Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies
Abstract
1. Introduction
1.1. Cognitive Manifestations of MMD
1.2. Epidemiology and Genetics of MMD
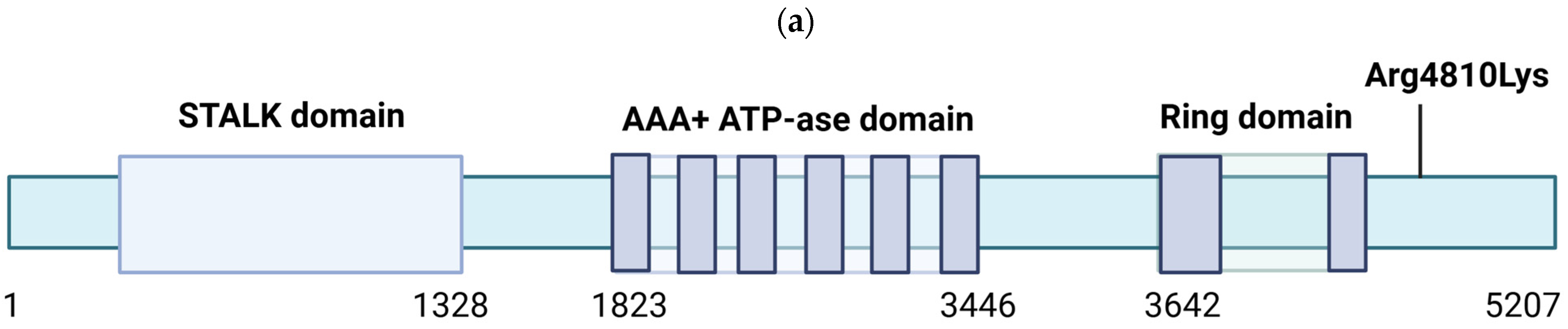
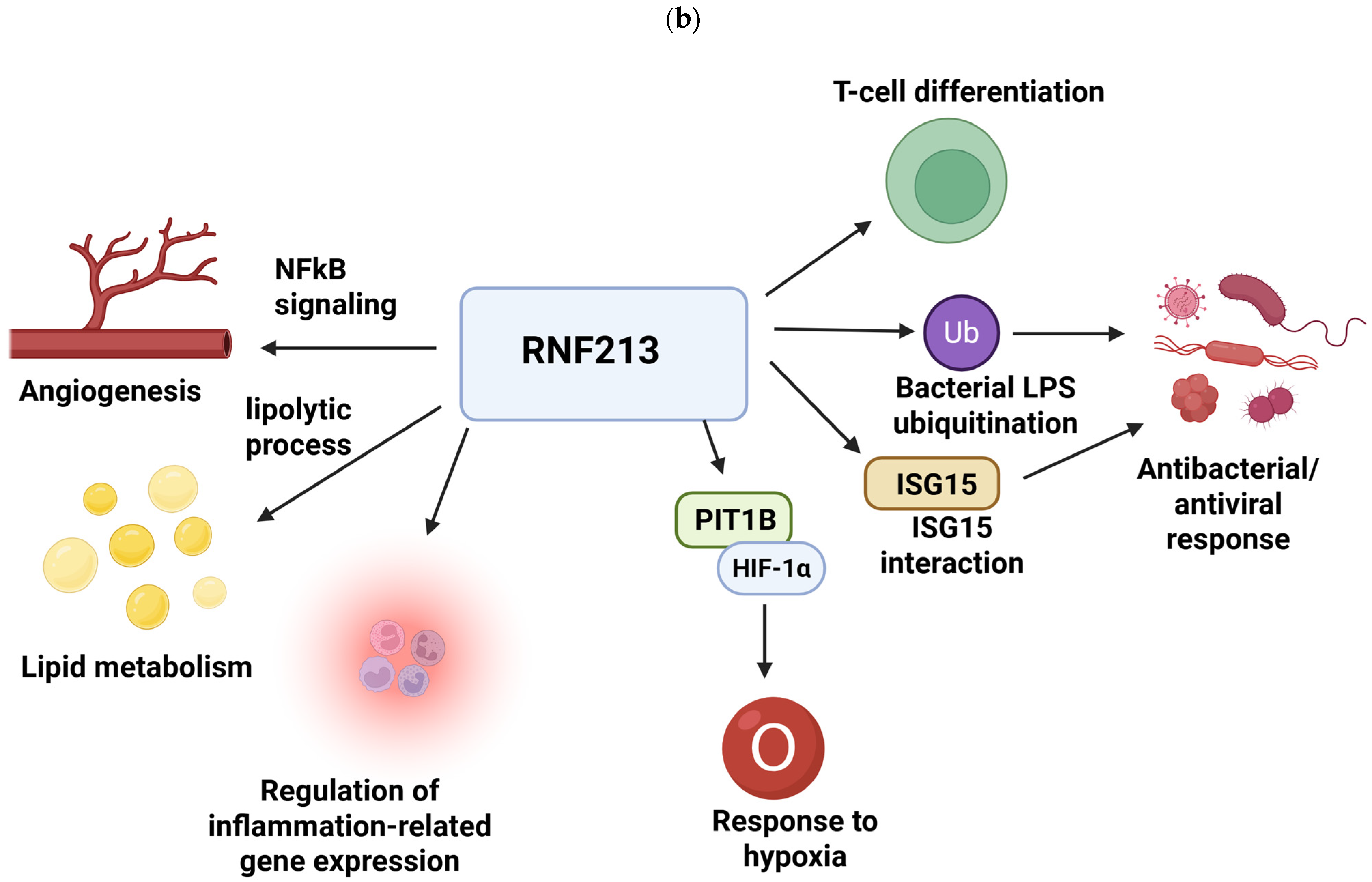
1.3. Clinical Course and Management of MMD
2. RNF213 Arg4810Lys and MMD: Biomarkers and Treatment Options
2.1. Korean Studies on Arg4810Lys and MMD
2.2. Japanese Studies on Arg4810Lys and MMD
2.3. Chinese and Other Asian Populations Studies on Arg4810Lys and MMD
2.4. Biomarkers and Treatment Strategies for MMD
3. Intracranial Artery Stenosis (ICAS) and Vascular Forms of Disease with RNF213 Arg4810Lys
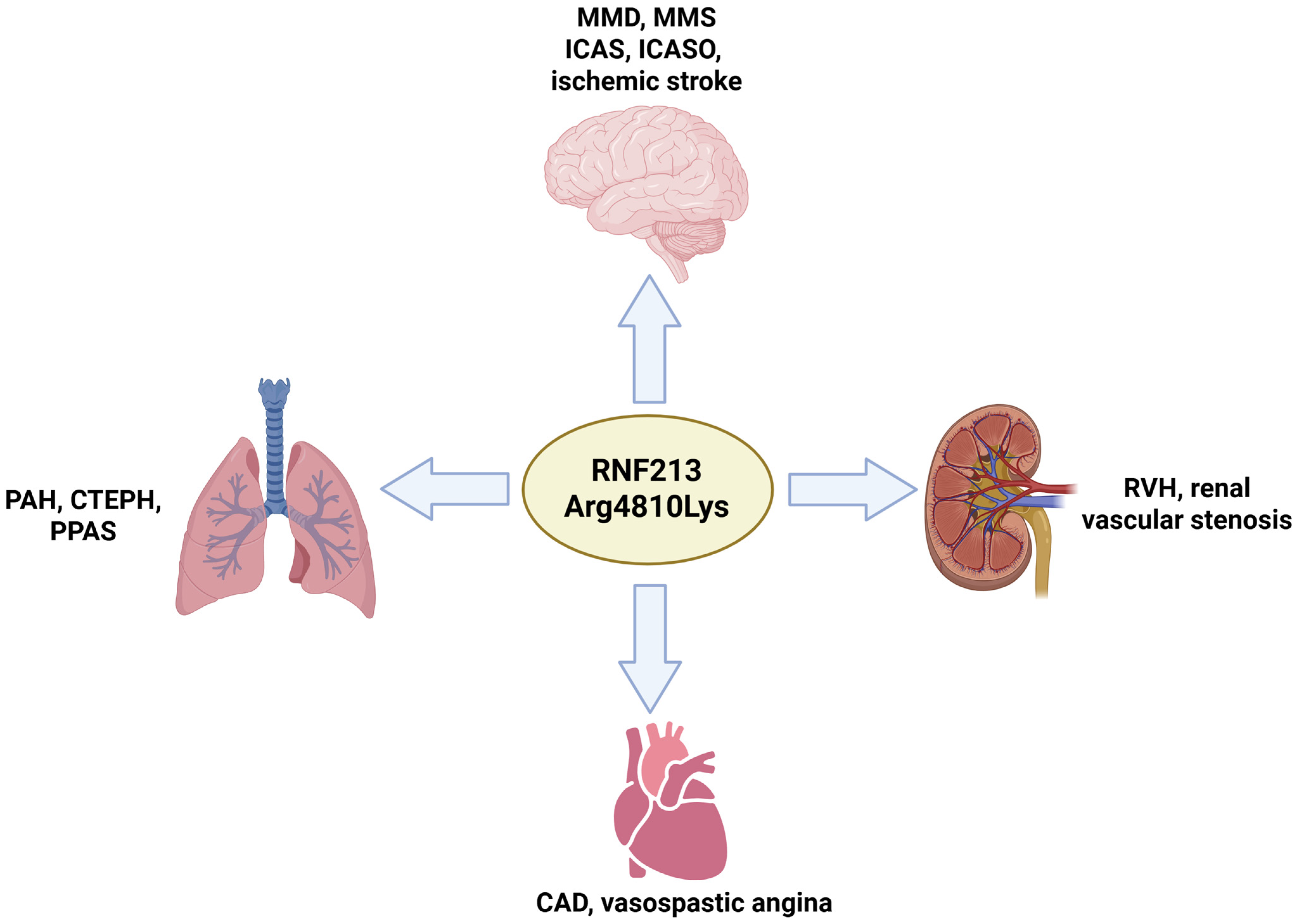
4. Non-Neurological Forms of RNF213 Arg4810Lys
4.1. RNF213 Arg4810Lys and Pulmonary Dysfunctions
4.2. RNF213 Arg4810Lys and Coronary Artery Disease (CAD) and Kidney Dysfunctions
5. RNF213 Arg418Lys and Modifier Factors
5.1. Compound Heterozygous Cases
5.2. RNF213 Arg4810Lys and Other Gene Mutations
5.3. RNF213 Arg4810Lys and Environmental Modifiers
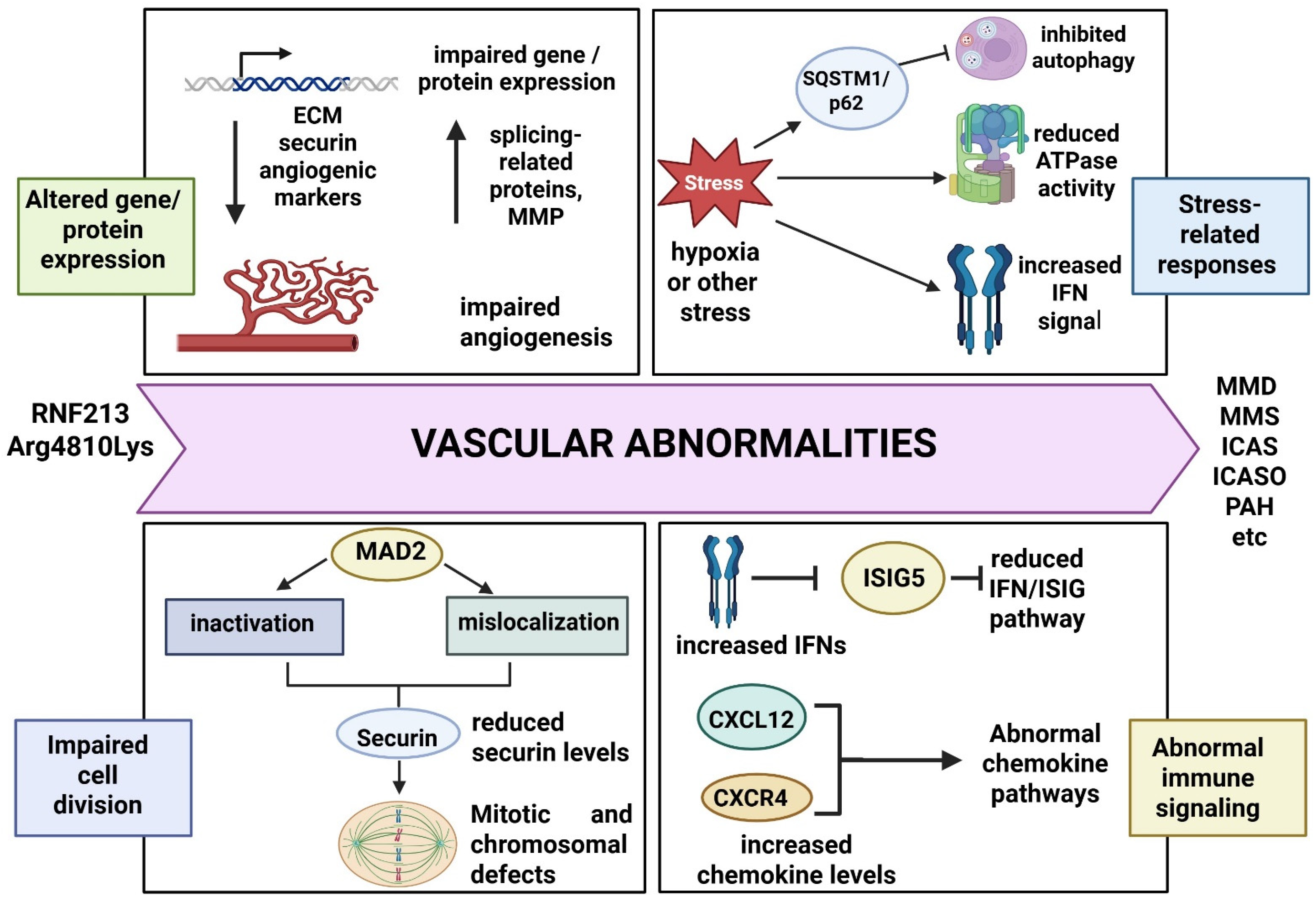
6. Functional Studies and Pathogenic Pathways of RNF213 Arg4810Lys
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takeda, K.; Sekine, A.; Tanabe, N.; Sugiura, T.; Shigeta, A.; Kitahara, S.; Imai, S.; Okaya, T.; Nagata, J.; Naito, A.; et al. Two cases of pulmonary arterial hypertension with specific vascular Malformations and unique eosinophilic inflammation in carriers of the RNF213 p. Arg4810Lys variant: Case series. Respir. Med. Case Rep. 2023, 42, 101829. [Google Scholar] [CrossRef]
- Koizumi, A.; Kobayashi, H.; Hitomi, T.; Harada, K.H.; Habu, T.; Youssefian, S. A new horizon of moyamoya disease and associated health risks explored through RNF213. Environ. Health Prev. Med. 2016, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Pollaci, G.; Gorla, G.; Potenza, A.; Carrozzini, T.; Canavero, I.; Bersano, A.; Gatti, L. Novel Multifaceted Roles for RNF213 Protein. Int. J. Mol. Sci. 2022, 23, 4492. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Zhang, Q.; Yu, L.B.; Zhao, J.Z. Role of Ring Finger Protein 213 in Moyamoya Disease. Chin. Med. J. 2016, 129, 2497–2501. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Y.; Jiang, L.; Liu, Y.; Zhang, L. The emerging role of E3 ubiquitin ligase RNF213 as an antimicrobial host determinant. Front. Cell. Infect. Microbiol. 2023, 13, 1205355. [Google Scholar] [CrossRef]
- Ahel, J.; Lehner, A.; Vogel, A.; Schleiffer, A.; Meinhart, A.; Haselbach, D.; Clausen, T. Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism. elife 2020, 9, e56185. [Google Scholar] [CrossRef]
- Durfee, L.A.; Lyon, N.; Seo, K.; Huibregtse, J.M. The ISG15 conjugation system broadly targets newly synthesized proteins: Implications for the antiviral function of ISG15. Mol. Cell 2010, 38, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Thery, F.; Martina, L.; Asselman, C.; Zhang, Y.; Vessely, M.; Repo, H.; Sedeyn, K.; Moschonas, G.D.; Bredow, C.; Teo, Q.W.; et al. Ring finger protein 213 assembles into a sensor for ISGylated proteins with antimicrobial activity. Nat. Commun. 2021, 12, 5772. [Google Scholar] [CrossRef]
- Sugihara, M.; Morito, D.; Ainuki, S.; Hirano, Y.; Ogino, K.; Kitamura, A.; Hirata, H.; Nagata, K. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J. Cell Biol. 2019, 218, 949–960. [Google Scholar] [CrossRef]
- Dofuku, S.; Sonehara, K.; Miyawaki, S.; Sakaue, S.; Imai, H.; Shimizu, M.; Hongo, H.; Shinya, Y.; Ohara, K.; Teranishi, Y.; et al. Genome-Wide Association Study of Intracranial Artery Stenosis Followed by Phenome-Wide Association Study. Transl. Stroke Res. 2023, 14, 322–333. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467, Erratum in Stroke 2025, 26, e483–e484. [Google Scholar] [CrossRef]
- Torazawa, S.; Miyawaki, S.; Imai, H.; Hongo, H.; Ishigami, D.; Shimizu, M.; Sakai, Y.; Ogawa, S.; Kiyofuji, S.; Koizumi, S.; et al. Association of rare variants in RNF213 with severe progression of intracranial artery stenosis in quasi-moyamoya disease. J. Neurosurg. 2024, 142, 394–403. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Cha, J.; Lee, M.J.; Yeon, J.Y.; Ki, C.S.; Jeon, P.; Kim, J.S.; Hong, S.C. A Polymorphism in RNF213 Is a Susceptibility Gene for Intracranial Atherosclerosis. PLoS ONE 2016, 11, e0156607. [Google Scholar] [CrossRef] [PubMed]
- Kamada, F.; Aoki, Y.; Narisawa, A.; Abe, Y.; Komatsuzaki, S.; Kikuchi, A.; Kanno, J.; Niihori, T.; Ono, M.; Ishii, N.; et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Hattori, Y.; Nishii, T.; Horinouchi, H.; Nakaoku, Y.; Ogata, S.; Inagaki, Y.; Asano, R.; Yoshimoto, T.; Nishimura, K.; et al. Relationship Between RNF213 p.R4810K and Echocardiographic Findings in Patients with Cerebrovascular Diseases: A Multicenter Prospective Cohort Study. J. Am. Heart Assoc. 2025, 14, e036333. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhou, Z.; Zhang, J.; Wang, Y.; He, S.; Wang, R. Cognitive dysfunction in Moyamoya disease: Latest developments and future directions. Front. Hum. Neurosci. 2024, 18, 1502318. [Google Scholar] [CrossRef]
- Kazumata, K.; Tha, K.K.; Narita, H.; Kusumi, I.; Shichinohe, H.; Ito, M.; Nakayama, N.; Houkin, K. Chronic ischemia alters brain microstructural integrity and cognitive performance in adult moyamoya disease. Stroke 2015, 46, 354–360. [Google Scholar] [CrossRef]
- Karzmark, P.; Zeifert, P.D.; Bell-Stephens, T.E.; Steinberg, G.K.; Dorfman, L.J. Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery 2012, 70, 634–638. [Google Scholar] [CrossRef]
- Tsunoda, S.; Inoue, T.; Ohwaki, K.; Takeuchi, N.; Shinkai, T.; Fukuda, A.; Segawa, M.; Kawashima, M.; Akabane, A.; Miyawaki, S.; et al. Association Between Frontal Lobe Hemodynamics and Neurocognitive Dysfunction in Adults with Moyamoya Disease: Retrospective Cohort Analysis. Neurosurgery 2023, 92, 547–556. [Google Scholar] [CrossRef]
- Maehara, N.; Nakamizo, A.; Arimura, K.; Yoshimoto, K. Memory, Executive, and Intellectual Functions in Adults with Moyamoya Disease. World Neurosurg. 2023, 180, e474–e483. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.G.; Rahme, R.J.; Aoun, S.G.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: Functional and neurocognitive outcomes in the pediatric and adult populations. Neurosurg. Focus. 2011, 30, E21. [Google Scholar] [CrossRef]
- Chan, E.; Gal, A.M.; Van Harskamp, N.; Adams, M.E.; Brown, M.M.; Werring, D.J.; Cipolotti, L.; Simister, R. Long-term study of the cognitive profile of Moyamoya Disease in adults. J. Stroke Cerebrovasc. Dis. 2023, 32, 107064. [Google Scholar] [CrossRef]
- Oakley, C.I.; Lanzino, G.; Klaas, J.P. Neuropsychiatric Symptoms of Moyamoya Disease: Considerations for the Clinician. Neuropsychiatr. Dis. Treat. 2024, 20, 663–669. [Google Scholar] [CrossRef]
- van Oort, S.; Beulens, J.W.J.; van Ballegooijen, A.J.; Burgess, S.; Larsson, S.C. Cardiovascular risk factors and lifestyle behaviours in relation to longevity: A Mendelian randomization study. J. Intern. Med. 2021, 289, 232–243. [Google Scholar] [CrossRef]
- Festa, J.R.; Schwarz, L.R.; Pliskin, N.; Cullum, C.M.; Lacritz, L.; Charbel, F.T.; Mathews, D.; Starke, R.M.; Connolly, E.S.; Marshall, R.S.; et al. Neurocognitive dysfunction in adult moyamoya disease. J. Neurol. 2010, 257, 806–815. [Google Scholar] [CrossRef]
- He, S.; Duan, R.; Liu, Z.; Ye, X.; Yuan, L.; Li, T.; Tan, C.; Shao, J.; Qin, S.; Wang, R. Characteristics of cognitive impairment in adult asymptomatic moyamoya disease. BMC Neurol. 2020, 20, 322. [Google Scholar] [CrossRef]
- Kronenburg, A.; van den Berg, E.; van Schooneveld, M.M.; Braun, K.P.J.; Calviere, L.; van der Zwan, A.; Klijn, C.J.M. Cognitive Functions in Children and Adults with Moyamoya Vasculopathy: A Systematic Review and Meta-Analysis. J. Stroke 2018, 20, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ito, M.; Isagawa, T.; Kuchimaru, T.; Takeda, N. RNF213 and cardiovascular disease: A review of histopathological, genetic perspectives, and potential molecular mechanisms. J. Cardiol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Niu, X.; Liang, F.; Dai, Y.; Liang, J.; Li, J.; Wu, X.; Zheng, H.; Qi, T.; Sheng, W. RNF213 loss-of-function promotes pathological angiogenesis in moyamoya disease via the Hippo pathway. Brain 2023, 146, 4674–4689. [Google Scholar] [CrossRef]
