Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review
Abstract
1. Introduction
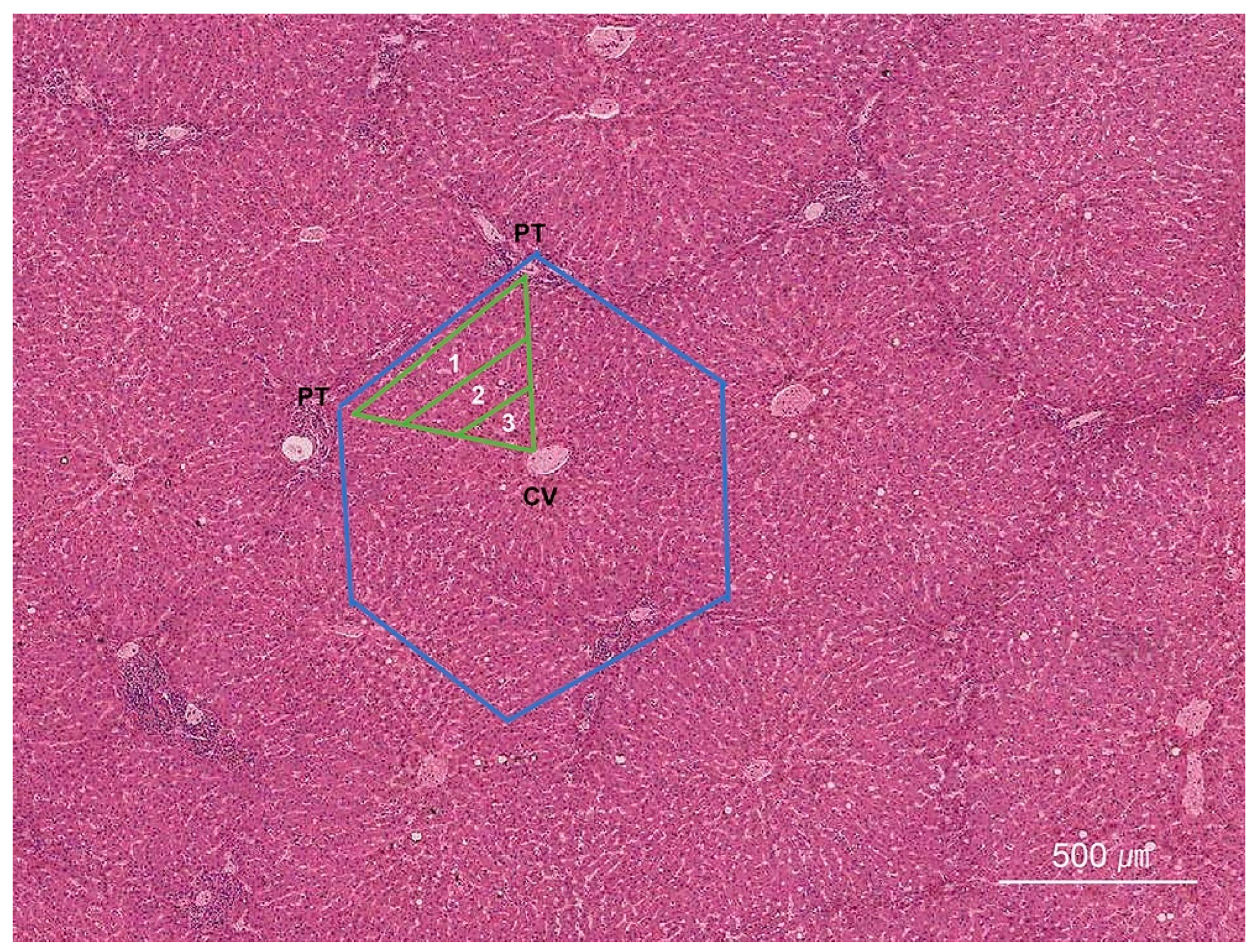
2. Normal Histology of the Liver
3. Types of Liver Biopsy Specimens
4. Handling of Liver Biopsy Specimens
5. Routine and Special Stains for Liver Biopsy Specimens
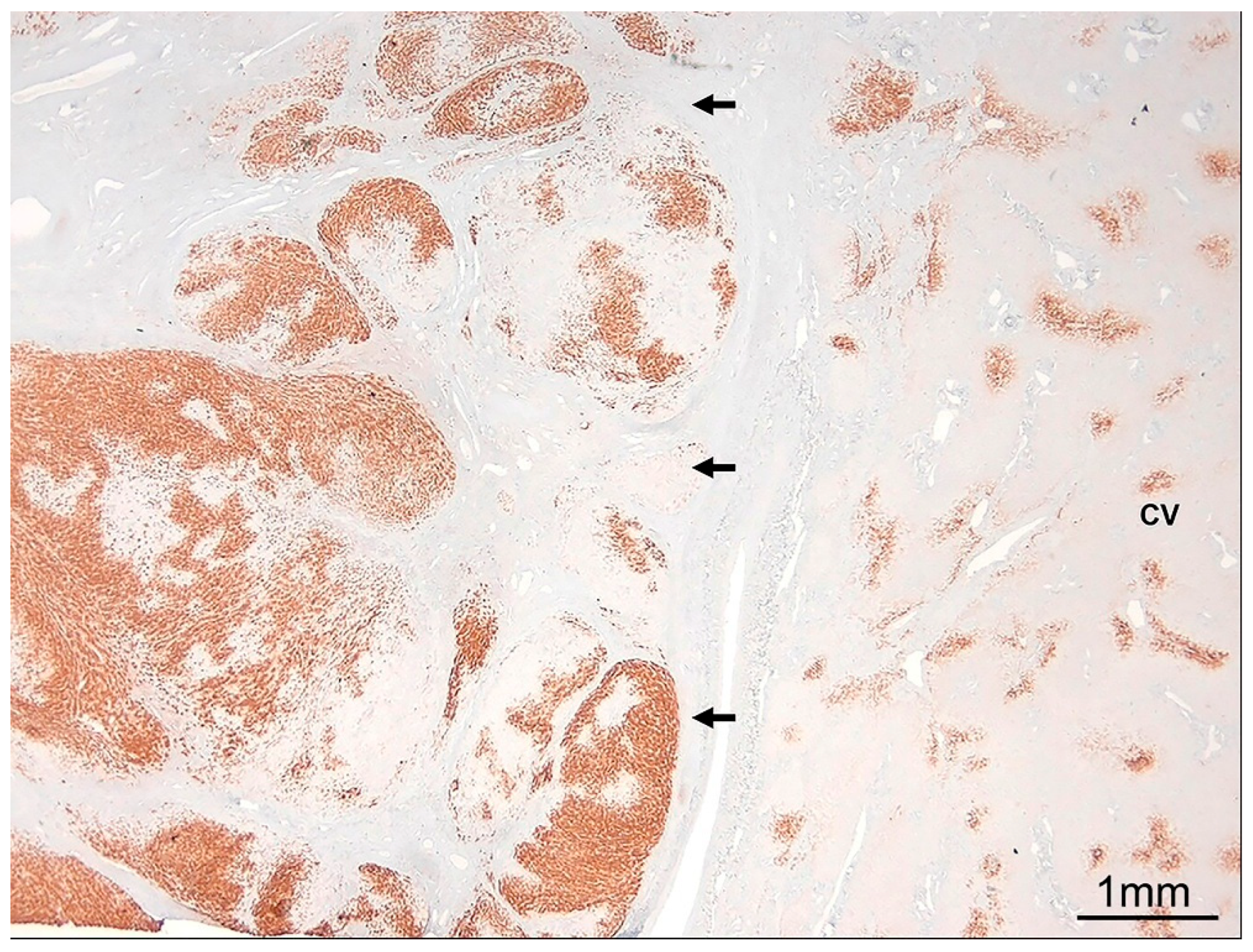
6. Immunohistochemistry
6.1. Non-Neoplastic Liver Diseases
6.2. Neoplastic Liver Diseases
7. Electron Microscopy
8. Molecular Analysis
9. Predominant Injury Pattern
9.1. Hepatitic Pattern
9.1.1. Acute and Chronic Hepatitis
9.1.2. Lobular Hepatitis
9.1.3. Neutrophilic Inflammation in the Lobules
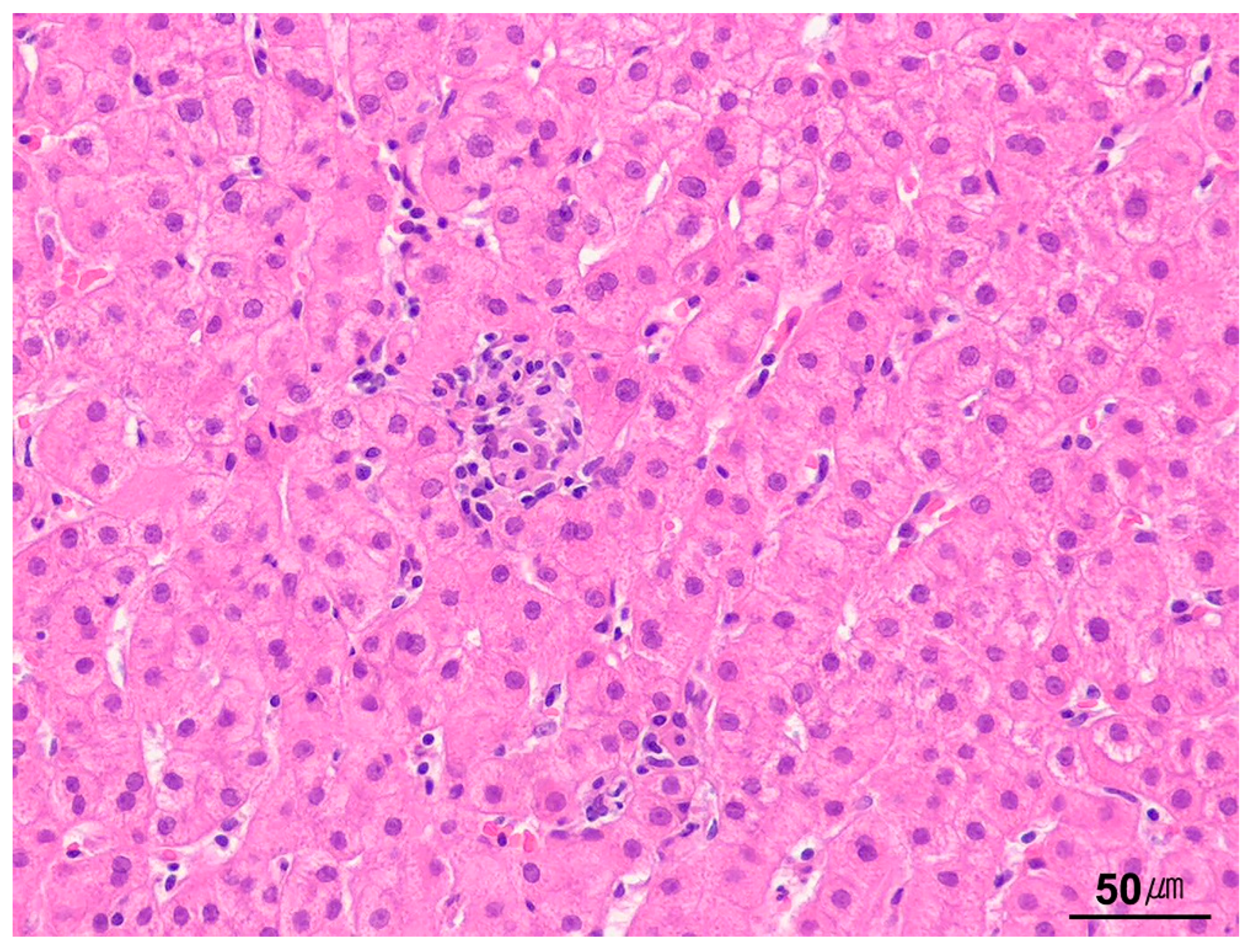
9.1.4. Portal Tract Inflammation
9.1.5. Interface Activity
9.1.6. Grading Hepatitis Activity
9.1.7. Resolving Hepatitis
9.2. Necrotic Pattern
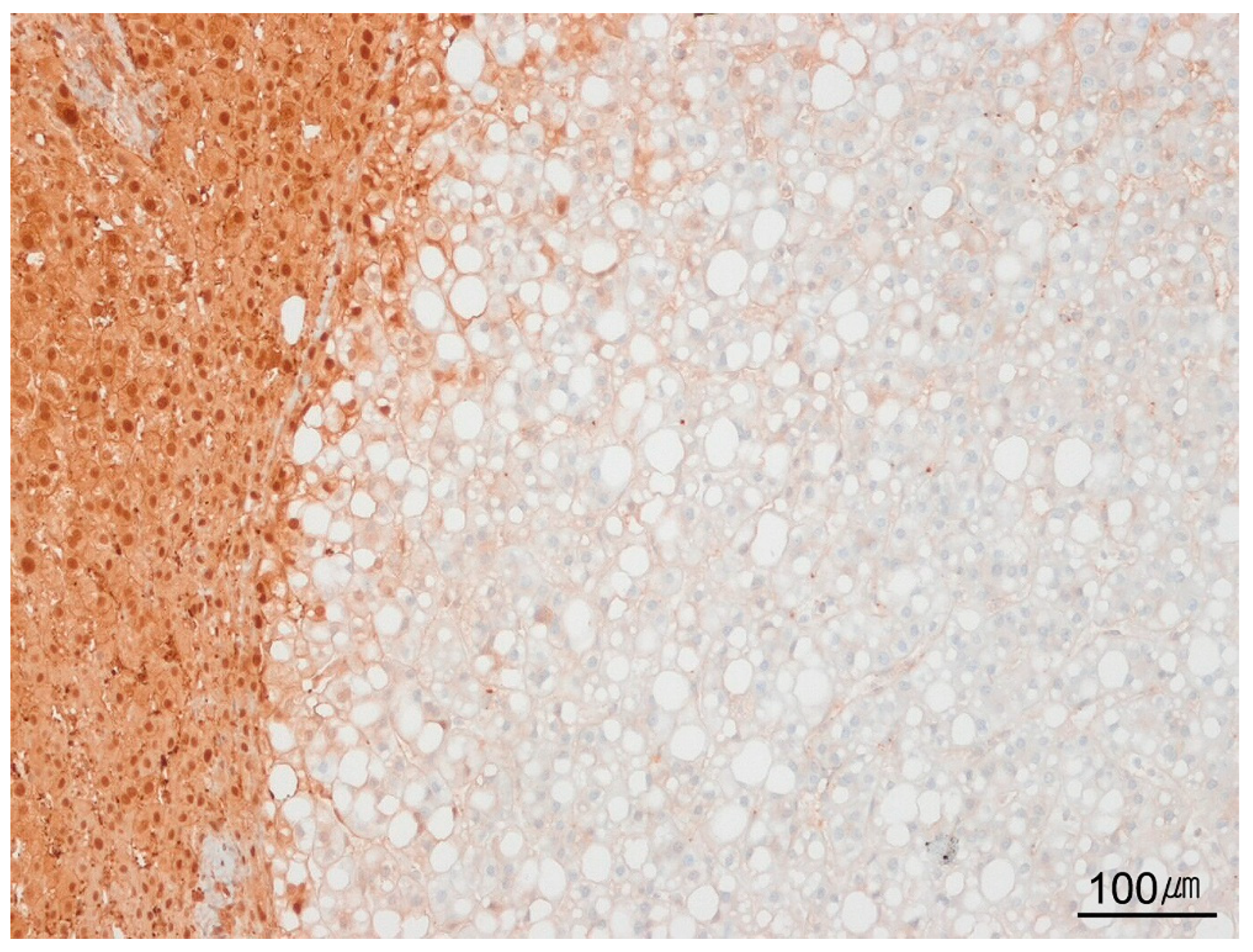
9.3. Steatotic Pattern
9.4. Cytoplasmic Changes and Inclusions in Hepatocytes
9.4.1. Glycogen Accumulation
9.4.2. Ballooned Hepatocytes
9.4.3. Giant-Cell Transformation
9.4.4. Hepatocyte Inclusions
9.5. Biliary/Cholestatic Patterns
9.5.1. Biliary Obstructive Pattern
9.5.2. Ductular Reaction
9.5.3. Ductopenia
9.5.4. Bland Lobular Cholestasis
9.5.5. Ascending Cholangitis
9.6. Vascular (Circulatory) Disease Patterns
9.6.1. Idiopathic Noncirrhotic Portal Hypertension
9.6.2. Veno-Occlusive Disease
9.6.3. Vascular Outflow Obstruction Disease
9.6.4. Peliosis Hepatis
9.7. Depositional/Storage Disorders
9.7.1. Iron
9.7.2. Copper
9.7.3. Amyloid
9.8. Granuloma
9.9. Fibrosis
9.9.1. Pericellular Fibrosis
9.9.2. Central Vein Fibrosis
9.9.3. Portal/Periportal Fibrosis
9.9.4. Bridging Fibrosis
9.9.5. Cirrhosis
10. Molecular Pathology in Liver Biopsies
11. Diagnostic Approach to Liver Pathology
11.1. Clinical and Laboratory Information
11.2. Pathological Diagnostic Approach
12. Future Perspectives
13. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amyloid A |
| AIH | Autoimmune hepatitis |
| AL | Amyloid light chain |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| B-HCA | β-catenin-activated hepatocellular adenoma |
