,51,52] |
| TMB | 10−1 µM | Seed germination | Decreased | [32] |
| TMB | 10−1 µM | α-amylase, biomolecules, lipase activity | Decreased | [32] |
| Hydroquinone | 45 µM, 90 µM, 180 µM | Seed germination, seedling length | Increased | [8] |
| 5,5-dimethyl-2(5H)-furanone | 1 µM, 10 µM, 100 µM, 1000 µM | Seed germination | Increased | [53] |
| (5RS)-5-ethyl-2(5H)-furanone | 1 µM, 10 µM, 100 µM, 1000 µM | Seed germination | Increased | [53] |
| Kangaroo paw (Anigozanthos manglesii Maund) | ||||
| Glyceronitrile | 1 µM, 5 µM, 10 µM, 20 µM, 50 µM | Seed germination | Increased | [54] |
| Karoo Frayreed (Rhodocoma arida H.P.Linder & Vlok) | ||||
| Glyceronitrile | 1 µM, 5 µM, 10 µM, 20 µM, 50 µM | Seed germination | Increased | [54] |
| Cat’s paw (Anigozanthos humilis Lindl.) | ||||
| Glyceronitrile | 1 µM, 5 µM, 10 µM, 20 µM, 50 µM | Seed germination | Increased | [54] |
| Tobacco (Nicotiana tabacum L.) | ||||
| Catechol | 10 µM | Root elongation, root hair elongation | Increased | [55] |
| Cucumber (Cucumis sativus L.) | ||||
| Catechol | 50 µM | Phenolic contents | Increased | [56] |
| Lemongrass (Cymbopogon flexuosus Steud.) | ||||
| Catechol | 5 µM | Seedling length, seedling weight | Increased | [57] |
| Catechol | 5 µM | Essential oil contents, essential oil yield, total chlorophyll content, total carotenoid content | Increased | [57] |
| Catechol | 5 µM | Proline contents | Decreased | [57] |
| Solution Applied | Solution Concentrations | Seed Germination (%) | Ref. |
|---|---|---|---|
| Chickpea | |||
| PDS | 2000 ppm | Increased | [25] |
| Cucumber | |||
| PDS | 5000, 10,000 ppm | Increased | [79] |
| Popcorn (Sapium sebiferum L. Roxb.) | |||
| KAR1 | 0.001 µM | Increased | [80] |
| Aubergine (Solanum melongena L.) | |||
| KAR | 0.1 µM | Increased | [81] |
| Western Australian plants | |||
| PDS | 10, 20, 30, 40, 50% | Increased | [82] |
| Melon (Cucumis melo L.) | |||
| KAR | 0.1 µM | Increased | [83] |
| Snotblom (Albuca pachychlamys Baker), Blue squill (Merwilla natalensis Planch. Speta.), Society garlic (Tulbaghia violacea Harv.) | |||
| PDS | 1000, 2000 ppm | Increased | [84] |
| Hairy cup flower (Angianthus tomentosus J.C.Wendl.), Dwarf cup flower (Gnephosis tenuissima Cass.), Large cupper wire daisy (Podolepis canescens A.Cunn. ex DC.), Yellow buttons (Myriocephalus guerinae F.Muell.) | |||
| KAR | 0.00667 µM, 0.0667 µM, 0.6667 µM | Increased | [85] |
| Orobanche (Orobanche minor Sm.), Striga (Striga hermonthica Delile Benth.) | |||
| KAR | 1 × 10−5 µM–10 µM | Increased | [86] |
| Medicinal plants | |||
| PDS | 2000 ppm | Increased | [87] |
| Wallaby grass (Austrodanthonia tenuior Steud.), Bentham’s love-grass (Eragrostis brownii Kunth.), Paddock love-grass (Eragrostis leptostachya R.Br. Steud.), Hairy panic grass (Panicum effusum R.Br.), Two color panic grass (Panicum simile Domin.) | |||
| PDS | 10% | Increased | [88] |
| Beans (Phaseolus vulgaris L.) | |||
| PDS | 0.5, 1, 1.5, 2% | Increased | [89] |
| Solution Applied | Solution Concentrations | Morphological, Physiological, and Biochemical Parameters Studied | Results | Ref. |
|---|---|---|---|---|
