Translational Control in Cardiac Pathophysiology and Therapeutic Development: When mRNA Meets the Heart
Abstract
1. Introduction
2. Techniques and Methods to Investigate mRNA Translation in the Heart
2.1. Biochemical Methods for Studying Translational Control
2.1.1. RNA-Binding Protein Immunoprecipitation (RIP) to Identify Bound Target RNAs
2.1.2. Crosslinking and Immunoprecipitation (CLIP) to Map RBP-Binding Sites on RNAs
2.1.3. In Vitro Pulldown of Interacting Proteins of Biotinylated RNA
2.1.4. Proximity Ligation Assay Associated with Immunoblot or Mass Spectrometry
2.1.5. Puromycin Incorporation ASSAY to Assess Global Translation Efficiency
| Technique and Method for Research in Translational Control | Purposes and Applications Related to Research in Cardiac Biology and Disease | Exemplary Reference |
|---|---|---|
| RBP immunoprecipitation (RIP)-seq | Identify target mRNAs of RBPs in cardiac cells | [13,14] |
| Crosslinking immunoprecipitation (CLIP)-seq | Map RNA binding sites recognized by RBP in cardiac cells | [15,19] |
| Biotinylated RNA pulldown | Identify RBPs interacting with RNA in vitro and in vivo | [21,22] |
| Proximity ligation assay | Identify protein-protein and protein-RNA interaction in cells | [24,25] |
| Puromycin incorporation assay | Measure nascent global protein synthesis in vitro and in vivo | [29,30] |
| Polysome profiling-seq | Determine mRNA translation efficiency in cardiac cells | [31,32,33] |
| Translating ribosome affinity purification-seq | Measure mRNA translation efficiency in specific cell types in vivo | [34,35,36] |
| Ribosome profiling (Ribo-seq) | Map ORFs and stalling sites in cardiac cells and whole hearts | [37,38,39] |
2.2. Deep Sequencing-Based Translatome Profiling in Cells and Animals
2.2.1. Polysome Profiling-Sequencing (Polysome-Seq)
2.2.2. Translating Ribosome Affinity Purification Sequencing (TRAP-Seq)
2.2.3. Translational Landscape in Human and Mouse Heart Failure Determined by Ribosome Profiling (Ribo-Seq)
2.3. Imaging-Based Techniques for Evaluating Translation Efficiency and Localized Translation in Cardiomyocytes
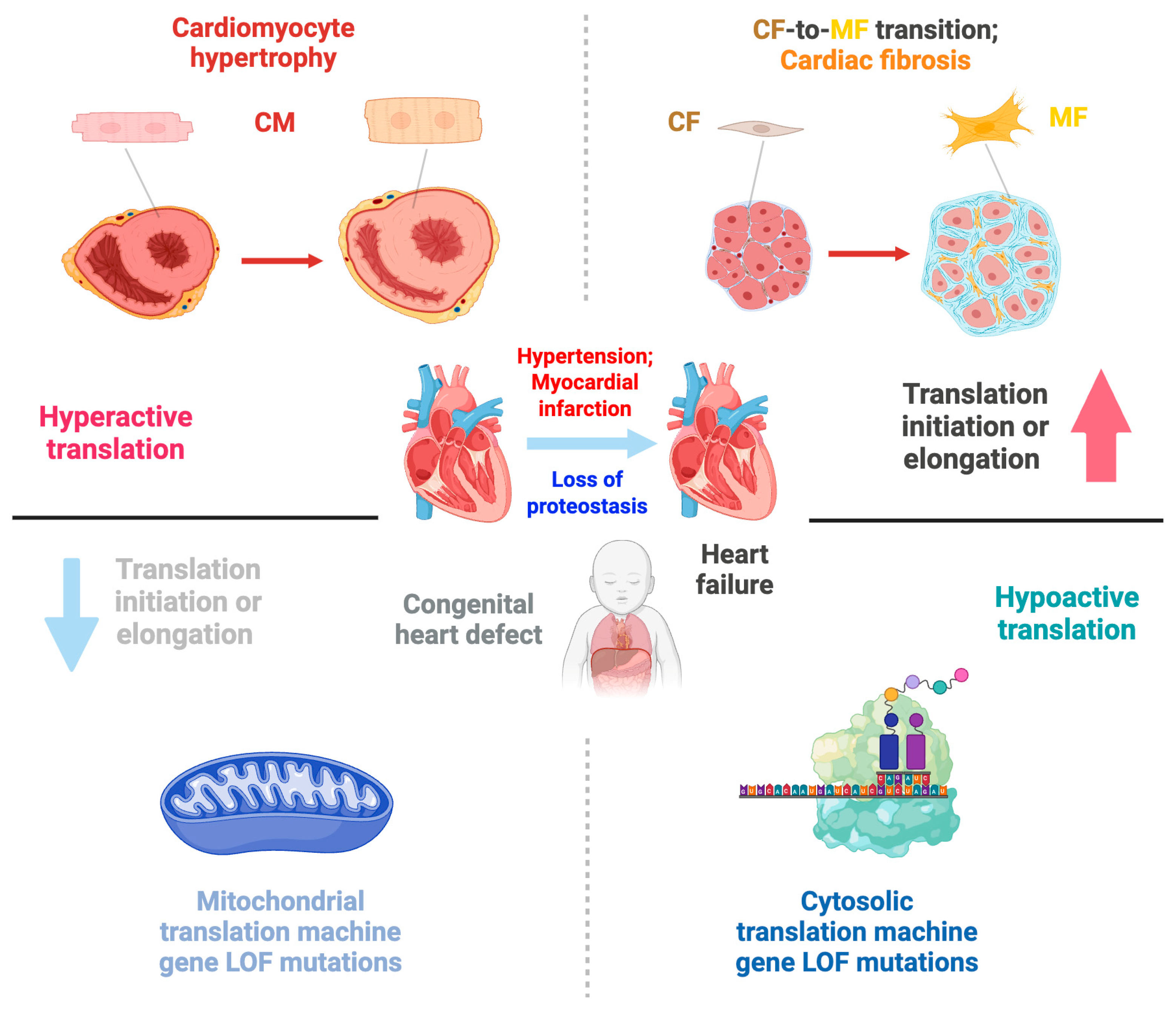
3. Translational Control in Cardiac Development and Congenital Heart Disease
3.1. Human Genetic Mutations in Translation Machinery and Congenital Heart Disease
3.1.1. Diamond Blackfan Anemia and Other Heart Disease-Causing Mutations in Cytoplasmic Translation Factors
3.1.2. Human Mutations in Mitochondrial Translation Machinery Lead to Genetic Cardiomyopathy
3.1.3. Loss-of-Function of PRRC2B-Mediated Translation Initiation Regulation Causes Congenital Cardiovascular Defect in Humans and Mice
3.1.4. eIF4E1C Regulates Cardiomyocyte Metabolism and Proliferation During Heart Regeneration in Zebrafish
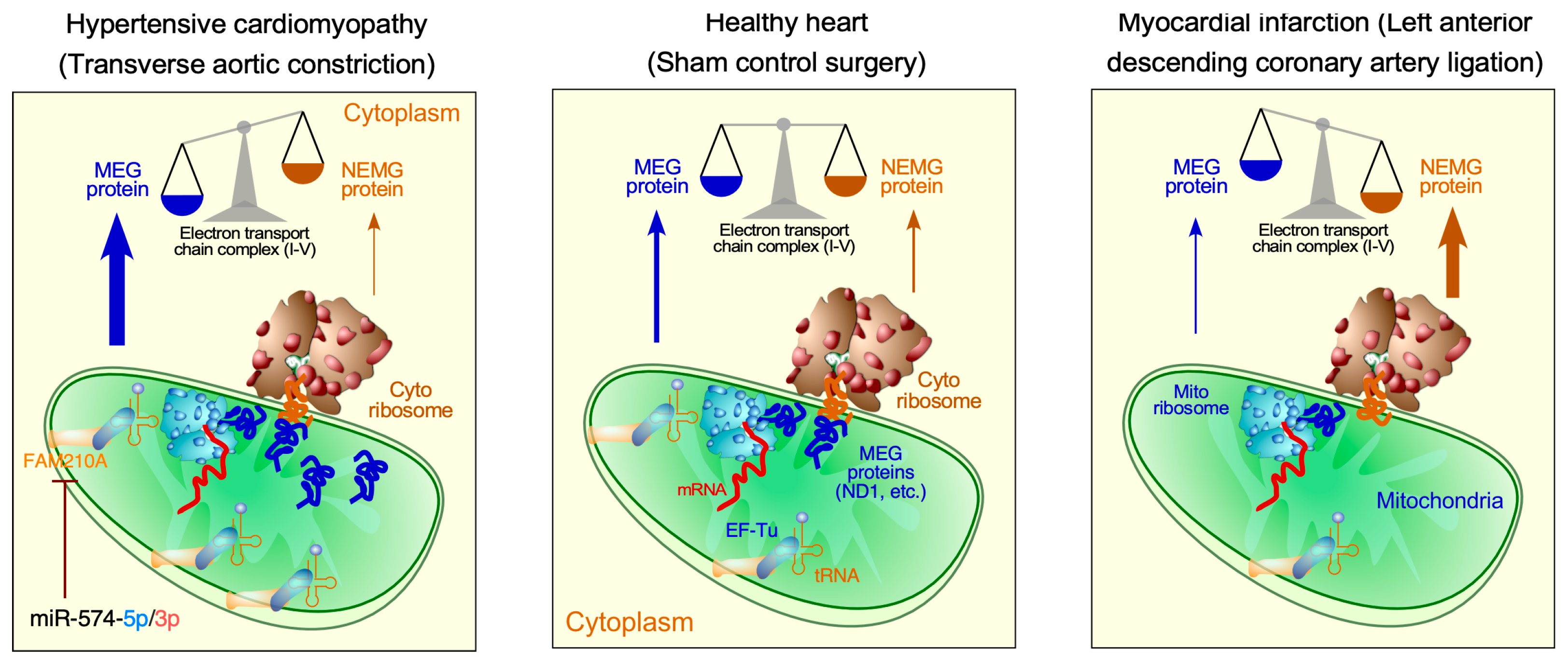
3.2. Translational Control in Mitochondrial Cardiomyopathy
3.3. Translational Regulation of Cardiac Cell Proliferation and Differentiation
4. Translational Control in Adult Cardiac Disease
4.1. Translational Control in Cardiomyocyte Hypertrophy
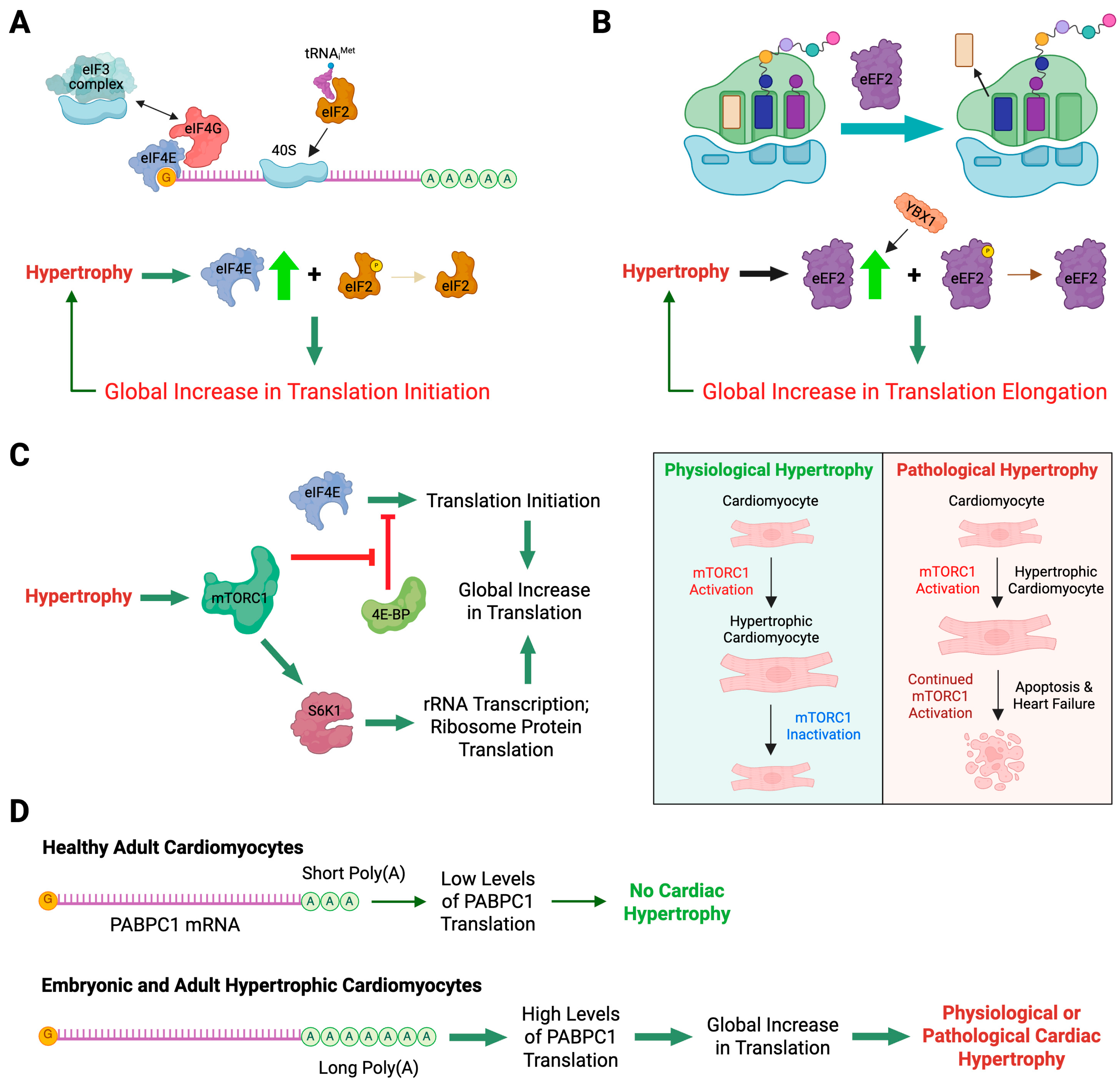
4.1.1. Role of Translation Initiation Factors in Cardiac Hypertrophy
4.1.2. Role of Translation Elongation Factors in Cardiac Hypertrophy
4.1.3. Genetic Loss-of-Function of mTORC1 Causes Heart Failure in Mice
4.1.4. PABPC1-Mediated Translational Control of Physiological and Pathological Cardiac Hypertrophy
4.1.5. Translational Control of Ybx1 Expression Regulates Cardiac Function in Response to Pressure Overload In Vivo
4.1.6. m6A-Dependent Translational Control in Maintaining Normal Cardiac Function
4.2. Translational Control in Cardiac Fibroblast Activation During Fibrosis
4.2.1. Translational Regulation in Human TGFβ-Activated Cardiac Fibroblasts
4.2.2. EPRS1 Promotes Cardiac Fibrosis by Enhancing Proline-Rich Extracellular Matrix Protein Translation
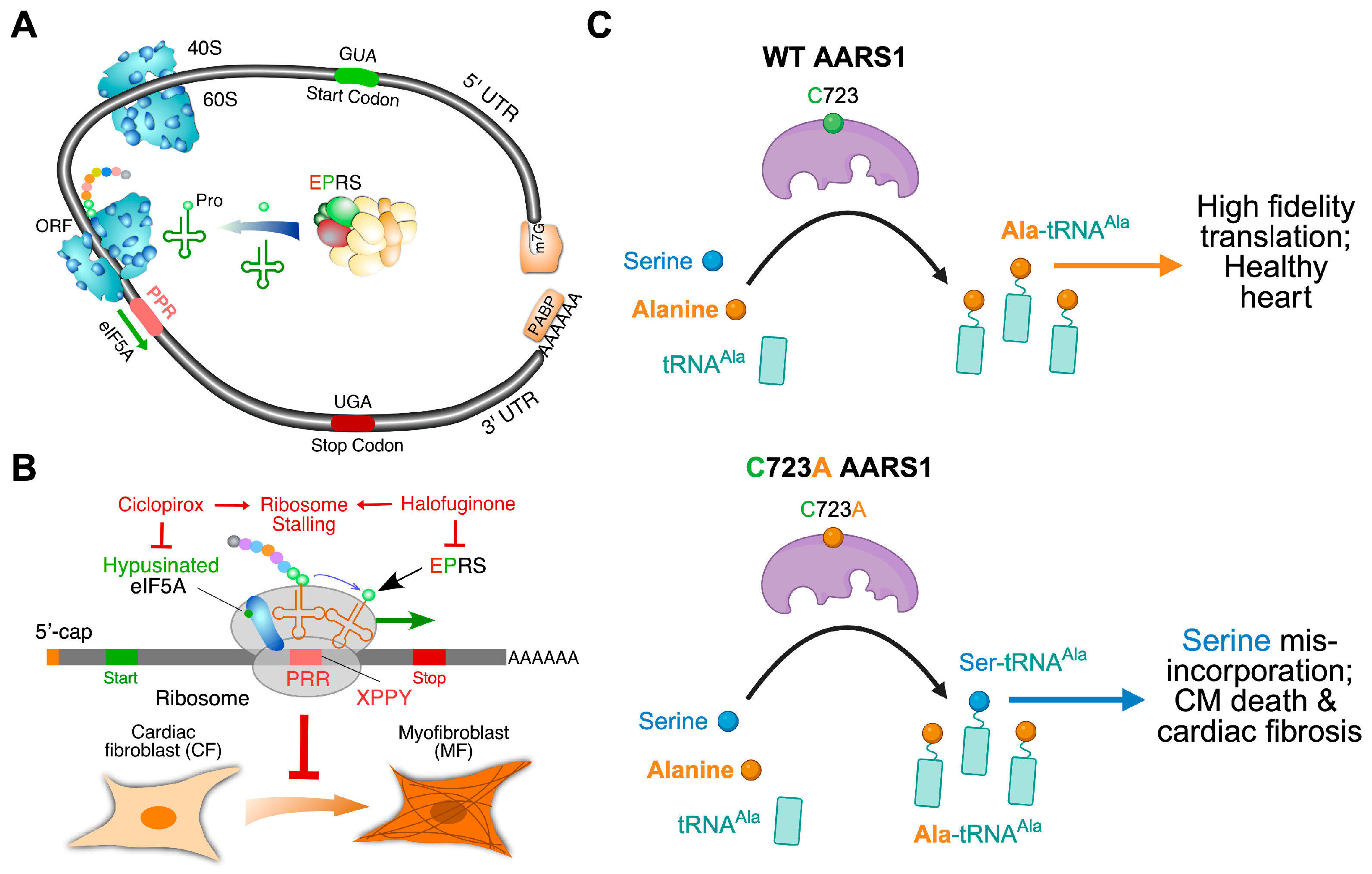
4.2.3. eIF5A: An Anti-Fibrosis Target Translation Elongation Factor
4.2.4. Editing-Defective Aars1 Mouse Shows Spontaneous Cardiac Proteinopathy and Fibrosis
5. Translation-Manipulating Therapeutics for Heart Disease Treatment
5.1. Translation-Targeted Medicines for Cardiac Disorders
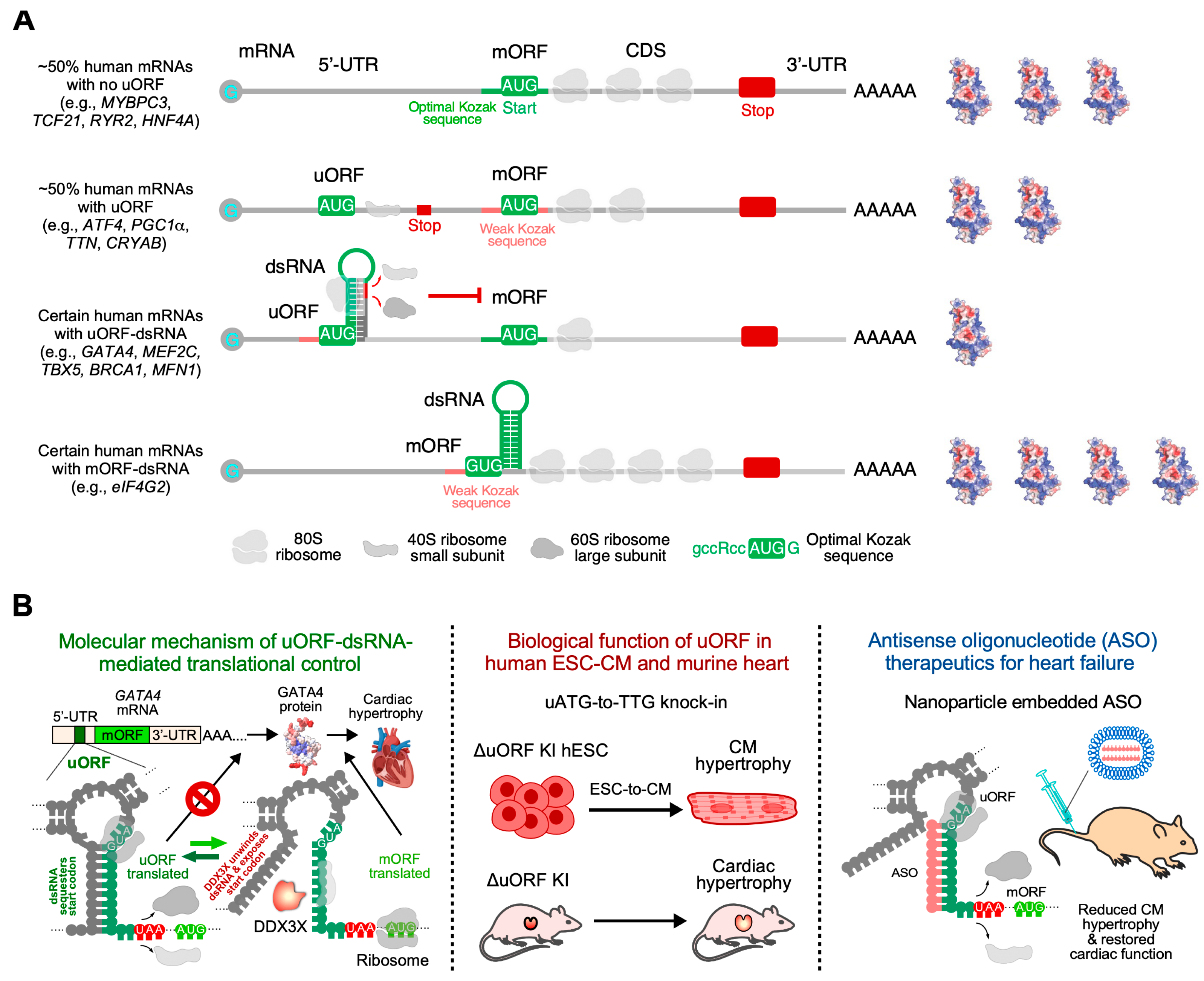
5.2. RNA Secondary Structure as a Potential Therapeutic Target for ASO Treatment of Cardiac Hypertrophy
5.3. Chemically Modified mRNA-Based CAR-T-Mediated Therapeutics for Cardiac Fibrosis
5.4. Chemically Modified mRNA-Based Therapeutics for Ischemic Heart Disease
6. Concluding Remarks and Future Perspective
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef]
- Schimmel, P. Aminoacyl tRNA synthetases: General scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu. Rev. Biochem. 1987, 56, 125–158. [Google Scholar] [CrossRef]
- Ibba, M.; Söll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Ibba, M. Quality control in aminoacyl-tRNA synthesis its role in translational fidelity. Adv. Protein Chem. Struct. Biol. 2012, 86, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Reynolds, N.; Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 2009, 63, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017, 552, 110–115. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y. mRNA Metabolism in Cardiac Development and Disease: Life After Transcription. Physiol. Rev. 2020, 100, 673–694. [Google Scholar] [CrossRef]
- Volkers, M.; Preiss, T.; Hentze, M.W. RNA-binding proteins in cardiovascular biology and disease: The beat goes on. Nat. Rev. Cardiol. 2024, 21, 361–378. [Google Scholar] [CrossRef]
- Hedaya, O.M.; Venkata Subbaiah, K.C.; Jiang, F.; Xie, L.H.; Wu, J.; Khor, E.S.; Zhu, M.; Mathews, D.H.; Proschel, C.; Yao, P. Secondary structures that regulate mRNA translation provide insights for ASO-mediated modulation of cardiac hypertrophy. Nat. Commun. 2023, 14, 6166. [Google Scholar] [CrossRef]
- Yao, P.; Potdar, A.A.; Arif, A.; Ray, P.S.; Mukhopadhyay, R.; Willard, B.; Xu, Y.; Yan, J.; Saidel, G.M.; Fox, P.L. Coding Region Polyadenylation Generates a Truncated tRNA Synthetase that Counters Translation Repression. Cell 2012, 149, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Varma, E.; Burghaus, J.; Schwarzl, T.; Sekaran, T.; Gupta, P.; Gorska, A.A.; Hofmann, C.; Stroh, C.; Jurgensen, L.; Kamuf-Schenk, V.; et al. Translational control of Ybx1 expression regulates cardiac function in response to pressure overload in vivo. Basic. Res. Cardiol. 2023, 118, 25. [Google Scholar] [CrossRef]
- Riechert, E.; Kmietczyk, V.; Stein, F.; Schwarzl, T.; Sekaran, T.; Jurgensen, L.; Kamuf-Schenk, V.; Varma, E.; Hofmann, C.; Rettel, M.; et al. Identification of dynamic RNA-binding proteins uncovers a Cpeb4-controlled regulatory cascade during pathological cell growth of cardiomyocytes. Cell Rep. 2021, 35, 109100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hedaya, O.M.; Khor, E.; Wu, J.; Auguste, M.; Yao, P. RNA binding protein PRRC2B mediates translation of specific mRNAs and regulates cell cycle progression. Nucleic Acids Res. 2023, 51, 5831–5846. [Google Scholar] [CrossRef] [PubMed]
- Anastasakis, D.G.; Apostolidi, M.; Garman, K.A.; Polash, A.H.; Umar, M.I.; Meng, Q.; Scutenaire, J.; Jarvis, J.E.; Wang, X.; Haase, A.D.; et al. Nuclear PKM2 binds pre-mRNA at folded G-quadruplexes and reveals their gene regulatory role. Mol. Cell 2024, 84, 3775–3789.E6. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, V.N. fCLIP-seq for transcriptomic footprinting of dsRNA-binding proteins: Lessons from DROSHA. Methods 2019, 152, 3–11. [Google Scholar] [CrossRef]