| BI-HCA | β-catenin-activated inflammatory hepatocellular adenoma |
| BRIC | Benign recurrent intrahepatic cholestasis |
| CCA | Cholangiocarcinoma |
| CEA | Carcinoembryonic antigen |
| CH HCC | Chromophobe hepatocellular carcinoma |
| CK | Cytokeratin |
| CMV | Cytomegalovirus |
| CRP | C-reactive protein |
| DILI | Drug-induced liver injury |
| EBV | Epstein–Barr virus |
| EUS | Endoscopic ultrasound |
| FL | Fibrolamellar |
| FNA | Fine-needle aspiration |
| GATA-3 | GATA-binding protein 3 |
| H&E | Hematoxylin and eosin |
| HBcAg | Hepatitis B core antigen |
| HBsAg | Hepatitis B surface antigen |
| HCA | Hepatocellular adenoma |
| H-HCA | Hepatocyte nuclear factor 1α-inactivated HCA |
| HCC | Hepatocellular carcinoma |
| ICCA | Intrahepatic cholangiocarcinoma |
| IHC | Immunohistochemistry |
| I-HCA | Inflammatory hepatocellular adenoma |
| INPH | Idiopathic noncirrhotic portal hypertension |
| ICP | Intrahepatic cholestasis of pregnancy |
| ISH | In situ hybridization |
| LFABP | Liver-fatty-acid-binding protein |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| METAVIR | Meta-analysis of histological data in viral hepatitis |
| MM HCC | Macrotrabecular massive hepatocellular carcinoma |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NGS | Next-generation sequencing |
| NRH | Nodular regenerative hyperplasia |
| PAS | Periodic acid–Schiff |
| PAS-D | Periodic acid–Schiff with diastase digestion |
| PAX8 | Paired box gene 8 |
| PBC | Primary biliary cholangitis |
| PCR | Polymerase chain reaction |
| PFIC | Progressive familial intrahepatic cholestasis |
| PSA | Prostate-specific antigen |
| PSC | Primary sclerosing cholangitis |
| SAA | Serum amyloid A |
| SATB2 | Special AT-rich sequence-binding protein 2 |
| SH-HCA | Sonic hedgehog-activated hepatocellular adenoma |
| SOS | Sinusoidal obstructive syndrome |
| TEM | Transmission electron microscopy |
| TTF-1 | Thyroid transcription factor-1 |
| Wnt | Wingless/Integrated |
References
