Chromosomal Aberrations in Induced Pluripotent Stem Cells: Identification of Breakpoints in the Large DCC Gene and HIST2 Histone Gene Cluster
Abstract
1. Introduction
2. Results
2.1. Sample Description
2.2. Incidence of Karyotype Abnormalities
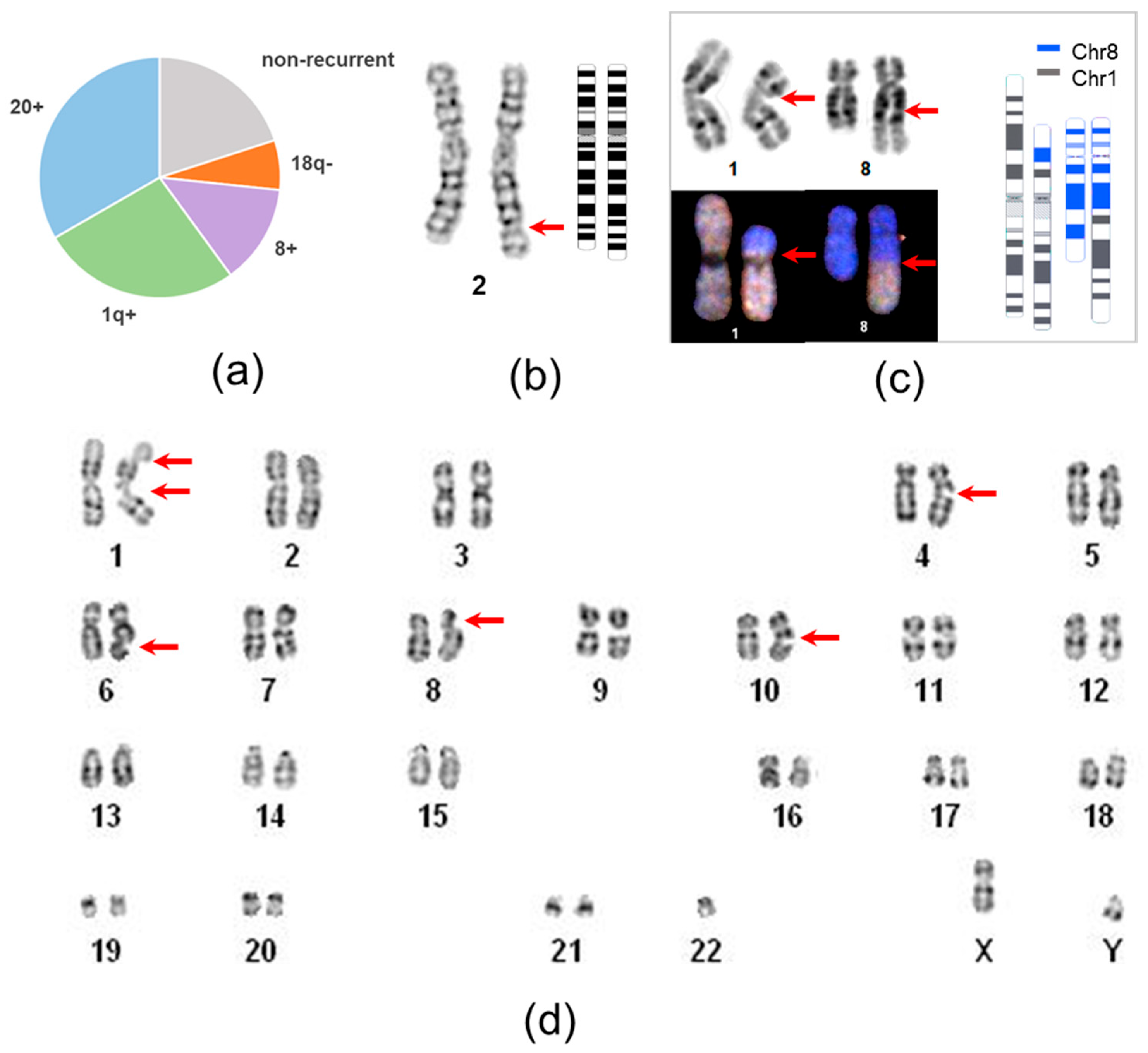
2.3. Recurrent and Non-Recurrent Aberrations
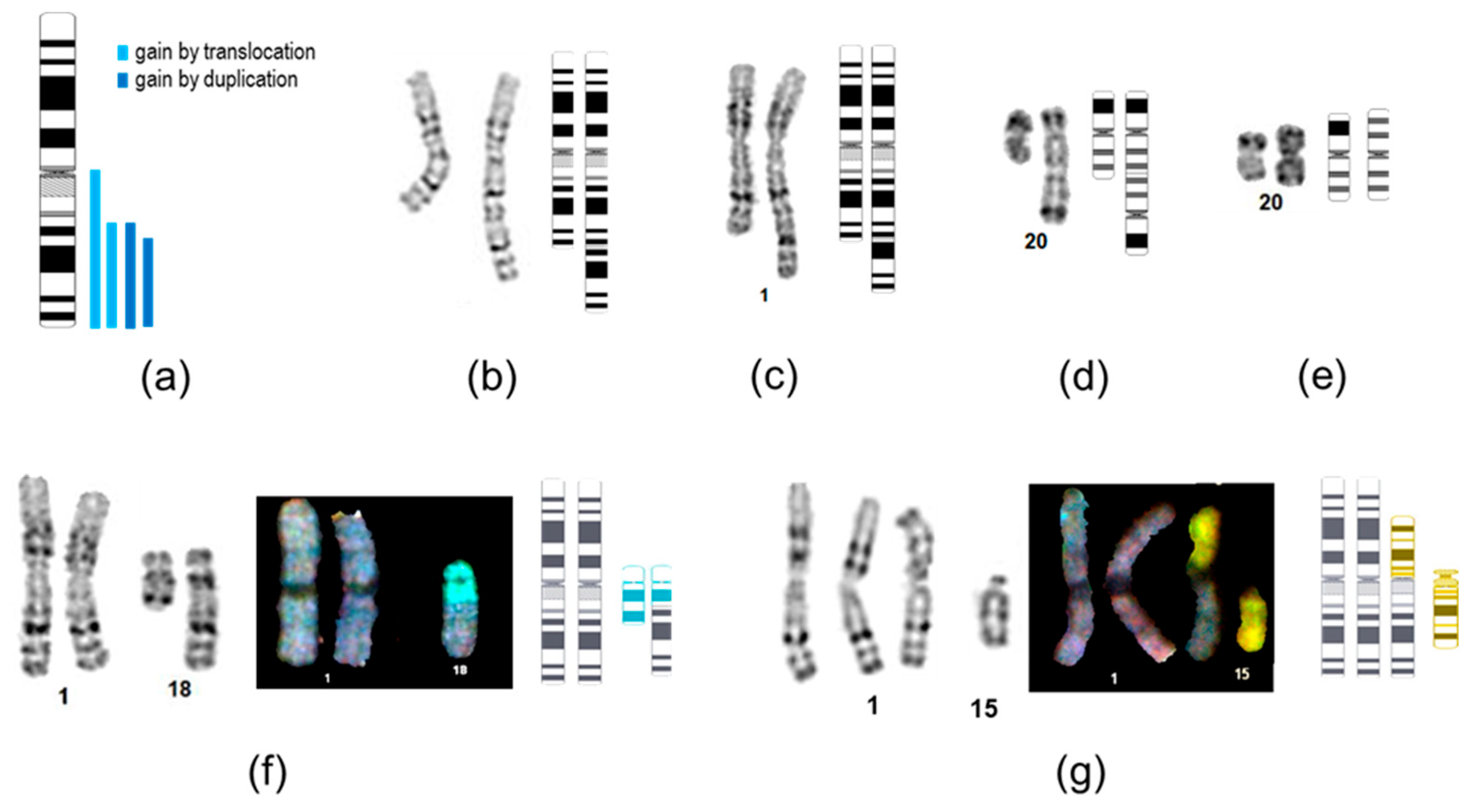
2.4. Structural Characterization of Recurrent Chromosomal Rearrangements
2.5. Dynamics of Abnormal Cell Clones Under Passaging
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Karyotyping
4.3. FISH and Spectral Karyotyping (SKY)
4.4. SNP Arrays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Manganelli, M.; Mazzoldi, E.L.; Ferraro, R.M.; Pinelli, M.; Parigi, M.; Aghel, S.A.M.; Bugatti, M.; Collo, G.; Stocco, G.; Vermi, W.; et al. Progesterone receptor is constitutively expressed in induced Pluripotent Stem Cells (iPSCs). Stem Cell Rev. Rep. 2024, 20, 2303–2317. [Google Scholar] [CrossRef]
- Milagre, I.; Pereira, C.; Oliveira, R.A. Compromised Mitotic Fidelity in Human Pluripotent Stem Cells. Int. J. Mol. Sci. 2023, 24, 11933. [Google Scholar] [CrossRef]
- Bárta, T.; Vinarský, V.; Holubcová, Z.; Doležalová, D.; Verner, J.; Pospíšilová, Š.; Dvořák, P.; Hampl, A.W. Human Embryonic Stem Cells Are Capable of Executing G1/S Checkpoint Activation. Stem Cells. 2010, 28, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, J.A.; Hoffmann, M.J.; Bingham, G.; Gagou, M.E.; Meuth, M.; Andrews, P.W. Human Embryonic Stem Cells Fail to Activate CHK1 and Commit to Apoptosis in Response to DNA Replication Stress. Stem Cells. 