- Iorio, C.; Marcotte, R.; Xu, Y.; Cojocari, D.; Rahman, A.A.; Pawling, J.; Zhang, W.; Sinha, A.; Rose, C.M.; Isasa, M.; et al. PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia. Nat. Cell Biol. 2016, 18, 803–813. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Antonelli, M.; Ueberheide, B.; Neel, B.G. Identification of a Novel Hypoxia-induced Inflammatory Cell Death Pathway. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, R.; Liu, W.; Huang, W.; Chen, X.; Gao, X.; Huang, Y.; Zhang, D. The role of the RING finger protein 213 gene in Moyamoya disease. Fluids Barriers CNS 2025, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, X.; Sheng, J.; Fu, Y.; Nie, D.; You, X.; Chen, Y.; Yang, X.; Ling, Q.; Zhang, H.; et al. RNF213 promotes Treg cell differentiation by facilitating K63-linked ubiquitination and nuclear translocation of FOXO1. Nat. Commun. 2024, 15, 5961. [Google Scholar] [CrossRef]
- Zhang, L.; Rashad, S.; Zhou, Y.; Niizuma, K.; Tominaga, T. RNF213 loss of function reshapes vascular transcriptome and spliceosome leading to disrupted angiogenesis and aggravated vascular inflammatory responses. J. Cereb. Blood Flow Metab. 2022, 42, 2107–2122. [Google Scholar] [CrossRef]
- Morito, D. Molecular structure and function of mysterin/RNF213. J. Biochem. 2024, 175, 495–505. [Google Scholar] [CrossRef]
- Lim, S.S.; Park, S.; Oh, B.H.; Jung, K.; Bae, J.W.; Bae, D.H. RNF213 vasculopathy manifested in various forms within a family: A case report. Medicine 2023, 102, e36627. [Google Scholar] [CrossRef]
- Cardoso, I.; Pinto, M.; Araújo, A.; Vila-Real, M. Rare RNF213 variant in adolescent with moyamoya disease. Rev. Neurol. 2023, 76, 177–181. (In English) [Google Scholar] [CrossRef]
- Zipfel, G.J.; Fox, D.J., Jr.; Rivet, D.J. Moyamoya disease in adults: The role of cerebral revascularization. Skull Base 2005, 15, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, X.; Ni, J. RNF213 in moyamoya disease: Genotype-phenotype association and the underlying mechanism. Chin. Med. J. 2024, 137, 2552–2560. [Google Scholar] [CrossRef]
- Bersano, A.; Guey, S.; Bedini, G.; Nava, S.; Hervé, D.; Vajkoczy, P.; Tatlisumak, T.; Sareela, M.; van der Zwan, A.; Klijn, C.J.; et al. European Moyamoya Disease Initiative. Research Progresses in Understanding the Pathophysiology of Moyamoya Disease. Cerebrovasc. Dis. 2016, 41, 105–118. [Google Scholar] [CrossRef]
- Moussouttas, M.; Rybinnik, I. A critical appraisal of bypass surgery in moyamoya disease. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420921092. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.S.; Wen, J.; Li, J.X.; Lai, R.; Wang, Y.F.; Liu, H.J.; Sheng, W.L. The association between the ring finger protein 213 (RNF213) polymorphisms and moyamoya disease susceptibility: A meta-analysis based on case-control studies. Mol. Genet. Genom. 2016, 291, 1193–1203. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Zhang, D.; Wang, R.; Zhang, Y.; Wang, S.; Yu, L.; Lu, C.; Liu, F.; Zhou, J.; et al. RNF213 as the major susceptibility gene for Chinese patients with moyamoya disease and its clinical relevance. J. Neurosurg. 2017, 126, 1106–1113. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.W.; Kim, D.H.; Won, H.H.; Yeon, J.Y.; Ki, C.S.; Shin, H.J.; Kim, J.S.; Hong, S.C.; Kim, D.K.; et al. Moyamoya Disease and Spectrums of RNF213 Vasculopathy. Transl. Stroke Res. 2020, 11, 580–589. [Google Scholar] [CrossRef]
- Sarkar, P.; Thirumurugan, K. In silico explanation for the causalities of deleterious RNF213 SNPs in Moyamoya disease and insulin resistance. Comput. Biol. Chem. 2021, 92, 107488. [Google Scholar] [CrossRef]
- Wang, Y.; Mambiya, M.; Li, Q.; Yang, L.; Jia, H.; Han, Y.; Liu, W. RNF213 p.R4810K Polymorphism and the Risk of Moyamoya Disease, Intracranial Major Artery Stenosis/Occlusion, and Quasi-Moyamoya Disease: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 2259–2270. [Google Scholar] [CrossRef]
- Park, M.G.; Shin, J.H.; Lee, S.W.; Park, H.R.; Park, K.P. RNF213 rs112735431 polymorphism in intracranial artery steno-occlusive disease and moyamoya disease in Koreans. J. Neurol. Sci. 2017, 375, 331–334. [Google Scholar] [CrossRef]
- Lin, J.; Sheng, W. RNF213 Variant Diversity Predisposes Distinct Populations to Dissimilar Cerebrovascular Diseases. BioMed Res. Int. 2018, 2018, 6359174. [Google Scholar] [CrossRef]
- Guey, S.; Kraemer, M.; Hervé, D.; Ludwig, T.; Kossorotoff, M.; Bergametti, F.; Schwitalla, J.C.; Choi, S.; Broseus, L.; Callebaut, I.; et al. FREX consortium. Rare RNF213 variants in the C-terminal region encompassing the RING-finger domain are associated with moyamoya angiopathy in Caucasians. Eur. J. Hum. Genet. 2017, 25, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, T.; Suzuki, H.; Momoi, M.; Shinya, Y.; Fukuda, K.; Kosaki, K.; Kataoka, M. RNF213-Associated Vascular Disease: A Concept Unifying Various Vasculopathies. Life 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Nie, F.; Li, Q.; Zhang, K.; Liu, M.; Yang, L.; Zhang, Q.; Liu, S.; Zeng, F.; et al. Association of Genetic Variants With Moyamoya Disease in 13 000 Individuals: A Meta-Analysis. Stroke 2020, 51, 1647–1655. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Ma, L.; Huang, S.; Li, H.; You, C. RNF213 polymorphism and Moyamoya disease: A systematic review and meta-analysis. Neurol. India 2013, 61, 35–39. [Google Scholar] [CrossRef]
- Liao, X.; Deng, J.; Dai, W.; Zhang, T.; Yan, J. Rare variants of RNF213 and moyamoya/non-moyamoya intracranial artery stenosis/occlusion disease risk: A meta-analysis and systematic review. Environ. Health Prev. Med. 2017, 22, 75. [Google Scholar] [CrossRef]
- Park, Y.S.; An, H.J.; Kim, J.O.; Kim, W.S.; Han, I.B.; Kim, O.J.; Kim, N.K.; Kim, D.S. The Role of RNF213 4810G>A and 4950G>A Variants in Patients with Moyamoya Disease in Korea. Int. J. Mol. Sci. 2017, 18, 2477. [Google Scholar] [CrossRef]
- Ok, T.; Jung, Y.H.; Kim, J.; Park, S.K.; Park, G.; Lee, S.; Lee, K.Y. RNF213 R4810K Variant in Suspected Unilateral Moyamoya Disease Predicts Contralateral Progression. J. Am. Heart Assoc. 2022, 11, e025676. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Morito, D.; Takashima, S.; Mineharu, Y.; Kobayashi, H.; Hitomi, T.; Hashikata, H.; Matsuura, N.; Yamazaki, S.; Toyoda, A.; et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 2011, 6, e22542. [Google Scholar] [CrossRef]
- Choi, E.H.; Lee, H.; Chung, J.W.; Seo, W.K.; Kim, G.M.; Ki, C.S.; Kim, Y.C.; Bang, O.Y. Ring Finger Protein 213 Variant and Plaque Characteristics, Vascular Remodeling, and Hemodynamics in Patients with Intracranial Atherosclerotic Stroke: A High-Resolution Magnetic Resonance Imaging and Hemodynamic Study. J. Am. Heart Assoc. 2019, 8, e011996. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Wang, X.; Zeng, F.; Zhang, K.; Zhang, Q.; Liu, M.; Liu, S.; Shang, M.; Li, Q.; et al. Meta-analysis of genotype and phenotype studies to confirm the predictive role of the RNF213 p.R4810K variant for moyamoya disease. Eur. J. Neurol. 2021, 28, 823–836. [Google Scholar] [CrossRef]
- Jang, M.A.; Shin, S.; Yoon, J.H.; Ki, C.S. Frequency of the moyamoya-related RNF213 p.Arg4810Lys variant in 1,516 Korean individuals. BMC Med. Genet. 2015, 16, 109. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, L.; Ai, S.; Xie, X.; Deng, J.; Jiang, Z.; Teng, B.; Liu, C.; Huang, H. Meta-analysis of the association between RNF213 polymorphisms and clinical features of moyamoya disease in Asian population. Clin. Neurol. Neurosurg. 2023, 231, 107801. [Google Scholar] [CrossRef]
- Oichi, Y.; Mineharu, Y.; Agawa, Y.; Morimoto, T.; Funaki, T.; Hitomi, T.; Kobayashi, H.; Todo, K.; Tani, S.; Imamura, H.; et al. Characterization of Moyamoya and Middle Cerebral Artery Diseases by Carotid Canal Diameter and RNF213 p.R4810K Genotype. J. Stroke Cerebrovasc. Dis. 2022, 31, 106481. [Google Scholar] [CrossRef]
- Strunk, D.; Bauer, P.; Keyvani, K.; Diehl, R.R.; Veltkamp, R.; Berlit, P.; Meuth, S.G.; Timmermann, L.; Schwitalla, J.C.; Kraemer, M. Moyamoya disease in Southeast Asians: Genetic and autopsy data, new cases, systematic review, and meta-analysis of all patients from the literature. J. Neurol. 2024, 271, 3328–3339. [Google Scholar] [CrossRef]