- Danan, C.; Manickavel, S.; Hafner, M. PAR-CLIP: A Method for Transcriptome-Wide Identification of RNA Binding Protein Interaction Sites, 2nd ed.; Humana Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Danan, C.; Manickavel, S.; Hafner, M. PAR-CLIP: A Method for Transcriptome-Wide Identification of RNA Binding Protein Interaction Sites, 3rd ed.; Humana Press: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Panda, A.C.; Martindale, J.L.; Gorospe, M. Affinity Pulldown of Biotinylated RNA for Detection of Protein-RNA Complexes. Bio-Protoc. 2016, 6, e2062. [Google Scholar] [CrossRef]
- Yao, P.; Potdar, A.A.; Ray, P.S.; Eswarappa, S.M.; Flagg, A.C.; Willard, B.; Fox, P.L. The HILDA complex coordinates a conditional switch in the 3′-untranslated region of the VEGFA mRNA. PLoS Biol. 2013, 11, e1001635. [Google Scholar] [CrossRef]
- Gerber, A.P. RNA-Centric Approaches to Profile the RNA-Protein Interaction Landscape on Selected RNAs. Non-Coding RNA 2021, 7, 11. [Google Scholar] [CrossRef]
- Sears, R.M.; May, D.G.; Roux, K.J. BioID as a Tool for Protein-Proximity Labeling in Living Cells. Methods Mol. Biol. 2019, 2012, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Wardman, R.; Keles, M.; Pachkiv, I.; Hemanna, S.; Grein, S.; Schwarz, J.; Stein, F.; Ola, R.; Dobreva, G.; Hentze, M.W.; et al. RNA-Binding Proteins Regulate Post-Transcriptional Responses to TGF-beta to Coordinate Function and Mesenchymal Activation of Murine Endothelial Cells. Arter. Thromb. Vasc. Biol. 2023, 43, 1967–1989. [Google Scholar] [CrossRef]
- George, J.; Mittal, S.; Kadamberi, I.P.; Pradeep, S.; Chaluvally-Raghavan, P. Optimized proximity ligation assay (PLA) for detection of RNA-protein complex interactions in cell lines. STAR Protoc. 2022, 3, 101340. [Google Scholar] [CrossRef] [PubMed]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A contaminant repository for affinity purification-mass spectrometry data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Ernlund, A.; Alard, A.; Ruggles, K.; Ueberheide, B.; Schneider, R.J. A widespread alternate form of cap-dependent mRNA translation initiation. Nat. Commun. 2018, 9, 3068. [Google Scholar] [CrossRef]
- Liakath-Ali, K.; Mills, E.W.; Sequeira, I.; Lichtenberger, B.M.; Pisco, A.O.; Sipila, K.H.; Mishra, A.; Yoshikawa, H.; Wu, C.C.; Ly, T.; et al. An evolutionarily conserved ribosome-rescue pathway maintains epidermal homeostasis. Nature 2018, 556, 376–380. [Google Scholar] [CrossRef]
- Hofmann, C.; Serafin, A.; Schwerdt, O.M.; Fischer, J.; Sicklinger, F.; Younesi, F.S.; Byrne, N.J.; Meyer, I.S.; Malovrh, E.; Sandmann, C.; et al. Transient Inhibition of Translation Improves Cardiac Function After Ischemia/Reperfusion by Attenuating the Inflammatory Response. Circulation 2024, 150, 1248–1267. [Google Scholar] [CrossRef]
- Grund, A.; Szaroszyk, M.; Korf-Klingebiel, M.; Malek Mohammadi, M.; Trogisch, F.; Schrameck, U.; Gigina, A.; Tiedje, C.; Gaestel, M.; Kraft, T.; et al. TIP 30 counteracts cardiac hypertrophy and failure by inhibiting translational elongation. EMBO Mol. Med. 2019, 11, e10018. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H.L.; Jiang, F.; Mickelson, D.J.; Myers, J.R.; Tang, W.H.; Yao, P. EPRS regulates proline-rich pro-fibrotic protein synthesis during cardiac fibrosis. bioRxiv 2019, 127, 827–846. [Google Scholar]
- Seimetz, J.; Arif, W.; Bangru, S.; Hernaez, M.; Kalsotra, A. Cell-type specific polysome profiling from mammalian tissues. Methods 2019, 155, 131–139. [Google Scholar] [CrossRef]
- Pereira, I.T.; Spangenberg, L.; Robert, A.W.; Amorin, R.; Stimamiglio, M.A.; Naya, H.; Dallagiovanna, B. Cardiomyogenic differentiation is fine-tuned by differential mRNA association with polysomes. BMC Genom. 2019, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Y.; Ma, Q.; Gu, F.; Day, D.S.; He, A.; Zhou, B.; Li, J.; Stevens, S.M.; Romo, D.; et al. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. Proc. Natl. Acad. Sci. USA 2013, 110, 15395–15400. [Google Scholar] [CrossRef]
- Doroudgar, S.; Hofmann, C.; Boileau, E.; Malone, B.; Riechert, E.; Gorska, A.A.; Jakobi, T.; Sandmann, C.; Jurgensen, L.; Kmietczyk, V.; et al. Monitoring Cell-Type-Specific Gene Expression Using Ribosome Profiling in Vivo During Cardiac Hemodynamic Stress. Circ. Res. 2019, 125, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Yang, L.; Su, T.; Morris, D.R.; McKnight, G.S.; Amieux, P.S. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13939–13944. [Google Scholar] [CrossRef]
- van Heesch, S.; Witte, F.; Schneider-Lunitz, V.; Schulz, J.F.; Adami, E.; Faber, A.B.; Kirchner, M.; Maatz, H.; Blachut, S.; Sandmann, C.-L.; et al. The Translational Landscape of the Human Heart. Cell 2019, 178, 242–260.e229. [Google Scholar] [CrossRef]
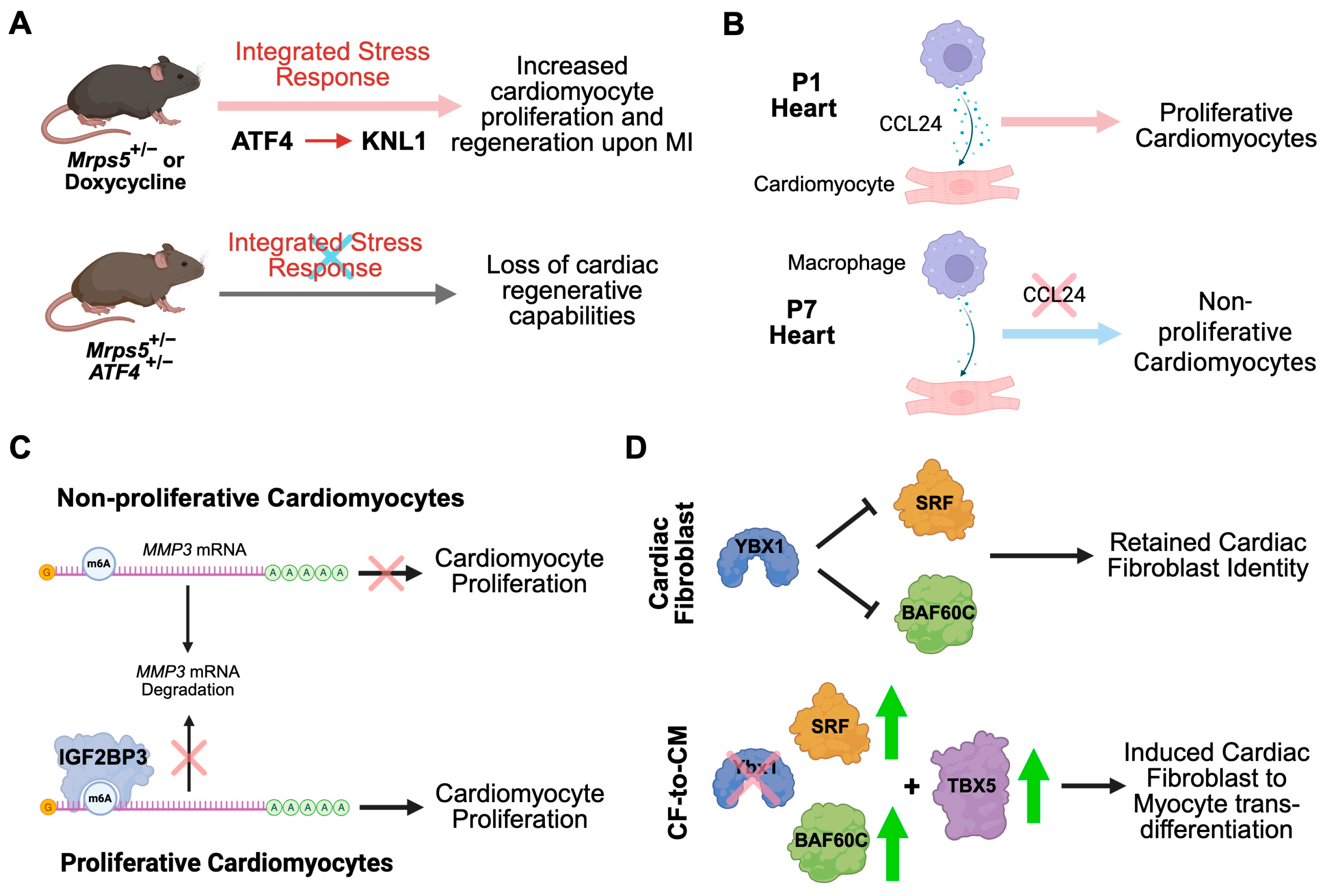
- Xie, Y.; Wang, Q.; Yang, Y.; Near, D.; Wang, H.; Colon, M.; Nguyen, C.; Slattery, C.; Keepers, B.; Farber, G.; et al. Translational landscape of direct cardiac reprogramming reveals a role of Ybx1 in repressing cardiac fate acquisition. Nat. Cardiovasc. Res. 2023, 2, 1060–1077. [Google Scholar] [CrossRef]
- Wu, J.; Subbaiah, K.C.V.; Hedaya, O.; Chen, S.; Munger, J.; Tang, W.H.W.; Yan, C.; Yao, P. FAM210A regulates mitochondrial translation and maintains cardiac mitochondrial homeostasis. Cardiovasc. Res. 2023, 119, 2441–2457. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.D.; Cipullo, M.; Atanassov, I.; Bratic, A.; Silva Ramos, E.; Schondorf, T.; Li, X.; Pearce, S.F.; Milenkovic, D.; Rorbach, J.; et al. MitoRibo-Tag Mice Provide a Tool for In Vivo Studies of Mitoribosome Composition. Cell Rep. 2019, 29, 1728–1738.E9. [Google Scholar] [CrossRef] [PubMed]
- Chothani, S.; Schäfer, S.; Adami, E.; Viswanathan, S.; Widjaja, A.A.; Langley, S.R.; Tan, J.; Wang, M.; Quaife, N.M.; Pua, C.J.; et al. Widespread Translational Control of Fibrosis in the Human Heart by RNA-Binding Proteins. Circulation 2019, 140, 937–951. [Google Scholar] [CrossRef]
- Lewis, Y.E.; Moskovitz, A.; Mutlak, M.; Heineke, J.; Caspi, L.H.; Kehat, I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J. Mol. Cell Cardiol. 2018, 116, 16–28. [Google Scholar] [CrossRef]
- Scarborough, E.A.; Uchida, K.; Vogel, M.; Erlitzki, N.; Iyer, M.; Phyo, S.A.; Bogush, A.; Kehat, I.; Prosser, B.L. Microtubules orchestrate local translation to enable cardiac growth. Nat. Commun. 2021, 12, 1547. [Google Scholar] [CrossRef]
- Bogdanov, V.; Soltisz, A.M.; Moise, N.; Sakuta, G.; Orengo, B.H.; Janssen, P.M.L.; Weinberg, S.H.; Davis, J.P.; Veeraraghavan, R.; Gyorke, S. Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes. Basic. Res. Cardiol. 2021, 116, 63. [Google Scholar] [CrossRef]
- Eichel, C.A.; Rios-Perez, E.B.; Liu, F.; Jameson, M.B.; Jones, D.K.; Knickelbine, J.J.; Robertson, G.A. A microtranslatome coordinately regulates sodium and potassium currents in the human heart. eLife 2019, 8, e52654. [Google Scholar] [CrossRef]
- Tominaga, M.; Hamanoue, S.; Goto, H.; Saito, T.; Nagai, J.I.; Masuno, M.; Umeda, Y.; Kurosawa, K. Diamond-Blackfan anemia caused by chromosome 1p22 deletion encompassing RPL5. Hum. Genome Var. 2019, 6, 36. [Google Scholar] [CrossRef]
- Ulirsch, J.C.; Verboon, J.M.; Kazerounian, S.; Guo, M.H.; Yuan, D.; Ludwig, L.S.; Handsaker, R.E.; Abdulhay, N.J.; Fiorini, C.; Genovese, G.; et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am. J. Hum. Genet. 2019, 104, 356. [Google Scholar] [CrossRef]
- Vlachos, A.; Osorio, D.S.; Atsidaftos, E.; Kang, J.; Lababidi, M.L.; Seiden, H.S.; Gruber, D.; Glader, B.E.; Onel, K.; Farrar, J.E.; et al. Increased Prevalence of Congenital Heart Disease in Children With Diamond Blackfan Anemia Suggests Unrecognized Diamond Blackfan Anemia as a Cause of Congenital Heart Disease in the General Population: A Report of the Diamond Blackfan Anemia Registry. Circ. Genom. Precis. Med. 2018, 11, e002044. [Google Scholar] [CrossRef]