2012, 30, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.K.; Jodkowska, K.; Teloni, F.; Bizard, A.H.; Zellweger, R.; Herrador, R.; Ortega, S.; Hickson, I.D.; Altmeyer, M.; Mendez, J.; et al. A short G1 phase imposes constitutive replication stress and fork remodelling in mouse embryonic stem cells. Nat. Commun. 2016, 7, 10660. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, H.; Lynch, P.J.; Chen, G.; Park, K.; Liu, Y.; Goehe, R.; Mallon, B.S.; Boehm, M.; Hursh, D.A. High Basal Levels of γH2AX in Human Induced Pluripotent Stem Cells Are Linked to Replication-Associated DNA Damage and Repair. Stem Cells 2018, 36, 1501–1513. [Google Scholar] [CrossRef]
- Krivec, N.; Couvreu de Deckersberg, E.; Lei, Y.; Al Delbany, D.; Regin, M.; Verhulst, S.; van Grunsven, L.A.; Sermon, K.; Spits, C. Gain of 1q confers an MDM4-driven growth advantage to undifferentiated and differentiating hESC while altering their differentiation capacity. Cell Death Dis. 2024, 15, 852. [Google Scholar] [CrossRef]
- Stavish, D.; Price, C.J.; Gelezauskaite, G.; Alsehli, H.; Leonhard, K.A.; Taapken, S.M.; McIntire, E.M.; Laing, O.; James, B.M.; Riley, J.J.; et al. Feeder-free culture of human pluripotent stem cells drives MDM4-mediated gain of chromosome 1q. Stem Cell Rep. 2024, 19, 1217–1232. [Google Scholar] [CrossRef]
- Vitillo, L.; Anjum, F.; Hewitt, Z.; Stavish, D.; Laing, O.; Baker, D.; Barbaric, I.; Coffey, P. The isochromosome 20q abnormality of pluripotent cells interrupts germ layer differentiation. Stem Cell Rep. 2023, 18, 782–797. [Google Scholar] [CrossRef]