- Liu, W.; Hitomi, T.; Kobayashi, H.; Harada, K.H.; Koizumi, A. Distribution of moyamoya disease susceptibility polymorphism p.R4810K in RNF213 in East and Southeast Asian populations. Neurol. Med. Chir. 2012, 52, 299–303. [Google Scholar] [CrossRef]
- Bersano, A.; Khan, N.; Fuentes, B.; Acerbi, F.; Canavero, I.; Tournier-Lasserve, E.; Vajcoczy, P.; Zedde, M.L.; Hussain, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Moyamoya angiopathy Endorsed by Vascular European Reference Network (VASCERN). Eur. Stroke J. 2023, 8, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Guey, S.; Schwitalla, J.C.; Bergametti, F.; Arnould, M.; Corpechot, M.; Hadjadj, J.; Riant, F.; Aloui, C.; Drunat, S.; et al. Clinical and Molecular Features of 5 European Multigenerational Families with Moyamoya Angiopathy. Stroke 2019, 50, 789–796, Erratum in Stroke 2021, 50, e50. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Brozman, M.; Kyselová, K.; Viszlayová, D.; Morimoto, T.; Roubec, M.; Školoudík, D.; Petrovičová, A.; Juskanič, D.; Strauss, J.; et al. RNF213 Rare Variants in Slovakian and Czech Moyamoya Disease Patients. PLoS ONE 2016, 11, e0164759. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, A.C.; Guo, D.; Ren, Z.; Flynn, K.; Santos-Cortez, R.L.; Leal, S.M.; Wang, G.T.; Regalado, E.S.; Steinberg, G.K.; Shendure, J.; et al. RNF213 rare variants in an ethnically diverse population with Moyamoya disease. Stroke 2014, 45, 3200–3207. [Google Scholar] [CrossRef]
- Jang, M.A.; Chung, J.W.; Yeon, J.Y.; Kim, J.S.; Hong, S.C.; Bang, O.Y.; Ki, C.S. Frequency and significance of rare RNF213 variants in patients with adult moyamoya disease. PLoS ONE 2017, 12, e0179689. [Google Scholar] [CrossRef]
- Santoro, C.; Mirone, G.; Zanobio, M.; Ranucci, G.; D’Amico, A.; Cicala, D.; Iascone, M.; Bernardo, P.; Piccolo, V.; Ronchi, A.; et al. Mystery(n) Phenotypic Presentation in Europeans: Report of Three Further Novel Missense RNF213 Variants Leading to Severe Syndromic Forms of Moyamoya Angiopathy and Literature Review. Int. J. Mol. Sci. 2022, 23, 8952. [Google Scholar] [CrossRef]
- Koganebuchi, K.; Sato, K.; Fujii, K.; Kumabe, T.; Haneji, K.; Toma, T.; Ishida, H.; Joh, K.; Soejima, H.; Mano, S.; et al. An analysis of the demographic history of the risk allele R4810K in RNF213 of moyamoya disease. Ann. Hum. Genet. 2021, 85, 166–177. [Google Scholar] [CrossRef]
- Shoemaker, L.D.; Clark, M.J.; Patwardhan, A.; Chandratillake, G.; Garcia, S.; Chen, R.; Morgan, A.A.; Leng, N.; Kirk, S.; Chen, R.; et al. Disease Variant Landscape of a Large Multiethnic Population of Moyamoya Patients by Exome Sequencing. G3 Genes Genomes Genet. 2015, 6, 41–49. [Google Scholar] [CrossRef]
- Kim, E.H.; Yum, M.S.; Ra, Y.S.; Park, J.B.; Ahn, J.S.; Kim, G.H.; Goo, H.W.; Ko, T.S.; Yoo, H.W. Importance of RNF213 polymorphism on clinical features and long-term outcome in moyamoya disease. J. Neurosurg. 2016, 124, 1221–1227. [Google Scholar] [CrossRef]
- Jee, T.K.; Yeon, J.Y.; Kim, S.M.; Bang, O.Y.; Kim, J.S.; Hong, S.C. Prospective Screening of Extracranial Systemic Arteriopathy in Young Adults with Moyamoya Disease. J. Am. Heart Assoc. 2020, 9, e016670. [Google Scholar] [CrossRef]
- Ok, T.; Jung, Y.H.; Lee, K.Y. Genotype-Phenotype Correlation of the RNF213 R4810K Variant in Moyamoya Disease. J. Stroke 2023, 25, 303–306. [Google Scholar] [CrossRef]
- An, S.; Kim, T.; Oh, C.W.; Bang, J.S.; Lee, S.U.; Heo, J. Author Correction: Vascular tortuosity of the internal carotid artery is related to the RNF213 c.14429G > A variant in moyamoya disease. Sci. Rep. 2020, 10, 4067, Erratum in Sci. Rep. 2019, 9, 8614. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Yamaguchi, K.; Akagawa, H.; Kawashima, A.; Moteki, Y.; Ishikawa, T.; Aihara, Y.; Saito, T.; Okada, Y.; Kawamata, T. Genotype-Phenotype Correlation in Long-Term Cohort of Japanese Patients with Moyamoya Disease. Cerebrovasc. Dis. 2019, 47, 105–111. [Google Scholar] [CrossRef]
- Miyatake, S.; Touho, H.; Miyake, N.; Ohba, C.; Doi, H.; Saitsu, H.; Taguri, M.; Morita, S.; Matsumoto, N. Sibling cases of moyamoya disease having homozygous and heterozygous c.14576G>A variant in RNF213 showed varying clinical course and severity. J. Hum. Genet. 2012, 57, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Miyake, N.; Touho, H.; Nishimura-Tadaki, A.; Kondo, Y.; Okada, I.; Tsurusaki, Y.; Doi, H.; Sakai, H.; Saitsu, H.; et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology 2012, 78, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Miyawaki, S.; Imai, H.; Hongo, H.; Kiyofuji, S.; Torazawa, S.; Koizumi, S.; Miyazawa, R.; Saito, N. Stroke Events and Risk Factors in Older Patients with Moyamoya Disease. World Neurosurg. 2024, 187, e405–e413. [Google Scholar] [CrossRef]
- Ishigami, D.; Miyawaki, S.; Imai, H.; Shimizu, M.; Hongo, H.; Dofuku, S.; Ohara, K.; Teranishi, Y.; Shimada, D.; Koizumi, S.; et al. RNF213 p.Arg4810Lys Heterozygosity in Moyamoya Disease Indicates Early Onset and Bilateral Cerebrovascular Events. Transl. Stroke Res. 2022, 13, 410–419, Erratum in Transl. Stroke Res. 2022, 13, 652. [Google Scholar] [CrossRef]
- Sasagasako, T.; Mineharu, Y.; Funaki, T.; Fushimi, Y.; Chihara, H.; Park, S.; Nakajima, K.; Matsui, Y.; Okawa, M.; Kikuchi, T.; et al. RNF213 Mutation Associated with the Progression from Middle Cerebral Artery Steno-Occlusive Disease to Moyamoya Disease. Transl. Stroke Res. 2024, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Mukawa, M.; Akagawa, H.; Thamamongood, T.; Inaji, M.; Tanaka, Y.; Maehara, T.; Kasuya, H.; Nariai, T. Absence of the RNF213 p.R4810K variant may indicate a severe form of pediatric moyamoya disease in Japanese patients. J. Neurosurg. Pediatr. 2021, 29, 48–56. [Google Scholar] [CrossRef]
- Inoue, T.; Murakami, N.; Sakadume, S.; Kido, Y.; Kikuchi, A.; Ichinoi, N.; Suzuki, K.; Kure, S.; Sakuta, R. Differing phenotypes of Moyamoya disease in a familial case involving heterozygous c.14429G > A variant in RNF213. Pediatr. Int. 2015, 57, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Moteki, Y.; Onda, H.; Kasuya, H.; Yoneyama, T.; Okada, Y.; Hirota, K.; Mukawa, M.; Nariai, T.; Mitani, S.; Akagawa, H. Systematic Validation of RNF213 Coding Variants in Japanese Patients with Moyamoya Disease. J. Am. Heart Assoc. 2015, 4, e001862. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Higashimoto, K.; Maeda, T.; Kawashima, M.; Matsuo, M.; Abe, T.; Matsushima, T.; Soejima, H. Differences in the Genotype Frequency of the RNF213 Variant in Patients with Familial Moyamoya Disease in Kyushu, Japan. Neurol. Med. Chir. 2017, 57, 607–611. [Google Scholar] [CrossRef]
- Cao, Y.; Kobayashi, H.; Morimoto, T.; Kabata, R.; Harada, K.H.; Koizumi, A. Frequency of RNF213 p.R4810K, a susceptibility variant for moyamoya disease, and health characteristics of carriers in the Japanese population. Environ. Health Prev. Med. 2016, 21, 387–390. [Google Scholar] [CrossRef]
- Torazawa, S.; Miyawaki, S.; Imai, H.; Hongo, H.; Ishigami, D.; Shimizu, M.; Ono, H.; Shinya, Y.; Sato, D.; Sakai, Y.; et al. RNF213 p.Arg4810Lys Wild Type is Associated with De Novo Hemorrhage in Asymptomatic Hemispheres with Moyamoya Disease. Transl. Stroke Res. 2024, 15, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Mineharu, Y.; Takagi, Y.; Takahashi, J.C.; Hashikata, H.; Liu, W.; Hitomi, T.; Kobayashi, H.; Koizumi, A.; Miyamoto, S. Rapid progression of unilateral moyamoya disease in a patient with a family history and an RNF213 risk variant. Cerebrovasc. Dis. 2013, 36, 155–157. [Google Scholar] [CrossRef]
- Aoyama, J.; Nariai, T.; Mukawa, M.; Inaji, M.; Tanaka, Y.; Maehara, T. Case of Familial Moyamoya Disease Presenting 10 Years After Initial Negative Magnetic Resonance Screening in Childhood. World Neurosurg. 2017, 105, 1035.e1–1035.e4. [Google Scholar] [CrossRef]
- Mineharu, Y.; Takagi, Y.; Koizumi, A.; Morimoto, T.; Funaki, T.; Hishikawa, T.; Araki, Y.; Hasegawa, H.; Takahashi, J.C.; Kuroda, S.; et al. SUPRA Japan Study Group. Posterior cerebral artery involvement in unilateral moyamoya disease is exclusively ipsilateral and influenced by RNF213 mutation gene dose: The SUPRA Japan study: PCA involvement in unilateral moyamoya. J. Stroke Cerebrovasc. Dis. 2024, 33, 107513. [Google Scholar] [CrossRef]