- Liu, Y.L.; Shibuya, A.; Glader, B.; Wilkes, M.C.; Barna, M.; Sakamoto, K.M. Animal models of Diamond-Blackfan anemia: Updates and challenges. Haematologica 2023, 108, 1222–1231. [Google Scholar] [CrossRef]
- Grimes, K.M.; Prasad, V.; Huo, J.; Kuwabara, Y.; Vanhoutte, D.; Baldwin, T.A.; Bowers, S.L.K.; Johansen, A.K.Z.; Sargent, M.A.; Lin, S.J.; et al. Rpl3l gene deletion in mice reduces heart weight over time. Front. Physiol. 2023, 14, 1054169. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, C.; Matsumoto, A.; Ichihara, K.; Yamamoto, T.; Yokoyama, T.; Mizoo, T.; Hatano, A.; Matsumoto, M.; Tanaka, Y.; Matsuura-Suzuki, E.; et al. RPL3L-containing ribosomes determine translation elongation dynamics required for cardiac function. Nat. Commun. 2023, 14, 2131. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, I.; Santos Vieira, H.G.; Lucas, M.C.; Ruiz-Orera, J.; Patone, G.; Kesteven, S.; Wu, J.; Feneley, M.; Espadas, G.; Sabido, E.; et al. Dynamic interplay between RPL3- and RPL3L-containing ribosomes modulates mitochondrial activity in the mammalian heart. Nucleic Acids Res. 2023, 51, 5301–5324. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.R.; Ganapathi, M.; Lee, T.M.; Fisher, J.M.; Patel, M.V.; Jayakar, P.; Buchanan, A.; Rippert, A.L.; Ahrens-Nicklas, R.C.; Nair, D.; et al. Recessive but damaging alleles of muscle-specific ribosomal protein gene RPL3L drive neonatal dilated cardiomyopathy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Methawasin, M.; Zhang, Y.; Gregorich, Z.R.; He, Y.; Liu, C.; Muldoon, J.; Hourani, Z.; Smith, J.E., 3rd; Granzier, H.; Guo, W. Reducing Granules Without Splicing Restoration Alleviates RBM20 Cardiomyopathy. Circ. Res. 2025, 136, 1134–1146. [Google Scholar] [CrossRef]
- Hosur, V.; Low, B.E.; Li, D.; Stafford, G.A.; Kohar, V.; Shultz, L.D.; Wiles, M.V. Genes adapt to outsmart gene-targeting strategies in mutant mouse strains by skipping exons to reinitiate transcription and translation. Genome Biol. 2020, 21, 168. [Google Scholar] [CrossRef]
- Smits, A.H.; Ziebell, F.; Joberty, G.; Zinn, N.; Mueller, W.F.; Clauder-Munster, S.; Eberhard, D.; Falth Savitski, M.; Grandi, P.; Jakob, P.; et al. Biological plasticity rescues target activity in CRISPR knock outs. Nat. Methods 2019, 16, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Luo, S.; Peng, G.; Lu, J.Y.; Cui, G.; Liu, L.; Yan, P.; Yin, Y.; Liu, W.; Wang, R.; et al. Mouse knockout models reveal largely dispensable but context-dependent functions of lncRNAs during development. J. Mol. Cell Biol. 2018, 10, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broeck, A.; Klinge, S. Principles of human pre-60S biogenesis. Science 2023, 381, eadh3892. [Google Scholar] [CrossRef]
- Witte, F.; Ruiz-Orera, J.; Mattioli, C.C.; Blachut, S.; Adami, E.; Schulz, J.F.; Schneider-Lunitz, V.; Hummel, O.; Patone, G.; Mucke, M.B.; et al. A trans locus causes a ribosomopathy in hypertrophic hearts that affects mRNA translation in a protein length-dependent fashion. Genome Biol. 2021, 22, 191. [Google Scholar] [CrossRef]
- Khalyfa, A.; Bourbeau, D.; Chen, E.; Petroulakis, E.; Pan, J.; Xu, S.; Wang, E. Characterization of elongation factor-1A (eEF1A-1) and eEF1A-2/S1 protein expression in normal and wasted mice. J. Biol. Chem. 2001, 276, 22915–22922. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Smith, L.L.; Padilla-Lopez, S.R.; Guida, B.S.; Blume, E.; Shi, J.; Morton, S.U.; Brownstein, C.A.; Beggs, A.H.; Kruer, M.C.; et al. Homozygous EEF1A2 mutation causes dilated cardiomyopathy, failure to thrive, global developmental delay, epilepsy and early death. Hum. Mol. Genet. 2017, 26, 3545–3552. [Google Scholar] [CrossRef]
- Feng, W.; Wang, L.; Veevers, J.; Liu, C.; Huang, T.; Chen, J. Loss of eEF1A2 (Eukaryotic Elongation Factor 1 A2) in Murine Myocardium Results in Dilated Cardiomyopathy. Circ. Heart Fail. 2021, 14, e008665. [Google Scholar] [CrossRef]
- Boczonadi, V.; Horvath, R. Mitochondria: Impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 2014, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Euro, L.; Konovalova, S.; Asin-Cayuela, J.; Tulinius, M.; Griffin, H.; Horvath, R.; Taylor, R.W.; Chinnery, P.F.; Schara, U.; Thorburn, D.R.; et al. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front. Genet. 2015, 6, 21. [Google Scholar] [CrossRef]
- Götz, A.; Tyynismaa, H.; Euro, L.; Ellonen, P.; Hyötyläinen, T.; Ojala, T.; Hämäläinen, R.H.; Tommiska, J.; Raivio, T.; Oresic, M.; et al. Exome Sequencing Identifies Mitochondrial Alanyl-tRNA Synthetase Mutations in Infantile Mitochondrial Cardiomyopathy. Am. J. Hum. Genet. 2011, 88, 635–642. [Google Scholar] [CrossRef]
- Sommerville, E.W.; Zhou, X.-L.; Oláhová, M.; Jenkins, J.; Euro, L.; Konovalova, S.; Hilander, T.; Pyle, A.; He, L.; Habeebu, S.; et al. Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum. Mol. Genet. 2019, 28, 258–268. [Google Scholar] [CrossRef]
- Rudler, D.L.; Hughes, L.A.; Perks, K.L.; Richman, T.R.; Kuznetsova, I.; Ermer, J.A.; Abudulai, L.N.; Shearwood, A.-M.J.; Viola, H.M.; Hool, L.C.; et al. Fidelity of translation initiation is required for coordinated respiratory complex assembly. Sci. Adv. 2019, 5, eaay2118. [Google Scholar] [CrossRef]
- Gebauer, F.; Schwarzl, T.; Valcarcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet. 2021, 22, 185–198. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Matthews, R.C.; Walcott, G.P.; Lu, Y.A.; Wei, Y.; Zhou, Y.; Zangi, L.; Zhang, J. CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts With Myocardial Infarction in Mice and Pigs. Circ. Res. 2023, 133, 484–504. [Google Scholar] [CrossRef]
- Bohlen, J.; Roiuk, M.; Neff, M.; Teleman, A.A. PRRC2 proteins impact translation initiation by promoting leaky scanning. Nucleic Acids Res. 2023, 51, 3391–3409. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Takahashi, K.; Yamamoto, T.; Iwasaki, M.; Narita, M.; Nakamura, M.; Rand, T.A.; Nakagawa, M.; Watanabe, A.; Yamanaka, S. Nat1 promotes translation of specific proteins that induce differentiation of mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2017, 114, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Kranzusch, P.J.; Doudna, J.A.; Cate, J.H. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 2016, 536, 96–99. [Google Scholar] [CrossRef]
- Lee, A.S.; Kranzusch, P.J.; Cate, J.H. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015, 522, 111–114. [Google Scholar] [CrossRef]
- Lamper, A.M.; Fleming, R.H.; Ladd, K.M.; Lee, A.S.Y. A phosphorylation-regulated eIF3d translation switch mediates cellular adaptation to metabolic stress. Science 2020, 370, 853–856. [Google Scholar] [CrossRef]
- Volta, V.; Perez-Baos, S.; de la Parra, C.; Katsara, O.; Ernlund, A.; Dornbaum, S.; Schneider, R.J. A DAP5/eIF3d alternate mRNA translation mechanism promotes differentiation and immune suppression by human regulatory T cells. Nat. Commun. 2021, 12, 6979. [Google Scholar] [CrossRef]
- Alard, A.; Katsara, O.; Rios-Fuller, T.; Parra, C.; Ozerdem, U.; Ernlund, A.; Schneider, R.J. Breast cancer cell mesenchymal transition and metastasis directed by DAP5/eIF3d-mediated selective mRNA translation. Cell Rep. 2023, 42, 112646. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Khor, E.S.; Jiang, F.; He, J.; Kawakami, Y.; Wainwright, L.; Hollinger, J.; Geiger, J.; Liu, H.; Meng, F.; et al. Loss-of-function of RNA-binding protein PRRC2B causes translational defects and congenital cardiovascular malformation. medRxiv 2024. [Google Scholar] [CrossRef]