| Mung bean (Vigna radiata L.) | ||||
| PDS | 1000, 2000 ppm | Roots initiation | Increased | [101] |
| Tomato (Solanum lycopersicum L.) | ||||
| KAR PDS | 10−4 µM–0.1 µM KAR 2000 ppm PDS | Radical emergence, hypocotyle elongation, seedling weight Plant height, number of leaves, stem thickness, number of fruit per plant, mean fruit weight, plant biomass, mean fruit diameter | Increased | [102,103] |
| Rice | ||||
| PDS | 1000, 2000 ppm | Seedling length, seedling weight | Increased | [16,104,105] |
| Wheat | ||||
| PDS | 100, 1000 ppm | Seedling length, seedling weight | Increased | [17] |
| Maize | ||||
| PDS | 400, 1600 ppm | Seedling length, seedling biomass | Increased | [37] |
| PDS | 400, 1600 ppm | Chlorophyll a, chlorophyll b, carotenoids, total soluble proteins | Increased | [37] |
| Wild garlic (Allium ursinum L.) | ||||
| PDS | 2000 ppm | Leaf length, root length, root number, seedling fresh biomass | Increased | [106] |
| PDS | 2000 ppm | phenolic, flavonoid, condensed tannin | Increased | [106] |
| Ipomoea cuttings | ||||
| PDS | 2000 ppm | Number of lateral roots, number of adventurous roots | increased | [22] |
| Maize | ||||
| PDS | 2000 ppm | Seedling length, fresh and dry weight | Increased | [18] |
| PDS | 2000 ppm | Photosynthetic pigments, carotenoids | Increased | [18] |
| PDS | 1000, 2000 ppm | ABA contents | Decreased | [107] |
| Bentgrass (Agrostis stolonifera L.) | ||||
| KAR1 | 0.1 µM | Chlorophyll contents, POD, SOD, APX activities | Increased | [108] |
| Horticulture crops | ||||
| PDS | PDS obtained from different plants | Root length, shoot length, seedling fresh/dry weight, seedling vigor | Increased | [109] |
| PDS | PDS obtained from different plants | Ion contents, photosynthetic pigments, α-amylase activity, N, P, K contents | Increased | [109] |
| PDS | PDS obtained from different plants | ABA | Decreased | [109] |
| Grass pea (Lathyrus sativus L.) | ||||
| PDS | 1, 5, 10, 20, 40% | Root length, shoot length, root dry weight, shoot dry weight, shoot width, number of branches, number of total nodule | Increased | [110] |
| PDS | 1, 5, 10, 20, 40% | Shoot protein contents, K, P, shoot and root oxalyldiaminopropionic acid (OPAD) | Increased | [110] |
| Carrot (Daucus carota L.) | ||||
| PDS and KAR1 | PDS treatments 25.8, 51.6, 103.2, 258.0 µg/L KAR1 treatments 0.00001 µM, 0.001 µM, 0.010 µM, 0.100 µM | Plant height, number of leaves, total leaf area, root length, root diameter, root fresh weight, root dry weight | Increased | [111] |
| PDS and KAR1 | PDS treatments 25.8, 51.6, 103.2, 258.0 µg/L KAR1 treatments 0.00001 µM, 0.001 µM, 0.010 µM, 0.100 µM | Total chlorophyll, total carotenoids, chlorophyll fluorescence, intercellular CO2 concentration, stomatal conductance, net photosynthetic rate, beta carotene, ascorbic acid | Increased | [111] |
| Cabbage | ||||
| KAR1 | 10 µM, 0.1 µM, 0.001 µM | Root/shoot fresh and dry weight | Increased | [112] |
| KAR 1 | 10 µM, 0.1 µM, 0.001 µM | Photosynthetic rate, leaf relative water contents, water potential, leaf osmotic potential, membrane stability index, total chlorophyll, total sugar, total carotenoids, intercellular CO2 concentrations, transpiration rate | Increased | [112] |