- Hirano, Y.; Miyawaki, S.; Imai, H.; Hongo, H.; Teranishi, Y.; Ishigami, D.; Sakai, Y.; Shimada, D.; Umekawa, M.; Segawa, M.; et al. Clinical and Genetic Characteristics of Patients with Moyamoya Disease who Experienced Both Ischemic and Hemorrhagic Events. World Neurosurg. 2023, 172, e438–e446. [Google Scholar] [CrossRef]
- Nomura, S.; Akagawa, H.; Yamaguchi, K.; Azuma, K.; Nakamura, A.; Fukui, A.; Matsuzawa, F.; Aihara, Y.; Ishikawa, T.; Moteki, Y.; et al. Difference in Clinical Phenotype, Mutation Position, and Structural Change of RNF213 Rare Variants Between Pediatric and Adult Japanese Patients with Moyamoya Disease. Transl. Stroke Res. 2024, 15, 1142–1153. [Google Scholar] [CrossRef]
- Yajima, H.; Miyawaki, S.; Sayama, S.; Kumasawa, K.; Ikemura, M.; Imai, H.; Hongo, H.; Hirano, Y.; Ishigami, D.; Torazawa, S.; et al. Hypertensive disorders of pregnancy in moyamoya disease: A single institution experience. J. Stroke Cerebrovasc. Dis. 2023, 32, 107377. [Google Scholar] [CrossRef] [PubMed]
- Uchino, H.; Ito, M.; Tokairin, K.; Tatezawa, R.; Sugiyama, T.; Kazumata, K.; Fujimura, M. Association of RNF213 polymorphism and cortical hyperintensity sign on fluid-attenuated inversion recovery images after revascularization surgery for moyamoya disease: Possible involvement of intrinsic vascular vulnerability. Neurosurg. Rev. 2023, 46, 119. [Google Scholar] [CrossRef]
- Kinoshita, T.; Tamada, N.; Hara, S.; Mukawa, M.; Shintaku, H.; Inaji, M.; Tanaka, Y.; Nariai, T.; Maehara, T. Two Postmortem Cases of Moyamoya Disease with Different RNF213 p.R4810K Variant Statuses. NMC Case Rep. J. 2024, 11, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, D.; Zhang, J.; Zhao, W. Association between the rs112735431 polymorphism of the RNF213 gene and moyamoya disease: A case-control study and meta-analysis. J. Clin. Neurosci. 2016, 32, 14–18. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wei, L.; Zhang, Q.; Zou, Z.; Yang, L.; Li, D.; Shang, M.; Han, C.; Mambiya, M.; et al. Predictive role of heterozygous p.R4810K of RNF213 in the phenotype of Chinese moyamoya disease. Neurology 2020, 94, e678–e686. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, X.; Zou, Z.; Ta, N.; Hao, F.; Yang, Y.; Li, D.; Liang, M.; Han, C.; et al. Validation and Extension Study Exploring the Role of RNF213 p.R4810K in 2,877 Chinese Moyamoya Disease Patients. J. Stroke Cerebrovasc. Dis. 2021, 30, 106071. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, H.; Zhang, L.; Xu, X.; Zhang, X.; Kang, Z.; Song, D.; Zhang, J.; Guan, M.; Gu, Y. Molecular analysis of RNF213 gene for moyamoya disease in the Chinese Han population. PLoS ONE 2012, 7, e48179. [Google Scholar] [CrossRef]
- Ge, P.; Ye, X.; Liu, X.; Deng, X.; Wang, R.; Zhang, Y.; Zhang, D.; Zhang, Q.; Zhao, J. Association Between p.R4810K Variant and Long-Term Clinical Outcome in Patients with Moyamoya Disease. Front. Neurol. 2019, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zeng, C.; Ge, P.; Liu, C.; Li, J.; Zhang, Y.; Zhang, D.; Zhang, Q.; Zhao, J. Association of RNF213 Variants with Periventricular Anastomosis in Moyamoya Disease. Stroke 2022, 53, 2906–2916. [Google Scholar] [CrossRef]
- Hao, F.; Gao, G.; Guo, Q.; Liu, S.; Wang, M.; Chang, Z.; Wang, H.; Lu, M.; Liu, S.; Zou, Z.; et al. Risk Factors for Massive Cerebral Infarction in Pediatric Patients with Moyamoya Disease. Pediatr. Neurol. 2024, 153, 159–165. [Google Scholar] [CrossRef]
- Hao, F.; Han, C.; Lu, M.; Wang, Y.; Gao, G.; Wang, Q.; Liu, S.; Liu, S.; Wang, M.; Ren, B.; et al. High-resolution MRI vessel wall enhancement in moyamoya disease: Risk factors and clinical outcomes. Eur. Radiol. 2024, 34, 5179–5189. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.Y.; Zhang, S.; Wang, W. Different phenotypes of moyamoya disease in a Chinese familial case involving heterozygous c.14429G>a variant in RNF213. Br. J. Neurosurg. 2023, 37, 1882–1885. [Google Scholar] [CrossRef]
- Liu, S.M.; Gao, G.; Hao, F.B.; Liu, S.T.; Yang, R.M.; Zhang, H.D.; Wang, M.J.; Zou, Z.X.; Yu, D.; Zhang, Q.; et al. Isolated anterior cerebral artery occlusion: An atypical form of moyamoya disease. Stroke Vasc. Neurol. 2024, 9, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chen, Y.F.; Fan, P.C.; Wang, K.C.; Wang, K.; Wang, J.; Kuo, M.F. Mutation genotypes of RNF213 gene from moyamoya patients in Taiwan. J. Neurol. Sci. 2015, 353, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.; Shafeeque, C.M.; Sudhir, J.B.; Banerjee, M.; Sylaja, P.N. Ethnic variation and the relevance of homozygous RNF 213 p.R4810.K variant in the phenotype of Indian Moya moya disease. PLoS ONE 2020, 15, e0243925, Erratum in PLoS ONE 2021, 16, e0249469. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, H.; Wu, X.; Niu, X.; Dai, Y.; Zhou, Z.; Ye, F. Neuregulin 1 as a potential biomarker for disease progression in moyamoya disease: A case-control study in Chinese population. J. Stroke Cerebrovasc. Dis. 2024, 33, 107581. [Google Scholar] [CrossRef]
- Odiete, O.; Hill, M.F.; Sawyer, D.B. Neuregulin in cardiovascular development and disease. Circ. Res. 2012, 111, 1376–1385. [Google Scholar] [CrossRef]
- Thamamongood, T.; Hara, S.; Akagawa, H.; Inaji, M.; Tanaka, Y.; Nariai, T.; Maehara, T. Synergistic Interaction of Thyroid Autoantibodies and Ring Finger Protein 213 Variant in Moyamoya Disease. Neurol. Med. Chir. 2024, 64, 43–49. [Google Scholar] [CrossRef]
- Bao, X.Y.; Tong, H.Y.; Wang, Q.N.; Wang, X.P.; Gao, G.; Zhang, Q.; Zou, Z.X.; Duan, L. A long-term study of posterior circulation changes after revascularization in patients with moyamoya disease. J. Neurosurg. 2023, 139, 1281–1286. [Google Scholar] [CrossRef]
- Nomura, S.; Akagawa, H.; Yamaguchi, K.; Kawashima, A.; Kawamata, T. Surgical Options and Genetic Screening of a Patient with Moyamoya Disease Harboring the RNF213 p.R4180 K Homozygous Variant. J. Child Neurol. 2020, 35, 621–622. [Google Scholar] [CrossRef]
- Kuroda, S.; Houkin, K.; Ishikawa, T.; Nakayama, N.; Iwasaki, Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery 2010, 66, 1093–1101; discussion 1101. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.; Xue, Y. Response to Letter to the Editor on “Clinical Features and Surgical Outcomes of Patients with Moyamoya Disease and the Homozygous RNF213 p.R4810 K Variant”. J. Child Neurol. 2020, 35, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ge, P.; Ma, Y.; Zhang, D.; Wang, R.; Zhang, Y.; Wang, S.; Cao, Y.; Zhao, M.; Zhao, J. Clinical Features and Surgical Outcomes of Patients with Moyamoya Disease and the Homozygous RNF213 p.R4810K Variant. J. Child Neurol. 2019, 34, 793–800. [Google Scholar] [CrossRef]
- Zheng, E.Y.; Hara, S.; Inaji, M.; Tanaka, Y.; Nariai, T.; Maehara, T. Regression of periventricular anastomosis after indirect revascularization in pediatric patients with moyamoya disease. J. Neurosurg. Pediatr. 2023, 32, 719–728. [Google Scholar] [CrossRef]
- Sato, D.; Miyawaki, S.; Imai, H.; Hongo, H.; Kiyofuji, S.; Koizumi, S.; Saito, N. Clinical Characteristics of Immediate Contralateral Ischemia Subsequent to Revascularization for Moyamoya Disease. World Neurosurg. 2024, 183, e355–e365. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, M.; Ito, M.; Uchino, H.; Sugiyama, T.; Fujimura, M. Impact of RNF213 p.R4810K variant on postoperative temporal muscle swelling used in encephalo-myo-synangiosis after combined revascularization for Moyamoya disease. Neurosurg. Rev. 2024, 48, 15. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, R.; Fujimura, M.; Katsuki, M.; Nishizawa, T.; Tomata, Y.; Niizuma, K.; Tominaga, T. Prolonged/delayed cerebral hyperperfusion in adult patients with moyamoya disease with RNF213 gene polymorphism c.14576G>A (rs112735431) after superficial temporal artery-middle cerebral artery anastomosis. J. Neurosurg. 2020, 135, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Ye, X.; Liu, X.; Deng, X.; Wang, J.; Wang, R.; Zhang, Y.; Zhang, D.; Zhang, Q.; Zhao, J. Association between p.R4810K Variant and Postoperative Collateral Formation in Patients with Moyamoya Disease. Cerebrovasc. Dis. 2019, 48, 77–84. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, B.J.; Lee, M.H.; Lee, H.B.; Lee, J.S.; Chang, D.I.; Choi-Kwon, S.; Chun, S.; Lee, J.K.; Kang, D.W.; et al. Are Genetic Variants Associated with the Location of Cerebral Arterial Lesions in Stroke Patients? Cerebrovasc. Dis. 2020, 49, 262–268. [Google Scholar] [CrossRef]