- Goldberg, N.; Bril, D.; Eisenstein, M.; Olender, T.; Savidor, A.; Bialik, S.; Pietrokovski, S.; Kimchi, A. The RNA-binding protein PRRC2B preserves 5′ TOP mRNA during starvation to maintain ribosome biogenesis during nutrient recovery. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, S.; Hu, W.; Gong, S.; Zhang, P.; Cheng, J.; Wang, S.; Wang, Y.; Shi, W.; Li, Q.; Wang, F.; et al. The Role of PRRC2B in Cerebral Vascular Remodeling Under Acute Hypoxia in Mice. Adv. Sci. 2023, 10, e2300892. [Google Scholar] [CrossRef]
- Rao, A.; Lyu, B.; Jahan, I.; Lubertozzi, A.; Zhou, G.; Tedeschi, F.; Jankowsky, E.; Kang, J.; Carstens, B.; Poss, K.D.; et al. The translation initiation factor homolog eif4e1c regulates cardiomyocyte metabolism and proliferation during heart regeneration. Development 2023, 150, dev201376. [Google Scholar] [CrossRef]
- Jobava, R.; Mao, Y.; Guan, B.-J.; Hu, D.; Krokowski, D.; Chen, C.-W.; Shu, X.E.; Chukwurah, E.; Wu, J.; Gao, Z.; et al. Adaptive translational pausing is a hallmark of the cellular response to severe environmental stress. Mol. Cell 2021, 81, 4191–4208.E8. [Google Scholar] [CrossRef]
- Santos-Ribeiro, D.; Godinas, L.; Pilette, C.; Perros, F. The integrated stress response system in cardiovascular disease. Drug Discov. Today 2018, 23, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef]
- Tanaka, K.-i.; Xue, Y.; Nguyen-Yamamoto, L.; Morris, J.A.; Kanazawa, I.; Sugimoto, T.; Wing, S.S.; Richards, J.B.; Goltzman, D. FAM210A is a novel determinant of bone and muscle structure and strength. Proc. Natl. Acad. Sci. USA 2018, 115, E3759–E3768. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Wu, J.; Venkata Subbaiah, K.C.; Jiang, F.; Hedaya, O.; Mohan, A.; Yang, T.; Welle, K.; Ghaemmaghami, S.; Tang, W.H.W.; Small, E.; et al. MicroRNA-574 regulates FAM210A expression and influences pathological cardiac remodeling. EMBO Mol. Med. 2020, 13, e12710. [Google Scholar] [CrossRef]
- Hollinger, J.; Wu, J.; Awayda, K.M.; O’Connell, M.R.; Yao, P. Expression and purification of the mitochondrial transmembrane protein FAM210A in Escherichia coli. Protein Expr. Purif. 2023, 210, 106322. [Google Scholar] [CrossRef]
- Mohamed, T.M.A.; Ang, Y.S.; Radzinsky, E.; Zhou, P.; Huang, Y.; Elfenbein, A.; Foley, A.; Magnitsky, S.; Srivastava, D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 2018, 173, 104–116.E12. [Google Scholar] [CrossRef] [PubMed]
- Aballo, T.J.; Roberts, D.S.; Bayne, E.F.; Zhu, W.; Walcott, G.; Mahmoud, A.I.; Zhang, J.; Ge, Y. Integrated proteomics reveals alterations in sarcomere composition and developmental processes during postnatal swine heart development. J. Mol. Cell Cardiol. 2023, 176, 33–40. [Google Scholar] [CrossRef]
- Zhao, M.; Nakada, Y.; Wei, Y.; Bian, W.; Chu, Y.; Borovjagin, A.V.; Xie, M.; Zhu, W.; Nguyen, T.; Zhou, Y.; et al. Cyclin D2 Overexpression Enhances the Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Myocardial Repair in a Swine Model of Myocardial Infarction. Circulation 2021, 144, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef]
- Bartsch, D.; Kalamkar, K.; Ahuja, G.; Lackmann, J.W.; Hescheler, J.; Weber, T.; Bazzi, H.; Clamer, M.; Mendjan, S.; Papantonis, A.; et al. mRNA translational specialization by RBPMS presets the competence for cardiac commitment in hESCs. Sci. Adv. 2023, 9, eade1792. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liang, T.; Lu, Y.W.; Pu, L.; Fu, X.; Dong, X.; Hong, T.; Zhang, F.; Liu, N.; Zhou, Y.; et al. Reduced Mitochondrial Protein Translation Promotes Cardiomyocyte Proliferation and Heart Regeneration. Circulation 2023, 148, 1887–1906. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, A.; Kennedy, L.; Yang, Y.; Patel, S.; Lin, Y.; Linask, K.L.; Fergusson, M.; Zhu, J.; Gucek, M.; Zou, J.; et al. The ribosomal prolyl-hydroxylase OGFOD1 decreases during cardiac differentiation and modulates translation and splicing. JCI Insight 2019, 5, e128496. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, M.; Shah, A.M.; Ye, W.; Tan, W.; Min, Y.L.; Botten, G.A.; Shelton, J.M.; Liu, N.; Bassel-Duby, R.; et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc. Natl. Acad. Sci. USA 2019, 116, 18455–18465. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Li, S.; Shen, S.; Xu, H.; Cai, S.; Yuan, X.; Wang, C.; Zhang, X.; Chen, S.; Chen, J.; Shi, D.L.; et al. IGF2BP3 promotes adult myocardial regeneration by stabilizing MMP3 mRNA through interaction with m6A modification. Cell Death Discov. 2023, 9, 164. [Google Scholar] [CrossRef]
- Caudron-Herger, M.; Jansen, R.E.; Wassmer, E.; Diederichs, S. RBP2GO: A comprehensive pan-species database on RNA-binding proteins, their interactions and functions. Nucleic Acids Res. 2021, 49, D425–D436. [Google Scholar] [CrossRef]
- Nakano, K.; Sadahiro, T.; Fujita, R.; Isomi, M.; Abe, Y.; Yamada, Y.; Akiyama, T.; Honda, S.; French, B.A.; Mizukami, H.; et al. Development of adeno-associated viral vectors targeting cardiac fibroblasts for efficient in vivo cardiac reprogramming. Stem Cell Rep. 2024, 19, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Sadahiro, T.; Yamada, Y.; Isomi, M.; Yamakawa, H.; Fujita, R.; Abe, Y.; Akiyama, T.; Nakano, K.; Kuze, Y.; et al. Direct Reprogramming Improves Cardiac Function and Reverses Fibrosis in Chronic Myocardial Infarction. Circulation 2023, 147, 223–238. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef]
- Kim, S.; Coulombe, P.A. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat. Rev. Mol. Cell Biol. 2010, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.D.; Jenkins, A.; Jenkins, A.K.; Brandenburger, Y. Cardiac hypertrophy: A matter of translation. Clin. Exp. Pharmacol. Physiol. 2003, 30, 517–527. [Google Scholar] [CrossRef]
- Chorghade, S.; Seimetz, J.; Emmons, R.; Yang, J.; Bresson, S.M.; Lisio, M.; Parise, G.; Conrad, N.K.; Kalsotra, A. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. eLife 2017, 6, e24139. [Google Scholar] [CrossRef]
- Gudbjarnason, S.; Telerman, M.; Chiba, C.; Wolf, P.L.; Bing, R.J. Myocardial Protein Synthesis in Cardiac Hypertrophy. J. Lab. Clin. Med. 1964, 63, 244–253. [Google Scholar]
- Gonzalez-Teran, B.; Lopez, J.A.; Rodriguez, E.; Leiva, L.; Martinez-Martinez, S.; Bernal, J.A.; Jimenez-Borreguero, L.J.; Redondo, J.M.; Vazquez, J.; Sabio, G. p38gamma and delta promote heart hypertrophy by targeting the mTOR-inhibitory protein DEPTOR for degradation. Nat. Commun. 2016, 7, 10477. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 2005, 115, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Brandenburger, Y.; Arthur, J.F.; Woodcock, E.A.; Du, X.J.; Gao, X.M.; Autelitano, D.J.; Rothblum, L.I.; Hannan, R.D. Cardiac hypertrophy in vivo is associated with increased expression of the ribosomal gene transcription factor UBF. FEBS Lett. 2003, 548, 79–84. [Google Scholar] [CrossRef]
- Luyken, J.; Hannan, R.D.; Cheung, J.Y.; Rothblum, L.I. Regulation of rDNA Transcription During Endothelin-1Induced Hypertrophy of Neonatal Cardiomyocytes. Circ. Res. 1996, 78, 354–361. [Google Scholar] [CrossRef]
- Hannan, R.D.; Luyken, J.; Rothblum, L.I. Regulation of ribosomal DNA transcription during contraction-induced hypertrophy of neonatal cardiomyocytes. J. Biol. Chem. 1996, 271, 3213–3220. [Google Scholar] [CrossRef]
- Pestova, T.V.; Kolupaeva, V.G.; Lomakin, I.B.; Pilipenko, E.V.; Shatsky, I.N.; Agol, V.I.; Hellen, C.U. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA 2001, 98, 7029–7036. [Google Scholar] [CrossRef]