| KAR 1 | 10 µM, 0.1 µM, 0.001 µM | Malondialdehyde (MDA), H2O2, electrolyte leakage | Decreased | [112] |
| Wheat | ||||
| PDS | 2000 ppm | Root/shoot length, seedling fresh weight | Increased | [17] |
| PDS | 1000, 2000 ppm | Root/shoot length, seedling fresh weight | Increased | [38] |
| PDS | 1000, 2000 ppm | H2O2, Thiobarbituric Acid Reactive Substances (TBARS) | Decreased | [38] |
| PDS | 1000, 2000 ppm | APX, POD, SOD | Increased | [38] |
| PDS | 2000, 4000 ppm | Seedling vigor, root length, leaf area, shoot length | Increased | [113] |
| PDS | 2000, 4000 ppm | Water potential, relative water, chlorophyll a, chlorophyll b, total chlorophyll, membrane stability | Increased | [113] |
| Redroot sage | ||||
| PDS and KAR1 | 1000 ppm PDS 0.001 µM KAR | Biosynthesis of flavonoids and terpenoids | Increased | [114] |
| Solution Applied | Solution Concentrations | Morphological and Biochemical Parameters Studied | Results | Ref. |
|---|---|---|---|---|
| Soybean | ||||
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | Main root length, total root fresh weight | Improved | [29] |
| Wheat | ||||
| PDS + Flooding | 2000 ppm PDS + Flooding | Root length, leaf length, leaf weight | Improved | [30] |
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | Root length, root weight, leaf length, leaf weight | Improved | [31] |
| Maize | ||||
| PDS + TIBA | 2000 ppm PDS + 24.78 µM TIBA | Plant height, leaf length, length of primary roots, number of secondary roots, root/shoot fresh weight | Improved | [98] |
| Rice | ||||
| PDS + Cadmium (Cd) | 1000 ppm PDS + 100 µM Cd 1000 ppm PDS + 200 µM Cd 1000 ppm PDS + 400 µM Cd | Seedling length, fresh and dry weight | Improved | [98] |
| Pea | ||||
| PDS + SiO2 NPs | 2000 ppm PDS + 665.6 µM SiO2 NPs | Seedling length, seedling fresh weight, number of leaves, number of secondary roots | Improved | [97] |
| Grapevine | ||||
| PDS + CdCl2 | 0.5% PDS + 54.5 µM CdCl2 1% PDS + 54.5 µM CdCl2 2% PDS + 54.5 µM CdCl2 | Root and shoot length/fresh weight, number of leaves | Improved | [131] |
| Cabbage | ||||
| KAR + Cd | 10 µM KAR1 + 44.5 µM Cd 0.1 µM KAR1 + 44.5 µM Cd 0.00001 µM KAR1 + 44.5 µM Cd | Seedling fresh/dry weight | Improved | [99] |
| Popcorn | ||||
| KAR1 + NaCl | 0.001 µM KAR1 + 150,000 µM NaCl | Shoot length, survival rate, root length, lateral root length, fresh weight | Improved | [80] |
| KAR1 + Osmotic stress | 0.001 µM KAR1 + 150,000 µM mannitol | Shoot length, survival rate, root length, lateral root length, fresh weight | Improved | [80] |
| Bentgrass | ||||
| KAR1 + Drought | 0.1 µM KAR1 + Drought | Electrolyte leakage, MDA, CAT activity | Decreased | [108] |
| KAR1 + Drought | 0.1 µM KAR1 + Drought | Relative water contents, chlorophyll, proline contents, APX, POD, SOD activities | Improved | [108] |
| KAR1 + Drought | 0.1 µM KAR1 + Drought | MAX2, KAI2, AFL1, ABF3, MYB13, DREB2A, Cu/Zn-SOD, APX2, CAT1, POD2 genes | Improved | [108] |
| KAR1 + Drought | 0.1 µM KAR1 + Drought | DLK2, KUF1, SMAX1, WRKY75, WRKY28, PPH, Chl-PRX genes | Deceased | [108] |