- Zedde, M.; Grisendi, I.; Assenza, F.; Napoli, M.; Moratti, C.; Pavone, C.; Bonacini, L.; Di Cecco, G.; D’Aniello, S.; Stoenoiu, M.S.; et al. RNF213 Polymorphisms in Intracranial Artery Dissection. Genes 2024, 15, 725. [Google Scholar] [CrossRef]
- Dofuku, S.; Miyawaki, S.; Imai, H.; Shimizu, M.; Hongo, H.; Shinya, Y.; Ohara, K.; Teranishi, Y.; Ono, H.; Nakatomi, H.; et al. RNF213 p.Arg4810Lys Variant Is Associated with Higher Stenosis Progression in Asymptomatic Intracranial Artery Stenosis. Transl. Stroke Res. 2024, 16, 1293–1300. [Google Scholar] [CrossRef]
- Suzuki, M.; Mineharu, Y.; Okawa, M.; Yoshida, K.; Nagata, M.; Yang, T.; Suzuki, K.; Takayama, N.; Yamamoto, Y.; Tabara, Y.; et al. Common and distinct risk profiles of asymptomatic extra- and intracranial atherosclerosis in the Nagahama cohort. J. Stroke Cerebrovasc. Dis. 2024, 33, 107782. [Google Scholar] [CrossRef]
- Ohara, M.; Yoshimoto, T.; Okazaki, S.; Gon, Y.; Todo, K.; Sasaki, T.; Takasugi, J.; Ohara, N.; Ihara, M.; Mochizuki, H. RNF213 p.R4810K Variant Carriers with Intracranial Arterial Stenosis Have a Low Atherosclerotic Burden. J. Atheroscler. Thromb. 2022, 29, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Hongo, H.; Miyawaki, S.; Imai, H.; Shimizu, M.; Yagi, S.; Mitsui, J.; Ishiura, H.; Yoshimura, J.; Doi, K.; Qu, W.; et al. Comprehensive investigation of RNF213 nonsynonymous variants associated with intracranial artery stenosis. Sci. Rep. 2020, 10, 11942. [Google Scholar] [CrossRef]
- Bang, O.Y.; Ryoo, S.; Kim, S.J.; Yoon, C.H.; Cha, J.; Yeon, J.Y.; Kim, K.H.; Kim, G.M.; Chung, C.S.; Lee, K.H.; et al. Adult Moyamoya Disease: A Burden of Intracranial Stenosis in East Asians? PLoS ONE 2015, 10, e0130663. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, E.H.; Chung, J.W.; Kim, J.H.; Kim, Y.S.; Seo, W.K.; Kim, G.M.; Bang, O.Y. Role of the RNF213 Variant in Vascular Outcomes in Patients with Intracranial Atherosclerosis. J. Am. Heart Assoc. 2021, 10, e017660. [Google Scholar] [CrossRef]
- Morimoto, T.; Mineharu, Y.; Kobayashi, H.; Harada, K.H.; Funaki, T.; Takagi, Y.; Sakai, N.; Miyamoto, S.; Koizumi, A. Significant Association of the RNF213 p.R4810K Polymorphism with Quasi-Moyamoya Disease. J. Stroke Cerebrovasc. Dis. 2016, 25, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Hongo, H.; Miyawaki, S.; Imai, H.; Shinya, Y.; Ono, H.; Mori, H.; Nakatomi, H.; Kunimatsu, A.; Saito, N. Smaller outer diameter of atherosclerotic middle cerebral artery associated with RNF213 c.14576G>A Variant (rs112735431). Surg. Neurol. Int. 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Shinya, Y.; Miyawaki, S.; Imai, H.; Hongo, H.; Ono, H.; Takenobu, A.; Nakatomi, H.; Teraoka, A.; Saito, N. Genetic Analysis of Ring Finger Protein 213 (RNF213) c.14576G>A in Intracranial Atherosclerosis of the Anterior and Posterior Circulations. J. Stroke Cerebrovasc. Dis. 2017, 26, 2638–2644. [Google Scholar] [CrossRef]
- Okazaki, S.; Morimoto, T.; Kamatani, Y.; Kamimura, T.; Kobayashi, H.; Harada, K.; Tomita, T.; Higashiyama, A.; Takahashi, J.C.; Nakagawara, J.; et al. Moyamoya Disease Susceptibility Variant RNF213 p.R4810K Increases the Risk of Ischemic Stroke Attributable to Large-Artery Atherosclerosis. Circulation 2019, 139, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Shimada, D.; Miyawaki, S.; Nakanishi, K.; Jono, T.; Maruoka, H.; Kawai, T.; Harada, Y.; Kono, T.; Komatsubara, K.; Nakauchi, J.; et al. RNF213 p.Arg4810Lys (c.14429G>A) is associated with extracranial arterial stenosis. Brain Commun. 2025, 7, fcaf049. [Google Scholar] [CrossRef]
- Fukushima, Y.; Miyawaki, S.; Inoue, T.; Shimizu, S.; Yoshikawa, G.; Imai, H.; Saito, N.; Tsutsumi, K. Repeated de novo aneurysm formation after anastomotic surgery: Potential risk of genetic variant RNF213 c.14576G>A. Surg. Neurol. Int. 2015, 6, 41. [Google Scholar] [CrossRef]
- Sun, X.; Luo, M.; Li, J.; Lai, R.; Lin, J.; Wang, Y.; Xu, X.; Wu, S.; Sheng, W. Prevalence of RNF213 variants in symptomatic intracranial arterial stenosis/occlusion in China. Mol. Genet. Genom. 2020, 295, 635–643. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, L.; Ge, P.; Ma, Y.; Zhang, D.; Zhang, Y.; Wang, R.; Wang, S.; Zhao, Y.; Cao, Y.; et al. Association of Ring Finger Protein 213 Gene, P.R4810k Polymorphism with Intracranial Major Artery Stenosis/Occlusion. J. Stroke Cerebrovasc. Dis. 2018, 27, 1556–1564. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.S.; Woo, M.H.; An, H.J.; Kim, J.O.; Park, H.S.; Ryu, C.S.; Kim, O.J.; Kim, N.K. Distribution of Intracranial Major Artery Stenosis/Occlusion According to RNF213 Polymorphisms. Int. J. Mol. Sci. 2020, 21, 1956. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, T.; Li, B.; Hu, S.; Liu, J.; Deng, J.; Tan, H.; Yan, J. Rare RNF213 variants and the risk of intracranial artery stenosis/occlusion disease in Chinese population: A case-control study. BMC Med. Genet. 2019, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, C.; Liao, X.; Xia, J.; Wang, X.; Deng, J.; Yan, J. Genetic analysis of RNF213 p.R4810K variant in non-moyamoya intracranial artery stenosis/occlusion disease in a Chinese population. Environ. Health Prev. Med. 2017, 22, 41. [Google Scholar] [CrossRef]
- Xue, S.; Cheng, W.; Wang, W.; Song, X.; Wu, J.; Song, H. The association between the ring finger protein 213 gene R4810K variant and intracranial major artery stenosis/occlusion in the Han Chinese population and high-resolution magnetic resonance imaging findings. Brain Circ. 2018, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Mineharu, Y.; Kimura, M.; Takagi, Y.; Kobayashi, H.; Hitomi, T.; Harada, K.H.; Uchihashi, Y.; Funaki, T.; Miyamoto, S.; et al. RNF213 p.R4810K Variant and Intracranial Arterial Stenosis or Occlusion in Relatives of Patients with Moyamoya Disease. J. Stroke Cerebrovasc. Dis. 2017, 26, 1841–1847. [Google Scholar] [CrossRef]
- Ogura, S.; Ohara, T.; Tanaka, E.; Ashida, S.; Maezono-Kandori, K.; Hanya, M.; Mizuta, I.; Mizuno, T. Clinical characteristics and intracranial arterial lesions of non-young adult ischemic stroke patients with RNF213 p.R4810K variant. J. Neurol. Sci. 2023, 452, 120775. [Google Scholar] [CrossRef] [PubMed]
- Nohara, A. What Can Be Seen From “Intracranial-Vascular”-Susceptibility Genetic Factor in “Cardiovascular-Susceptible” Familial Hypercholesterolemia: A New Clue. JACC Asia 2023, 3, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, T.; Okazaki, S.; Morimoto, T.; Kobayashi, H.; Harada, K.; Tomita, T.; Higashiyama, A.; Yoshimoto, T.; Takahashi, J.C.; Nakagawara, J.; et al. Prevalence of RNF213 p.R4810K Variant in Early-Onset Stroke with Intracranial Arterial Stenosis. Stroke 2019, 50, 1561–1563. [Google Scholar] [CrossRef]
- Okazaki, S.; Yoshimoto, T.; Ohara, M.; Takagaki, M.; Nakamura, H.; Watanabe, K.; Gon, Y.; Todo, K.; Sasaki, T.; Araki, H.; et al. Effect of the RNF213 p.R4810K Variant on the Progression of Intracranial Artery Stenosis: A 15-Year Follow-up Study. Neurol. Genet. 2022, 8, e200029. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.B.; Kwon, H.S. RNF213 Polymorphism in Intracranial Artery Dissection. J. Stroke 2018, 20, 404–406. [Google Scholar] [CrossRef]
- Shinya, Y.; Miyawaki, S.; Nakatomi, H.; Shin, M.; Teraoka, A.; Saito, N. Hemorrhagic Onset Intracranial Artery Dissection of Middle Cerebral Artery Followed by Progressive Arterial Stenosis with Genetic Variant RNF213 p.Arg4810Lys (rs112735431). World Neurosurg. 2020, 141, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Yu, L.; Duan, R.; Ma, Y.; Ge, P.; Zhang, D.; Zhang, Y.; Wang, R.; Wang, S.; et al. The Association of the RNF213 p.R4810K Polymorphism with Quasi-Moyamoya Disease and a Review of the Pertinent Literature. World Neurosurg. 2017, 99, 701–708.e1. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.T.E.; Mizuta, I.; Watanabe-Hosomi, A.; Yokote, A.; Koizumi, T.; Mukai, M.; Kinoshita, M.; Ohara, T.; Mizuno, T. RNF213-related susceptibility of Japanese CADASIL patients to intracranial arterial stenosis. J. Hum. Genet. 2018, 63, 687–690. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Mitra, P.; Mishra, S.; Roy, A.; Podder, G.; Ray, B.K.; Biswas, A.; Hui, S.P.; Banerjee, T.K.; Biswas, A. Arg4810Lys mutation in RNF213 among Eastern Indian non-MMD ischemic stroke patients: A genotype-phenotype correlation. Neurol. Sci. 2024, 45, 315–319. [Google Scholar] [CrossRef]