- Lei, Y.; Al Delbany, D.; Krivec, N.; Regin, M.; de Deckersberg, E.C.; Janssens, C.; Ghosh, M.; Sermon, K.; Spits, C. SALL3 mediates the loss of neuroectodermal differentiation potential in human embryonic stem cells with chromosome 18q loss. Stem Cell Rep. 2024, 19, 562–578. [Google Scholar] [CrossRef]
- Al Delbany, D.; Ghosh, M.S.; Krivec, N.; Huyghebaert, A.; Regin, M.; Duong, M.C.; Lei, Y.; Sermon, K.; Olsen, C.; Spits, C. De Novo Cancer Mutations Frequently Associate with Recurrent Chromosomal Abnormalities during Long-Term Human Pluripotent Stem Cell Culture. Cells 2024, 13, 1395. [Google Scholar] [CrossRef]
- Assou, S.; Girault, N.; Plinet, M.; Bouckenheimer, J.; Sansac, C.; Combe, M.; Mianné, J.; Bourguignon, C.; Fieldes, M.; Ahmed, E.; et al. Recurrent Genetic Abnormalities in Human Pluripotent Stem Cells: Definition and Routine Detection in Culture Supernatant by Targeted Droplet Digital PCR. Stem Cell Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Baker, D.; Hirst, A.J.; Gokhale, P.J.; Juarez, M.A.; Williams, S.; Wheeler, M.; Bean, K.; Allison, T.F.; Moore, H.D.; Andrews, P.W.; et al. Detecting Genetic Mosaicism in Cultures of Human Pluripotent Stem Cells. Stem Cell Rep. 2016, 7, 998–1012. [Google Scholar] [CrossRef]
- Yoshida, S.; Kato, T.M.; Sato, Y.; Umekage, M.; Ichisaka, T.; Tsukahara, M.; Takasu, N.; Yamanaka, S. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 2023, 4, 51–66.e10. [Google Scholar] [CrossRef] [PubMed]