- Gill, R.M.; Kakar, S. Liver and gallbladder. In Robbins & Cotran Pathologic Basis of Disease, 10th ed.; Kumar, V., Abbas, A.K., Aster, J.C., Eds.; Elsevier: Philadelphia, PA, USA, 2021; pp. 823–831. [Google Scholar]
- Lee, R.G. General principles. In Diagnostic Liver Pathology, 1st ed.; Lee, R.G., Ed.; Mosby: St. Louis, MO, USA, 1994; pp. 1–19. [Google Scholar]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef]
- Crawford, J.M. Evidence-based interpretation of liver biopsies. Lab. Investig. 2006, 86, 326–334. [Google Scholar] [CrossRef]
- Menghini, G. One-second needle biopsy of the liver. Gastroenterology 1958, 35, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.E.; Czaja, A.J.; Rakela, J.; Ludwig, J. The nature of unexplained chronic aminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology 1989, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.M.; Baker, A.L.; Riddell, R.H.; Rochman, H.; Boyer, J.L. Nonalcoholic liver disease. Overlooked causes of liver injury in patients with heavy alcohol consumption. Am. J. Med. 1979, 66, 429–434. [Google Scholar] [CrossRef]
- Van Ness, M.M.; Diehl, A.M. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann. Intern. Med. 1989, 111, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Lok, A.S. Use of Liver Imaging and Biopsy in Clinical Practice. N. Engl. J. Med. 2017, 377, 756–768. [Google Scholar] [CrossRef]
- Siegel, C.A.; Silas, A.M.; Suriawinata, A.A.; van Leeuwen, D.J. Liver biopsy 2005: When and how? Cleve. Clin. J. Med. 2005, 72, 199–224, 206, 208 passim. [Google Scholar] [CrossRef]
- Rockey, D.C. Liver Biopsy—An Essential LiFT in the Diagnosis of Unexplained Liver Disease. Dig. Dis. Sci. 2025, 70, 1274–1276. [Google Scholar] [CrossRef]
- Shi, R.; Yang, F.; Wu, H.; Liu, Y. The Diagnostic Value of Liver Biopsy for Unexplained Liver Dysfunction: A Retrospective Study. J. Multidiscip. Healthc. 2024, 17, 2399–2407. [Google Scholar] [CrossRef]
