Understanding the Pre-Clinical Stages of Parkinson’s Disease: Where Are We in Clinical and Research Settings?
Abstract
1. Introduction
2. What We Know About PD: The Clinical Readouts
2.1. Post-Diagnostic Phase
2.1.1. Prodromal Phase
| Marker Under Evaluation | Rational | Sampling | Supportive Molecular and/or Pathologic Evidences | References |
|---|---|---|---|---|
| αSyn presence | Higher in early PD patients. Suggested negative correlation with cognitive decline (CSF). GI: based on Braakt hypothesis. | Blood, CSF, GI | Still debated if its plasma level in PD subject is higher than in healthy individuals. CSF: usually reported lower in PD subject vs. healthy controls. GI: detected. | [10,39,122,128,134,135,136,137,148] |
| Uric acid | Higher levels are associated with a significantly decreased risk of PD. | Blood and urine | Anti-oxidant action. | [128] |
| DJ1 | Higer in early PD patients. | CSF | Plays a role in mitochondrial dysfunction, oxidative stress, and chaperons activity. | [128] |
| HMOX1 | Higher in early-on-set PD patients. | Plasma and saliva | Sensor of redox stress. Tentative protection by bilirubin production. By the other side: potentially dangerous by producing iron. | [68,131,132,133,149] |
| Bilirubin | Higher serum bilirubin level in early-stage PD. | Serum, plasma | Elevated serum or plasma bilirubin levels in PD may result from the overexpression of HMOX1, which leads to anti-oxidant and anti-inflammatory effects and may contribute to neuroprotection. | [150,151,152] |
| DA | Detecting DOPAn initial suffering that preceed DOPAn loss and the appearance of cardinal motor signs. | DAT-scan | Decrease in DA transporter binding. | [10,122,123] |
| Additional information from PD models | ||||
| DA | Retrograde degeneration of DA in the nigrostriatal system. | In models |
| [153,154,155] |
| NE, SE, Chol reduced levels in the extra nigrostriatal areas | Hyposmia, REM sleep deficits and disturbances. | In models | Suggested dysfunction of the neurological circuits. Correlation among symptoms and neurotransmitter’s level. | [156,157,158,159,160,161,162,163] |
| Occurrence of gastrointestinal symptoms in prodromal and frank PD | Constipation, reduced intestinal motility. | In models | Alterations in the resident neuronal populations of the GI tract. Increased αSyn detected. | [164,165,166] |
2.1.2. Pre-Clinical Phase
2.1.3. Risk Phase
3. Where We Are in Understanding the Early Stages of PD Through Research Models
| Reserpine | Inhibition of VMAT2, which is expressed on synaptic vesicles of DOPAn, NE, and SE neurons and regulates the release of neurotransmitters [196]. Accordingly, dysfunction of these circuits in PD has been reported [161,189,197]. This model reproduces motor signs [161,198,199,200]; DA loss [189,199,200]; non-motor signs [161]; αSyn presence [199]; mitochondrial dysfunction and redox stress [155,161,199,201]; autophagy [155,161,199,202]; and inflammation [161,199]. |
| 6-OHDA | Production of ROS and inhibition of mitochondrial respiratory chain complexes I and IV [203]. Reduces GSH and SOD reduction. Increases glutamate (Glut); astrogliosis, autophagy, and proteasomal dysfunction induction [161,204]. Does not cross the blood–brain barrier (BBB), so intracranial injections are necessary. High mortality rate when administered bilaterally. Endogenous production of 6-OHDA has been reported in the brains of PD patients [55,189,203,205]. This model reproduces motor signs [55,156,161,189,198,199,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219]; DA loss [55,156,189,198,199,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222]; non-motor signs [156,160,161,207,211,212,213,218,223,224]; non-DA loss [156,160,189,212,213,214,216]; αSyn presence [208] and non-detected [55,189,199,205,206]; mitochondrial dysfunction and redox stress [161,199,209,210,221,223,225,226,227,228,229,230,231,232,233]; autophagy [161,199,234]; and inflammation [161,199,207,217,219,220,235]. |
| MPTP | MPTP crosses the BBB due to its lipophilic nature. In the brain MPTP is converted to MPTP+ by the glial MAO-B, spontaneously oxidized to MPP+, then taken up into DA neurons by DAT [198,236,237]. MPTP may also be administered, but the positive charge reduces its brain bioavailability. Stereotaxic injection provide better results [193]. In cells MPP+ acts by accumulating in VMAT2 vesicles, leading to DA release and toxic auto-oxidation. MPP+ also binds to mitochondrial NADH, decreasing ATP production and inhibiting complex I with ROS production [208]. MPTP increases Glut, induces astrogliosis, microgliosis and cytokine release [238,239]. Rats are resistant to MPTP, thus requiring higher doses, and presenting high mortality rates secondary to massive loss of neurons [193,208,240]. Acute doses in MPTP models like mice, rats, zebrafish, and monkeys lead to a quick decrease in ATP, potentially resulting in rapid DOPAn death by apoptosis and necrosis [189,205,208,237], possibly being too rapid to allow proper access to each stage. Chronic administration of low doses in PD features in a small percentage of animals [189,193,197]. This model reproduces motor signs [156,161,189,199,205,208,214,237,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256]; DA loss [55,156,189,198,199,205,206,208,214,238,241,242,243,244,245,246,248,249,250,251,252,254,255,256,257,258]; non-motor signs [156,160,161,241,242,250,255,256,259,260,261,262] and non-detected [249]; non-DA loss: [156,160,189,214,242,247,248,252,255,261,262] and non-detected [249,250]; αSyn presence [55,189,198,199,206,208,241,242,261,262] and non-detected [205,249]; absence of LBs [205,249,261,262]; mitochondrial dysfunction/redox stress [161,189,199,242,244,245,257,263,264,265,266,267,268,269,270]; autophagy [161,199,234]; inflammation [161,199,238,241,242,243,257,258,261,262,267,269]; and GI [164]. |
| Paraquat | Agricultural herbicide linked to PD in farmers; not DA-specific. Paraquat induces mitochondrial dysfunction and ROS production [84], reduces GSH and thioredoxin, leads to lipid, protein, and DNA damage, inflammation, autophagy, and proteasomal dysfunction [189,197,205,237]. Potentially toxic to the liver, kidney, and lungs; high mortality rate [208]. This model reproduces motor signs [55,156,161,198,199,205,206,208,271,272,273,274]; DA loss [55,156,198,199,205,206,208,272,273,274]; non-motor signs [161,272]; non-DA loss [156,271,274]; αSyn presence [55,199,206] and non-detection [205,208]; but with the absence of LBs [55,189]; mitochondrial dysfunction and redox stress [161,189,199,263,271,274,275,276,277]; autophagy [161,199]; and inflammation [161,199]. |
| Rotenone | Agricultural herbicide and insecticide linked to PD. Acts similarly to paraquat on mitochondria, redox state [79,84,161,189,193,205,237,278,279], and inhibiting proteasome activity [208], but it does not activate the caspase pathway [280]. Recently, low doses have been shown to have a pro-inflammatory effect [281,282,283]. It is water and alcohol insoluble, rapidly degraded by light, and metabolized in the liver and gastric mucosa. Thus, its usage in vivo is somehow difficult [192]. Acute doses may lead to systemic toxicity and necrosis in the brain [284], with potential lethality [192]. Heterogeneity in the time scale of symptom development and low percentage of animals developing the disease are scientific and ethic problems, overstepped recently by the use of older animals and adjuvants [192,195,285]. Rotenone reproduces motor signs [55,156,161,189,198,199,205,206,208,282,283,285,286,287,288,289]; DA loss: [55,156,189,198,199,205,206,208,282,283,285,286,287,288,289]; non-motor signs [156,161,208]; non-DA loss [156,206]; αSyn presence [55,189,199,206,282,285,288] and not detected [205,208]; LBs [189,288]; mitochondrial dysfunction and redox stress [161,199,263,266,286,289,290,291]; autophagy [161,199,292,293,294,295,296]; inflammation [154,161,199,282,283,288,289,290,294,297]; and gastro intestinal manifestations (GI) [156,165,166]. |
| LPS | Pro-inflammatory [193], but also induces mitochondrial dysfunction and redox stress [161,194,199]. Largely used in recent years to explore the most recent hypotheses that inflammation is important in PD outcome. LPS reproduces motor signs [67,161,194,199,298,299,300,301,302,303,304,305,306,307]; DA loss [194,199,298,299,302,303,304,305,306,307,308,309]; non-motor signs [161,194,300,301]; non-DA loss [194,298,299,300,301,302,305]; αSyn presence [194,199,302,305,309] and non-detected [194]; mitochondrial dysfunction and redox stress [161,199,298,300,301,305,306,308]; autophagy [161,199]; inflammation: [67,161,199,298,299,302,303,304,305,306,307,308]; and GI [307] |
| αSyn | Protein physiologically present. It can be oxidated, modifying its folding. Misfolding provokes the formation of amyloid fibrils that will result in forming the LBs [45,53,54,55,56], motor signs [310,311,312]; DA loss [54,310,311,312]; αSyn presence [54,310,311,312]; LBs [310]; inflammation [54,310,312]. |
| Genetic models | Multiple models based on multiple genetic variants for each PD gene exist. Large variability of the PD-like features is reached in all genetic animal models. The variability is due both to the genetic variant reproduced and the promoter that controls the gene of interest expression [199,313,314,315]. PARK1/4(SNCA) * has been reported to reproduce motor signs [55,161,189,198,199,205,206,313,314]; DA loss [55,160,198,314] and non-detected [189,199]; non-motor signs [160,161,314]; non-DA loss [160,313]; αSyn presence [55,199,206,313,314] and non-detected [206,313,314]; mitochondrial dysfunction [161,199]; redox stress [161]; autophagy [161,199]; GI [314,316]; inflammation [161,199]. PARK8 (LRRK2) ** has been reported to reproduce motor signs [55,189,198,206,313] and non-detected [161,199,205,314]; DA loss [198,314] and non-detected [199,205]; non-motor signs [161]; non-DA loss [313]; αSyn presence [198] and non-detected [55,189,205,313]; absence of LBs [206,313]; mitochondrial dysfunction [161,199]; redox stress [161]; autophagy [161,199]; GI [314,317]; and inflammation [161,199]. PARK2 (PRKN) *** has been reported to reproduce motor signs [199,313,314]; absence of DA loss [314]; non-motor signs; non-DA loss [189,199,206,314]; αSyn presence [314] and non-detected [189,206,313]; redox stress [199]; GI [199]; and inflammation [189]. PARK7 (DJ1) has been reported to reproduce motor signs [313,314] and non-detected [199,205,206]; DA loss [314] and non-detected [189,199,205,206]; non-DA loss [313]; αSyn presence [314] and non-detected [199,205,206]; absence of LBs [313]; mitochondrial dysfunction [199]; inflammation [199]. PARK5 (UCHL1) has been reported to reproduce motor signs [198,199,206]; DA loss [198]; non-motor signs [314]; absence of αSyn [199]; absence of LB [206]. PARK6 (PINK1) has been reported to reproduce motor signs [313,314] and non-detected [205]; DA loss [189,314] and non-detected [205]; non-motor signs [314]; non-DA loss (only in mice) [313]; αSyn presence [314] and non-detected [189,205,313]; absence of LBs [313]. VMAT2 has been reported to reproduce motor signs [160]; DA loss [160]; non-motor signs [160]; non-DA loss [160]. |
3.1. Modeling PD
3.1.1. Modeling the Early Stages of Clinical PD
| DA Loss/Involvement | Non-DA Loss/Involvement | Motor Deficits | Non-Motor Deficits | αSyn | LBs | Inflammation | Mitochondria and Redox | Ref. and Short Description | |
|---|---|---|---|---|---|---|---|---|---|
| Post-diagnosis phase | Y | Y | Y | Y | Y | ° | Y | ° | [241] Mice, MPTP |
| Y | Y | Y | Y | Y | ° | Y | Y | [242] Mice, MPTP | |
| Y | Y | Y | ° | ° | ° | Y | Y | [298] Rat, LPS | |
| Y | Y | Y | ° | ° | ° | Y | ° | [299] Rat, LPS | |
| ° | Y | Y | Y | ° | ° | ° | Y | [300,301] Rat, LPS | |
| Y | Y | Y | ° | Y | ° | Y | ° | [302] Mice, LPS | |
| ° | Y | Y | ° | Y | No | Y | ° | [261,262] NHP, MPTP | |
| Y | ° | Y | Y | ° | ° | Y | ° | [207] Mice, 6-OHDA | |
| Y | ° | Y | ° | ° | ° | ° | Y | [209] Rat, 6-OHDA | |
| Y | ° | Y | ° | ° | ° | Y | ° | [219] Mice, 6-OHDA * | |
| Y | ° | Y | ° | ° | ° | Y | ° | [243] Mice, MPTP | |
| Y | ° | Y | ° | ° | ° | ° | Y | [210] Mice, 6-OHDA | |
| Y | ° | ° | ° | ° | ° | Y | ° | [220] Rat, 6-OHDA * | |
| Y | ° | ° | ° | ° | ° | ° | Y | [221] Zebrafish, 6-OHDA | |
| Y | ° | ° | ° | ° | ° | Y | Y | [257] Mice, MPTP * | |
| Y | ° | ° | ° | ° | ° | Y | ° | [258] Mice, MPTP | |
| Y | ° | ° | ° | ° | ° | Y | ° | [238] Mice, MPTP | |
| Y | ° | Y | ° | ° | ° | ° | Y | [244] Mice, MPTP * | |
| Y | ° | Y | ° | ° | ° | ° | Y | [245] Zebrafish, MPTP * | |
| Y | ° | Y | ° | ° | ° | ° | Y | [286] Rat, rotenone | |
| Y | ° | Y | ° | ° | ° | Y | ° | [283] Mice, rotenone | |
| Y | ° | Y | ° | ° | ° | ° | Y | [271] Rat, paraquat | |
| Y | ° | Y | ° | ° | ° | Y | ° | [303] Rat, LPS | |
| Y | ° | Y | GI | ° | ° | Y | ° | [307] Rat, LPS | |
| Y | ° | ° | ° | Y | ° | Y | ° | [54] Rat, αSyn * | |