- Vaz, I.M.; Borgonovo, T.; Kasai-Brunswick, T.H.; Santos, D.S.D.; Mesquita, F.C.P.; Vasques, J.F.; Gubert, F.; Rebelatto, C.L.K.; Senegaglia, A.C.; Brofman, P.R.S. Chromosomal aberrations after induced pluripotent stem cells reprogramming. Genet. Mol. Biol. 2021, 44, e20200147. [Google Scholar] [CrossRef]
- Jacobs, K.; Zambelli, F.; Mertzanidou, A.; Smolders, I.; Geens, M.; Nguyen, H.T.; Barbé, L.; Sermon, K.; Spits, C. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and Genome Instability. Stem Cell Rep. 2016, 6, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Geens, M.; Mertzanidou, A.; Jacobs, K.; Heirman, C.; Breckpot, K.; Spits, C. Gain of 20q11.21 in human embryonic stem cells improves cell survival by increased expression of Bcl-xL. Mol. Hum. Reprod. 2014, 20, 168–177. [Google Scholar] [CrossRef]
- Trusler, O.; Huang, Z.; Goodwin, J.; Laslett, A.L. Cell surface markers for the identification and study of human naive pluripotent stem cells. Stem Cell Res. 2018, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shilova, N.V.; Minzhenkova, M.E.; Markova, Z.G.; Vasiliev, P.A.; Tabakov, V.Y.; Matuschenko, G.N. Tissue specificity of somatic mosaicism in constitutional trisomy of chromosome 8. Med. Genet. 2024, 23, 50–57. (In Russian) [Google Scholar]
- McIntire, E.; Taapken, S.; Nisler, B.; Leonhard, K. Recurrent Duplications of the Long Arm of Chromosome 1 in Human Pluripotent Stem Cell Lines; WiCell: Madison, WI, USA, 2012. Available online: www.wicell.org/media.acux/c1a2eba9-f127-42e7-aecc-2b23b97d0a7f (accessed on 7 August 2025).