- Torbenson, M.; Schirmacher, P. Liver cancer biopsy-back to the future? Hepatology 2015, 61, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.; Bruix, J. Biopsy for liver cancer: How to balance research needs with evidence-based clinical practice. Hepatology 2015, 61, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Theodossi, A.; Skene, A.M.; Portmann, B.; Knill-Jones, R.P.; Patrick, R.S.; Tate, R.A.; Kealey, W.; Jarvis, K.J.; O’Brian, D.J.; Williams, R. Observer variation in assessment of liver biopsies including analysis by kappa statistics. Gastroenterology 1980, 79, 232–241. [Google Scholar] [CrossRef]
- Suriawinata, A.A.; Thung, S.N. Approach to liver specimens, normal, minor, and structural alterations. In Liver Pathology. An Atlas and Concise Guide, 1st ed.; Suriawinata, A.A., Thung, S.N., Eds.; Demos Medical: New York, NY, USA, 2011; pp. 1–29. [Google Scholar]
- Gerber, M.A.; Thung, S.N. Histology of the liver. Am. J. Surg. Pathol. 1987, 11, 709–722. [Google Scholar] [CrossRef]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.M.; Theise, N.D. Rappaport, Glisson, Hering, and Mall-Champions of Liver Microanatomy: Microscopic and Ultramicroscopic Anatomy of the Liver into the Modern Age. Clin. Liver Dis. 2021, 18, 76–92. [Google Scholar] [CrossRef]
- Kudryavtsev, B.N.; Kudryavtseva, M.V.; Sakuta, G.A.; Stein, G.I. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch. B Cell. Pathol. Incl. Mol. Pathol. 1993, 64, 387–393. [Google Scholar] [CrossRef]
- Suriawinata, A.A.; Thung, S.N. Liver. In Histology for pathologists, 4th ed.; Mills, S.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 733–756. [Google Scholar]
- Petrelli, M.; Scheuer, P.J. Variation in subcapsular liver structure and its significance in the interpretation of wedge biopsies. J. Clin. Pathol. 1967, 20, 743–748. [Google Scholar] [CrossRef]
- Colombo, M.; Del Ninno, E.; de Franchis, R.; De Fazio, C.; Festorazzi, S.; Ronchi, G.; Tommasini, M.A. Ultrasound-assisted percutaneous liver biopsy: Superiority of the Tru-Cut over the Menghini needle for diagnosis of cirrhosis. Gastroenterology 1988, 95, 487–489. [Google Scholar] [CrossRef]
- Piccinino, F.; Sagnelli, E.; Pasquale, G.; Giusti, G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J. Hepatol. 1986, 2, 165–173. [Google Scholar] [CrossRef]