| Y | Y | Y | Y | ° | ° | ° | ° | [211] Rat, 6-OHDA * | |
| Y | Y | Y | Y | ° | ° | ° | ° | [212] Rat, 6-OHDA * | |
| Y | Y | Y | Y | ° | ° | ° | ° | [213] Rat, 6-OHDA * | |
| Y | Y | Y | ° | ° | ° | ° | ° | [214] Zebrafish, 6-OHDA or MPTP * | |
| Y | ° | Y | ° | ° | ° | ° | ° | [246] NHP, MPTP * | |
| Y | Y | Y | ° | ° | ° | ° | ° | [247] NHP, MPTP | |
| Y | Y | Y | ° | ° | ° | ° | ° | [248] NHP, MPTP | |
| Y | No | Y | No | No | No | ° | ° | [249] NHP, MPTP * | |
| Y | Y | Y | Y | ° | ° | ° | ° | [250] Mice, MPTP * | |
| Y | ° | Y | ° | ° | ° | ° | ° | [251] Zebrafish, MPTP | |
| Y | Y | Y | ° | ° | ° | ° | ° | [252] Zebrafish, MPTP | |
| ° | ° | Y | ° | ° | ° | ° | ° | [253] Zebrafish, MPTP | |
| Y | ° | Y | ° | Y | ° | ° | ° | [285] Rat, rotenone | |
| Y | ° | Y | ° | ° | ° | ° | ° | [287] Rat, rotenone | |
| Y | ° | Y | Y | ° | ° | ° | ° | [272] Zebrafish, paraquat | |
| Y | ° | Y | ° | ° | ° | ° | ° | [273] Rat, paraquat * | |
| Prodromal to post-diagnosis | Y | ° | Y | ° | ° | ° | Y | ° | [346] DJ1 mice genetic model * |
| Y | ° | Y | ° | ° | ° | ° | ° | [246] NHP, MPTP * | |
| Y | ° | Y | ° | ° | ° | ° | ° | [200] Mice, reserpine * | |
| Y | ° | Y | ° | ° | ° | Y | ° | [346] Park2 mice genetic model | |
| Y | ° | Y | ° | No | No | Y | ° | [347] Parkin rat genetic model * | |
| Y | ° | Y | ° | Y | Y | Y | ° | [310] Mice, αSyn * | |
| Y | ° | ° | ° | ° | ° | ° | ° | [222] Rat, 6-OHDA * | |
| Y | ° | Y | ° | Y | ° | ° | ° | [311] Mice, LPS and αSyn * | |
| ° | ° | Y | ° | ° | ° | Y | ° | [67] Rat, LPS | |
| Y | ° | Y | ° | ° | ° | Y | ° | [304] Mice, LPS * | |
| Y | Y | Y | ° | ° | ° | ° | Y | [274] Zebrafish, paraquat * | |
| Y | Y | Y | ° | Y | ° | Y | Y | [305] Rat, LPS * | |
| Y | ° | Y | ° | Y | Y | Y | ° | [288] Mice, rotenone * | |
| Y | ° | Y | ° | ° | ° | Y | Y | [289] Mice, rotenone * | |
| Y | ° | Y | ° | Y | ° | Y | ° | [282] Rat, rotenone * | |
| Y | ° | Y | ° | ° | ° | Y | ° | [254] NHP, MPTP | |
| Y | ° | Y | ° | ° | ° | ° | ° | [215] Rat, 6-OHDA | |
| Y | Y | Y | ° | ° | ° | ° | ° | [216] Rat, 6-OHDA | |
| Y | ° | Y | Y | ° | ° | ° | ° | [255] Rat, 6-OHDA * [255] Rat, MPTP * | |
| Y | Y | Y | Y | ° | ° | ° | ° | ||
| Y | ° | Y | ° | weak | ° | Y | ° | [312] Mice, αSyn | |
| Y | ° | Y | ° | ° | ° | Y | ° | [217] Zebrafish, 6-OHDA * | |
| Prodromal phase | Y | No | Y | Y | ° | ° | ° | ° | [218] Rat, 6-OHDA |
| ° | ° | ° | Y | ° | ° | ° | Y | [223] Rat, 6-OHDA | |
| Y | ° | Y | Y | ° | ° | ° | ° | [256] NHP, MPTP | |
| Risk phase | Y | ° | ° | ° | ° | ° | Y | Y | [306] Rat, LPS * |
| Out of classification based on the clinical cardinal symptoms | Y | ° | ° | ° | Y | Y | Y | ° | [348] αSyn mice genetic model, LPS |
| ° | ° | ° | ° | ° | ° | Y | Y | [269] Mice, MPTP * | |
| Y | ° | ° | ° | Y | ° | ° | ° | [309] Mice, LPS * | |
| Y | ° | ° | ° | ° | ° | Y | Y | [308] Mice, LPS |
3.1.2. Modeling the Late Stages of Clinical PD
| Inflammation | Redox Stress | Autophagy, lysosome, αSyn | Apoptosis | Ref. and Short Description | |
|---|---|---|---|---|---|
| Post-diagnosis phase (cell death of at least 50%) | ° | ° | ° | ° | [357] OBCs, reserpine |
| ° | Y | Y | Y | [155] SH-SY5Y, reserpine | |
| ° | ° | ° | Y | [358] OBCs, 6-OHDA | |
| ° | ° | Y § | ° | [343] SH-SY5Y, 6-OHDA § | |
| ° | ° | ° | Y | [359] SH-SY5Y, 6-OHDA | |
| ° | Y | ° | Y | [225] SH-SY5Y, 6-OHDA | |
| ° | ° | ° | Y | [359] PC12, 6-OHDA | |
| ° | Y | ° | Y | [229] PC12, 6-OHDA | |
| ° | Y | ° | ° | [230] PC12, 6-OHDA | |
| ° | ° | ° | Y | [360] LUHMES, 6-OHDA | |
| ° | Y | ° | Y | [276] SH-SY5Y, paraquat and physical stretch | |
| ° | Y | ° | Y | [277] SH-SY5Y, paraquat | |
| ° | ° | ° | ° | [361] SH-SY5Y, paraquat | |
| ° | Y | ° | Y | [263] SH-SY5Y, paraquat * | |
| ° | ° | ° | Y | [354] OBCs, rotenone | |
| ° | Y | Y | Y | [293] MN9D, rotenone | |
| Y | ° | Y | Y | [294] MN9D, rotenone | |
| ° | ° | Y | Y | [295] MN9D, rotenone | |
| Y | ° | Y | Y | [290] LUHMES, rotenone | |
| ° | Y | ° | Y | [266] PC12, rotenone | |
| ° | ° | Y | ° | [330] SH-SY5Y, MPTP | |
| ° | Y | ° | Y | [264] SH-SY5Y, MPTP | |
| ° | ° | ° | Y | [360] SH-SY5Y, MPTP | |
| ° | Y | Y | Y | [362] SH-SY5Y, MPTP | |
| ° | Y | ° | Y | [265] SH-SY5Y, MPTP * | |
| ° | Y | ° | Y | [266] PC12, MPTP | |
| Y | Y | ° | Y | [363] PC12, MPTP | |
| ° | Y | ° | Y | [270] PC12, MPTP | |
| Y | Y | ° | Y | [267] MN9D, MPTP | |
| ° | ° | ° | Y | [364] MN9D, MPTP | |
| ° | Y | ° | Y | [268] MN9D, MPTP | |
| Prodromal to post-diagnosis phase | Y | Y | ° | Y | [297] OBCs, rotenone * |
| Y | Y | ° | Y | [154] OBCs, rotenone * | |
| ° | Y | ° | Y | [263] SH-SY5Y, rotenone *,# | |
| ° | Y | ° | Y | [263] SH-SY5Y, MPTP *,# | |
| ° | ° | ° | Y | [280] MN9D, rotenone # | |
| ° | ° | ° | ° | [345] SH-SY5Y, MPTP | |
| Out of classification based on the clinical cardinal symptoms (no data on cell viability, studied only the molecular mechanisms) | ° | ° | Y | ° | [202] PC12, reserpine |
| ° | Y | ° | ° | [201] PC12, reserpine | |
| ° | Y | ° | ° | [227] SH-SY5Y, 6-OHDA * | |
| ° | Y | ° | Y | [228] SH-SY5Y, 6-OHDA | |
| Y | ° | ° | ° | [235] SH-SY5Y, 6-OHDA | |
| ° | ° | ° | Y | [340] PC12, 6-OHDA | |
| ° | Y | ° | Y | [226] MN9D, 6-OHDA | |
| ° | ° | ° | Y | [232] MN9D, 6-OHDA | |
| ° | ° | Y | ° | [234] MN9D, 6-OHDA | |
| ° | Y | ° | Y | [233] MN9D, 6-OHDA * | |
| ° | Y | ° | Y | [231] MN9D, 6-OHDA | |
| ° | ° | ° | ° | [365] LUHMES, 6-OHDA | |
| ° | Y | ° | Y | [275] OBCs, paraquat | |
| ° | ° | Y | Y | [292] SH-SY5Y, rotenone | |
| Y | Y | ° | Y | [366] SH-SY5Y, rotenone | |
| ° | ° | Y | Y | [296] PC12, rotenone | |
| ° | ° | ° | ° | [329] PC12, rotenone | |
| ° | ° | ° | ° | [153] OBCs, MPTP | |
| ° | ° | ° | ° | [356] OBCs, MPTP | |
| ° | Y | ° | Y | [367] SH-SY5Y, MPTP | |
| ° | ° | ° | ° | [329] PC12, MPTP | |
| ° | Y | Y | Y | [368] PC12, MPTP | |
| ° | ° | Y | ° | [234] MN9D, MPTP |
3.2. Modeling the Prodromal Phase
3.3. Modeling the Risk Phase
4. Conclusions
- Acute schemes approach does not model consistently the etiopathogenesis of human PD. This limits the translational relevance in the understanding of the disease and therapies screening.
- Focus on synucleopathy. The role of synucleopathy is debated, possibly relevant only to a subtype of PD. Possibly a late event.
- Up to now, large attention to the late (after diagnosis) molecular events. Need for discovery approaches, necessity in exploring the early stages.
- Disease complexity. Multiple mechanisms and neurotransmitters involved, in a different time scale. Need for experimental schemes exploring the different phases in a single study.
- Need for complex models. The temporal scale of human PD is too long for experimental studies. Models are necessary and fundamental.
- Need for solid, reproducible and shared models. The past studies build the basis on knowledge in how to properly mimic human PD. Using this background to pursuit the objectives suggested in the previous points by creating slow degenerative models, by using shared, uniform protocols, making data comparable and potentially complementary might be a plus.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT 2A, 1A | serotonin 2A, 1A receptor |
| 6-OHDA | 6-Hydroxydopamine |
| ADA | adenosine 2A receptor |
| AMP | cyclic adenosine monophosphate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| αSyn | αSynuclein |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2 Associated X |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| Chol | choline/cholinergic |
| CNS | central nervous system |
| COX | cyclooxygenase |
| CSF | cerebrospinal fluid |
| CXCL12 | cytokine C-X-C motif chemokine ligand 12 |
| D1, 2 | dopamine receptor 1, 2 |
| DA | dopamine |
| DAT | dopamine transporter |
| DAT SPECT | dopamine transporter single-photon emission computed tomography |
| DJ1 | deglycase J1 (PARK7) |
| DOPAn | dopaminergic neurons |
| FTH1 | ferritin heavy chain 1 |
| GABA | gamma-aminobutyric acid receptor |
| GDNF | glial-derived neurotrophic factor |
| GI | gastrointestinal |
| Glut | glutamate |
| GPe | external globus pallidus |
| Gpi | internal globus pallidus |
| GSH | reduced glutathione |
| H&Y | Hoehn & Yahr score |
| HDAC4 | histone deacetylase 4 |
| HMOX1 | heme oxygenase 1 |
| HOTAIRM1 | HOXA transcript antisense RNA, myeloid-specific 1 |
| IFNγ | interferon-γ |
| IGFBP5 | insulin growth factor binding protein 5 |
| IL | interleukin |
| LBs | Lewy body |
| lncRNA | long non-coding RNA |
| LPS | lipopolysaccharides |
| LRRK2 | leucine-rich repeat kinase 2 |
| M1, 2, 4 | muscarinic acetylcholine receptor 1, 2, 4 |
| MALAT1 | metastasis associated lung adenocarcinoma transcript 1 |
| MAO-B | glial monoamine oxidase B |
| MAPK | mitogen-activated protein kinases |
| mGluR5 | metabotropic glutamate receptor 5 |
| MHCII | major histocompatibility complex class II |
| miR | micro-RNA |
| MPTP | 1-Methyl-4-phenyl-1,2178,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MRI | magnetic resonance imaging |
| nAChR | nicotinic acetylcholine receptors |
| NADH | nicotinamide adenine dinucleotide-coenzyme 1 |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHPs | non-human primates |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| NMDA | N-methyl-D-aspartate receptor |
| NOS | nitrogen oxygen species |
| NOX4 | NADPH oxidase 4 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OBCs | organotypic brain cultures |
| PD | Parkinson’s disease |
| PET | positron emission tomography scan |
| PINK1 | PTEN-induced kinase 1 |
| PKA | protein kinase A |
| PPARs | peroxisome proliferator-activated receptors |
| PRKN | parkin RBR E3 ubiquitin protein ligase |
| RBD | REM sleep behavior disorders |
| ROS | reactive oxygen species |
| SN | substantia nigra |
| SNCA | synuclein alpha gene |
| SNHG14 | small nucleolar RNA host gene 14 |
| SNpc | substantia nigra pars compacta |
| SNpr | substantia nigra pars reticulata |
| SOD | superoxide dismutase |
| TLR4 | toll-like receptor 4 |
| TNFα | tumor necrosis factor alpha |
| UCHL1 | ubiquitin carboxyl-terminal hydrolase isozyme L1 |
| UPDRS | unified PD rating scale |
| VMAT2 | vesicular monoamine transporter 2 |
| WT | wild-type |
| α2A, 2C | α2A, 2C adrenergic receptor |
References
- Dommershuijsen, L.J.; Boon, A.J.W.; Ikram, M.K. Probing the Pre-diagnostic Phase of Parkinson’s Disease in Population-Based Studies. Front. Neurol. 2021, 12, 702502. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Rocca, W.A.; Ahlskog, J.E. When does Parkinson disease start? Arch. Neurol. 2010, 67, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Liang, L.; DeLong, M.R.; Papa, S.M. Inversion of Dopamine Responses in Striatal Medium Spiny Neurons and Involuntary Movements. J. Neurosci. 2008, 28, 7537–7547. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Hirano, S. Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol. Med. 2021, 27, 40. [Google Scholar] [CrossRef]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef]
- Noyce, A.J.; Lees, A.J.; Schrag, A.-E. The prediagnostic phase of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 871–878. [Google Scholar] [CrossRef]
- Breen, D.P.; Lang, A.E. Tracking the course of prodromal Parkinson’s disease. Brain 2017, 140, 259–262. [Google Scholar] [CrossRef]
- Siderowf, A.; Stern, M.B. Preclinical diagnosis of Parkinson’s disease: Are we there yet? Curr. Neurol. Neurosci. Rep. 2006, 6, 295–301. [Google Scholar] [CrossRef]
- Caproni, S.; Colosimo, C. Diagnosis and Differential Diagnosis of Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 13–24. [Google Scholar] [CrossRef]
- Moretti, R.; Torre, P.; Antonello, R.M. Parkinson’s Disease: Behavioural and Cognitive Aspects: Behavioural & Cognitive Aspects, 1st ed.; Nova Biomedical: New York, NY, USA, 2013; ISBN 978-1-62257-778-1. [Google Scholar]
- Mantri, S.; Morley, J.F.; Siderowf, A.D. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat. Disord. 2019, 64, 20–28. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Brück, A.; Aalto, S.; Nurmi, E.; Vahlberg, T.; Bergman, J.; Rinne, J.O. Striatal subregional 6-[18F]fluoro-L-dopa uptake in early Parkinson’s disease: A two-year follow-up study. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Greffard, S.; Verny, M.; Bonnet, A.-M.; Beinis, J.-Y.; Gallinari, C.; Meaume, S.; Piette, F.; Hauw, J.-J.; Duyckaerts, C. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol. 2006, 63, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Nandhagopal, R.; Kuramoto, L.; Schulzer, M.; Mak, E.; Cragg, J.; Lee, C.S.; McKenzie, J.; McCormick, S.; Samii, A.; Troiano, A.; et al. Longitudinal progression of sporadic Parkinson’s disease: A multi-tracer positron emission tomography study. Brain J. Neurol. 2009, 132, 2970–2979. [Google Scholar] [CrossRef]
- Hawkes, C.H. Parkinson’s disease and aging: Same or different process? Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 47–53. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.V.; Halliday, G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef]