- Halliwell, J.A.; Baker, D.; Judge, K.; Quail, M.A.; Oliver, K.; Betteridge, E.; Skelton, J.; Andrews, P.W.; Barbaric, I. Nanopore Sequencing Indicates That Tandem Amplification of Chromosome 20q11.21 in Human Pluripotent Stem Cells Is Driven by Break-Induced Replication. Stem Cells Dev. 2021, 30, 578–586. [Google Scholar] [CrossRef]
- Pasi, C.E.; Dereli-Öz, A.; Negrini, S.; Friedli, M.; Fragola, G.; Lombardo, A.; Van Houwe, G.; Naldini, L.; Casola, S.; Testa, G.; et al. Genomic instability in induced stem cells. Cell Death Differ. 2011, 18, 745–753. [Google Scholar] [CrossRef]
- Halliwell, J.A.; Frith, T.J.; Laing, O.; Price, C.J.; Bower, O.J.; Stavish, D.; Gokhale, P.J.; Hewitt, Z.; El-Khamisy, S.F.; Barbaric, I.; et al. Nucleosides Rescue Replication-Mediated Genome Instability of Human Pluripotent Stem Cells. Stem Cell Rep. 2020, 14, 1009–1017. [Google Scholar] [CrossRef]
- Kislova, A.V.; Zheglo, D.; Pozhitnova, V.O.; Sviridov, P.S.; Gadzhieva, E.P.; Voronina, E.S. Replication stress causes delayed mitotic entry and chromosome 12 fragility at the ANKS1B large neuronal gene in human induced pluripotent stem cells. Chromosome Res. 2023, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Tena, A.; Zhang, Y.; Kyritsis, N.; Devorak, A.; Zurita, J.; Wei, P.C.; Alt, F.W. Induction of recurrent break cluster genes in neural progenitor cells differentiated from embryonic stem cells in culture. Proc. Natl. Acad. Sci. USA 2020, 117, 10541–10546. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.H.; Faryabi, R.B.; Callén, E.; Wong, N.; Malhowski, A.; Chen, H.T.; Gutierrez-Cruz, G.; Sun, H.W.; McKinnon, P.; Wright, G.; et al. Identification of Early Replicating Fragile Sites that Contribute to Genome Instability. Cell 2013, 152, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Kondrateva, E.; Adilgereeva, E.; Amelina, E.; Tabakov, V.; Demchenko, A.; Ustinov, K.; Yasinovsky, M.; Voronina, E.; Lavrov, A.; Smirnikhina, S. Generation of induced pluripotent stem cell line (RCMGi001-A) from human skin fibroblasts of a cystic fibrosis patient with p.F508del mutation. Stem Cell Res. 2020, 48, 101933. [Google Scholar] [CrossRef]
- Kondrateva, E.; Grigorieva, O.; Panchuk, I.; Bychkov, I.; Zakharova, E.; Tabakov, V.; Pozhitnova, V.; Voronina, E.; Shchagina, O.; Lavrov, A.; et al. Generation of induced pluripotent stem cell line (RCMGi012-A) from fibroblasts of patient with mucopolysaccharidosis type VI. Stem Cell Res. 2023, 73, 103259. [Google Scholar] [CrossRef]
- Kondrateva, E.; Demchenko, A.; Slesarenko, Y.; Yasinovsky, M.; Amelina, E.; Tabakov, V.; Voronina, E.; Lavrov, A.; Smirnikhina, S. Derivation of iPSC line (RCMGi002-A) from dermal fibroblasts of a cystic fibrosis female patient with homozygous F508del mutation. Stem Cell Res. 2021, 53, 102251. [Google Scholar] [CrossRef]
- Kondrateva, E.; Panchuk, I.; Demchenko, A.; Grigorieva, O.; Zheglo, D.; Voronina, E.; Erofeeva, A.; Tabakov, V.; Orlova, M.; Lavrov, A.; et al. Generation of induced pluripotent stem cell line (RCMGi008-A) from human skin fibroblasts of a cystic fibrosis patient with compound heterozygous F508del/CFTRdele2.3 mutations in CFTR gene. Stem Cell Res. 2022, 63, 102854. [Google Scholar] [CrossRef]