- Abdi, W.; Millan, J.C.; Mezey, E. Sampling variability on percutaneous liver biopsy. Arch. Intern. Med. 1979, 139, 667–669. [Google Scholar] [CrossRef]
- Babb, R.R.; Jackman, R.J. Needle biopsy of the liver. A critique of four currently available methods. West. J. Med. 1989, 150, 39–42. [Google Scholar] [PubMed]
- Maharaj, B.; Maharaj, R.J.; Leary, W.P.; Cooppan, R.M.; Naran, A.D.; Pirie, D.; Pudifin, D.J. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986, 1, 523–525. [Google Scholar] [CrossRef] [PubMed]
- McAfee, J.H.; Keeffe, E.B.; Lee, R.G.; Rösch, J. Transjugular liver biopsy. Hepatology 1992, 15, 726–732. [Google Scholar] [CrossRef]
- Glenthøj, A.; Sehested, M.; Torp-Pedersen, S. Diagnostic reliability of histological and cytological fine needle biopsies from focal liver lesions. Histopathology 1989, 15, 375–383. [Google Scholar] [CrossRef]
- Hajdu, S.I.; D’Ambrosio, F.G.; Fields, V.; Lightdale, C.J. Aspiration and brush cytology of the liver. Semin. Diagn. Pathol. 1986, 3, 227–238. [Google Scholar] [PubMed]
- Perry, M.D.; Johnston, W.W. Needle biopsy of the liver for the diagnosis of nonneoplastic liver diseases. Acta Cytol. 1985, 29, 385–390. [Google Scholar]
- Crawford, J.M. Liver tissue processing and normal histology. In Odze and Goldman Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 4th ed.; Odze, R.D., Goldblum, J.R., Eds.; Elsevier: Philadelphia, PA, USA, 2023; pp. 1352–1368. [Google Scholar]
- Torbenson, M.S. Liver. In Surgical Pathology Dissection. An Illustrated Guide, 2nd ed.; Westra, W.H., Hruban, R.H., Phelps, T.H., Isacson, C., Eds.; Springer: New York, NY, USA, 2003; pp. 76–81. [Google Scholar]
- Compton, C.C.; Robb, J.A.; Anderson, M.W.; Berry, A.B.; Birdsong, G.G.; Bloom, K.J.; Branton, P.A.; Crothers, J.W.; Cushman-Vokoun, A.M.; Hicks, D.G.; et al. Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch. Pathol. Lab. Med. 2019, 143, 1346–1363. [Google Scholar] [CrossRef]