- Lang, A.E. The progression of Parkinson disease. Neurology 2007, 68, 948–952. [Google Scholar] [CrossRef]
- Cohen, G. Oxidative stress, mitochondrial respiration, and Parkinson’s disease. Ann. N. Y. Acad. Sci. 2000, 899, 112–120. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Belizaire, R.; Isacson, O.; Jenner, P.; Olanow, C.W. Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 2003, 179, 38–46. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S.; Damier, P.; Faucheux, B. Glial cells and inflammation in parkinson’s disease: A role in neurodegeneration? Ann. Neurol. 1998, 44, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Tieu, K.; Choi, D.-K.; Wu, D.-C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Mattissek, C.; Teis, D. The role of the endosomal sorting complexes required for transport (ESCRT) in tumorigenesis. Mol. Membr. Biol. 2014, 31, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Tieu, K.; Cohen, O.; Choi, D.-K.; Wu, D.C.; Marks, D.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Pathogenic role of glial cells in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 121–129. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Koziorowski, D.; Figura, M.; Milanowski, Ł.M.; Szlufik, S.; Alster, P.; Madetko, N.; Friedman, A. Mechanisms of Neurodegeneration in Various Forms of Parkinsonism-Similarities and Differences. Cells 2021, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Pathoanatomy of Parkinson’s disease. J. Neurol. 2000, 247, II3–II10. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Dauer, W.T.; Vonsattel, J.P.G. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann. Neurol. 2008, 64, 485–491. [Google Scholar] [CrossRef]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.A.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Lebouvier, T.; Neunlist, M.; Bruley des Varannes, S.; Coron, E.; Drouard, A.; N’Guyen, J.-M.; Chaumette, T.; Tasselli, M.; Paillusson, S.; Flamand, M.; et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE 2010, 5, e12728. [Google Scholar] [CrossRef]
- Bellomo, G.; De Luca, C.M.G.; Paoletti, F.P.; Gaetani, L.; Moda, F.; Parnetti, L. α-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 2022, 99, 195–205. [Google Scholar] [CrossRef]
- Waqar, S.; Khan, H.; Zulfiqar, S.K.; Ahmad, A. Skin Biopsy as a Diagnostic Tool for Synucleinopathies. Cureus 2023, 15, e47179. [Google Scholar] [CrossRef]
- Yulug, B.; Ozansoy, M.; Cankaya, S. A different view on the pathophysiology of Parkinson’s disease: A descendent neurochemical hypothesis? Neural Regen. Res. 2019, 14, 1717–1718. [Google Scholar] [CrossRef]
- Jellinger, K.A. Is Braak staging valid for all types of Parkinson’s disease? J. Neural Transm. Vienna Austria 1996 2019, 126, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; McLean, P.J.; Lozano, A.M.; Lang, A.E. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 2013, 73, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; De Cecco, E.; Aguzzi, A. The Hidden Cell-to-Cell Trail of α-Synuclein Aggregates. J. Mol. Biol. 2023, 435, 167930. [Google Scholar] [CrossRef]
- Mikolaenko, I.; Pletnikova, O.; Kawas, C.H.; O’Brien, R.; Resnick, S.M.; Crain, B.; Troncoso, J.C. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: Evidence from the Baltimore Longitudinal Study of Aging (BLSA). J. Neuropathol. Exp. Neurol. 2005, 64, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Klos, K.J.; Ahlskog, J.E.; Josephs, K.A.; Apaydin, H.; Parisi, J.E.; Boeve, B.F.; DeLucia, M.W.; Dickson, D.W. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 2006, 66, 1100–1102. [Google Scholar] [CrossRef]
- Ding, Z.-T.; Wang, Y.; Jiang, Y.-P.; Hashizume, Y.; Yoshida, M.; Mimuro, M.; Inagaki, T.; Iwase, T. Characteristics of alpha-synucleinopathy in centenarians. Acta Neuropathol. 2006, 111, 450–458. [Google Scholar] [CrossRef]
- Parkkinen, L.; Pirttilä, T.; Alafuzoff, I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008, 115, 399–407. [Google Scholar] [CrossRef]
- Doherty, K.M.; Silveira-Moriyama, L.; Parkkinen, L.; Healy, D.G.; Farrell, M.; Mencacci, N.E.; Ahmed, Z.; Brett, F.M.; Hardy, J.; Quinn, N.; et al. Parkin disease: A clinicopathologic entity? JAMA Neurol. 2013, 70, 571–579. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Tarsy, D. Parkinson’s Disease: Hoehn and Yahr Scale. In Movement Disorders: A Video Atlas: A Video Atlas; Bhidayasiri, R., Tarsy, D., Eds.; Current Clinical Neurology; Humana Press: Totowa, NJ, USA, 2012; pp. 4–5. ISBN 978-1-60327-426-5. [Google Scholar]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Netherlands Brain Bank; Rozemuller, A.J.M.; van de Berg, W.D.J. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1244–1251. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Harms, A.S.; Delic, V.; Thome, A.D.; Bryant, N.; Liu, Z.; Chandra, S.; Jurkuvenaite, A.; West, A.B. α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol. Commun. 2017, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998, 152, 879–884. [Google Scholar] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; McCann, H. The progression of pathology in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2010, 1184, 188–195. [Google Scholar] [CrossRef]
- Selikhova, M.; Williams, D.R.; Kempster, P.A.; Holton, J.L.; Revesz, T.; Lees, A.J. A clinico-pathological study of subtypes in Parkinson’s disease. Brain J. Neurol. 2009, 132, 2947–2957. [Google Scholar] [CrossRef]
- Kempster, P.A.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Lees, A.J. Relationships between age and late progression of Parkinson’s disease: A clinico-pathological study. Brain J. Neurol. 2010, 133, 1755–1762. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R.; Tiribelli, C.; Gazzin, S. Bilirubin: A Promising Therapy for Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6223. [Google Scholar] [CrossRef]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The Role of Innate and Adaptive Immunity in Parkinson’s Disease. J. Park. Dis. 2013, 3, 493–514. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R.; Tiribelli, C.; Gazzin, S. Bilirubin and inflammation in neurodegenerative and other neurological diseases. Neuroimmunol. Neuroinflamm. 2020, 7, 92–108. [Google Scholar] [CrossRef]
- Phani, S.; Loike, J.D.; Przedborski, S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S207–S209. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- De Lella Ezcurra, A.L.; Chertoff, M.; Ferrari, C.; Graciarena, M.; Pitossi, F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol. Dis. 2010, 37, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Jayanti, S.; Dalla Verde, C.; Tiribelli, C.; Gazzin, S. Inflammation, Dopaminergic Brain and Bilirubin. Int. J. Mol. Sci. 2023, 24, 11478. [Google Scholar] [CrossRef]
- Pajares, M.; I. Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2016, 136, 10–17. [Google Scholar] [CrossRef]
- Kouchaki, E.; Kakhaki, R.D.; Tamtaji, O.R.; Dadgostar, E.; Behnam, M.; Nikoueinejad, H.; Akbari, H. Increased serum levels of TNF-α and decreased serum levels of IL-27 in patients with Parkinson disease and their correlation with disease severity. Clin. Neurol. Neurosurg. 2018, 166, 76–79. [Google Scholar] [CrossRef]
- Menza, M.; Dobkin, R.D.; Marin, H.; Mark, M.H.; Gara, M.; Bienfait, K.; Dicke, A.; Kusnekov, A. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson’s disease. Psychosomatics 2010, 51, 474–479. [Google Scholar]
- Rocha, N.P.; Teixeira, A.L.; Scalzo, P.L.; Barbosa, I.G.; de Sousa, M.S.; Morato, I.B.; Vieira, E.L.M.; Christo, P.P.; Palotás, A.; Reis, H.J. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 527–531. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Imamura, K.; Hishikawa, N.; Sawada, M.; Nagatsu, T.; Yoshida, M.; Hashizume, Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003, 106, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Lutters, B.; Foley, P.; Koehler, P.J. The centennial lesson of encephalitis lethargica. Neurology 2018, 90, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Limphaibool, N.; Iwanowski, P.; Holstad, M.J.V.; Kobylarek, D.; Kozubski, W. Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications. Front. Neurol. 2019, 10, 652. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Lenhard, J.M.; Oliver, B.B.; Ringold, G.M.; Kliewer, S.A. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1997, 272, 3406–3410. [Google Scholar] [CrossRef]
- Jimenez-Ferrer, I.; Swanberg, M. Immunogenetics of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]; Codon Publications: Brisbane, Australia, 2018; pp. 27–44. [Google Scholar] [CrossRef]
- Yao, L.; Wu, J.; Koc, S.; Lu, G. Genetic Imaging of Neuroinflammation in Parkinson’s Disease: Recent Advancements. Front. Cell Dev. Biol. 2021, 9, 655819. [Google Scholar] [CrossRef]
- Arena, G.; Sharma, K.; Agyeah, G.; Krüger, R.; Grünewald, A.; Fitzgerald, J.C. Neurodegeneration and Neuroinflammation in Parkinson’s Disease: A Self-Sustained Loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Kondo, S.; Le, W.; Jankovic, J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain J. Neurol. 2008, 131, 1969–1978. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Yoo, M.S.; Chun, H.S.; Son, J.J.; DeGiorgio, L.A.; Kim, D.J.; Peng, C.; Son, J.H. Oxidative stress regulated genes in nigral dopaminergic neuronal cells: Correlation with the known pathology in Parkinson’s disease. Mol. Brain Res. 2003, 110, 76–84. [Google Scholar] [CrossRef]
- Schipper, H.M.; Liberman, A.; Stopa, E.G. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp. Neurol. 1998, 150, 60–68. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef]
- Schipper, H.M. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 2004, 3, 265–301. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme oxygenase-1: Role in brain aging and neurodegeneration. Exp. Gerontol. 2000, 35, 821–830. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.; Song, W.; Cui, X.; Pan, M.; Wang, Q.; Feng, Y.; Xu, Y. Elevated Heme Oxygenase-1 Correlates with Increased Brain Iron Deposition Measured by Quantitative Susceptibility Mapping and Decreased Hemoglobin in Patients with Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 656626. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhou, H.; Hong, J.-S. Oxidative Stress, Neuroinflammation, and Neurodegeneration. In Neuroinflammation and Neurodegeneration; Peterson, P.K., Toborek, M., Eds.; Springer: New York, NY, USA, 2014; pp. 81–104. ISBN 978-1-4939-1071-7. [Google Scholar]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Broadfoot, C.K.; Abur, D.; Hoffmeister, J.D.; Stepp, C.E.; Ciucci, M.R. Research-based Updates in Swallowing and Communication Dysfunction in Parkinson Disease: Implications for Evaluation and Management. Perspect. ASHA Spec. Interest Groups 2019, 4, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Buddhala, C.; Loftin, S.K.; Kuley, B.M.; Cairns, N.J.; Campbell, M.C.; Perlmutter, J.S.; Kotzbauer, P.T. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann. Clin. Transl. Neurol. 2015, 2, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z. Mapping neuromodulatory systems in Parkinson’s disease: Lessons learned beyond dopamine. Curr. Med. 2022, 1, 15. [Google Scholar] [CrossRef]
- Agid, Y.; Cervera, P.; Hirsch, E.; Javoy-Agid, F.; Lehericy, S.; Raisman, R.; Ruberg, M. Biochemistry of Parkinson’s disease 28 years later: A critical review. Mov. Disord. Off. J. Mov. Disord. Soc. 1989, 4 (Suppl. S1), S126–S144. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef]
- Hilker, R.; Thomas, A.V.; Klein, J.C.; Weisenbach, S.; Kalbe, E.; Burghaus, L.; Jacobs, A.H.; Herholz, K.; Heiss, W.D. Dementia in Parkinson disease: Functional imaging of cholinergic and dopaminergic pathways. Neurology 2005, 65, 1716–1722. [Google Scholar] [CrossRef]
- Rinne, J.O.; Ma, S.Y.; Lee, M.S.; Collan, Y.; Röyttä, M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat. Disord. 2008, 14, 553–557. [Google Scholar] [CrossRef]
- Thannickal, T.C.; Lai, Y.-Y.; Siegel, J.M. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain J. Neurol. 2007, 130, 1586–1595. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Parkinson’s Disease and Parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs 2013, 73, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Latapi, P.; Bhowmick, S.S.; Saranza, G.; Fox, S.H. Non-Dopaminergic Treatments for Motor Control in Parkinson’s Disease: An Update. CNS Drugs 2020, 34, 1025–1044. [Google Scholar] [CrossRef]
- Coelho, M.; Ferreira, J.J. Late-stage Parkinson disease. Nat. Rev. Neurol. 2012, 8, 435–442. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Paredes-Rodriguez, E.; Vegas-Suarez, S.; Morera-Herreras, T.; De Deurwaerdere, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Kinnerup, M.B.; Sommerauer, M.; Damholdt, M.F.; Schaldemose, J.L.; Ismail, R.; Terkelsen, A.J.; Stær, K.; Hansen, A.; Fedorova, T.D.; Knudsen, K.; et al. Preserved noradrenergic function in Parkinson’s disease patients with rest tremor. Neurobiol. Dis. 2021, 152, 105295. [Google Scholar] [CrossRef]