- Panchuk, I.O.; Grigorieva, O.V.; Kondrateva, E.V.; Kurshakova, E.V.; Tabakov, V.; Bychkov, I.O.; Zakharova, E.; Orlova, M.D.; Voronina, E.S.; Pozhitnova, V.O.; et al. Generation of two iPSC lines from patient with Mucopolysaccharidosis IV B type and autosomal recessive non-syndromic hearing loss 12. Stem Cell Res. 2023, 71, 103183. [Google Scholar] [CrossRef]
- Panchuk, I.; Kondrateva, E.; Demchenko, A.; Grigorieva, O.; Erofeeva, A.; Amelina, E.; Tabakov, V.; Orlova, M.; Voronina, E.; Pozhitnova, V.; et al. Generation of two induced pluripotent stem cell lines (RCMGi005-A/B) from human skin fibroblasts of a cystic fibrosis patient with homozygous F508del mutation in CFTR gene. Stem Cell Res. 2022, 64, 102896. [Google Scholar] [CrossRef]
- Demchenko, A.; Balyasin, M.; Nazarova, A.; Grigorieva, O.; Panchuk, I.; Kondrateva, E.; Tabakov, V.; Schagina, O.; Amelina, E.; Smirnikhina, S. Human Induced Lung Organoids: A Promising Tool for Cystic Fibrosis Drug Screening. Int. J. Mol. Sci. 2025, 26, 437. [Google Scholar] [CrossRef]
- Kondrateva, E.V.; Grigorieva, O.V.; Kurshakova, E.V.; Panchuk, I.O.; Pozhitnova, V.O.; Voronina, E.S.; Tabakov, V.Y.; Nikishina, I.P.; Arsenyeva, S.V.; Matkava, V.G.; et al. Creation of Induced Pluripotent Stem Cells RCMGi014-A Using Reprogramming of Urine Cells of a Patient with Fibrodysplasia Ossificans Progressiva Associated with Heterozygous Mutation in the ACVR1 Gene. Russ. J. Dev. Biol. 2024, 55, 34–38. [Google Scholar] [CrossRef]
- Kondrateva, E.; Demchenko, A.; Slesarenko, Y.; Pozhitnova, V.; Yasinovsky, M.; Amelina, E.; Tabakov, V.; Voronina, E.; Lavrov, A.; Smirnikhina, S. Generation of two induced pluripotent stem cell lines (RCMGi004-A and -B) from human skin fibroblasts of a cystic fibrosis patient with compound heterozygous F508del/W1282X mutations in CFTR gene. Stem Cell Res. 2021, 52, 102232. [Google Scholar] [CrossRef]
- Kondrateva, E.; Grigorieva, O.; Kurshakova, E.; Panchuk, I.; Pozhitnova, V.; Voronina, E.; Tabakov, V.; Orlova, M.; Lavrov, A.; Smirnikhina, S.; et al. Generation of induced pluripotent stem cell line (RCMGi009-A) from urine cells of patient with fibrodysplasia ossificans progressiva. Stem Cell Res. 2023, 70, 103133. [Google Scholar] [CrossRef]
- Mikhailova, V.; Kondratyev, N.; Alfimova, M.; Kaleda, V.; Lezheiko, T.; Ublinsky, M.; Ushakov, V.; Lebedeva, I.; Galiakberova, A.; Artyuhov, A.; et al. The SLC6A1 Mutation Schizophrenia case—A Comprehensive Case Study With iPSC Generation. Eur. Psychiatr. 2024, 67, S764–S765. [Google Scholar] [CrossRef]
- Alsalloum, A.; Mityaeva, O.; Kegeles, E.; Khavina, E.; Volchkov, P. Generation of two human induced pluripotent stem cell lines (ABi001-A and ABi002-A) from cone dystrophy with supernormal rod response patients caused by KCNV2 mutation. Stem Cell Res. 2023, 69, 103099. [Google Scholar] [CrossRef] [PubMed]
- Gornostal, E.; Alsalloum, A.; Mityaeva, O.; Volchkov, P. Generation of induced pluripotent stem line (MIPTi001-A) derived from patient with X-linked adrenoleukodystrophy (X-ALD). Stem Cell Res. 2024, 74, 103298. [Google Scholar] [CrossRef] [PubMed]
| Diagnoses | No of Donors | No of Lines |
|---|---|---|
| cystic fibrosis | 8 | 16 |
| fibrodysplasia ossificans progressiva | 3 | 6 |
| glycogen storage disease I | 2 | 3 |
| multiple endocrine neoplasia 1 | 2 | 6 |
| mucopolysaccharidosis type IVb | 1 | 3 |
| Maroteaux–Lamy syndrome | 1 | 2 |
| Leber’s hereditary optic neuropathy | 1 | 3 |
| Duchenne muscular dystrophy | 1 | 3 |