- Torbenson, M.S. General approach to biopsy specimens. In Biopsy Interpretation of the Liver, 4th ed.; Torbenson, M.S., Ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020; pp. 1–12. [Google Scholar]
- Lefkowitch, J.H. Special stains in diagnostic liver pathology. Semin. Diagn. Pathol. 2006, 23, 190–198. [Google Scholar] [CrossRef]
- Jungermann, K.; Kietzmann, T. Oxygen: Modulator of metabolic zonation and disease of the liver. Hepatology 2000, 31, 255–260. [Google Scholar] [CrossRef]
- Burke, Z.D.; Reed, K.R.; Phesse, T.J.; Sansom, O.J.; Clarke, A.R.; Tosh, D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 2009, 136, 2316–2324.e3. [Google Scholar] [CrossRef]
- Clark, I.; Torbenson, M.S. Immunohistochemistry and Special Stains in Medical Liver Pathology. Adv. Anat. Pathol. 2017, 24, 99–109. [Google Scholar] [CrossRef]
- Torbenson, M.; Ng, I.O.; Park, Y.N.; Roncalli, M.; Sakamoto, M. Hepatocellular carcinoma. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 229–239. [Google Scholar]
- Kim, H.; Choi, G.H.; Na, D.C.; Ahn, E.Y.; Kim, G.I.; Lee, J.E.; Cho, J.Y.; Yoo, J.E.; Choi, J.S.; Park, Y.N. Human hepatocellular carcinomas with “Stemness”-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology 2011, 54, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Durnez, A.; Verslype, C.; Nevens, F.; Fevery, J.; Aerts, R.; Pirenne, J.; Lesaffre, E.; Libbrecht, L.; Desmet, V.; Roskams, T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006, 49, 138–151. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Kakar, S.; Nault, I.C. Focal nodular hyperplasia of the liver. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 221–223. [Google Scholar]
- Bioulac-Sage, P.; Kakar, S.; Nault, J.C. Hepatocellular adenoma. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 224–228. [Google Scholar]
- Choi, J.H.; Thung, S.N. Pathology and diagnostic approaches to well-differentiated hepatocellular lesions: A narrative review. J. Yeungnam Med. Sci. 2025, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, S.E.; Kakar, S. Evaluating Liver Biopsies with Well-Differentiated Hepatocellular Lesions. Surg. Pathol. Clin. 2023, 16, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Wu, E.; Weiss, L.M. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod. Pathol. 2000, 13, 962–972. [Google Scholar] [CrossRef]