- Doppler, C.E.J.; Smit, J.A.M.; Hommelsen, M.; Seger, A.; Horsager, J.; Kinnerup, M.B.; Hansen, A.K.; Fedorova, T.D.; Knudsen, K.; Otto, M.; et al. Microsleep disturbances are associated with noradrenergic dysfunction in Parkinson’s disease. Sleep 2021, 44, zsab040. [Google Scholar] [CrossRef]
- Helmich, R.C.; Lehéricy, S. Dying-back of ascending noradrenergic projections in Parkinson’s disease. Brain 2021, 144, 2562–2564. [Google Scholar] [CrossRef]
- Müller, M.L.T.M.; Bohnen, N.I. Cholinergic Dysfunction in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 377. [Google Scholar] [CrossRef]
- Trujillo, P.; Song, A.K.; Hay, K.R.; Aumann, M.; Yan, Y.; Kang, H.; Donahue, M.J.; Claassen, D.O. Dopamine-induced changes to thalamic GABA concentration in impulsive Parkinson disease patients. Npj Park. Dis. 2022, 8, 37. [Google Scholar] [CrossRef]
- Terkelsen, M.H.; Hvingelby, V.S.; Pavese, N. Molecular Imaging of the GABAergic System in Parkinson’s Disease and Atypical Parkinsonisms. Curr. Neurol. Neurosci. Rep. 2022, 22, 867–879. [Google Scholar] [CrossRef]
- Alharbi, B.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Alharbi, H.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Role of GABA pathway in motor and non-motor symptoms in Parkinson’s disease: A bidirectional circuit. Eur. J. Med. Res. 2024, 29, 205. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Agnati, L.; Fuxe, K.; Chen, J.-F.; Morelli, M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006, 29, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Le, W.; Jankovic, J. Preclinical Biomarkers of Parkinson Disease. Arch. Neurol. 2011, 68, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Fazio, P.; Svenningsson, P.; Cselényi, Z.; Halldin, C.; Farde, L.; Varrone, A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 592–599. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Chan, P.; Stoessl, A.J. The underlying mechanism of prodromal PD: Insights from the parasympathetic nervous system and the olfactory system. Transl. Neurodegener. 2017, 6, 4. [Google Scholar] [CrossRef]
- Ahadiat, S.-A.; Hosseinian, Z. A look back at the prodromal findings in Parkinson’s disease. Bull. Natl. Res. Cent. 2023, 47, 167. [Google Scholar] [CrossRef]
- Landers, M.R.; Johnson, K.N.; Johnson, S.; Ormsby, T.; Salgo, D.C.; Zorn, J.B.; Lyle, J.; Murtishaw, A.S.; Salazar, A.M.; Kinney, J.W. Pre-diagnosis physical activity habits are associated with age of diagnosis in Parkinson’s disease. Clin. Park. Relat. Disord. 2019, 1, 25–30. [Google Scholar] [CrossRef]
- Landers, M.R.; Navalta, J.W.; Murtishaw, A.S.; Kinney, J.W.; Pirio Richardson, S. A High-Intensity Exercise Boot Camp for Persons With Parkinson Disease: A Phase II, Pragmatic, Randomized Clinical Trial of Feasibility, Safety, Signal of Efficacy, and Disease Mechanisms. J. Neurol. Phys. Ther. JNPT 2019, 43, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Moccia, M.; Spina, E.; Barone, P.; Pellecchia, M.T. Biomarkers of Parkinson’s disease: Recent insights, current challenges, and future prospects. J. Park. Restless Legs Syndr. 2016, 6, 1–13. [Google Scholar]
- Fazlollahi, A.; Zahmatyar, M.; Alizadeh, H.; Noori, M.; Jafari, N.; Nejadghaderi, S.A.; Sullman, M.J.M.; Gharagozli, K.; Kolahi, A.-A.; Safiri, S. Association between gout and the development of Parkinson’s disease: A systematic review and meta-analysis. BMC Neurol. 2022, 22, 383. [Google Scholar] [CrossRef]
- Liu, L.; Han, Y.; Zhang, Z.; Wang, Y.; Hu, Y.; Kaznacheyeva, E.; Ding, J.; Guo, D.; Wang, G.; Li, B.; et al. Loss of DJ-1 function contributes to Parkinson’s disease pathogenesis in mice via RACK1-mediated PKC activation and MAO-B upregulation. Acta Pharmacol. Sin. 2023, 44, 1948–1961. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, J.; Ma, J.; Wang, Z.; Shi, X.; Li, M.; Huang, S.; Hu, S.; Zhao, Z.; Li, D. Increased Plasma Heme Oxygenase-1 Levels in Patients With Early-Stage Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 621508. [Google Scholar] [CrossRef]
- Song, W.; Kothari, V.; Velly, A.M.; Cressatti, M.; Liberman, A.; Gornitsky, M.; Schipper, H.M. Evaluation of salivary heme oxygenase-1 as a potential biomarker of early Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 583–591. [Google Scholar] [CrossRef]
- Galindez, J.M.; Juwara, L.; Cressatti, M.; Gornitsky, M.; Velly, A.M.; Schipper, H.M. Salivary Heme Oxygenase-1: A Potential Biomarker for Central Neurodegeneration. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029114. [Google Scholar] [CrossRef]
- Du, T.; Wang, L.; Liu, W.; Zhu, G.; Chen, Y.; Zhang, J. Biomarkers and the Role of α-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 645996. [Google Scholar] [CrossRef]
- Yilmaz, R.; Hopfner, F.; van Eimeren, T.; Berg, D. Biomarkers of Parkinson’s disease: 20 years later. J. Neural Transm. Vienna Austria 1996 2019, 126, 803–813. [Google Scholar] [CrossRef]
- Knudsen, K.; Krogh, K.; Østergaard, K.; Borghammer, P. Constipation in parkinson’s disease: Subjective symptoms, objective markers, and new perspectives. Mov. Disord. 2017, 32, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, P.; Hoffman, M.; Badiali, D.; Colosimo, C. Constipation: An emerging risk factor for Parkinson’s disease? Eur. J. Neurol. 2016, 23, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Simonet, C.; Bestwick, J.; Jitlal, M.; Waters, S.; Ben-Joseph, A.; Marshall, C.R.; Dobson, R.; Marrium, S.; Robson, J.; Jacobs, B.M.; et al. Assessment of Risk Factors and Early Presentations of Parkinson Disease in Primary Care in a Diverse UK Population. JAMA Neurol. 2022, 79, 359–369. [Google Scholar] [CrossRef]
- Patel, B.; Chiu, S.; Armstrong, M.J. Identifying Parkinson Risk Markers in Primary Care-Old Associations and New Insights. JAMA Neurol. 2022, 79, 331–333. [Google Scholar] [CrossRef]
- Miller-Patterson, C.; Hsu, J.Y.; Willis, A.W.; Hamedani, A.G. Functional Impairment in Individuals With Prodromal or Unrecognized Parkinson Disease. JAMA Neurol. 2023, 80, 200–204. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Gasperi, A.; Willeit, P.; Kiechl, S.; Stockner, H.; Willeit, J.; Rungger, G.; Sawires, M.; Nocker, M.; Rastner, V.; et al. Prodromal Parkinson’s disease as defined per MDS research criteria in the general elderly community. Mov. Disord. Off. J. Mov. Disord. Soc. 2016, 31, 1405–1408. [Google Scholar] [CrossRef]
- Marini, K.; Mahlknecht, P.; Tutzer, F.; Stockner, H.; Gasperi, A.; Djamshidian, A.; Willeit, P.; Kiechl, S.; Willeit, J.; Rungger, G.; et al. Application of a Simple Parkinson’s Disease Risk Score in a Longitudinal Population-Based Cohort. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1658–1662. [Google Scholar] [CrossRef]
- Pilotto, A.; Heinzel, S.; Suenkel, U.; Lerche, S.; Brockmann, K.; Roeben, B.; Schaeffer, E.; Wurster, I.; Yilmaz, R.; Liepelt-Scarfone, I.; et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 1025–1034. [Google Scholar] [CrossRef]
- Plouvier, A.O.A.; Hameleers, R.J.M.G.; van den Heuvel, E.A.J.; Bor, H.H.; Olde Hartman, T.C.; Bloem, B.R.; van Weel, C.; Lagro-Janssen, A.L.M. Prodromal symptoms and early detection of Parkinson’s disease in general practice: A nested case-control study. Fam. Pract. 2014, 31, 373–378. [Google Scholar] [CrossRef]
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B. MDS Task Force on the Definition of Parkinson’s Disease Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Noyce, A.J.; Bestwick, J.P.; Silveira-Moriyama, L.; Hawkes, C.H.; Knowles, C.H.; Hardy, J.; Giovannoni, G.; Nageshwaran, S.; Osborne, C.; Lees, A.J.; et al. PREDICT-PD: Identifying risk of Parkinson’s disease in the community: Methods and baseline results. J. Neurol. Neurosurg. Psychiatry 2014, 85, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.D.; Xue, C.; Kolachalama, V.B.; Donald, W.A. Interpretable Machine Learning on Metabolomics Data Reveals Biomarkers for Parkinson’s Disease. ACS Cent. Sci. 2023, 9, 1035–1045. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease: MDS Criteria for Prodromal PD. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef]
- Llido, J.P.; Jayanti, S.; Tiribelli, C.; Gazzin, S. Bilirubin and Redox Stress in Age-Related Brain Diseases. Antioxidants 2023, 12, 1525. [Google Scholar] [CrossRef]
- Macías-García, D.; Méndez-Del Barrio, C.; Jesús, S.; Labrador, M.A.; Adarmes-Gómez, A.; Vargas-González, L.; Carrillo, F.; Gómez-Garre, P.; Mir, P. Increased bilirubin levels in Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 63, 213–216. [Google Scholar] [CrossRef]
- Moccia, M.; Picillo, M.; Erro, R.; Longo, K.; Amboni, M.; Santangelo, G.; Palladino, R.; Allocca, R.; Caporale, O.; Triassi, M.; et al. Increased bilirubin levels in de novo Parkinson’s disease. Eur. J. Neurol. 2015, 22, 954–959. [Google Scholar] [CrossRef]
- Albillos, S.M.; Montero, O.; Calvo, S.; Solano-Vila, B.; Trejo, J.M.; Cubo, E. Plasma acyl-carnitines, bilirubin, tyramine and tetrahydro-21-deoxycortisol in Parkinson’s disease and essential tremor. A case control biomarker study. Parkinsonism Relat. Disord. 2021, 91, 167–172. [Google Scholar] [CrossRef]
- Neely, M.D.; Schmidt, D.E.; Deutch, A.Y. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience 2007, 149, 457–464. [Google Scholar] [CrossRef]
- Dal Ben, M.; Bongiovanni, R.; Tuniz, S.; Fioriti, E.; Tiribelli, C.; Moretti, R.; Gazzin, S. Earliest Mechanisms of Dopaminergic Neurons Sufferance in a Novel Slow Progressing Ex Vivo Model of Parkinson Disease in Rat Organotypic Cultures of Substantia Nigra. Int. J. Mol. Sci. 2019, 20, 2224. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Wang, B.; Shen, T.; Luo, W.; Liu, T. Preclinical reserpine models recapitulating motor and non-motor features of Parkinson’s disease: Roles of epigenetic upregulation of alpha-synuclein and autophagy impairment. Front. Pharmacol. 2022, 13, 944376. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Shao, D.; Song, C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson’s disease. Physiol. Behav. 2009, 98, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, T.; Bubser, M.; Ferguson, M.C.; Neely, M.D.; Goodwin, J.S.; Montine, T.J.; Deutch, A.Y.; Ansah, T.A. Cortical serotonin and norepinephrine denervation in parkinsonism: Preferential loss of the beaded serotonin innervation. Eur. J. Neurosci. 2009, 30, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Choudhury, M.E.; Yamanishi, Y.; Kyaw, W.T.; Kubo, M.; Kannou, M.; Nishikawa, N.; Tanaka, J.; Nomoto, M.; Nagai, M. Modafinil alleviates levodopa-induced excessive nighttime sleepiness and restores monoaminergic systems in a nocturnal animal model of Parkinson’s disease. J. Pharmacol. Sci. 2018, 136, 266–271. [Google Scholar] [CrossRef]
- Decourt, M.; Jiménez-Urbieta, H.; Benoit-Marand, M.; Fernagut, P.-O. Neuropsychiatric and Cognitive Deficits in Parkinson’s Disease and Their Modeling in Rodents. Biomedicines 2021, 9, 684. [Google Scholar] [CrossRef]
- Lama, J.; Buhidma, Y.; Fletcher, E.J.R.; Duty, S. Animal models of Parkinson’s disease: A guide to selecting the optimal model for your research. Neuronal Signal. 2021, 5, NS20210026. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. The Rotenone Models Reproducing Central and Peripheral Features of Parkinson’s Disease. NeuroSci 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Taguchi, T.; Ikuno, M.; Yamakado, H.; Takahashi, R. Animal Model for Prodromal Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1961. [Google Scholar] [CrossRef]
- Chaumette, T.; Lebouvier, T.; Aubert, P.; Lardeux, B.; Qin, C.; Li, Q.; Accary, D.; Bézard, E.; Bruley Des Varannes, S.; Derkinderen, P.; et al. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol. Motil. 2009, 21, 215–222. [Google Scholar] [CrossRef]
- Drolet, R.E.; Cannon, J.R.; Montero, L.; Greenamyre, J.T. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol. Dis. 2009, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.G.; Noorian, A.R.; Srinivasan, S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp. Neurol. 2009, 218, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Real, C.C.; Binda, K.H.; Thomsen, M.B.; Lillethorup, T.P.; Brooks, D.J.; Landau, A.M. Selecting the Best Animal Model of Parkinson’s Disease for Your Research Purpose: Insight from in vivo PET Imaging Studies. Curr. Neuropharmacol. 2023, 21, 1241–1272. [Google Scholar] [CrossRef]
- Sulzer, D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007, 30, 244–250. [Google Scholar] [CrossRef]
- Noyce, A.J.; Bestwick, J.P.; Silveira-Moriyama, L.; Hawkes, C.H.; Giovannoni, G.; Lees, A.J.; Schrag, A. Meta-Analysis of Early Nonmotor Features and Risk Factors for Parkinson Disease. Ann. Neurol. 2012, 72, 893–901. [Google Scholar] [CrossRef]