- Inoue, H.; Oomura, M.; Nishikawa, Y.; Mase, M.; Matsukawa, N. Aplastic or twig-like middle cerebral artery and cardiogenic cerebral embolism mimicking moyamoya disease with RNF213 polymorphism: A case report. Interv. Neuroradiol. 2022, 28, 634–638. [Google Scholar] [CrossRef]
- Phi, J.H.; Choi, J.W.; Seong, M.W.; Kim, T.; Moon, Y.J.; Lee, J.; Koh, E.J.; Ryu, S.K.; Kang, T.H.; Bang, J.S.; et al. Association between moyamoya syndrome and the RNF213 c.14576G>A variant in patients with neurofibromatosis Type 1. J. Neurosurg. Pediatr. 2016, 17, 717–722. [Google Scholar] [CrossRef]
- Zhou, H.; Jing, J.; Pu, Y.; Li, W.; Meng, X.; Wang, A.; Zuo, Y.; Xu, Z.; Xu, Q.; Suo, Y.; et al. Detailed phenotype of RNF213 p.R4810K variant identified by the Chinese patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc. Neurol. 2023, 8, 503–510. [Google Scholar] [CrossRef]
- Lin, T.C.; Uchino, H.; Ito, M.; Yamaguchi, S.; Ishi, Y.; Fujimura, M. Moyamoya syndrome after proton beam therapy in a pediatric patient with a pineal germ cell tumor and a germline polymorphism in RNF213. Child’s Nerv. Syst. 2024, 40, 3873–3878. [Google Scholar] [CrossRef]
- Eto, F.; Yoshimoto, T.; Okazaki, S.; Nishimura, K.; Ogura, S.; Yamaguchi, E.; Fukuma, K.; Saito, S.; Washida, K.; Koga, M.; et al. RNF213 p.R4810K (c.14429G > A) Variant Determines Anatomical Variations of the Circle of Willis in Cerebrovascular Disease. Front. Aging Neurosci. 2021, 13, 681743. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, S.; Yoshimoto, T.; Ihara, M. A case of hemichorea in RNF213-related vasculopathy. BMC Neurol. 2021, 21, 32. [Google Scholar] [CrossRef]
- Torazawa, S.; Miyawaki, S.; Shinya, Y.; Kawashima, M.; Hasegawa, H.; Dofuku, S.; Uchikawa, H.; Kin, T.; Shin, M.; Nakatomi, H.; et al. De Novo Development of Moyamoya Disease after Stereotactic Radiosurgery for Brain Arteriovenous Malformation in a Patient with RNF213 p.Arg4810Lys (rs112735431). World Neurosurg. 2020, 140, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Echizenya, I.; Tokairin, K.; Kawabori, M.; Kazumata, K.; Houkin, K. Reversible Cerebral Angiopathy after Viral Infection in a Pediatric Patient with Genetic Variant of RNF213. J. Stroke Cerebrovasc. Dis. 2020, 29, 104549. [Google Scholar] [CrossRef] [PubMed]
- Iwanishi, M.; Azuma, C.; Tezuka, Y.; Yamamoto, Y.; Ito-Kobayashi, J.; Washiyama, M.; Kusakabe, T.; Kikugawa, S. Observation of p.R4810K, a Polymorphism of the Mysterin Gene, the Susceptibility Gene for Moyamoya Disease, in Two Female Japanese Diabetic Patients with Familial Partial Lipodystrophy 1. Intern. Med. 2020, 59, 2529–2537. [Google Scholar] [CrossRef]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kabata, R.; Kinoshita, H.; Morimoto, T.; Ono, K.; Takeda, M.; Choi, J.; Okuda, H.; Liu, W.; Harada, K.H.; et al. Rare variants in RNF213, a susceptibility gene for moyamoya disease, are found in patients with pulmonary hypertension and aggravate hypoxia-induced pulmonary hypertension in mice. Pulm. Circ. 2018, 8, 2045894018778155. [Google Scholar] [CrossRef]
- Hiraide, T.; Kataoka, M.; Suzuki, H.; Aimi, Y.; Chiba, T.; Isobe, S.; Katsumata, Y.; Goto, S.; Kanekura, K.; Yamada, Y.; et al. Poor outcomes in carriers of the RNF213 variant (p.Arg4810Lys) with pulmonary arterial hypertension. J. Heart Lung Transplant. 2020, 39, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A.; Song, J.S.; Park, T.K.; Yang, J.H.; Kwon, W.C.; Kim, S.R.; Kim, S.M.; Cha, J.; Jang, S.Y.; Cho, Y.S.; et al. Nonsyndromic Peripheral Pulmonary Artery Stenosis Is Associated With Homozygosity of RNF213 p.Arg4810Lys Regardless of Co-occurrence of Moyamoya Disease. Chest 2018, 153, 404–413. [Google Scholar] [CrossRef]
- Kanezawa, M.; Shimokawahara, H.; Tsuji, M.; Suruga, K.; Miyagi, A.; Marunaka, M.; Mukai, T.; Kawaguchi, T.; Yang, T.Y.; Yamaguchi, I.; et al. The results of genetic analysis and clinical outcomes after stent deployment in adult patients with isolated peripheral pulmonary artery stenosis. Eur. Respir. J. 2023, 62, 2301511. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Minatsuki, S.; Fujita, K.; Takeda, N.; Hatano, M.; Komuro, I. Two Siblings with Peripheral Pulmonary Arterial Stenosis: Pulmonary Angiography of Advanced and Early Stages. Chest 2022, 161, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakamura, J.; Sakiyama, S.; Nakaya, T.; Sato, T.; Watanabe, T.; Ohira, H.; Makita, K.; Tomaru, U.; Ishizu, A.; et al. A histopathological report of a 16-year-old male with peripheral pulmonary artery stenosis and Moyamoya disease with a homozygous RNF213 mutation. Respir. Med. Case Rep. 2019, 29, 100977. [Google Scholar] [CrossRef]
- Fukushima, H.; Takenouchi, T.; Kosaki, K. Homozygosity for moyamoya disease risk allele leads to moyamoya disease with extracranial systemic and pulmonary vasculopathy. Am. J. Med. Genet. Part A 2016, 170, 2453–2456. [Google Scholar] [CrossRef]
- Kanezawa, M.; Shimokawahara, H.; Miyagi, A.; Matsubara, H. Stenting of Isolated Pulmonary Artery Stenosis in an Adult Patient with RNF213 p.Arg4810Lys Variant. Can. J. Cardiol. 2024, 40, 2255–2258. [Google Scholar] [CrossRef]
- Kiko, T.; Asano, R.; Ishibashi, T.; Endo, H.; Nishi, N.; Hayashi, H.; Ueda, J.; Aoki, T.; Tsuji, A.; Nakaoka, Y.; et al. Prevalence and Clinical Characteristics of Heterozygous RNF213 p.Arg4810Lys Variant Carriers Diagnosed with Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2024, 13, e035009. [Google Scholar] [CrossRef]
- Ozaki, D.; Endo, H.; Tashiro, R.; Sugimura, K.; Tatebe, S.; Yasuda, S.; Tomata, Y.; Endo, T.; Tominaga, K.; Niizuma, K.; et al. Association between RNF213 c.14576G>A Variant (rs112735431) and Peripheral Pulmonary Artery Stenosis in Moyamoya Disease. Cerebrovasc. Dis. 2022, 51, 282–287. [Google Scholar] [CrossRef]
- Morimoto, T.; Mineharu, Y.; Ono, K.; Nakatochi, M.; Ichihara, S.; Kabata, R.; Takagi, Y.; Cao, Y.; Zhao, L.; Kobayashi, H.; et al. Significant association of RNF213 p.R4810K, a moyamoya susceptibility variant, with coronary artery disease. PLoS ONE 2017, 12, e0175649. [Google Scholar] [CrossRef]
- Nomura, S.; Aihara, Y.; Akagawa, H.; Chiba, K.; Yamaguchi, K.; Kawashima, A.; Okada, Y.; Kawamata, T. Can Moyamoya Disease Susceptibility Gene Affect Extracranial Systemic Artery Stenosis? J. Stroke Cerebrovasc. Dis. 2020, 29, 104532. [Google Scholar] [CrossRef]
- Koizumi, A.; Kobayashi, H.; Liu, W.; Fujii, Y.; Senevirathna, S.T.; Nanayakkara, S.; Okuda, H.; Hitomi, T.; Harada, K.H.; Takenaka, K.; et al. P.R4810K, a polymorphism of RNF213, the susceptibility gene for moyamoya disease, is associated with blood pressure. Environ. Health Prev. Med. 2013, 18, 121–129. [Google Scholar] [CrossRef]
- Hikino, K.; Koyama, S.; Ito, K.; Koike, Y.; Koido, M.; Matsumura, T.; Kurosawa, R.; Tomizuka, K.; Ito, S.; Liu, X.; et al. RNF213 Variants, Vasospastic Angina, and Risk of Fatal Myocardial Infarction. JAMA Cardiol. 2024, 9, 723–731. [Google Scholar] [CrossRef]
- Ishiyama, H.; Tanaka, T.; Yoshimoto, T.; Takahashi, A.; Ogata, S.; Nishimura, K.; Asano, Y.; Koizumi, A.; Noguchi, T.; Ihara, M. RNF213 p.R4810K Variant Increases the Risk of Vasospastic Angina. JACC Asia 2023, 3, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Yamada, H.; Setoh, K.; Matsukawa, M.; Takahashi, M.; Kawaguchi, T.; Nakayama, T.; Matsuda, F.; Kosugi, S. The association between the Moyamoya disease susceptible gene RNF213 variant and incident cardiovascular disease in a general population: The Nagahama study. J. Hypertens. 2021, 39, 2521–2526. [Google Scholar] [CrossRef]