- Gebauer, F.; Hentze, M.W. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 2004, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lazaris-Karatzas, A.; Montine, K.S.; Sonenberg, N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 1990, 345, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Ventoso, I.; Blanco, R.; Perales, C.; Carrasco, L. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc. Natl. Acad. Sci. USA 2001, 98, 12966. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Ivester, C.T.; Carabello, B.A.; Cooper, G.; McDermott, P.J. Translational Initiation Factor eIF-4E: A LINK BETWEEN CARDIAC LOAD AND PROTEIN SYNTHESIS. J. Biol. Chem. 1996, 271, 8359–8364. [Google Scholar] [CrossRef]
- Tuxworth, W.J.; Wada, H.; Ishibashi, Y.; McDermott, P.J. Role of load in regulating eIF-4F complex formation in adult feline cardiocytes. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H1273–H1282. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.A.; Namboodiri, A.M.S.; McDermott, P.J. Transcriptional regulation of the rat eIF4E gene in cardiac muscle cells: The role of specific elements in the promoter region. Gene 2001, 267, 1–12. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Carabello, B.A.; Hamawaki, M.; Nemoto, S.; Matsuo, T.; McDermott, P.J. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H2176–H2184. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.A.; McDermott, P.J. Increased expression of eukaryotic initiation factor 4E during growth of neonatal rat cardiocytes in vitro. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H2133–H2142. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.-C.; Raught, B.; Sonenberg, N. eIF4 Initiation Factors: Effectors of mRNA Recruitment to Ribosomes and Regulators of Translation. Annu. Rev. Biochem. 1999, 68, 913–963. [Google Scholar] [CrossRef]
- Saghir, A.N.; Tuxworth, W.J., Jr.; Hagedorn, C.H.; McDermott, P.J. Modifications of eukaryotic initiation factor 4F (eIF4F) in adult cardiocytes by adenoviral gene transfer: Differential effects on eIF4F activity and total protein synthesis rates. Biochem. J. 2001, 356, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hardt, S.E.; Tomita, H.; Katus, H.A.; Sadoshima, J. Phosphorylation of Eukaryotic Translation Initiation Factor 2Bε by Glycogen Synthase Kinase-3β Regulates β-Adrenergic Cardiac Myocyte Hypertrophy. Circ. Res. 2004, 94, 926–935. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Fassett, J.; Kwak, D.; Liu, X.; Hu, X.; Wang, H.; Guo, H.; Xu, D.; Yan, S.; et al. Loss of the Eukaryotic Initiation Factor 2α Kinase General Control Nonderepressible 2 Kinase Protects Mice From Pressure Overload-Induced Congestive Heart Failure Without Affecting Ventricular Hypertrophy. Hypertension 2013, 63, 128–135. [Google Scholar] [CrossRef]
- Kimball, S.R. Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 1999, 31, 25–29. [Google Scholar] [CrossRef]
- Rani, S.; Sreenivasaiah, P.K.; Cho, C.; Kim, D.H. Salubrinal Alleviates Pressure Overload-Induced Cardiac Hypertrophy by Inhibiting Endoplasmic Reticulum Stress Pathway. Mol. Cells 2017, 40, 66–72. [Google Scholar] [CrossRef]
- James, C.C.; Smyth, J.W. Alternative mechanisms of translation initiation: An emerging dynamic regulator of the proteome in health and disease. Life Sci. 2018, 212, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Salas, E.; Piñeiro, D.; Fernández, N. Alternative Mechanisms to Initiate Translation in Eukaryotic mRNAs. Comp. Funct. Genom. 2012, 2012, 391546. [Google Scholar] [CrossRef]
- Basheer, W.A.; Xiao, S.; Epifantseva, I.; Fu, Y.; Kleber, A.G.; Hong, T.; Shaw, R.M. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ. Res. 2017, 121, 1069–1080. [Google Scholar] [CrossRef]
- Peters Nicholas, S.; Coromilas, J.; Severs Nicholas, J.; Wit Andrew, L. Disturbed Connexin43 Gap Junction Distribution Correlates with the Location of Reentrant Circuits in the Epicardial Border Zone of Healing Canine Infarcts That Cause Ventricular Tachycardia. Circulation 1997, 95, 988–996. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013, 5, 611–618. [Google Scholar] [CrossRef]
- Ul-Hussain, M.; Olk, S.; Schoenebeck, B.; Wasielewski, B.; Meier, C.; Prochnow, N.; May, C.; Galozzi, S.; Marcus, K.; Zoidl, G.; et al. Internal ribosomal entry site (IRES) activity generates endogenous carboxyl-terminal domains of Cx43 and is responsive to hypoxic conditions. J. Biol. Chem. 2014, 289, 20979–20990. [Google Scholar] [CrossRef]
- Schiavi, A.; Hudder, A.; Werner, R. Connexin43 mRNA contains a functional internal ribosome entry site. FEBS Lett. 1999, 464, 118–122. [Google Scholar] [CrossRef]
- Salat-Canela, C.; Sesé, M.; Peula, C.; Ramón y Cajal, S.; Aasen, T. Internal translation of the connexin 43 transcript. Cell Commun. Signal. 2014, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.R.P.; Garland, G.; Pöyry, T.; Mead, E.; Vlahov, N.; Sfakianos, A.; Grosso, S.; De-Lima-Hedayioglu, F.; Mallucci, G.R.; von der Haar, T.; et al. Control of translation elongation in health and disease. Dis. Model. Mech. 2020, 13, dmm043208. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, R.M.; Ramakrishnan, V. Structural Basis of the Translational Elongation Cycle. Annu. Rev. Biochem. 2013, 82, 203–236. [Google Scholar] [CrossRef]
- Faller, W.J.; Jackson, T.J.; Knight, J.R.P.; Ridgway, R.A.; Jamieson, T.; Karim, S.A.; Jones, C.; Radulescu, S.; Huels, D.J.; Myant, K.B.; et al. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature 2015, 517, 497–500. [Google Scholar] [CrossRef]
- Delaidelli, A.; Jan, A.; Herms, J.; Sorensen, P.H. Translational control in brain pathologies: Biological significance and therapeutic opportunities. Acta Neuropathol. 2019, 137, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Kameshima, S.; Okada, M.; Ikeda, S.; Watanabe, Y.; Yamawaki, H. Coordination of changes in expression and phosphorylation of eukaryotic elongation factor 2 (eEF2) and eEF2 kinase in hypertrophied cardiomyocytes. Biochem. Biophys. Rep. 2016, 7, 218–224. [Google Scholar] [CrossRef]
- Everett, A.D.; Stoops, T.D.; Nairn, A.C.; Brautigan, D. Angiotensin II regulates phosphorylation of translation elongation factor-2 in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H161–H167. [Google Scholar] [CrossRef]
- Wang, L.; Proud, C.G. Regulation of the phosphorylation of elongation factor 2 by MEK-dependent signalling in adult rat cardiomyocytes. FEBS Lett. 2002, 531, 285–289. [Google Scholar] [CrossRef] [PubMed]
- McLeod, L.E.; Wang, L.; Proud, C.G. beta-Adrenergic agonists increase phosphorylation of elongation factor 2 in cardiomyocytes without eliciting calcium-independent eEF2 kinase activity. FEBS Lett. 2001, 489, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Pattan, G.; Rafeequi, T. Eukaryotic elongation factor-2 (eEF2): Its regulation and peptide chain elongation. Cell Biochem. Funct. 2011, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pittman, Y.; Kandl, K.; Lewis, M.; Valente, L.; Kinzy, T. Coordination of Eukaryotic Translation Elongation Factor 1A (eEF1A) Function in Actin Organization and Translation Elongation by the Guanine Nucleotide Exchange Factor eEF1B. J. Biol. Chem. 2009, 284, 4739–4747. [Google Scholar] [CrossRef]
- He, H.; Chen, M.; Scheffler, N.K.; Gibson Bradford, W.; Spremulli Linda, L.; Gottlieb Roberta, A. Phosphorylation of Mitochondrial Elongation Factor Tu in Ischemic Myocardium. Circ. Res. 2001, 89, 461–467. [Google Scholar] [CrossRef]
- Borutaite, V.; Mildaziene, V.; Brown, G.C.; Brand, M.D. Control and kinetic analysis of ischemia-damaged heart mitochondria: Which parts of the oxidative phosphorylation system are affected by ischemia? Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 1995, 1272, 154–158. [Google Scholar] [CrossRef]