- Foubert-Samier, A.; Helmer, C.; Perez, F.; Le Goff, M.; Auriacombe, S.; Elbaz, A.; Dartigues, J.-F.; Tison, F. Past exposure to neuroleptic drugs and risk of Parkinson disease in an elderly cohort. Neurology 2012, 79, 1615–1621. [Google Scholar] [CrossRef]
- Goldman, S.M.; Quinlan, P.J.; Ross, G.W.; Marras, C.; Meng, C.; Bhudhikanok, G.S.; Comyns, K.; Korell, M.; Chade, A.R.; Kasten, M.; et al. Solvent Exposures and Parkinson’s Disease Risk in Twins. Ann. Neurol. 2012, 71, 776–784. [Google Scholar] [CrossRef]
- Adams, J.D. Possible causes of Parkinson’s disease. Front. Biosci. Landmark Ed. 2021, 26, 387–394. [Google Scholar] [CrossRef]
- Chen, H.; Jacobs, E.; Schwarzschild, M.A.; McCullough, M.L.; Calle, E.E.; Thun, M.J.; Ascherio, A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann. Neurol. 2005, 58, 963–967. [Google Scholar] [CrossRef]
- Martin-de-Pablos, A.; Córdoba-Fernández, A.; Fernández-Espejo, E. Analysis of neurotrophic and antioxidant factors related to midbrain dopamine neuronal loss and brain inflammation in the cerebrospinal fluid of the elderly. Exp. Gerontol. 2018, 110, 54–60. [Google Scholar] [CrossRef]
- Leite, F.; Ribeiro, L. Dopaminergic Pathways in Obesity-Associated Inflammation. J. Neuroimmune Pharmacol. 2020, 15, 93–113. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of sGut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Kalampokini, S.; Becker, A.; Fassbender, K.; Lyros, E.; Unger, M.M. Nonpharmacological Modulation of Chronic Inflammation in Parkinson’s Disease: Role of Diet Interventions. Park. Dis. 2019, 2019, e7535472. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; DeStefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014, 46, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Trinh, J.; Farrer, M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013, 9, 445–454. [Google Scholar] [CrossRef]
- Magistrelli, L.; Contaldi, E.; Vignaroli, F.; Gallo, S.; Colombatto, F.; Cantello, R.; Comi, C. Immune Response Modifications in the Genetic Forms of Parkinson’s Disease: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3476. [Google Scholar] [CrossRef]
- Furuyashiki, T. Roles of dopamine and inflammation-related molecules in behavioral alterations caused by repeated stress. J. Pharmacol. Sci. 2012, 120, 63–69. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Zhou, L.; Sun, X.; Yang, Y.; Zhang, C.; Wang, T.; Fu, F. Dopamine, a co-regulatory component, bridges the central nervous system and the immune system. Biomed. Pharmacother. 2022, 145, 112458. [Google Scholar] [CrossRef]
- Marchetti, B.; Giachino, C.; Tirolo, C.; Serapide, M.F. “Reframing” dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications. Aging Cell 2022, 21, e13575. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Immunomodulatory Effects of Dopamine in Inflammatory Diseases. Front. Immunol. 2021, 12, 663102. [Google Scholar] [CrossRef] [PubMed]
- Nolan, Y.M.; Sullivan, A.M.; Toulouse, A. Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol. Med. 2013, 19, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.-P.; Cheng, Z.-Y.; He, L. The modulatory role of dopamine receptors in brain neuroinflammation. Int. Immunopharmacol. 2019, 76, 105908. [Google Scholar] [CrossRef]
- Melis, M.; Carta, G.; Pistis, M.; Banni, S. Physiological Role of Peroxisome Proliferator-Activated Receptors Type Alpha on Dopamine Systems. CNS Neurol. Disord.-Drug Targets-CNS Neurol. Disord. 2013, 12, 70–77. [Google Scholar] [CrossRef]
- Pingale, T.; Gupta, G.L. Classic and evolving animal models in Parkinson’s disease. Pharmacol. Biochem. Behav. 2020, 199, 173060. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene–environment interactions. NeuroToxicology 2015, 46, 101–116. [Google Scholar] [CrossRef]
- Innos, J.; Hickey, M.A. Using Rotenone to Model Parkinson’s Disease in Mice: A Review of the Role of Pharmacokinetics. Chem. Res. Toxicol. 2021, 34, 1223–1239. [Google Scholar] [CrossRef]
- Meredith, G.E.; Sonsalla, P.K.; Chesselet, M.-F. Animal models of Parkinson’s disease progression. Acta Neuropathol. 2008, 115, 385–398. [Google Scholar] [CrossRef]
- Deng, I.; Corrigan, F.; Zhai, G.; Zhou, X.-F.; Bobrovskaya, L. Lipopolysaccharide animal models of Parkinson’s disease: Recent progress and relevance to clinical disease. Brain Behav. Immun.-Health 2020, 4, 100060. [Google Scholar] [CrossRef]
- Klæstrup, I.H.; Just, M.K.; Holm, K.L.; Alstrup, A.K.O.; Romero-Ramos, M.; Borghammer, P.; Van Den Berge, N. Impact of aging on animal models of Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 909273. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of vesicular monoamine transporter 2 (VMAT2) inhibitors in tardive dyskinesia: Reducing dopamine leads to less “go” and more “stop” from the motor striatum for robust therapeutic effects. CNS Spectr. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Neha, S.; Ahmad, M.; Kumari, B.; Ali, M.Z.; Dholaniya, P.S. Early Diagnosis of Parkinson’s Disease: Utility of Animal Models. In Parkinson’s Disease—Animal Models, Current Therapies and Clinical Trials; IntechOpen: London, UK, 2022; ISBN 978-1-80356-489-0. [Google Scholar]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Dovonou, A.; Bolduc, C.; Soto Linan, V.; Gora, C.; Peralta, M.R., III; Lévesque, M. Animal models of Parkinson’s disease: Bridging the gap between disease hallmarks and research questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef]
- de Freitas, C.M.; Busanello, A.; Schaffer, L.F.; Peroza, L.R.; Krum, B.N.; Leal, C.Q.; Ceretta, A.P.C.; da Rocha, J.B.T.; Fachinetto, R. Behavioral and neurochemical effects induced by reserpine in mice. Psychopharmacology 2016, 233, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Drukarch, B.; Jongenelen, C.A.; Schepens, E.; Langeveld, C.H.; Stoof, J.C. Glutathione is involved in the granular storage of dopamine in rat PC 12 pheochromocytoma cells: Implications for the pathogenesis of Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 6038–6045. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Sullivan, P.; Cooney, A.; Jinsmaa, Y.; Sullivan, R.; Gross, D.J.; Holmes, C.; Kopin, I.J.; Sharabi, Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells: Relevance to the pathogenesis of Parkinson’s disease. J. Neurochem. 2012, 123, 932–943. [Google Scholar] [CrossRef]
- Glinka, Y.; Gassen, M.; Youdim, M.B. Mechanism of 6-hydroxydopamine neurotoxicity. J. Neural Transm. Suppl. 1997, 50, 55–66. [Google Scholar]
- Henning, J.; Strauss, U.; Wree, A.; Gimsa, J.; Rolfs, A.; Benecke, R.; Gimsa, U. Differential astroglial activation in 6-hydroxydopamine models of Parkinson’s disease. Neurosci. Res. 2008, 62, 246–253. [Google Scholar] [CrossRef]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef]
- Hisahara, S.; Shimohama, S. Toxin-Induced and Genetic Animal Models of Parkinson’s Disease. Park. Dis. 2010, 2011, 951709. [Google Scholar] [CrossRef]
- Antunes, M.S.; Cattelan Souza, L.; Ladd, F.V.L.; Ladd, A.A.B.L.; Moreira, A.L.; Bortolotto, V.C.; Silva, M.R.P.; Araújo, S.M.; Prigol, M.; Nogueira, C.W.; et al. Hesperidin Ameliorates Anxiety-Depressive-Like Behavior in 6-OHDA Model of Parkinson’s Disease by Regulating Striatal Cytokine and Neurotrophic Factors Levels and Dopaminergic Innervation Loss in the Striatum of Mice. Mol. Neurobiol. 2020, 57, 3027–3041. [Google Scholar] [CrossRef]
- Khan, E.; Hasan, I.; Haque, M.E. Parkinson’s Disease: Exploring Different Animal Model Systems. Int. J. Mol. Sci. 2023, 24, 9088. [Google Scholar] [CrossRef]
- Berger, K.; Przedborski, S.; Cadet, J.L. Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res. Bull. 1991, 26, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Cousins, M.S.; Salamone, J.D. Involvement of ventrolateral striatal dopamine in movement initiation and execution: A microdialysis and behavioral investigation. Neuroscience 1996, 70, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.M.; Barbiero, J.; Gradowski, R.W.; Bochen, S.; Lima, M.M.S.; Da Cunha, C.; Andreatini, R.; Vital, M.A.B.F. Induction of depressive-like behavior by intranigral 6-OHDA is directly correlated with deficits in striatal dopamine and hippocampal serotonin. Behav. Brain Res. 2014, 259, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tadaiesky, M.T.; Dombrowski, P.A.; Figueiredo, C.P.; Cargnin-Ferreira, E.; Da Cunha, C.; Takahashi, R.N. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience 2008, 156, 830–840. [Google Scholar] [CrossRef]
- Anichtchik, O.V.; Kaslin, J.; Peitsaro, N.; Scheinin, M.; Panula, P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 2004, 88, 443–453. [Google Scholar] [CrossRef]
- Lindner, M.D.; Plone, M.A.; Francis, J.M.; Blaney, T.J.; Salamone, J.D.; Emerich, D.F. Rats with partial striatal dopamine depletions exhibit robust and long-lasting behavioral deficits in a simple fixed-ratio bar-pressing task. Behav. Brain Res. 1997, 86, 25–40. [Google Scholar] [CrossRef]
- Kamińska, K.; Lenda, T.; Konieczny, J.; Czarnecka, A.; Lorenc-Koci, E. Depressive-like neurochemical and behavioral markers of Parkinson’s disease after 6-OHDA administered unilaterally to the rat medial forebrain bundle. Pharmacol. Rep. 2017, 69, 985–994. [Google Scholar] [CrossRef]
- Feng, C.-W.; Wen, Z.-H.; Huang, S.-Y.; Hung, H.-C.; Chen, C.-H.; Yang, S.-N.; Chen, N.-F.; Wang, H.-M.; Hsiao, C.-D.; Chen, W.-F. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef]
- Loiodice, S.; Wing Young, H.; Rion, B.; Méot, B.; Montagne, P.; Denibaud, A.-S.; Viel, R.; Drieu La Rochelle, C. Implication of nigral dopaminergic lesion and repeated L-dopa exposure in neuropsychiatric symptoms of Parkinson’s disease. Behav. Brain Res. 2019, 360, 120–127. [Google Scholar] [CrossRef]
- Na, S.J.; DiLella, A.G.; Lis, E.V.; Jones, K.; Levine, D.M.; Stone, D.J.; Hess, J.F. Molecular profiling of a 6-hydroxydopamine model of Parkinson’s disease. Neurochem. Res. 2010, 35, 761–772. [Google Scholar] [CrossRef]
- Walsh, S.; Finn, D.P.; Dowd, E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience 2011, 175, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Parng, C.; Roy, N.M.; Ton, C.; Lin, Y.; McGrath, P. Neurotoxicity assessment using zebrafish. J. Pharmacol. Toxicol. Methods 2007, 55, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Oertel, W.H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 1994, 59, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Mokhemer, E.; Abuirmeileh, A.N. Beneficial effect of etazolate on depression-like behavior and, learning, and memory impairment in a model of Parkinson’s disease. Behav. Brain Res. 2018, 350, 109–115. [Google Scholar] [CrossRef]
- Vo, Q.; Gilmour, T.P.; Venkiteswaran, K.; Fang, J.; Subramanian, T. Polysomnographic Features of Sleep Disturbances and REM Sleep Behavior Disorder in the Unilateral 6-OHDA Lesioned Hemiparkinsonian Rat. Park. Dis. 2014, 2014, 852965. [Google Scholar] [CrossRef]
- Jordán, J.; Galindo, M.F.; Tornero, D.; González-García, C.; Ceña, V. Bcl-xL blocks mitochondrial multiple conductance channel activation and inhibits 6-OHDA-induced death in SH-SY5Y cells. J. Neurochem. 2004, 89, 124–133. [Google Scholar] [CrossRef]
- Choi, W.-S.; Eom, D.-S.; Han, B.S.; Kim, W.K.; Han, B.H.; Choi, E.-J.; Oh, T.H.; Markelonis, G.J.; Cho, J.W.; Oh, Y.J. Phosphorylation of p38 MAPK Induced by Oxidative Stress Is Linked to Activation of Both Caspase-8- and -9-mediated Apoptotic Pathways in Dopaminergic Neurons. J. Biol. Chem. 2004, 279, 20451–20460. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Effect of 6-hydroxydopamine increase the glutathione level in SH-SY5Y human neuroblastoma cells. Acta Biochim. Pol. 2023, 70, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Shen, X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2006, 97, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Han, J.; Wu, P.; Wu, C.; Fan, Y.; Zhao, L.; Hao, X.; Chen, D.; Zhu, M. Sorting Nexin 5 Plays an Important Role in Promoting Ferroptosis in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 5463134. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, J.; Hao, X.; Li, H.; Zhang, G.; Liu, X.; Li, X.; Zhao, C.; Kuang, W.; Chen, D.; et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6-OHDA Model of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1796–1812. [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, S.H.; Shin, D.-I.; Hwang, J.-Y.; Park, B.; Park, Y.-J.; Lee, T.H.; Chae, H.Z.; Jin, B.K.; Oh, T.H.; et al. Oxidative Modification of Peroxiredoxin Is Associated with Drug-induced Apoptotic Signaling in Experimental Models of Parkinson Disease. J. Biol. Chem. 2008, 283, 9986–9998. [Google Scholar] [CrossRef]
- Oh, C.-K.; Choi, Y.K.; Hwang, I.-Y.; Ko, Y.U.; Chung, I.K.; Yun, N.; Oh, Y.J. RING-finger protein 166 plays a novel pro-apoptotic role in neurotoxin-induced neurodegeneration via ubiquitination of XIAP. Cell Death Dis. 2020, 11, 939. [Google Scholar] [CrossRef]