- Hara, S.; Shimizu, K.; Nariai, T.; Kishino, M.; Kudo, T.; Umemoto, T.; Inaji, M.; Maehara, T. De Novo Renal Artery Stenosis Developed in Initially Normal Renal Arteries during the Long-Term Follow-Up of Patients with Moyamoya Disease. J. Stroke Cerebrovasc. Dis. 2020, 29, 104786. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, H. Renovascular hypertension and RNF213 p.R4810K variant in Korean children with Moyamoya disease. Clin. Nephrol. 2021, 96, 105–111. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Liu, W.; Xiong, Y.; Sun, W.; Huang, X.; Jiang, Y.; Ni, G.; Sun, W.; Zhou, L.; et al. Impacts and interactions of PDGFRB, MMP-3, TIMP-2, and RNF213 polymorphisms on the risk of Moyamoya disease in Han Chinese human subjects. Gene 2013, 526, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Fan, Z.; Cheng, J.; Zhong, L.; Lin, Y.; Qu, X. Development of atherosclerotic-moyamoya syndrome with genetic variant of RNF213 p.R4810K and p.T1727M: A case report. Clin. Neurol. Neurosurg. 2018, 168, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, Q.; Yuan, S.; Shen, Y.; Feng, Y.; Liu, L.; Zhu, Y.; Zhao, Y.; Cui, J.; Qin, J.; et al. Recurrent Cerebral Infarction Due to Moyamoya Disease Complicated with Systemic Lupus Erythematosus: A Case Report and Literature Review. Neurologist 2024, 29, 4–13. [Google Scholar] [CrossRef]
- Nomura, S.; Akagawa, H.; Yamaguchi, K.; Ishikawa, T.; Kawashima, A.; Kasuya, H.; Mukawa, M.; Nariai, T.; Maehara, T.; Okada, Y.; et al. Rare and Low-Frequency Variants in RNF213 Confer Susceptibility to Moyamoya Syndrome Associated with Hyperthyroidism. World Neurosurg. 2019, 127, e460–e466. [Google Scholar] [CrossRef]
- Ryu, B.; Kawamata, T.; Yamaguchi, K.; Kawashima, A.; Ono, M.; Okada, Y. Moyamoya disease concurrent with Graves’ disease treated by direct bypass: Clinical features and treatment strategies. Acta Neurochir. 2015, 157, 1095–1102. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kaku, Y.; Koga, H. Case Report: RNF213 variant and choroidal anastomosis as potential risk factors for early stroke in moyamoya syndrome associated with Down syndrome. Front. Pediatr. 2023, 11, 1289554. [Google Scholar] [CrossRef]
- Chong, P.F.; Ogata, R.; Kobayashi, H.; Koizumi, A.; Kira, R. Early onset of moyamoya syndrome in a Down syndrome patient with the genetic variant RNF213 p.R4810K. Brain Dev. 2015, 37, 822–824. [Google Scholar] [CrossRef]
- Park, J.; Jang, W.; Han, J.Y. Differing disease phenotypes of Duchenne muscular dystrophy and Moyamoya disease in female siblings of a Korean family. Mol. Genet. Genom. Med. 2019, 7, e862. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kuwahara, M.; Saigoh, K.; Ishiura, H.; Yamagishi, Y.; Hamano, Y.; Samukawa, M.; Suzuki, H.; Hirano, M.; Mitsui, Y.; et al. The novel de novo mutation of KIF1A gene as the cause for Spastic paraplegia 30 in a Japanese case. eNeurologicalSci 2018, 14, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Chida-Nagai, A.; Tonoki, H.; Makita, N.; Ishiyama, H.; Ihara, M.; Maruo, Y.; Tsujioka, T.; Sasaki, D.; Izumi, G.; Yamazawa, H.; et al. A Noonan-like pediatric patient with a de novo CBL pathogenic variant and an RNF213 polymorphism p.R4810K presenting with cardiopulmonary arrest due to left main coronary artery ostial atresia. Am. J. Med. Genet. Part A 2023, 191, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kitayama, J.; Sahara, N.; Okata, T.; Miyake, N.; Matsumoto, N.; Kitazono, T.; Ago, T. Filamin A Variant as a Possible Second-Hit Gene Promoting Moyamoya Disease-like Vascular Formation Associated with RNF213 p.R4810K Variant. Neurol. Genet. 2022, 8, e200017. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, M.J.; Kim, S.J.; Lee, J.E.; Chae, J.H.; Ko, J.M. A case of CHOPS syndrome accompanied with moyamoya disease and systemic vasculopathy. Brain Dev. 2021, 43, 454–458. [Google Scholar] [CrossRef]
- Noda, K.; Hattori, Y.; Hori, M.; Harada-Shiba, M.; Ihara, M. A Case of Multiple Intracranial Major Artery Stenoses With Coexisting PCSK9 p.E32K and RNF213 p.R4810K Variants. Neurol. Genet. 2023, 9, e200099, Erratum in Neurol. Genet. 2024, 10, e200218. [Google Scholar] [CrossRef]
- Noda, K.; Hattori, Y.; Hori, M.; Nakaoku, Y.; Tanaka, A.; Yoshimoto, T.; Nishimura, K.; Yokota, T.; Harada-Shiba, M.; Ihara, M. Amplified Risk of Intracranial Artery Stenosis/Occlusion Associated with RNF213 p.R4810K in Familial Hypercholesterolemia. JACC Asia 2023, 3, 625–633. [Google Scholar] [CrossRef]
- Saito, S.; Hosoki, S.; Yamaguchi, E.; Ishiyama, H.; Abe, S.; Yoshimoto, T.; Tanaka, T.; Hattori, Y.; Liao, Y.C.; Lee, Y.C.; et al. Blended Phenotype of NOTCH3 and RNF213 Variants with Accelerated Large and Small Artery Crosstalk: A Case Report and Literature Review. Neurol. Genet. 2024, 10, e200176. [Google Scholar] [CrossRef]
- Xu, J.; Zou, Z.; Liu, W.; Zhang, Q.; Shen, J.; Hao, F.; Chen, G.; Yu, D.; Li, Y.; Zhang, Z.; et al. HAPLN3 p.T34A contributes to incomplete penetrance of moyamoya disease in Chinese carrying RNF213 p.R4810K. Eur. J. Neurol. 2024, 31, e16473. [Google Scholar] [CrossRef]
- Tashiro, R.; Niizuma, K.; Khor, S.S.; Tokunaga, K.; Fujimura, M.; Sakata, H.; Endo, H.; Inoko, H.; Ogasawara, K.; Tominaga, T. Identification of HLA-DRB1*04, 10 allele as risk allele for Japanese moyamoya disease and its association with autoimmune thyroid disease: A case-control study. PLoS ONE 2019, 14, e0220858. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsuda, Y.; Hitomi, T.; Okuda, H.; Shioi, H.; Matsuda, T.; Imai, H.; Sone, M.; Taura, D.; Harada, K.H.; et al. Biochemical and Functional Characterization of RNF213 (Mysterin) R4810K, a Susceptibility Mutation of Moyamoya Disease, in Angiogenesis In Vitro and In Vivo. J. Am. Heart Assoc. 2015, 4, e002146. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, J.; Wang, A.; Zhao, X. Association Between Low-Density Lipoprotein-Cholesterol Level and Risk of Intracranial Atherosclerotic Stenosis: Results from the APAC Study. Neurologist 2023, 28, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.M.; Arellano, J.; Grose, C.; Pearson, R. Recurrent encephalitis and stroke following cessation of acyclovir prophylaxis in a patient with neonatal herpes simplex virus with RNF213 mutation. Ann. Child Neurol. Soc. 2024, 2, 235–241. [Google Scholar] [CrossRef]
- Tan, B.Y.Q.; Kok, C.H.P.; Ng, M.B.J.; Loong, S.; Jou, E.; Yeo, L.L.L.; Han, W.; Anderson, C.D.; Khor, C.C.; Lai, P.S. Exploring RNF213 in Ischemic Stroke and Moyamoya Disease: From Cellular Models to Clinical Insights. Biomedicines 2024, 13, 17. [Google Scholar] [CrossRef]
- Hamauchi, S.; Shichinohe, H.; Uchino, H.; Yamaguchi, S.; Nakayama, N.; Kazumata, K.; Osanai, T.; Abumiya, T.; Houkin, K.; Era, T. Cellular Functions and Gene and Protein Expression Profiles in Endothelial Cells Derived from Moyamoya Disease-Specific iPS Cells. PLoS ONE 2016, 11, e0163561. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Brodeur, A.; Touzel Deschênes, L.; Dupré, N.; Gros-Louis, F. RNF213 Loss-of-Function Promotes Angiogenesis of Cerebral Microvascular Endothelial Cells in a Cellular State Dependent Manner. Cells 2022, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, J.H.; Phi, J.H.; Kang, H.S.; Kim, J.E.; Chae, J.H.; Kim, S.J.; Kim, Y.H.; Kim, Y.Y.; Cho, B.K.; et al. Decreased level and defective function of circulating endothelial progenitor cells in children with moyamoya disease. J. Neurosci. Res. 2010, 88, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Enmi, J.I.; Hattori, Y.; Iguchi, S.; Saito, S.; Harada, K.H.; Okuda, H.; Mineharu, Y.; Takagi, Y.; Youssefian, S.; et al. Dysregulation of RNF213 promotes cerebral hypoperfusion. Sci. Rep. 2018, 8, 3607. [Google Scholar] [CrossRef]
- Hitomi, T.; Habu, T.; Kobayashi, H.; Okuda, H.; Harada, K.H.; Osafune, K.; Taura, D.; Sone, M.; Asaka, I.; Ameku, T.; et al. Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients. Biochem. Biophys. Res. Commun. 2013, 438, 13–19. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Huang, L.; Chen, Y.; Luo, H.; Zhang, S.; Chen, H. Generation of an induced pluripotent stem cell line HUSTTJi001-A from a Moyamoya disease patient with RNF213 gene mutation. Stem Cell Res. 2021, 57, 102575. [Google Scholar] [CrossRef]
- Hitomi, T.; Habu, T.; Kobayashi, H.; Okuda, H.; Harada, K.H.; Osafune, K.; Taura, D.; Sone, M.; Asaka, I.; Ameku, T.; et al. The moyamoya disease susceptibility variant RNF213 R4810K (rs112735431) induces genomic instability by mitotic abnormality. Biochem. Biophys. Res. Commun. 2013, 439, 419–426. [Google Scholar] [CrossRef]
- Tashiro, R.; Niizuma, K.; Kasamatsu, J.; Okuyama, Y.; Rashad, S.; Kikuchi, A.; Fujimura, M.; Kure, S.; Ishii, N.; Tominaga, T. Dysregulation of Rnf 213 gene contributes to T cell response via antigen uptake, processing, and presentation. J. Cell Physiol. 2021, 236, 7554–7564. [Google Scholar] [CrossRef]