| Grapevine | ||||
| PDS + CdCl2 | 0.5% PDS + 54.5 µM CdCl2 1% PDS + 54.5 µM CdCl2 2% PDS + 54.5 µM CdCl2 | Chlorophyll, relative water, stomatal conductance, leaf temperature, total phenolics contents, SOD, APX, CAT activities, electrolyte leakages (%), proline, MDA | Improved | [131] |
| Wheat | ||||
| PDS + Flooding | 2000 ppm PDS + Flooding | RuBisCO activase and RuBisCO large/small subunits, photosynthetic pigments, glutamine, glutamic acid, aspartic acid, serine | Improved | [30] |
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | APX, Ubiquitin, ATP | Decreased | [31] |
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | H+-ATPase | Improved | [31] |
| Soybean | ||||
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | Glycoproteins, H+ ATPase | Improved | [29] |
| PDS + NaCl | 2000 ppm PDS + 100,000 µM NaCl | Osmotin, alcohol dehydrogenase, sucrose synthase, cellulose synthase, xyloglucan endotransglucosylase/hydrolase | Decreased | [29] |
| Rice | ||||
| PDS + Cd | 1000 ppm PDS + 100 µM Cd, 1000 ppm PDS + 200 µM Cd 1000 ppm PDS + 400 µM Cd | Cell membrane stability, photosynthetic pigments, Na+, K+, Ca++ | Improved | [99] |
| PDS + Cd | 1000 ppm PDS + 100 µM Cd 1000 ppm PDS + 200 µM Cd 1000 ppm PDS + 400 µM Cd | Proline, total soluble sugar, POD, CAT, Cd | Decreased | [99] |
| Cabbage | ||||
| KAR + Cd | 10 µM KAR1 + 44.5 µM Cd 0.01 µM KAR1 + 44.5 µM Cd 0.00001 µM KAR1 + 44.5 µM Cd | Water potential, leaf osmotic potential, membrane stability index, metal tolerance index, chlorophyll a, chlorophyll b, total chlorophyll, carotenoid, soluble sugars, intercellular CO2 concentrations, stomatal conductance, transpiration rate, DHAR, MDHAR, GST, CAT, GR, GPX, APX, SOD, POD, proline | Improved | [112] |
| KAR + Cd | 10 µM KAR1 + 44.5 µM Cd 0.01 µM KAR1 + 44.5 µM Cd 0.00001 µM KAR1 + 44.5 µM Cd | Root Cd, shoot Cd, translocation factor, MDA, H2O2, electrolyte leakage | Decreased | [112] |
| Popcorn | ||||
| KAR1 + NaCl | 0.001 µM KAR1 + 150,000 µM NaCl | Electrolyte leakage, MDA, H2O2, APX, POD | Improved | [80] |
| KAR1 + NaCl | 0.001 µM KAR1 + 150,000 µM NaCl | SOD, CAT | Deceased | [80] |
| KAR + Osmotic stress | 0.001 µM KAR1 + 200,000 µM mannitol | MDA, H2O2, SOD CAT, APX, POD, ABA | Decreased | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, A.; Aslam, M.M.; Komatsu, S. Role of Plant-Derived Smoke Solution on Plants Under Stress. Int. J. Mol. Sci. 2025, 26, 7911. https://doi.org/10.3390/ijms26167911
Khatoon A, Aslam MM, Komatsu S. Role of Plant-Derived Smoke Solution on Plants Under Stress. International Journal of Molecular Sciences. 2025; 26(16):7911. https://doi.org/10.3390/ijms26167911
Chicago/Turabian StyleKhatoon, Amana, Muhammad Mudasar Aslam, and Setsuko Komatsu. 2025. "Role of Plant-Derived Smoke Solution on Plants Under Stress" International Journal of Molecular Sciences 26, no. 16: 7911. https://doi.org/10.3390/ijms26167911
APA StyleKhatoon, A., Aslam, M. M., & Komatsu, S. (2025). Role of Plant-Derived Smoke Solution on Plants Under Stress. International Journal of Molecular Sciences, 26(16), 7911. https://doi.org/10.3390/ijms26167911