- Han, B.S.; Hong, H.-S.; Choi, W.-S.; Markelonis, G.J.; Oh, T.H.; Oh, Y.J. Caspase-Dependent and -Independent Cell Death Pathways in Primary Cultures of Mesencephalic Dopaminergic Neurons after Neurotoxin Treatment. J. Neurosci. 2003, 23, 5069–5078. [Google Scholar] [CrossRef]
- Jang, H.J.; Chung, K.C. The ubiquitin-proteasome system and autophagy mutually interact in neurotoxin-induced dopaminergic cell death models of Parkinson’s disease. FEBS Lett. 2022, 596, 2898–2913. [Google Scholar] [CrossRef]
- Lei, C.; Zhongyan, Z.; Wenting, S.; Jing, Z.; Liyun, Q.; Hongyi, H.; Juntao, Y.; Qing, Y. Identification of necroptosis-related genes in Parkinson’s disease by integrated bioinformatics analysis and experimental validation. Front. Neurosci. 2023, 17, 1097293. [Google Scholar] [CrossRef]
- Gerlach, M.; Riederer, P.; Przuntek, H.; Youdim, M.B. MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur. J. Pharmacol. 1991, 208, 273–286. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Blesa, J.; Przedborski, S. Animal models of Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, S183–S185. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, Q.; Du, X.; Xu, S.; Li, M.; Ma, S. Early activation of Egr-1 promotes neuroinflammation and dopaminergic neurodegeneration in an experimental model of Parkinson’s disease. Exp. Neurol. 2018, 302, 145–154. [Google Scholar] [CrossRef]
- Lofrumento, D.D.; Saponaro, C.; Cianciulli, A.; De Nuccio, F.; Mitolo, V.; Nicolardi, G.; Panaro, M.A. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation 2011, 18, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, A.; Sieber, B.A.; Heikkila, R.E.; Sonsalla, P.K. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J. Pharmacol. Exp. Ther. 1994, 270, 1000–1007. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, Y.-K.; Ahn, S.; Hwang, T.-Y.; Lee, H.; Park, H.-J. A Comprehensive Phenotype of Non-motor Impairments and Distribution of Alpha-Synuclein Deposition in Parkinsonism-Induced Mice by a Combination Injection of MPTP and Probenecid. Front. Aging Neurosci. 2020, 12, 599045. [Google Scholar] [CrossRef]
- Cunha, M.P.; Pazini, F.L.; Lieberknecht, V.; Budni, J.; Oliveira, Á.; Rosa, J.M.; Mancini, G.; Mazzardo, L.; Colla, A.R.; Leite, M.C.; et al. MPP+-Lesioned Mice: An Experimental Model of Motor, Emotional, Memory/Learning, and Striatal Neurochemical Dysfunctions. Mol. Neurobiol. 2017, 54, 6356–6377. [Google Scholar] [CrossRef]
- Shi, J.; Cai, Q.; Zhang, J.; He, X.; Liu, Y.; Zhu, R.; Jin, L. AM1241 alleviates MPTP-induced Parkinson’s disease and promotes the regeneration of DA neurons in PD mice. Oncotarget 2017, 8, 67837–67850. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Zhao, L.; Yang, C.; Pan, L.; Li, C.; Liu, K.; Bai, G.; Gao, H.; Yan, Z. Metabolic Disturbances in the Striatum and Substantia Nigra in the Onset and Progression of MPTP-Induced Parkinsonism Model. Front. Neurosci. 2018, 12, 90. [Google Scholar] [CrossRef]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Parkinson’s disease model in zebrafish using intraperitoneal MPTP injection. Front. Neurosci. 2023, 17, 1236049. [Google Scholar] [CrossRef]
- Bezard, E.; Imbert, C.; Deloire, X.; Bioulac, B.; Gross, C.E. A chronic MPTP model reproducing the slow evolution of Parkinson’s disease: Evolution of motor symptoms in the monkey. Brain Res. 1997, 766, 107–112. [Google Scholar] [CrossRef]
- Pifl, C.; Schingnitz, G.; Hornykiewicz, O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 1991, 44, 591–605. [Google Scholar] [CrossRef]
- Graham, W.C.; Robertson, R.G.; Sambrook, M.A.; Crossman, A.R. Injection of excitatory amino acid antagonists into the medial pallidal segment of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated primate reverses motor symptoms of parkinsonism. Life Sci. 1990, 47, PL91–PL97. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Syed, E.; Jackson, M.J.; Johnston, L.C.; Smith, L.A.; Jenner, P. A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur. J. Neurosci. 2005, 21, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Vucković, M.G.; Wood, R.I.; Holschneider, D.P.; Abernathy, A.; Togasaki, D.M.; Smith, A.; Petzinger, G.M.; Jakowec, M.W. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol. Dis. 2008, 32, 319–327. [Google Scholar] [CrossRef]
- Wen, L.; Wei, W.; Gu, W.; Huang, P.; Ren, X.; Zhang, Z.; Zhu, Z.; Lin, S.; Zhang, B. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 2008, 314, 84–92. [Google Scholar] [CrossRef]
- Lam, C.S.; Korzh, V.; Strahle, U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur. J. Neurosci. 2005, 21, 1758–1762. [Google Scholar] [CrossRef]
- Sarath Babu, N.; Murthy, C.L.N.; Kakara, S.; Sharma, R.; Brahmendra Swamy, C.V.; Idris, M.M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease in zebrafish. Proteomics 2016, 16, 1407–1420. [Google Scholar] [CrossRef]
- Tang, L.; Xu, N.; Huang, M.; Yi, W.; Sang, X.; Shao, M.; Li, Y.; Hao, Z.; Liu, R.; Shen, Y.; et al. A primate nigrostriatal atlas of neuronal vulnerability and resilience in a model of Parkinson’s disease. Nat. Commun. 2023, 14, 7497. [Google Scholar] [CrossRef]
- Ferro, M.M.; Bellissimo, M.I.; Anselmo-Franci, J.A.; Angellucci, M.E.M.; Canteras, N.S.; Da Cunha, C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurochemical, motor and memory alterations. J. Neurosci. Methods 2005, 148, 78–87. [Google Scholar] [CrossRef]
- Schneider, J.S.; Roeltgen, D.P. Delayed matching-to-sample, object retrieval, and discrimination reversal deficits in chronic low dose MPTP-treated monkeys. Brain Res. 1993, 615, 351–354. [Google Scholar] [CrossRef]
- Mandel, S.; Grünblatt, E.; Maor, G.; Youdim, M.B.H. Early and Late Gene Changes in MPTP Mice Model of Parkinson’s Disease Employing cDNA Microarray. Neurochem. Res. 2002, 27, 1231–1243. [Google Scholar] [CrossRef]
- Benner, E.J.; Mosley, R.L.; Destache, C.J.; Lewis, T.B.; Jackson-Lewis, V.; Gorantla, S.; Nemachek, C.; Green, S.R.; Przedborski, S.; Gendelman, H.E. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Barraud, Q.; Lambrecq, V.; Forni, C.; McGuire, S.; Hill, M.; Bioulac, B.; Balzamo, E.; Bezard, E.; Tison, F.; Ghorayeb, I. Sleep disorders in Parkinson’s disease: The contribution of the MPTP non-human primate model. Exp. Neurol. 2009, 219, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Verhave, P.S.; Jongsma, M.J.; Van den Berg, R.M.; Vis, J.C.; Vanwersch, R.A.P.; Smit, A.B.; Van Someren, E.J.W.; Philippens, I.H.C.H.M. REM Sleep Behavior Disorder in the Marmoset MPTP Model of Early Parkinson Disease. Sleep 2011, 34, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Forno, L.S.; Langston, J.W.; DeLanney, L.E.; Irwin, I.; Ricaurte, G.A. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann. Neurol. 1986, 20, 449–455. [Google Scholar] [CrossRef]
- Forno, L.S.; DeLanney, L.E.; Irwin, I.; Langston, J.W. Similarities and differences between MPTP-induced parkinsonsim and Parkinson’s disease. Neuropathologic considerations. Adv. Neurol. 1993, 60, 600–608. [Google Scholar]
- Martins, J.B.; Bastos, M.D.L.; Carvalho, F.; Capela, J.P. Differential Effects of Methyl-4-Phenylpyridinium Ion, Rotenone, and Paraquat on Differentiated SH-SY5Y Cells. J. Toxicol. 2013, 2013, 347312. [Google Scholar] [CrossRef]
- Dai, H.-Y.; Chang, M.-X.; Sun, L. HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway. Transl. Neurosci. 2023, 14, 20220296. [Google Scholar] [CrossRef]
- Krug, A.K.; Gutbier, S.; Zhao, L.; Pöltl, D.; Kullmann, C.; Ivanova, V.; Förster, S.; Jagtap, S.; Meiser, J.; Leparc, G.; et al. Transcriptional and metabolic adaptation of human neurons to the mitochondrial toxicant MPP+. Cell Death Dis. 2014, 5, e1222. [Google Scholar] [CrossRef]
- Shindo, Y.; Yamanaka, R.; Suzuki, K.; Hotta, K.; Oka, K. Altered expression of Mg2+ transport proteins during Parkinson’s disease-like dopaminergic cell degeneration in PC12 cells. Biochim. Biophys. Acta 2016, 1863, 1979–1984. [Google Scholar] [CrossRef]
- Xiao, X.; Tan, Z.; Jia, M.; Zhou, X.; Wu, K.; Ding, Y.; Li, W. Long Noncoding RNA SNHG1 Knockdown Ameliorates Apoptosis, Oxidative Stress and Inflammation in Models of Parkinson’s Disease by Inhibiting the miR-125b-5p/MAPK1 Axis. Neuropsychiatr. Dis. Treat. 2021, 17, 1153–1163. [Google Scholar]
- He, S.; Wang, Q.; Chen, L.; He, Y.J.; Wang, X.; Qu, S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson’s disease model via regulation of Nox4/ROS/Nrf2 signaling. J. Transl. Med. 2023, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Grünblatt, E.; Levites, Y.; Maor, G.; Mandel, S. Early and late molecular events in neurodegeneration and neuroprotection in Parkinson’s disease MPTP model as assessed by cDNA microarray; the role of iron. Neurotox. Res. 2002, 4, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, P.; Yin, H.; Wang, X.; Lv, J.; Yuan, J.; Zhu, J.; Wang, Y. Rapamycin reverses ferroptosis by increasing autophagy in MPTP/MPP+-induced models of Parkinson’s disease. Neural Regen. Res. 2023, 18, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, Y.; Sun, X. Toxic influence of chronic oral administration of paraquat on nigrostriatal dopaminergic neurons in C57BL/6 mice. Chin. Med. J. 2009, 122, 2366–2371. [Google Scholar] [PubMed]
- Bortolotto, J.W.; Cognato, G.P.; Christoff, R.R.; Roesler, L.N.; Leite, C.E.; Kist, L.W.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Long-term exposure to paraquat alters behavioral parameters and dopamine levels in adult zebrafish (Danio rerio). Zebrafish 2014, 11, 142–153. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Leahy, S.; Harrison, K.; Sikorska, M.; Sandhu, J.K.; Cohen, J.; Keshan, C.; Lopatin, D.; Miller, H.; Borowy-Borowski, H.; et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: Potential for therapeutic application in Parkinson’s disease. BMC Neurosci. 2014, 15, 21. [Google Scholar] [CrossRef]
- Nellore, J.; Nandita, P. Paraquat exposure induces behavioral deficits in larval zebrafish during the window of dopamine neurogenesis. Toxicol. Rep. 2015, 2, 950–956. [Google Scholar] [CrossRef]
- Vornov, J.J.; Park, J.; Thomas, A.G. Regional vulnerability to endogenous and exogenous oxidative stress in organotypic hippocampal culture. Exp. Neurol. 1998, 149, 109–122. [Google Scholar] [CrossRef]
- Wang, F.; Franco, R.; Skotak, M.; Hu, G.; Chandra, N. Mechanical stretch exacerbates the cell death in SH-SY5Y cells exposed to paraquat: Mitochondrial dysfunction and oxidative stress. NeuroToxicology 2014, 41, 54–63. [Google Scholar] [CrossRef]
- Zuo, Y.; Xie, J.; Li, X.; Li, Y.; Thirupathi, A.; Zhang, J.; Yu, P.; Gao, G.; Chang, Y.; Shi, Z. Ferritinophagy-Mediated Ferroptosis Involved in Paraquat-Induced Neurotoxicity of Dopaminergic Neurons: Implication for Neurotoxicity in PD. Oxid. Med. Cell. Longev. 2021, 2021, 9961628. [Google Scholar] [CrossRef]
- Ascherio, A.; Chen, H.; Weisskopf, M.G.; O’Reilly, E.; McCullough, M.L.; Calle, E.E.; Schwarzschild, M.A.; Thun, M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006, 60, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Counsell, C.; Mozzoni, P.; et al. Environmental risk factors for Parkinson’s disease and parkinsonism: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Han, B.-S.; Kim, S.-J.; Oh, Y.J. Mechanisms to prevent caspase activation in rotenone-induced dopaminergic neurodegeneration: Role of ATP depletion and procaspase-9 degradation. Apoptosis 2012, 17, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, X.; Jinjun, Q.; Minfang, X.; Jing, Q.; Juan, X.; Rui, M.; Li, Z.; Jing, G. Effects of Low Concentrations of Rotenone upon Mitohormesis in SH-SY5Y Cells. Dose-Response Publ. Int. Hormesis Soc. 2013, 11, 270–280. [Google Scholar] [CrossRef]
- Van Laar, A.D.; Webb, K.R.; Keeney, M.T.; Van Laar, V.S.; Zharikov, A.; Burton, E.A.; Hastings, T.G.; Glajch, K.E.; Hirst, W.D.; Greenamyre, J.T.; et al. Transient exposure to rotenone causes degeneration and progressive parkinsonian motor deficits, neuroinflammation, and synucleinopathy. Npj Park. Dis. 2023, 9, 121. [Google Scholar] [CrossRef]
- Xu, L.; Hao, L.-P.; Yu, J.; Cheng, S.-Y.; Li, F.; Ding, S.-M.; Zhang, R. Curcumin protects against rotenone-induced Parkinson’s disease in mice by inhibiting microglial NLRP3 inflammasome activation and alleviating mitochondrial dysfunction. Heliyon 2023, 9, e16195. [Google Scholar] [CrossRef]
- Sanfeliu, C.; Bartra, C.; Suñol, C.; Rodríguez-Farré, E. New insights in animal models of neurotoxicity-induced neurodegeneration. Front. Neurosci. 2023, 17, 1248727. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Liao, Z.; Wang, B.; Gong, S.; Wang, C.; Zhang, M.; Wang, G.; Cai, H.; Liao, F.-F.; et al. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol. Neurodegener. 2015, 10, 4. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Nicklas, W.J.; Vyas, I.; Duvoisin, R.C. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci. Lett. 1985, 62, 389–394. [Google Scholar] [CrossRef]