- Ohkubo, K.; Sakai, Y.; Inoue, H.; Akamine, S.; Ishizaki, Y.; Matsushita, Y.; Sanefuji, M.; Torisu, H.; Ihara, K.; Sardiello, M.; et al. Moyamoya disease susceptibility gene RNF213 links inflammatory and angiogenic signals in endothelial cells. Sci. Rep. 2015, 5, 13191. [Google Scholar] [CrossRef]
- Shirozu, N.; Ohgidani, M.; Hata, N.; Tanaka, S.; Inamine, S.; Sagata, N.; Kimura, T.; Inoue, I.; Arimura, K.; Nakamizo, A.; et al. Angiogenic and inflammatory responses in human induced microglia-like (iMG) cells from patients with Moyamoya disease. Sci. Rep. 2023, 13, 14842. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, G.H.; Choi, E.S.; Park, S.Y.; Kim, D.S.; Chang, J.; Hong, J.M. RNF213 variant and autophagic impairment: A pivotal link to endothelial dysfunction in moyamoya disease. J. Cereb. Blood Flow Metab. 2024, 44, 1801–1815. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Banh, R.S.; Zhang, W.; Sidhu, S.S.; Neel, B.G. MMD-associated RNF213 SNPs encode dominant-negative alleles that globally impair ubiquitylation. Life Sci. Alliance 2022, 5, e202000807. [Google Scholar] [CrossRef]
- Kanoke, A.; Fujimura, M.; Niizuma, K.; Ito, A.; Sakata, H.; Sato-Maeda, M.; Morita-Fujimura, Y.; Kure, S.; Tominaga, T. Temporal profile of the vascular anatomy evaluated by 9.4-tesla magnetic resonance angiography and histological analysis in mice with the R4859K mutation of RNF213, the susceptibility gene for moyamoya disease. Brain Res. 2015, 1624, 497–505. [Google Scholar] [CrossRef]
- Hiraide, T.; Tsuda, N.; Momoi, M.; Shinya, Y.; Sano, M.; Fukuda, K.; Shibahara, J.; Kuramoto, J.; Kanai, Y.; Kosaki, K.; et al. CXCL12/CXCR4 pathway as a novel therapeutic target for RNF213-associated pulmonary arterial hypertension. Sci. Rep. 2024, 14, 26604. [Google Scholar] [CrossRef]
- Xu, S.; Chen, T.; Yu, J.; Wan, L.; Zhang, J.; Chen, J.; Wei, W.; Li, X. Insights into the regulatory role of epigenetics in moyamoya disease: Current advances and future prospectives. Mol. Ther. Nucleic Acids 2024, 35, 102281. [Google Scholar] [CrossRef]
- Fujimura, M. Precision Medicine for Moyamoya Disease. No. Shinkei. Geka. 2022, 50, 216–221. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Y.; Fan, Y.N.; Wang, Q.N.; Wang, X.P.; Yang, R.M.; Zou, Z.X.; Zhang, Q.; Li, D.S.; Duan, L.; Yu, X.G. The Potential Mechanism Behind Native and Therapeutic Collaterals in Moyamoya. Front. Neurol. 2022, 13, 861184. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Yamamoto, Y.; Hattori, Y.; Liu, W.; Kobayashi, H.; Ishiyama, H.; Yoshimoto, T.; Miyawaki, S.; Clausen, T.; Bang, O.Y.; et al. Moyamoya disease: Diagnosis and interventions. Lancet Neurol. 2022, 21, 747–758. [Google Scholar] [CrossRef]
- Fujimura, M.; Sonobe, S.; Nishijima, Y.; Niizuma, K.; Sakata, H.; Kure, S.; Tominaga, T. Genetics and Biomarkers of Moyamoya Disease: Significance of RNF213 as a Susceptibility Gene. J. Stroke 2014, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Luo, J.; Chen, Q. The Susceptibility Pathogenesis of Moyamoya Disease. World Neurosurg. 2017, 101, 731–741. [Google Scholar] [CrossRef]
- Mineharu, Y.; Takagi, Y.; Koizumi, A.; Morimoto, T.; Funaki, T.; Hishikawa, T.; Araki, Y.; Hasegawa, H.; Takahashi, J.C.; Kuroda, S.; et al. Genetic and nongenetic factors for contralateral progression of unilateral moyamoya disease: The first report from the SUPRA Japan Study Group. J. Neurosurg. 2021, 136, 1005–1014, Erratum in J. Neurosurg. 2021, 136, 1207. [Google Scholar] [CrossRef]
- Lawal, A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 2017, 270, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, Y.; Kaito, H.; Yoshida, M.; Hara, S.; Tanaka, R. Moyamoya disease with refractory hypertension associated with peripheral arterial stenosis in the renal parenchyma. CEN Case Rep. 2021, 10, 506–509. [Google Scholar] [CrossRef]
- Gerlevik, U.; Saygı, C.; Cangül, H.; Kutlu, A.; Çaralan, E.F.; Topçu, Y.; Özören, N.; Sezerman, O.U. Computational analysis of missense filamin-A variants, including the novel p.Arg484Gln variant of two brothers with periventricular nodular heterotopia. PLoS ONE 2022, 17, e0265400. [Google Scholar] [CrossRef]
- Guo, Q.; Feng, X.; Zhou, Y. PCSK9 Variants in Familial Hypercholesterolemia: A Comprehensive Synopsis. Front. Genet. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Nair, A.; Greeny, A.; Rajendran, R.; Abdelgawad, M.A.; Ghoneim, M.M.; Raghavan, R.P.; Sudevan, S.T.; Mathew, B.; Kim, H. KIF1A-Associated Neurological Disorder: An Overview of a Rare Mutational Disease. Pharmaceuticals 2023, 16, 147. [Google Scholar] [CrossRef]
- Torazawa, S.; Miyawaki, S.; Imai, H.; Hongo, H.; Ono, H.; Ogawa, S.; Sakai, Y.; Kiyofuji, S.; Koizumi, S.; Komura, D.; et al. Association of Genetic Variants with Postoperative Donor Artery Development in Moyamoya Disease: RNF213 and Other Moyamoya Angiopathy-Related Gene Analysis. Transl. Stroke Res. 2025, 16, 679–689. [Google Scholar] [CrossRef]
- Brunet, T.; Zott, B.; Lieftüchter, V.; Lenz, D.; Schmidt, A.; Peters, P.; Kopajtich, R.; Zaddach, M.; Zimmermann, H.; Hüning, I.; et al. De novo variants in RNF213 are associated with a clinical spectrum ranging from Leigh syndrome to early-onset stroke. Genet. Med. 2024, 26, 101013. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Asano, R.; Ishibashi, T.; Endo, H.; Fujisaki, S.; Takano, R.; Akao, M.; Nishi, N.; Hayashi, H.; Kotoku, A.; et al. Balloon Pulmonary Angioplasty in Heterozygous RNF213 p.Arg4810Lys Variant Carriers Diagnosed with Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2025, 14, e039002. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, C.; Zhao, Y.; Song, H.; Li, Z.; Jin, F.; Cui, C. RNF213 gene silencing upregulates transforming growth factor β1 expression in bone marrow-derived mesenchymal stem cells and is involved in the onset of Moyamoya disease. Exp. Ther. Med. 2021, 22, 1024. [Google Scholar] [CrossRef]
| Heterozygous Arg4810Lys | Homozygous Arg4810Lys | |
|---|---|---|
| Penetrance | Lower | Higher |
| Vascular features | Less severe | More extensive stenosis |
| Stroke outcome | Increased risk of ischemic stroke; prognosis is less severe | Higher risk of recurrent strokes; prognosis is worse |
| Age at onset | Later onset | Earlier onset |
| Disease progression | Relatively slow | Faster disease progression |
| Treatment response | Good response to revascularization surgery or other treatment | Needs early and aggressive management; outcome may be poor |
| Disease | Zygosity | Clinical Phenotypes | Prognosis |
|---|---|---|---|
| MMD | Heterozygous/homozygous | TIAs, stroke, hemorrhage, seizures, and cognitive decline | Revascularization surgery may be successful |
| ICAS/ICASO | Heterozygous | Stroke, tandem lesions, and anterior circulation stenosis | Risk of progression to MMD |
| MMS | Heterozygous | Atypical MMD + comorbidities | Variable severity |
| Dissections | Heterozygous | Sudden stroke and MCA dissection | Early onset and treatable |
| Organ | Disease | Key Symptoms/Risks | Prognostic Outcome |
|---|---|---|---|
| Lungs | PAH and PPAS | Dyspnea and poor therapy response | Lung transplant in severe cases |
| Heart | CAD and VSA | Angina and vasospasm | Higher mortality in males/homozygous cases |
| Kidney | RVA and RVS | Hypertension and ischemia | Rare but possible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagyinszky, E.; Yang, Y.; An, S.S.A. Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies. Int. J. Mol. Sci. 2025, 26, 7864. https://doi.org/10.3390/ijms26167864
Bagyinszky E, Yang Y, An SSA. Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies. International Journal of Molecular Sciences. 2025; 26(16):7864. https://doi.org/10.3390/ijms26167864
Chicago/Turabian StyleBagyinszky, Eva, YoungSoon Yang, and Seong Soo A. An. 2025. "Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies" International Journal of Molecular Sciences 26, no. 16: 7864. https://doi.org/10.3390/ijms26167864
APA StyleBagyinszky, E., Yang, Y., & An, S. S. A. (2025). Multisystemic Impact of RNF213 Arg4810Lys: A Comprehensive Review of Moyamoya Disease and Associated Vasculopathies. International Journal of Molecular Sciences, 26(16), 7864. https://doi.org/10.3390/ijms26167864