- Selves, J.; Long-Mira, E.; Mathieu, M.C.; Rochaix, P.; Ilié, M. Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. Cancers 2018, 10, 108. [Google Scholar] [CrossRef]
- Conner, J.R.; Hornick, J.L. Metastatic carcinoma of unknown primary: Diagnostic approach using immunohistochemistry. Adv. Anat. Pathol. 2015, 22, 149–167. [Google Scholar] [CrossRef]
- Spycher, M.A. Electron microscopy: A method for the diagnosis of inherited metabolic storage diseases. Electron microscopy in diagnosis. Pathol. Res. Pract. 1980, 167, 118–135. [Google Scholar] [CrossRef]
- Pennelli, N.; Scaravilli, F.; Zacchello, F. The morphogenesis of Gaucher cells investigated by electron microscopy. Blood 1969, 34, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E. Detection and identification of viruses by electron microscopy. J. Electron. Microsc. Tech. 1986, 4, 265–301. [Google Scholar] [CrossRef]
- Phillips, M.J.; Blendis, L.M.; Poucell, S.; Patterson, J.; Petric, M.; Roberts, E.; Levy, G.A.; Superina, R.A.; Greig, P.D.; Cameron, R.; et al. Syncytial giant-cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course, and paramyxoviral features. N. Engl. J. Med. 1991, 324, 455–460. [Google Scholar] [CrossRef]
- Poucell, S.; Ireton, J.; Valencia-Mayoral, P.; Downar, E.; Larratt, L.; Patterson, J.; Blendis, L.; Phillips, M.J. Amiodarone-associated phospholipidosis and fibrosis of the liver. Light, immunohistochemical, and electron microscopic studies. Gastroenterology 1984, 86, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Lloreta-Trull, J.; Serrano, S. The current role of electron microscopy in the diagnosis of epithelial and epithelioid tumors. Semin. Diagn. Pathol. 2003, 20, 46–59. [Google Scholar] [PubMed]