- Rocha, S.M.; Bantle, C.M.; Aboellail, T.; Chatterjee, D.; Smeyne, R.J.; Tjalkens, R.B. Rotenone induces regionally distinct α-synuclein protein aggregation and activation of glia prior to loss of dopaminergic neurons in C57Bl/6 mice. Neurobiol. Dis. 2022, 167, 105685. [Google Scholar] [CrossRef]
- Broome, S.T.; Musumeci, G.; Castorina, A. PACAP and VIP Mitigate Rotenone-Induced Inflammation in BV-2 Microglial Cells. J. Mol. Neurosci. 2022, 72, 2163–2175. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harishchandra, D.S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B.N.; Rokad, D.; Jin, H.; Anantharam, V.; et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. Npj Park. Dis. 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Guo, Y.; Lü, P.; Gong, X.; Chen, K.; Li, X.; Tang, M. Rotenone-induced PINK1/Parkin-mediated mitophagy: Establishing a silkworm model for Parkinson’s disease potential. Front. Mol. Neurosci. 2024, 17, 1359294. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Han, C.; Jiang, H.; Ma, K.; Guo, S.; Xia, Y.; Wan, F.; Huang, J.; Xiong, N.; et al. Histone Deacetylase 4 Inhibition Reduces Rotenone-Induced Alpha-Synuclein Accumulation via Autophagy in SH-SY5Y Cells. Brain Sci. 2023, 13, 670. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wang, M.-H.; Yang, H.-C.; Tian, T.; Sun, G.-F.; Ji, Y.-F.; Hu, W.-T.; Liu, X.; Wang, J.-P.; Lu, H. Dopaminergic neuron injury in Parkinson’s disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/α-synuclein pathway. Aging 2019, 11, 9264–9279. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zou, Y.; Li, S.; Qi, R.; Yu, H.; Li, J. MALAT1 Mediates α-Synuclein Expression through miR-23b-3p to Induce Autophagic Impairment and the Inflammatory Response in Microglia to Promote Apoptosis in Dopaminergic Neuronal Cells. Mediators Inflamm. 2023, 2023, 4477492. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Zhang, R.; Wang, H.; Li, Y.; Liu, Z.; Xie, W.; Geng, D.; Wang, L. circ-Pank1 promotes dopaminergic neuron neurodegeneration through modulating miR-7a-5p/α-syn pathway in Parkinson’s disease. Cell Death Dis. 2022, 13, 477. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Cooney, A.; Jinsmaa, Y.; Kopin, I.J.; Sharabi, Y. Rotenone decreases intracellular aldehyde dehydrogenase activity: Implications for the pathogenesis of Parkinson’s disease. J. Neurochem. 2015, 133, 14–25. [Google Scholar] [CrossRef]
- Jayanti, S.; Moretti, R.; Tiribelli, C.; Gazzin, S. Bilirubin Prevents the TH+ Dopaminergic Neuron Loss in a Parkinson’s Disease Model by Acting on TNF-α. Int. J. Mol. Sci. 2022, 23, 14276. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, W.-Y.; Huang, H.; Li, W.-Z.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A Protects Against Lipopolysaccharide-Induced Damage of Dopaminergic Neurons Both In Vivo and In Vitro via Inhibition of Microglial Activation. Neurotox. Res. 2016, 30, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Gorgan, L.D. Intranigral lipopolysaccharide induced anxiety and depression by altered BDNF mRNA expression in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 126–132. [Google Scholar] [CrossRef]
- Hritcu, L.; Ciobica, A. Intranigral lipopolysaccharide administration induced behavioral deficits and oxidative stress damage in laboratory rats: Relevance for Parkinson’s disease. Behav. Brain Res. 2013, 253, 25–31. [Google Scholar] [CrossRef]
- He, Q.; Yu, W.; Wu, J.; Chen, C.; Lou, Z.; Zhang, Q.; Zhao, J.; Wang, J.; Xiao, B. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS ONE 2013, 8, e78418. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2018, 19, 12. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Liu, M.; Hunter, R.L.; Cass, W.A.; Pandya, J.D.; Sullivan, P.G.; Shin, E.-J.; Kim, H.-C.; Gash, D.M.; Bing, G. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS ONE 2009, 4, e5482. [Google Scholar] [CrossRef]
- Ling, Z.; Gayle, D.A.; Ma, S.Y.; Lipton, J.W.; Tong, C.W.; Hong, J.-S.; Carvey, P.M. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov. Disord. Off. J. Mov. Disord. Soc. 2002, 17, 116–124. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Zhang, Y.; Chen, C.-L.; Song, J.; Fan, R.-F.; Cai, Q.-Q.; Wang, Z.-Y.; Zhu, J.-X. Alterations in TH- and ChAT-immunoreactive neurons in the DMV and gastric dysmotility in an LPS-induced PD rat model. Auton. Neurosci. Basic Clin. 2013, 177, 194–198. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Y.; Hong, J.-S.; Crews, F.T. NADPH oxidase and aging drive microglial activation, oxidative stress and dopaminergic neurodegeneration following systemic LPS administration. Glia 2013, 61, 855–868. [Google Scholar] [CrossRef]
- Zheng, H.-F.; Yang, Y.-P.; Hu, L.-F.; Wang, M.-X.; Wang, F.; Cao, L.-D.; Li, D.; Mao, C.-J.; Xiong, K.-P.; Wang, J.-D.; et al. Autophagic impairment contributes to systemic inflammation-induced dopaminergic neuron loss in the midbrain. PLoS ONE 2013, 8, e70472. [Google Scholar] [CrossRef]
- Lai, T.T.; Kim, Y.J.; Nguyen, P.T.; Koh, Y.H.; Nguyen, T.T.; Ma, H.-I.; Kim, Y.E. Temporal Evolution of Inflammation and Neurodegeneration With Alpha-Synuclein Propagation in Parkinson’s Disease Mouse Model. Front. Integr. Neurosci. 2021, 15, 715190. [Google Scholar] [CrossRef]
- Oliveras-Salvá, M.; Van der Perren, A.; Casadei, N.; Stroobants, S.; Nuber, S.; D’Hooge, R.; Van den Haute, C.; Baekelandt, V. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 2013, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Sznejder-Pachołek, A.; Joniec-Maciejak, I.; Wawer, A.; Ciesielska, A.; Mirowska-Guzel, D. The effect of α-synuclein on gliosis and IL-1α, TNFα, IFNγ, TGFβ expression in murine brain. Pharmacol. Rep. 2017, 69, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2016, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Breger, L.S.; Fuzzati Armentero, M.T. Genetically engineered animal models of Parkinson’s disease: From worm to rodent. Eur. J. Neurosci. 2019, 49, 533–560. [Google Scholar] [CrossRef]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Qadirifard, M.S.; Kosari, M.; Naghsh, N.; Asgari taei, A.; Klegeris, A.; Dehghani, M.; Bahrami, A.; et al. Pre-clinical Studies Identifying Molecular Pathways of Neuroinflammation in Parkinson’s Disease: A Systematic Review. Front. Aging Neurosci. 2022, 14, 855776. [Google Scholar] [CrossRef]
- Devine, M.J.; Ryten, M.; Vodicka, P.; Thomson, A.J.; Burdon, T.; Houlden, H.; Cavaleri, F.; Nagano, M.; Drummond, N.J.; Taanman, J.-W.; et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat. Commun. 2011, 2, 440. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Byers, B.; Cord, B.; Shcheglovitov, A.; Byrne, J.; Gujar, P.; Kee, K.; Schüle, B.; Dolmetsch, R.E.; Langston, W.; et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011, 8, 267–280. [Google Scholar] [CrossRef]
- Razali, K.; Othman, N.; Mohd Nasir, M.H.; Doolaanea, A.A.; Kumar, J.; Ibrahim, W.N.; Mohamed Ibrahim, N.; Mohamed, W.M.Y. The Promise of the Zebrafish Model for Parkinson’s Disease: Today’s Science and Tomorrow’s Treatment. Front. Genet. 2021, 12, 655550. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Lones, M.A.; Bedder, M.; Currie, P.D.; Smith, S.L.; Pownall, M.E. Machine learning discriminates a movement disorder in a zebrafish model of Parkinson’s disease. Dis. Model. Mech. 2020, 13, dmm045815. [Google Scholar] [CrossRef] [PubMed]
- Wasel, O.; Freeman, J.L. Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5981. [Google Scholar] [CrossRef] [PubMed]
- Makhija, D.T.; Jagtap, A.G. Studies on sensitivity of zebrafish as a model organism for Parkinson’s disease: Comparison with rat model. J. Pharmacol. Pharmacother. 2014, 5, 39–46. [Google Scholar] [CrossRef]
- Thomas Broome, S.; Castorina, A. Systemic Rotenone Administration Causes Extra-Nigral Alterations in C57BL/6 Mice. Biomedicines 2022, 10, 3174. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Lv, D.-J.; Li, L.-X.; Chen, J.; Wei, S.-Z.; Wang, F.; Hu, H.; Xie, A.-M.; Liu, C.-F. Sleep deprivation caused a memory defects and emotional changes in a rotenone-based zebrafish model of Parkinson’s disease. Behav. Brain Res. 2019, 372, 112031. [Google Scholar] [CrossRef]
- Ramli, M.D.B.C.; Hashim, N.H.B.; Uzid, M.B.M.; Weinheimeri, A.Z. Zebrafish parkinson’s model: The effects of tocotrienol rich fraction towards rotenone induced zebrafish. Int. J. Med. Toxicol. Leg. Med. 2020, 23, 1–7. [Google Scholar] [CrossRef]
- Ding, F.; Luan, L.; Ai, Y.; Walton, A.; Gerhardt, G.A.; Gash, D.M.; Grondin, R.; Zhang, Z. Development of a stable, early stage unilateral model of Parkinson’s disease in middle-aged rhesus monkeys. Exp. Neurol. 2008, 212, 431–439. [Google Scholar] [CrossRef]
- Atack, J.R.; Shook, B.C.; Rassnick, S.; Jackson, P.F.; Rhodes, K.; Drinkenburg, W.H.; Ahnaou, A.; Te Riele, P.; Langlois, X.; Hrupka, B.; et al. JNJ-40255293, a novel adenosine A2A/A1 antagonist with efficacy in preclinical models of Parkinson’s disease. ACS Chem. Neurosci. 2014, 5, 1005–1019. [Google Scholar] [CrossRef]
- Ryu, E.J.; Harding, H.P.; Angelastro, J.M.; Vitolo, O.V.; Ron, D.; Greene, L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 10690–10698. [Google Scholar] [CrossRef]
- Xie, H.; Hu, H.; Chang, M.; Huang, D.; Gu, X.; Xiong, X.; Xiong, R.; Hu, L.; Li, G. Identification of chaperones in a MPP+-induced and ATRA/TPA-differentiated SH-SY5Y cell PD model. Am. J. Transl. Res. 2016, 8, 5659–5671. [Google Scholar]
- Cenci, M.A.; Björklund, A. Animal models for preclinical Parkinson’s research: An update and critical appraisal. Prog. Brain Res. 2020, 252, 27–59. [Google Scholar]
- Nair, V.D.; McNaught, K.S.P.; González-Maeso, J.; Sealfon, S.C.; Olanow, C.W. p53 mediates nontranscriptional cell death in dopaminergic cells in response to proteasome inhibition. J. Biol. Chem. 2006, 281, 39550–39560. [Google Scholar] [CrossRef]
- Lopes, U.G.; Erhardt, P.; Yao, R.; Cooper, G.M. p53-dependent induction of apoptosis by proteasome inhibitors. J. Biol. Chem. 1997, 272, 12893–12896. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, S.; Solesio, M.E.; Manzanares, J.; Jordán, J.; Galindo, M.F. Lactacystin requires reactive oxygen species and Bax redistribution to induce mitochondria-mediated cell death. Br. J. Pharmacol. 2009, 158, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Han, E.S.; Park, E.S.; Bang, H. Inhibition of MG132-induced mitochondrial dysfunction and cell death in PC12 cells by 3-morpholinosydnonimine. Brain Res. 2005, 1036, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bir, A.; Sen, O.; Anand, S.; Khemka, V.K.; Banerjee, P.; Cappai, R.; Sahoo, A.; Chakrabarti, S. α-synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: Implications in the pathogenesis of Parkinson’s disease. J. Neurochem. 2014, 131, 868–877. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sawada, H.; Izumi, Y.; Kume, T.; Katsuki, H.; Shimohama, S.; Akaike, A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: Relevance to Parkinson disease. J. Biol. Chem. 2007, 282, 4364–4372. [Google Scholar] [CrossRef]
- Sacino, A.N.; Brooks, M.; Thomas, M.A.; McKinney, A.B.; Lee, S.; Regenhardt, R.W.; McGarvey, N.H.; Ayers, J.I.; Notterpek, L.; Borchelt, D.R.; et al. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10732–10737. [Google Scholar] [CrossRef]
- Sacino, A.N.; Brooks, M.; McKinney, A.B.; Thomas, M.A.; Shaw, G.; Golde, T.E.; Giasson, B.I. Brain injection of α-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 12368–12378. [Google Scholar] [CrossRef] [PubMed]
- Canal, M.; Martín-Flores, N.; Pérez-Sisqués, L.; Romaní-Aumedes, J.; Altas, B.; Man, H.-Y.; Kawabe, H.; Alberch, J.; Malagelada, C. Loss of NEDD4 contributes to RTP801 elevation and neuron toxicity: Implications for Parkinson’s disease. Oncotarget 2016, 7, 58813–58831. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sisqués, L.; Sancho-Balsells, A.; Solana-Balaguer, J.; Campoy-Campos, G.; Vives-Isern, M.; Soler-Palazón, F.; Anglada-Huguet, M.; López-Toledano, M.-Á.; Mandelkow, E.-M.; Alberch, J.; et al. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021, 12, 616. [Google Scholar] [CrossRef]
- Falkenburger, B.H.; Saridaki, T.; Dinter, E. Cellular models for Parkinson’s disease. J. Neurochem. 2016, 139, 121–130. [Google Scholar] [CrossRef]
- Xicoy, H.; Brouwers, J.F.; Kalnytska, O.; Wieringa, B.; Martens, G.J.M. Lipid Analysis of the 6-Hydroxydopamine-Treated SH-SY5Y Cell Model for Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 848–859. [Google Scholar] [CrossRef]
- Amo, T.; Oji, Y.; Saiki, S.; Hattori, N. Metabolomic analysis revealed mitochondrial dysfunction and aberrant choline metabolism in MPP+-exposed SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2019, 519, 540–546. [Google Scholar] [CrossRef]
- Amo, T.; Oji, Y.; Saiki, S.; Hattori, N. Metabolomic analysis data of MPP+-exposed SH-SY5Y cells using CE-TOFMS. Data Brief 2021, 34, 106707. [Google Scholar] [CrossRef]