- Cai, Y.C.; Bullard, J.; Thompson, N.; Spremulli, L. Interaction of Mitochondrial Elongation Factor Tu with Aminoacyl-tRNA and Elongation Factor Ts. J. Biol. Chem. 2000, 275, 20308–20314. [Google Scholar] [CrossRef]
- Lippmann, C.; Lindschau, C.; Buchner, K.; Erdmann, V.A. Phosphorylation of Elongation Factor Tu in Vitro and in Vivo. In Cellular Regulation by Protein Phosphorylation; Heilmeyer, L.M.G., Ed.; NATO ASI Series; Springer: Berlin/Heidelberg, Germany, 1991; pp. 441–445. [Google Scholar] [CrossRef]
- Brandenburger, Y.; Jenkins, A.; Autelitano, D.J.; Hannan, R.D. Increased expression of UBF is a critical determinant for rRNA synthesis and hypertrophic growth of cardiac myocytes. FASEB J. 2001, 15, 2051–2053. [Google Scholar] [CrossRef]
- Hannan, R.D.; Luyken, J.; Rothblum, L.I. Regulation of rDNA Transcription Factors during Cardiomyocyte Hypertrophy Induced by Adrenergic Agents. J. Biol. Chem. 1995, 270, 8290–8297. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Izumo, S. Rapamycin Selectively Inhibits Angiotensin II–Induced Increase in Protein Synthesis in Cardiac Myocytes In Vitro. Circ. Res. 1995, 77, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Fujio, Y.; Kunisada, K.; Hirota, H.; Matsui, H.; Kishimoto, T.; Yamauchi-Takihara, K. Activation of Phosphatidylinositol 3-Kinase through Glycoprotein 130 Induces Protein Kinase B and p70 S6 Kinase Phosphorylation in Cardiac Myocytes. J. Biol. Chem. 1998, 273, 9703–9710. [Google Scholar] [CrossRef]
- Volkers, M.; Toko, H.; Doroudgar, S.; Din, S.; Quijada, P.; Joyo, A.Y.; Ornelas, L.; Joyo, E.; Thuerauf, D.J.; Konstandin, M.H.; et al. Pathological hypertrophy amelioration by PRAS40-mediated inhibition of mTORC1. Proc. Natl. Acad. Sci. USA 2013, 110, 12661–12666. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef]
- Robitaille, A.M.; Christen, S.; Shimobayashi, M.; Cornu, M.; Fava, L.L.; Moes, S.; Prescianotto-Baschong, C.; Sauer, U.; Jenoe, P.; Hall, M.N. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013, 339, 1320–1323. [Google Scholar] [CrossRef]
- Hannan, K.M.; Brandenburger, Y.; Jenkins, A.; Sharkey, K.; Cavanaugh, A.; Rothblum, L.; Moss, T.; Poortinga, G.; McArthur, G.A.; Pearson, R.B.; et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell Biol. 2003, 23, 8862–8877. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pires, K.M.; Whitehead, K.J.; Olsen, C.D.; Wayment, B.; Zhang, Y.C.; Bugger, H.; Ilkun, O.; Litwin, S.E.; Thomas, G.; et al. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. PLoS ONE 2013, 8, e54221. [Google Scholar] [CrossRef] [PubMed]
- Mazelin, L.; Panthu, B.; Nicot, A.S.; Belotti, E.; Tintignac, L.; Teixeira, G.; Zhang, Q.; Risson, V.; Baas, D.; Delaune, E.; et al. mTOR inactivation in myocardium from infant mice rapidly leads to dilated cardiomyopathy due to translation defects and p53/JNK-mediated apoptosis. J. Mol. Cell Cardiol. 2016, 97, 213–225. [Google Scholar] [CrossRef]
- Shende, P.; Plaisance, I.; Morandi, C.; Pellieux, C.; Berthonneche, C.; Zorzato, F.; Krishnan, J.; Lerch, R.; Hall, M.N.; Ruegg, M.A.; et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation 2011, 123, 1073–1082. [Google Scholar] [CrossRef]
- Zhang, D.; Contu, R.; Latronico, M.V.; Zhang, J.; Rizzi, R.; Catalucci, D.; Miyamoto, S.; Huang, K.; Ceci, M.; Gu, Y.; et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Investig. 2010, 120, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Shioi, T.; McMullen, J.R.; Tarnavski, O.; Converso, K.; Sherwood, M.C.; Manning, W.J.; Izumo, S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 2003, 107, 1664–1670. [Google Scholar] [CrossRef]
- McMullen, J.R.; Sherwood, M.C.; Tarnavski, O.; Zhang, L.; Dorfman, A.L.; Shioi, T.; Izumo, S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 2004, 109, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, Y.; Nie, J.; Liu, H.; Lu, S.; Hu, X.; Zhu, J.; Zhao, X.; Chen, J.; Chen, X.; et al. Genetic and pharmacological inhibition of Rheb1-mTORC1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am. J. Pathol. 2013, 182, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Kmietczyk, V.; Oelschlager, J.; Gupta, P.; Varma, E.; Hartl, S.; Furkel, J.; Konstandin, M.; Marx, A.; Loewenthal, Z.; Kamuf-Schenk, V.; et al. Ythdf2 regulates cardiac remodeling through its mRNA target transcripts. J. Mol. Cell Cardiol. 2023, 181, 57–66. [Google Scholar] [CrossRef]
- Berulava, T.; Buchholz, E.; Elerdashvili, V.; Pena, T.; Islam, M.R.; Lbik, D.; Mohamed, B.A.; Renner, A.; von Lewinski, D.; Sacherer, M.; et al. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. Eur. J. Heart Fail. 2020, 22, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis--a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Davis, J.; Molkentin, J.D. Myofibroblasts: Trust your heart and let fate decide. J. Mol. Cell. Cardiol. 2014, 70, 9–18. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Obana, M.; Maeda, M.; Takeda, K.; Hayama, A.; Mohri, T.; Yamashita, T.; Nakaoka, Y.; Komuro, I.; Takeda, K.; Matsumiya, G.; et al. Therapeutic Activation of Signal Transducer and Activator of Transcription 3 by Interleukin-11 Ameliorates Cardiac Fibrosis After Myocardial Infarction. Circulation 2010, 121, 684–691. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Lim, W.W.; Viswanathan, S.; Chothani, S.; Corden, B.; Dasan, C.M.; Goh, J.W.T.; Lim, R.; Singh, B.K.; Tan, J.; et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 2024, 632, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Lunitz, V.; Ruiz-Orera, J.; Hubner, N.; van Heesch, S. Multifunctional RNA-binding proteins influence mRNA abundance and translational efficiency of distinct sets of target genes. PLoS Comput. Biol. 2021, 17, e1009658. [Google Scholar] [CrossRef]
- Yao, P.; Fox, P.L. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol. Med. 2013, 5, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Mazumder, B.; Seshadri, V.; Gerber, C.A.; Chavatte, L.; Kinter, M.; Ting, S.M.; Dignam, J.D.; Kim, S.; Driscoll, D.M.; et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: Gene-specific silencing of translation. Cell 2004, 119, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.S.; Jia, J.; Yao, P.; Majumder, M.; Hatzoglou, M.; Fox, P.L. A stress-responsive RNA switch regulates VEGFA expression. Nature 2009, 457, 915–919. [Google Scholar] [CrossRef]
- Mendes, M.I.; Gutierrez Salazar, M.; Guerrero, K.; Thiffault, I.; Salomons, G.S.; Gauquelin, L.; Tran, L.T.; Forget, D.; Gauthier, M.S.; Waisfisz, Q.; et al. Bi-allelic Mutations in EPRS, Encoding the Glutamyl-Prolyl-Aminoacyl-tRNA Synthetase, Cause a Hypomyelinating Leukodystrophy. Am. J. Hum. Genet. 2018, 102, 676–684. [Google Scholar] [CrossRef]
- Khan, D.; Ramachandiran, I.; Vasu, K.; China, A.; Khan, K.; Cumbo, F.; Halawani, D.; Terenzi, F.; Zin, I.; Long, B.; et al. Homozygous EPRS1 missense variant causing hypomyelinating leukodystrophy-15 alters variant-distal mRNA m6A site accessibility. Nat. Commun. 2024, 15, 1–24. [Google Scholar] [CrossRef]
- Da, M.; Feng, Y.; Xu, J.; Hu, Y.; Lin, Y.; Ni, B.; Qian, B.; Hu, Z.; Mo, X. Association of aminoacyl-tRNA synthetases gene polymorphisms with the risk of congenital heart disease in the Chinese Han population. PLoS ONE 2014, 9, e110072. [Google Scholar] [CrossRef]
- Rau, C.D.; Romay, M.C.; Tuteryan, M.; Wang, J.J.; Santolini, M.; Ren, S.; Karma, A.; Weiss, J.N.; Wang, Y.; Lusis, A.J. Systems Genetics Approach Identifies Gene Pathways and Adamts2 as Drivers of Isoproterenol-Induced Cardiac Hypertrophy and Cardiomyopathy in Mice. Cell Syst. 2017, 4, 121–128.E4. [Google Scholar] [CrossRef]