| Cone dystrophy with supernormal rod response | 2 | 2 |
| maturity-onset diabetes of the young type 3 | 1 | 2 |
| maturity-onset diabetes of the young type 10 | 1 | 1 |
| maturity-onset diabetes of the young type 12 | 1 | 2 |
| X-linked adrenoleukodystrophy | 1 | 1 |
| lysosomal acid lipase deficiency | 1 | 3 |
| schizophrenia (de novo SLC6A1 variant) | 1 | 1 |
| Subtotal (diseased) | 27 (82%) | 54 (83%) |
| Healthy/unaffected carrier | 6 | 11 |
| Total | 33 | 65 |
| Aberration | Culture-Acquired | Cell Lines | Recurrent Rearrangement Type | Percentage in Present Study |
|---|---|---|---|---|
| trisomy 20 | nd | P4L5 | chr20 gain | 8.6% of all tests, 38.5% of unique aberrant lines |
| trisomy 20 | yes | P12L3 | ||
| trisomy 20 | nd | P13L10 | ||
| i(20q) | nd | MNA cl3 | ||
| i(20q) | nd | MNA e67 | ||
| psu idic(20) | yes | P16L2 * | ||
| 1q duplication | 1q gain | 7.2% of all tests, 30.8% of unique aberrant lines | ||
| dup(1)(q23q44) | nd | P5Lmix | ||
| dup(1)(q25q44) * | nd | P16L2 ** | ||
| 1q translocation-duplication | ||||
| der(18)t(1;18)(q23;q21.2) *** | nd | HAS cl38 | 1q gain, 18q loss | |
| der(18)t(1;18)(q23;q21.2) *** | nd | HAS cl51 | ||
| der(1)t(1;15)(p11;q11.2) | yes | MAK-F | 1q gain | |
| trisomy 8 | nd | P19L7 | chr8 gain | 2.9% of all tests, 15.4% of unique aberrant lines |
| trisomy 8 | nd | P22L1 | ||
| balanced translocation | non-recurrent | 5.8% of all tests, 23.1% of unique aberrant lines | ||
| t(1;8)(p21;q22) | nd | KTV cl3 | ||
| t(1;8)(p21;q22) | nd | KTV cl11 | ||
| dup(2)(q32;q33) | nd | P8L1 | ||
| multiple chromatide breaks (chtb) | nd | P5L5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheglo, D.; Pozhitnova, V.O.; Kislova, A.V.; Markova, Z.G.; Kiselev, D.; Sviridov, P.S.; Sviridova, V.; Gumerova, L.I.; Smirnikhina, S.A.; Alsalloum, A.; et al. Chromosomal Aberrations in Induced Pluripotent Stem Cells: Identification of Breakpoints in the Large DCC Gene and HIST2 Histone Gene Cluster. Int. J. Mol. Sci. 2025, 26, 7728. https://doi.org/10.3390/ijms26167728
Zheglo D, Pozhitnova VO, Kislova AV, Markova ZG, Kiselev D, Sviridov PS, Sviridova V, Gumerova LI, Smirnikhina SA, Alsalloum A, et al. Chromosomal Aberrations in Induced Pluripotent Stem Cells: Identification of Breakpoints in the Large DCC Gene and HIST2 Histone Gene Cluster. International Journal of Molecular Sciences. 2025; 26(16):7728. https://doi.org/10.3390/ijms26167728
Chicago/Turabian StyleZheglo, Diana, Victoria O. Pozhitnova, Anastasiia V. Kislova, Zhanna G. Markova, Danila Kiselev, Philipp S. Sviridov, Valeria Sviridova, Lyajsan I. Gumerova, Svetlana A. Smirnikhina, Almaqdad Alsalloum, and et al. 2025. "Chromosomal Aberrations in Induced Pluripotent Stem Cells: Identification of Breakpoints in the Large DCC Gene and HIST2 Histone Gene Cluster" International Journal of Molecular Sciences 26, no. 16: 7728. https://doi.org/10.3390/ijms26167728
APA StyleZheglo, D., Pozhitnova, V. O., Kislova, A. V., Markova, Z. G., Kiselev, D., Sviridov, P. S., Sviridova, V., Gumerova, L. I., Smirnikhina, S. A., Alsalloum, A., Pylina, S. V., Kutsev, S. I., & Voronina, E. S. (2025). Chromosomal Aberrations in Induced Pluripotent Stem Cells: Identification of Breakpoints in the Large DCC Gene and HIST2 Histone Gene Cluster. International Journal of Molecular Sciences, 26(16), 7728. https://doi.org/10.3390/ijms26167728