- Rousseaux, M.W.C.; Marcogliese, P.C.; Qu, D.; Hewitt, S.J.; Seang, S.; Kim, R.H.; Slack, R.S.; Schlossmacher, M.G.; Lagace, D.C.; Mak, T.W.; et al. Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 15918–15923. [Google Scholar] [CrossRef]
- Van Rompuy, A.-S.; Lobbestael, E.; Van der Perren, A.; Van den Haute, C.; Baekelandt, V. Long-term overexpression of human wild-type and T240R mutant Parkin in rat substantia nigra induces progressive dopaminergic neurodegeneration. J. Neuropathol. Exp. Neurol. 2014, 73, 159–174. [Google Scholar] [CrossRef]
- Gao, H.-M.; Zhang, F.; Zhou, H.; Kam, W.; Wilson, B.; Hong, J.-S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 2011, 119, 807–814. [Google Scholar] [CrossRef]
- Vaz, R.L.; Sousa, S.; Chapela, D.; van der Linde, H.C.; Willemsen, R.; Correia, A.D.; Outeiro, T.F.; Afonso, N.D. Identification of antiparkinsonian drugs in the 6-hydroxydopamine zebrafish model. Pharmacol. Biochem. Behav. 2020, 189, 172828. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.M.; Campos, F.L.; Coimbra, B.; Pêgo, J.M.; Rodrigues, C.; Lima, R.; Rodrigues, A.J.; Sousa, N.; Salgado, A.J. Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol. Neurodegener. 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Masilamoni, G.J.; Smith, Y. Chronic MPTP administration regimen in monkeys: A model of dopaminergic and non-dopaminergic cell loss in Parkinson’s disease. J. Neural Transm. 2018, 125, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.-M. Involvement of Non-Dopaminergic Pathways in Parkinson’s Disease. CNS Drugs 2000, 13, 351–364. [Google Scholar] [CrossRef]
- Levy, R.; Herrero, M.T.; Ruberg, M.; Villares, J.; Faucheux, B.; Guridi, J.; Guillen, J.; Luquin, M.R.; Javoy-Agid, F.; Obeso, J.A. Effects of nigrostriatal denervation and L-dopa therapy on the GABAergic neurons in the striatum in MPTP-treated monkeys and Parkinson’s disease: An in situ hybridization study of GAD67 mRNA. Eur. J. Neurosci. 1995, 7, 1199–1209. [Google Scholar] [CrossRef]
- Ullrich, C.; Humpel, C. Rotenone induces cell death of cholinergic neurons in an organotypic co-culture brain slice model. Neurochem. Res. 2009, 34, 2147–2153. [Google Scholar] [CrossRef]
- Cui, K.; Yang, F.; Tufan, T.; Raza, M.U.; Zhan, Y.; Fan, Y.; Zeng, F.; Brown, R.W.; Price, J.B.; Jones, T.C.; et al. Restoration of Noradrenergic Function in Parkinson’s Disease Model Mice. ASN Neuro 2021, 13, 17590914211009730. [Google Scholar] [CrossRef]
- Zhao, F.; Li, C.; Zhuang, Y.; Yan, Y.; Gao, Y.; Behnisch, T. Apoptosis signal-regulating kinase 1 (Ask1) deficiency alleviates MPP+-induced impairment of evoked dopamine release in the mouse hippocampus. Front. Cell. Neurosci. 2024, 18, 1288991. [Google Scholar] [CrossRef]
- Kroener, S.; Chandler, L.J.; Phillips, P.E.M.; Seamans, J.K. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS ONE 2009, 4, e6507. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, L.; Wang, X.-L.; Gao, L.; Li, Z.-Z.; Gao, G.-D.; Yang, Q. Firing pattern modulation through SK channel current increase underlies neuronal survival in an organotypic slice model of Parkinson’s disease. Mol. Neurobiol. 2015, 51, 424–436. [Google Scholar] [CrossRef]
- Guo, S.; Lei, Q.; Yang, Q.; Chen, R. IGFBP5 Promotes Neuronal Apoptosis in a 6-OHDA-Toxicant Model of Parkinson’s Disease by Inhibiting the Sonic Hedgehog Signaling Pathway. Med. Princ. Pract. 2024, 33, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gaceb, A.; Barbariga, M.; Paul, G. An In Vitro Partial Lesion Model of Differentiated Human Mesencephalic Neurons: Effect of Pericyte Secretome on Phenotypic Markers. J. Mol. Neurosci. MN 2020, 70, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, J.; Li, B.-X.; Sun, Y. Wnt1 silencing enhances neurotoxicity induced by paraquat and maneb in SH-SY5Y cells. Exp. Ther. Med. 2019, 18, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Yin, M.; Zhang, M.-H. Cell-based assays for Parkinson’s disease using differentiated human LUHMES cells. Acta Pharmacol. Sin. 2014, 35, 945–956. [Google Scholar] [CrossRef]
- Ji, L.-L.; Huang, T.-T.; Mao, L.-L.; Xu, Y.-F.; Chen, W.-Y.; Wang, W.-W.; Wang, L.-H. The gut microbiota metabolite butyrate mitigates MPTP/MPP+-induced Parkinson’s disease by inhibiting the JAK2/STAT3 signaling pathway. Kaohsiung J. Med. Sci. 2023, 39, 1002–1010. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.; Li, R. lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2. Am. J. Transl. Res. 2018, 10, 563. [Google Scholar]
- Stępkowski, T.M.; Wasyk, I.; Grzelak, A.; Kruszewski, M. 6-OHDA-Induced Changes in Parkinson’s Disease-Related Gene Expression are not Affected by the Overexpression of PGAM5 in In Vitro Differentiated Embryonic Mesencephalic Cells. Cell. Mol. Neurobiol. 2015, 35, 1137–1147. [Google Scholar] [CrossRef]
- Alegre-Cortés, E.; Muriel-González, A.; Canales-Cortés, S.; Uribe-Carretero, E.; Martínez-Chacón, G.; Aiastui, A.; López de Munain, A.; Niso-Santano, M.; Gonzalez-Polo, R.A.; Fuentes, J.M.; et al. Toxicity of Necrostatin-1 in Parkinson’s Disease Models. Antioxidants 2020, 9, 524. [Google Scholar] [CrossRef]
- Ito, K.; Eguchi, Y.; Imagawa, Y.; Akai, S.; Mochizuki, H.; Tsujimoto, Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Xu, Z.; Patterson, T.A.; Wren, J.D.; Han, T.; Shi, L.; Duhart, H.; Ali, S.F.; Slikker, W. A microarray study of MPP+-treated PC12 Cells: Mechanisms of toxicity (MOT) analysis using bioinformatics tools. BMC Bioinform. 2005, 6, S8. [Google Scholar] [CrossRef] [PubMed]
- Prediger, R.D.S.; Rial, D.; Medeiros, R.; Figueiredo, C.P.; Doty, R.L.; Takahashi, R.N. Risk is in the Air. Ann. N. Y. Acad. Sci. 2009, 1170, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.S.; Targa, A.D.S.; Noseda, A.C.D.; Aurich, M.F.; Da Cunha, C.; Lima, M.M.S. Olfactory impairment in the rotenone model of Parkinson’s disease is associated with bulbar dopaminergic D2 activity after REM sleep deprivation. Front. Cell. Neurosci. 2014, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Pan-Montojo, F.; Anichtchik, O.; Dening, Y.; Knels, L.; Pursche, S.; Jung, R.; Jackson, S.; Gille, G.; Spillantini, M.G.; Reichmann, H.; et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 2010, 5, e8762. [Google Scholar] [CrossRef]
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.-N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2. [Google Scholar] [CrossRef]
- Doty, R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012, 46, 527–552. [Google Scholar] [CrossRef]
- Arshamian, A.; Iravani, B.; Lundström, J.N. Is congenital anosmia protective for Parkinson’s disease triggered by pathogenic entrance through the nose? Npj Park. Dis. 2022, 8, 152. [Google Scholar] [CrossRef]
- Bagnoli, E.; Trotier, A.; McMahon, J.; Quinlan, L.R.; Biggs, M.; Pandit, A.; FitzGerald, U. Prodromal Parkinson’s disease and the catecholaldehyde hypothesis: Insight from olfactory bulb organotypic cultures. FASEB J. 2023, 37, e23272. [Google Scholar] [CrossRef]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic Animal Models of Parkinson’s Disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef]
- Sallinen, V.; Kolehmainen, J.; Priyadarshini, M.; Toleikyte, G.; Chen, Y.-C.; Panula, P. Dopaminergic cell damage and vulnerability to MPTP in Pink1 knockdown zebrafish. Neurobiol. Dis. 2010, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lev, N.; Barhum, Y.; Ben-Zur, T.; Melamed, E.; Steiner, I.; Offen, D. Knocking Out DJ-1 Attenuates Astrocytes Neuroprotection Against 6-Hydroxydopamine Toxicity. J. Mol. Neurosci. 2013, 50, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Cabin, D.E.; Fang, F.; Reijo Pera, R.A. Parkinson’s Disease: Overview of Transcription Factor Regulation, Genetics, and Cellular and Animal Models. Front. Neurosci. 2022, 16, 894620. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.F.; Lockhart, P.J.; Farrer, M.J. Parkin genetics: One model for Parkinson’s disease. Hum. Mol. Genet. 2004, 13, R127–R133. [Google Scholar] [CrossRef]
- Frank-Cannon, T.C.; Tran, T.; Ruhn, K.A.; Martinez, T.N.; Hong, J.; Marvin, M.; Hartley, M.; Treviño, I.; O’Brien, D.E.; Casey, B.; et al. Parkin Deficiency Increases Vulnerability to Inflammation-Related Nigral Degeneration. J. Neurosci. 2008, 28, 10825–10834. [Google Scholar] [CrossRef]
- Wang, A.; Zhong, G.; Ying, M.; Fang, Z.; Chen, Y.; Wang, H.; Wang, C.; Liu, C.; Guo, Y. Inhibition of NLRP3 inflammasome ameliorates LPS-induced neuroinflammatory injury in mice via PINK1/Parkin pathway. Neuropharmacology 2024, 257, 110063. [Google Scholar] [CrossRef]
- Milanese, C.; Sager, J.J.; Bai, Q.; Farrell, T.C.; Cannon, J.R.; Greenamyre, J.T.; Burton, E.A. Hypokinesia and Reduced Dopamine Levels in Zebrafish Lacking β- and γ1-Synucleins. J. Biol. Chem. 2012, 287, 2971–2983. [Google Scholar] [CrossRef]
- Robea, M.-A.; Balmus, I.-M.; Ciobica, A.; Strungaru, S.; Plavan, G.; Gorgan, L.D.; Savuca, A.; Nicoara, M. Parkinson’s Disease-Induced Zebrafish Models: Focussing on Oxidative Stress Implications and Sleep Processes. Oxid. Med. Cell. Longev. 2020, 2020, 1370837. [Google Scholar] [CrossRef]
- O’Donnell, K.C.; Lulla, A.; Stahl, M.C.; Wheat, N.D.; Bronstein, J.M.; Sagasti, A. Axon degeneration and PGC-1α-mediated protection in a zebrafish model of α-synuclein toxicity. Dis. Model. Mech. 2014, 7, 571–582. [Google Scholar]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Abdelmotilib, H.; Liu, Z.; Stoyka, L.; Daher, J.P.L.; Milnerwood, A.J.; Unni, V.K.; Hirst, W.D.; Yue, Z.; Zhao, H.T.; et al. G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons. J. Neurosci. 2016, 36, 7415–7427. [Google Scholar] [CrossRef]
- Bose, A.; Petsko, G.A.; Studer, L. Induced pluripotent stem cells: A tool for modeling Parkinson’s disease. Trends Neurosci. 2022, 45, 608–620. [Google Scholar] [CrossRef]
- Hunter, R.L.; Dragicevic, N.; Seifert, K.; Choi, D.Y.; Liu, M.; Kim, H.-C.; Cass, W.A.; Sullivan, P.G.; Bing, G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007, 100, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.; Ojha, U.; Bhurtel, S.; Bing, G.; Choi, D.-Y. Lipopolysaccharide-induced functional and structural injury of the mitochondria in the nigrostriatal pathway. Neurosci. Res. 2017, 114, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Cheng, B.; Choi, D.-Y.; Liu, M.; Liu, S.; Cass, W.A.; Bing, G. Intrastriatal lipopolysaccharide injection induces parkinsonism in C57/B6 mice. J. Neurosci. Res. 2009, 87, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, Y.; wai Tong, C.; Snyder, J.A.; Lipton, J.W.; Carvey, P.M. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp. Neurol. 2006, 199, 499–512. [Google Scholar] [CrossRef]
- Capuano, A.W.; Wilson, R.S.; Honer, W.G.; Petyuk, V.A.; Leurgans, S.E.; Yu, L.; Gatchel, J.R.; Arnold, S.; Bennett, D.A.; Arvanitakis, Z. Brain IGFBP-5 Modifies the Relation of Depressive Symptoms to Decline in Working Memory in Older Persons. J. Affect. Disord. 2019, 250, 313–318. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Boyd, G.W. Role of the Insulin-like Growth Factor System in Neurodegenerative Disease. Int. J. Mol. Sci. 2024, 25, 4512. [Google Scholar] [CrossRef]
- Yu, H.; Sun, T.; He, X.; Wang, Z.; Zhao, K.; An, J.; Wen, L.; Li, J.-Y.; Li, W.; Feng, J. Association between Parkinson’s Disease and Diabetes Mellitus: From Epidemiology, Pathophysiology and Prevention to Treatment. Aging Dis. 2022, 13, 1591–1605. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Russo, T.; Riessland, M. Age-Related Midbrain Inflammation and Senescence in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 917797. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef]
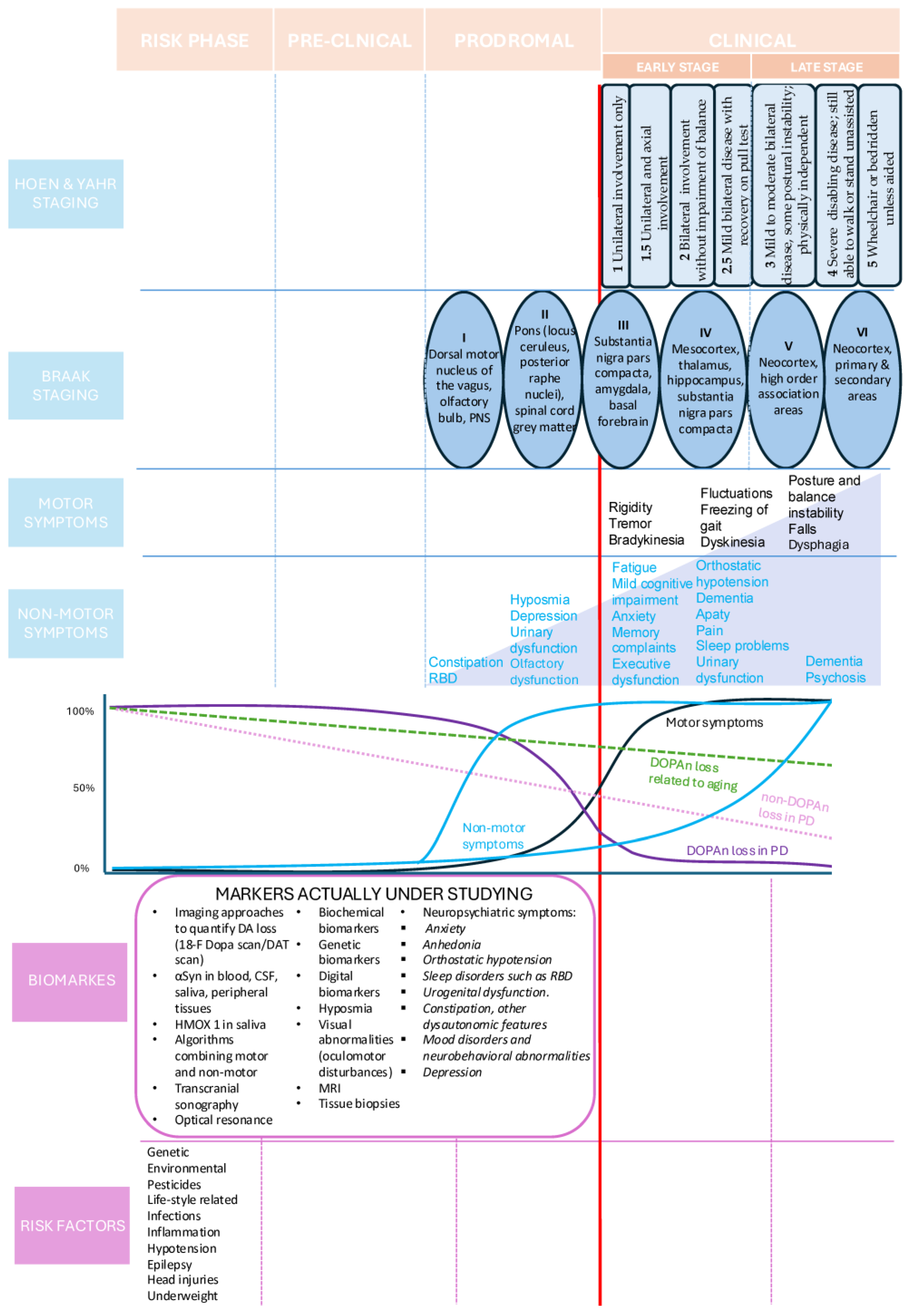
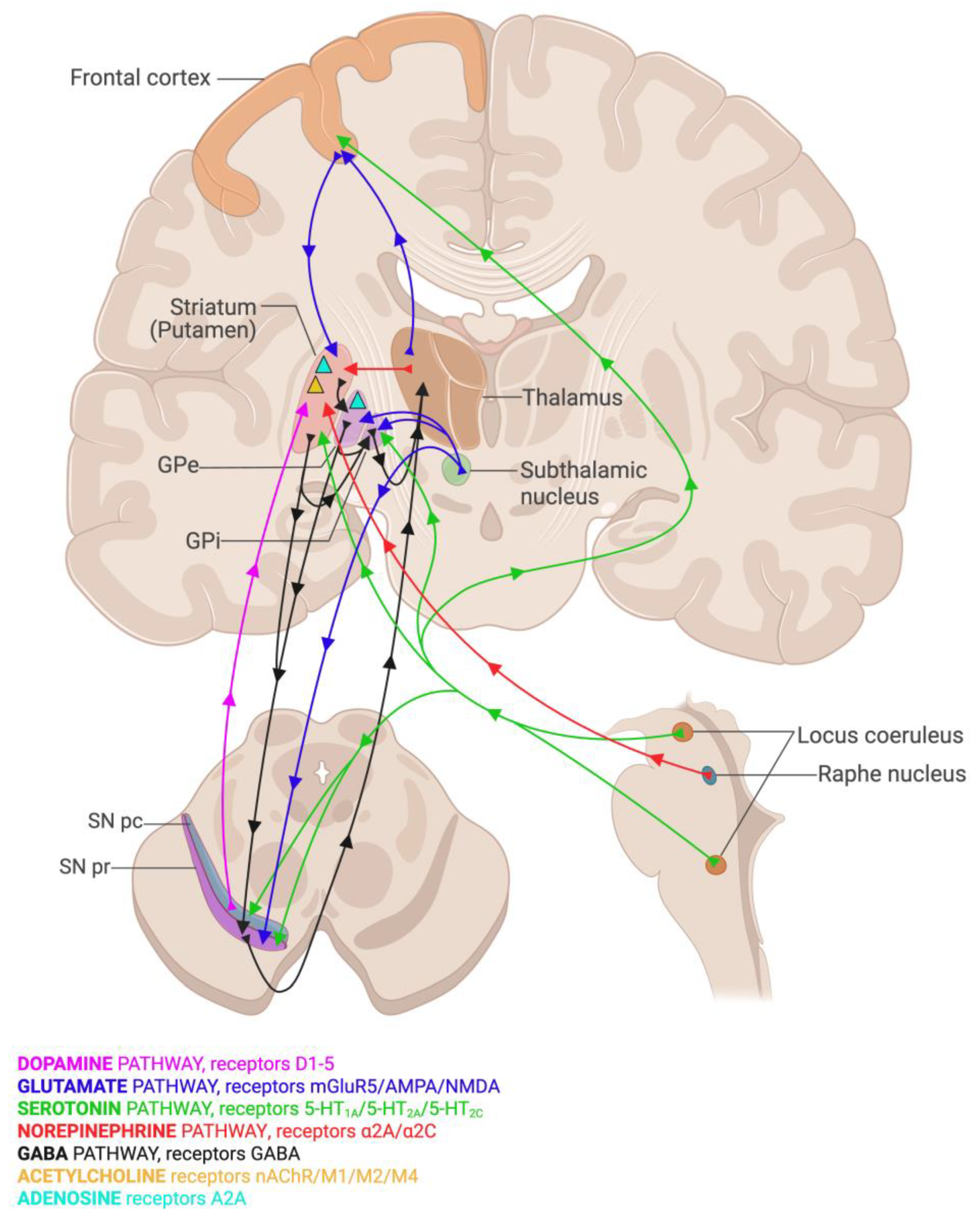
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Verde, C.; Jayanti, S.; El Khobar, K.; Stanford, J.A.; Tiribelli, C.; Gazzin, S. Understanding the Pre-Clinical Stages of Parkinson’s Disease: Where Are We in Clinical and Research Settings? Int. J. Mol. Sci. 2025, 26, 6881. https://doi.org/10.3390/ijms26146881
Dalla Verde C, Jayanti S, El Khobar K, Stanford JA, Tiribelli C, Gazzin S. Understanding the Pre-Clinical Stages of Parkinson’s Disease: Where Are We in Clinical and Research Settings? International Journal of Molecular Sciences. 2025; 26(14):6881. https://doi.org/10.3390/ijms26146881
Chicago/Turabian StyleDalla Verde, Camilla, Sri Jayanti, Korri El Khobar, John A. Stanford, Claudio Tiribelli, and Silvia Gazzin. 2025. "Understanding the Pre-Clinical Stages of Parkinson’s Disease: Where Are We in Clinical and Research Settings?" International Journal of Molecular Sciences 26, no. 14: 6881. https://doi.org/10.3390/ijms26146881
APA StyleDalla Verde, C., Jayanti, S., El Khobar, K., Stanford, J. A., Tiribelli, C., & Gazzin, S. (2025). Understanding the Pre-Clinical Stages of Parkinson’s Disease: Where Are We in Clinical and Research Settings? International Journal of Molecular Sciences, 26(14), 6881. https://doi.org/10.3390/ijms26146881