- Galindo, C.L.; Skinner, M.A.; Errami, M.; Olson, L.D.; Watson, D.A.; Li, J.; McCormick, J.F.; McIver, L.J.; Kumar, N.M.; Pham, T.Q.; et al. Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure. BMC Physiol. 2009, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Ting, J.G.W.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef]
- Harigaya, Y.; Parker, R. No-go decay: A quality control mechanism for RNA in translation. Wiley Interdiscip. Rev. RNA 2010, 1, 132–141. [Google Scholar] [CrossRef]
- Wu, J.; Hollinger, J.; Bonanno, E.; Jiang, F.; Yao, P. Cardiomyocyte-Specific Loss of Glutamyl-prolyl-tRNA Synthetase Leads to Disturbed Protein Homeostasis and Dilated Cardiomyopathy. Cells 2023, 13, 35. [Google Scholar] [CrossRef]
- Doerfel, L.K.; Wohlgemuth, I.; Kothe, C.; Peske, F.; Urlaub, H.; Rodnina, M.V. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 2013, 339, 85–88. [Google Scholar] [CrossRef]
- Schuller, A.P.; Wu, C.C.; Dever, T.E.; Buskirk, A.R.; Green, R. eIF5A Functions Globally in Translation Elongation and Termination. Mol. Cell 2017, 66, 194–205.E5. [Google Scholar] [CrossRef]
- Lassak, J.; Wilson, D.N.; Jung, K. Stall no more at polyproline stretches with the translation elongation factors EF-P and IF-5A. Mol. Microbiol. 2016, 99, 219–235. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids 2016, 48, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Mirmira, R.G.; Jaume, J.C. Eukaryotic translation initiation factor 5A inhibition alters physiopathology and immune responses in a “humanized” transgenic mouse model of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E791–E798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subbaiah, K.C.V.; Wu, J.; Tang, W.H.W.; Yao, P. Ciclopirox Inhibition of eIF5A Hypusination Attenuates Fibroblast Activation and Cardiac Fibrosis. J. Cardiovasc. Dev. Dis. 2023, 10, 52. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Jani, V.P.; Yoo, E.J.; Binek, A.; Guo, A.; Kim, J.S.; Aguilan, J.; Keykhaei, M.; Jenkin, S.R.; Sidoli, S.; Sharma, K.; et al. Myocardial Proteome in Human Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2025, 14, e038945. [Google Scholar] [CrossRef]
- Goldfarb, L.; Dalakas, M. Tragedy in a heartbeat: Malfunctioning desmin causes skeletal and cardiac muscle disease. J. Clin. Investig. 2011, 119, 1806–1813. [Google Scholar] [CrossRef]
- McLendon, P.; Robbins, J. Desmin-related cardiomyopathy: An unfolding story. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1220–H1228. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Schimmel, P.; Kim, S. Aminoacyl tRNA synthetases and their connections to disease. Proc. Natl. Acad. Sci. USA 2008, 105, 11043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Satz, J.; Vo, M.-N.; Nangle, L.; Schimmel, P.; Ackerman, S. Deficiencies in tRNA synthetase editing activity cause cardioproteinopathy. Proc. Natl. Acad. Sci. USA 2014, 111, 17570–17575. [Google Scholar] [CrossRef]
- Yao, P.; Zhu, B.; Jaeger, S.; Eriani, G.; Wang, E.D. Recognition of tRNALeu by Aquifex aeolicus leucyl-tRNA synthetase during the aminoacylation and editing steps. Nucleic Acids Res. 2008, 36, 2728–2738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beebe, K.; Pouplana, L.; Schimmel, P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 2003, 22, 668–675. [Google Scholar] [CrossRef]
- Lee, j.-w.; Beebe, K.; Nangle, L.; Jang, J.; Guess, C.; Cook, S.; Davisson, M.; Sundberg, J.; Schimmel, P.; Ackerman, S. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 2006, 443, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Nangle, L.; Motta, C.; Schimmel, P. Global Effects of Mistranslation from an Editing Defect in Mammalian Cells. Chem. Biol. 2006, 13, 1091–1100. [Google Scholar] [CrossRef]
- Huang, Q.; Yao, P.; Eriani, G.; Wang, E.D. In vivo identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain. Nucleic Acids Res. 2012, 40, 10463–10477. [Google Scholar] [CrossRef][Green Version]
- Zuhlke, V.; de Rochemont, W.D.M.; Gudbjarnason, S.; Bing, R.J. Inhibition of protein synthesis in cardiac hypertrophy and its relation to myocardial failure. Circ. Res. 1966, 18, 558–572. [Google Scholar] [CrossRef]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, L.; Yang, X.L.; Schimmel, P. ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature 2013, 494, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Arabacilar, P.; Bernard, R.E.; Bao, W.; Olzinski, A.R.; Guo, Y.; Lal, H.; Eisennagel, S.H.; Platchek, M.C.; Xie, W.; et al. Activation of the Amino Acid Response Pathway Blunts the Effects of Cardiac Stress. J. Am. Heart Assoc. 2017, 6, e004453. [Google Scholar] [CrossRef]
- Sundrud, M.S.; Koralov, S.B.; Feuerer, M.; Calado, D.P.; Kozhaya, A.E.; Rhule-Smith, A.; Lefebvre, R.E.; Unutmaz, D.; Mazitschek, R.; Waldner, H.; et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 2009, 324, 1334–1338. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Y.; Hu, Z.; Xing, W.; Kun, L.V.; Wang, D.; Hu, N. Small-Molecule Integrated Stress Response Inhibitor Reduces Susceptibility to Postinfarct Atrial Fibrillation in Rats via the Inhibition of Integrated Stress Responses. J. Pharmacol. Exp. Ther. 2021, 378, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Onat, U.I.; Yildirim, A.D.; Tufanli, O.; Cimen, I.; Kocaturk, B.; Veli, Z.; Hamid, S.M.; Shimada, K.; Chen, S.; Sin, J.; et al. Intercepting the Lipid-Induced Integrated Stress Response Reduces Atherosclerosis. J. Am. Coll. Cardiol. 2019, 73, 1149–1169. [Google Scholar] [CrossRef]
- Hedaya, O.M.; Jiang, F.; Baliga, U.; Ivanov, A.; Chen, S.; Schwartz, J.L.; Kawakami, Y.; Yan, C.; Yao, P. Upstream open reading frame inactivation augments GATA4 translation and cardiomyocyte hypertrophy in mice. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Losson, R.; Lacroute, F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc. Natl. Acad. Sci. USA 1979, 76, 5134–5137. [Google Scholar] [CrossRef]
- Hug, N.; Longman, D.; Caceres, J.F. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 2016, 44, 1483–1495. [Google Scholar] [CrossRef]
- He, F.; Jacobson, A. Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes. Dev. 1995, 9, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Sebat, J.; Wilkinson, M.F. Cell type- and factor-specific nonsense-mediated RNA decay. Nucleic Acids Res. 2025, 53, gkaf395. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Li, X.; Serin, G.; Maquat, L.E. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 2001, 106, 607–617. [Google Scholar] [CrossRef]
- Le Hir, H.; Gatfield, D.; Izaurralde, E.; Moore, M.J. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001, 20, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Deniaud, A.; Boehm, V.; Gehring, N.H.; Schaffitzel, C.; Cusack, S. Structural and functional analysis of the three MIF4G domains of nonsense-mediated decay factor UPF2. Nucleic Acids Res. 2014, 42, 2673–2686. [Google Scholar] [CrossRef]
- Maquat, L.E.; Kinniburgh, A.J.; Rachmilewitz, E.A.; Ross, J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell 1981, 27, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V.; The, C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Coller, J. Codon optimality-mediated mRNA degradation: Linking translational elongation to mRNA stability. Mol. Cell 2022, 82, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Pelechano, V.; Wei, W.; Steinmetz, L.M. Widespread Co-translational RNA Decay Reveals Ribosome Dynamics. Cell 2015, 161, 1400–1412. [Google Scholar] [CrossRef]
- Harigaya, Y.; Parker, R. Codon optimality and mRNA decay. Cell Res. 2016, 26, 1269–1270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiang, Y.; Huang, W.; Tan, L.; Chen, T.; He, Y.; Irving, P.S.; Weeks, K.M.; Zhang, Q.C.; Dong, X. Pervasive downstream RNA hairpins dynamically dictate start-codon selection. Nature 2023, 621, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Guenther, U.P.; Weinberg, D.E.; Zubradt, M.M.; Tedeschi, F.A.; Stawicki, B.N.; Zagore, L.L.; Brar, G.A.; Licatalosi, D.D.; Bartel, D.P.; Weissman, J.S.; et al. The helicase Ded1p controls use of near-cognate translation initiation codons in 5′ UTRs. Nature 2018, 559, 130–134. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, S.; Zhang, Z.; Morgens, D.W.; Hayes, L.R.; Lee, S.; Portz, B.; Xie, Y.; Nguyen, B.V.; Haney, M.S.; et al. CRISPR-Cas9 Screens Identify the RNA Helicase DDX3X as a Repressor of C9ORF72 (GGGGCC)n Repeat-Associated Non-AUG Translation. Neuron 2019, 104, 885–898.E8. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Rurik, J.G.; Tombacz, I.; Yadegari, A.; Mendez Fernandez, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Aditham, A.; Guo, J.; Huang, J.; Kostas, F.; Maher, K.; Friedrich, M.J.; Xavier, R.J.; Zhang, F.; et al. Chemical and topological design of multicapped mRNA and capped circular RNA to augment translation. Nat. Biotechnol. 2024, 43, 1128–1143. [Google Scholar] [CrossRef]
- Anttila, V.; Saraste, A.; Knuuti, J.; Hedman, M.; Jaakkola, P.; Laugwitz, K.L.; Krane, M.; Jeppsson, A.; Sillanmaki, S.; Rosenmeier, J.; et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol. Ther. 2023, 31, 866–874. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Liang, X.H.; Shen, W.; Sun, H.; Migawa, M.T.; Vickers, T.A.; Crooke, S.T. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat. Biotechnol. 2016, 34, 875–880. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, T.; Gong, J.; Shan, H. Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy. Molecules 2025, 30, 962. [Google Scholar] [CrossRef]
- Philippou, S.; Mastroyiannopoulos, N.P.; Tomazou, M.; Oulas, A.; Ackers-Johnson, M.; Foo, R.S.; Spyrou, G.M.; Phylactou, L.A. Selective Delivery to Cardiac Muscle Cells Using Cell-Specific Aptamers. Pharm 2023, 16, 1264. [Google Scholar] [CrossRef]
- Narayan, C.; Lin, L.H.; Barros, M.N.; Gilbert, T.C.; Brown, C.R.; Reddin, D.; London, B.; Chen, Y.; Wilson, M.E.; Streeter, J.; et al. Identification of In Vivo Internalizing Cardiac-Specific RNA Aptamers. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.; Allen, E.C.; Bharti, N.; Davyt, M.; Joshi, D.; Perez-Garcia, C.G.; Santos, L.; Mukthavaram, R.; Delgado-Toscano, M.A.; Molina, B.; et al. Engineered tRNAs suppress nonsense mutations in cells and in vivo. Nature 2023, 618, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2023, 41, 262–272. [Google Scholar] [CrossRef]
- Carrieri, C.; Cimatti, L.; Biagioli, M.; Beugnet, A.; Zucchelli, S.; Fedele, S.; Pesce, E.; Ferrer, I.; Collavin, L.; Santoro, C.; et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 2012, 491, 454–457. [Google Scholar] [CrossRef]
- Arnoldi, M.; Zarantonello, G.; Espinoza, S.; Gustincich, S.; Di Leva, F.; Biagioli, M. Design and Delivery of SINEUP: A New Modular Tool to Increase Protein Translation. Methods Mol. Biol. 2022, 2434, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.W.; Narasimhan, M.; Bennett, E.J. Ubiquitin-dependent translation control mechanisms: Degradation and beyond. Cell Rep. 2024, 43, 115050. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights Into the Role of mTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef] [PubMed]
- McGirr, T.; Onar, O.; Jafarnejad, S.M. Dysregulated ribosome quality control in human diseases. FEBS J. 2025, 292, 936–959. [Google Scholar] [CrossRef]
- Ford, P.W.; Garshott, D.M.; Narasimhan, M.; Ge, X.; Jordahl, E.M.; Subramanya, S.; Bennett, E.J. RNF10 and RIOK3 facilitate 40S ribosomal subunit degradation upon 60S biogenesis disruption or amino acid starvation. Cell Rep. 2025, 44, 115371. [Google Scholar] [CrossRef] [PubMed]
- Garzia, A.; Meyer, C.; Tuschl, T. The E3 ubiquitin ligase RNF10 modifies 40S ribosomal subunits of ribosomes compromised in translation. Cell Rep. 2021, 36, 109468. [Google Scholar] [CrossRef]
- Clemens, M.J. Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog. Mol. Subcell. Biol. 2001, 27, 57–89. [Google Scholar] [CrossRef]
- Vanhoutte, D.; Schips, T.G.; Vo, A.; Grimes, K.M.; Baldwin, T.A.; Brody, M.J.; Accornero, F.; Sargent, M.A.; Molkentin, J.D. Thbs1 induces lethal cardiac atrophy through PERK-ATF4 regulated autophagy. Nat. Commun. 2021, 12, 3928. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, W.; Duan, X.; Zhang, Q.; Cao, L.; Liu, C.; Li, G.; Wang, Z.; Zhang, J.; Li, J.; et al. Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway. Cardiovasc. Diabetol. 2024, 23, 19. [Google Scholar] [CrossRef]
- Li, R.J.; He, K.L.; Li, X.; Wang, L.L.; Liu, C.L.; He, Y.Y. Salubrinal protects cardiomyocytes against apoptosis in a rat myocardial infarction model via suppressing the dephosphorylation of eukaryotic translation initiation factor 2alpha. Mol. Med. Rep. 2015, 12, 1043–1049. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Yao, F.; Su, Q.; Liu, D.; Xue, R.; Dai, G.; Fang, R.; Zeng, J.; Chen, Y.; et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch. Biochem. Biophys. 2014, 558, 79–86. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhu, H.; Li, H.; Zou, M.H.; Xie, Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013, 62, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Du, L. Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes Obes. Metab. 2011, 13, 289–301. [Google Scholar] [CrossRef]
- Kanamori, H.; Naruse, G.; Yoshida, A.; Minatoguchi, S.; Watanabe, T.; Kawaguchi, T.; Yamada, Y.; Mikami, A.; Kawasaki, M.; Takemura, G.; et al. Metformin Enhances Autophagy and Provides Cardioprotection in delta-Sarcoglycan Deficiency-Induced Dilated Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005418. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Zhang, G.; Li, Q.; Song, W.; Wang, X.; Cook, J.A.; van der Stoel, M.; Wright, B.W.; Altamirano, F.; et al. IRE1alpha Mediates the Hypertrophic Growth of Cardiomyocytes Through Facilitating the Formation of Initiation Complex to Promote the Translation of TOP-Motif Transcripts. Circulation 2024, 150, 1010–1029. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliga, U.K.; Yang, L.; Ivanov, A.; Schwartz, J.L.; Jiang, F.; Khor, E.-S.; Das, D.; Wainwright, L.; Yao, P. Translational Control in Cardiac Pathophysiology and Therapeutic Development: When mRNA Meets the Heart. Int. J. Mol. Sci. 2025, 26, 7863. https://doi.org/10.3390/ijms26167863
Baliga UK, Yang L, Ivanov A, Schwartz JL, Jiang F, Khor E-S, Das D, Wainwright L, Yao P. Translational Control in Cardiac Pathophysiology and Therapeutic Development: When mRNA Meets the Heart. International Journal of Molecular Sciences. 2025; 26(16):7863. https://doi.org/10.3390/ijms26167863
Chicago/Turabian StyleBaliga, Uday K., Liuqing Yang, Aleksandr Ivanov, Jack L. Schwartz, Feng Jiang, Eng-Soon Khor, Debojyoti Das, Lindsey Wainwright, and Peng Yao. 2025. "Translational Control in Cardiac Pathophysiology and Therapeutic Development: When mRNA Meets the Heart" International Journal of Molecular Sciences 26, no. 16: 7863. https://doi.org/10.3390/ijms26167863
APA StyleBaliga, U. K., Yang, L., Ivanov, A., Schwartz, J. L., Jiang, F., Khor, E.-S., Das, D., Wainwright, L., & Yao, P. (2025). Translational Control in Cardiac Pathophysiology and Therapeutic Development: When mRNA Meets the Heart. International Journal of Molecular Sciences, 26(16), 7863. https://doi.org/10.3390/ijms26167863