- Tickoo, S.K.; Zee, S.Y.; Obiekwe, S.; Xiao, H.; Koea, J.; Robiou, C.; Blumgart, L.H.; Jarnagin, W.; Ladanyi, M.; Klimstra, D.S. Combined hepatocellular-cholangiocarcinoma: A histopathologic, immunohistochemical, and in situ hybridization study. Am. J. Surg. Pathol. 2002, 26, 989–997. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Erickson, L.A.; Burgart, L.J.; Lloyd, R.V. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am. J. Surg. Pathol. 2000, 24, 177–182. [Google Scholar] [CrossRef]
- Saito, K.; Sullivan, D.; Haruna, Y.; Theise, N.D.; Thung, S.N.; Gerber, M.A. Detection of hepatitis C virus RNA sequences in hepatocellular carcinoma and its precursors by microdissection polymerase chain reaction. Arch. Pathol. Lab. Med. 1997, 121, 400–403. [Google Scholar]
- Hoshida, Y.; Villanueva, A.; Kobayashi, M.; Peix, J.; Chiang, D.Y.; Camargo, A.; Gupta, S.; Moore, J.; Wrobel, M.J.; Lerner, J.; et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 1995–2004. [Google Scholar] [CrossRef]
- Asselah, T.; Bièche, I.; Paradis, V.; Bedossa, P.; Vidaud, M.; Marcellin, P. Genetics, genomics, and proteomics: Implications for the diagnosis and the treatment of chronic hepatitis C. Semin. Liver. Dis. 2007, 27, 13–27. [Google Scholar] [CrossRef]
- Feng, G.; Li, X.P.; Niu, C.Y.; Liu, M.L.; Yan, Q.Q.; Fan, L.P.; Li, Y.; Zhang, K.L.; Gao, J.; Qian, M.R.; et al. Bioinformatics analysis reveals novel core genes associated with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Gene 2020, 742, 144549. [Google Scholar] [CrossRef]
- Chen, D.B.; Xie, X.W.; Zhao, Y.J.; Wang, X.Y.; Liao, W.J.; Chen, P.; Deng, K.J.; Fei, R.; Qin, W.Y.; Wang, J.H.; et al. RFX5 promotes the progression of hepatocellular carcinoma through transcriptional activation of KDM4A. Sci. Rep. 2020, 10, 14538. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Andersen, J.B. Next-generation sequencing: Application in liver cancer-past, present and future? Biology 2012, 1, 383–394. [Google Scholar] [CrossRef]
- Torbenson, M.; Karkar, S.; Washington, K. Core pathology patterns in medical liver specimens. In Non-Neoplastic Diseases of the Liver: AFIP Atlas of Tumor Pathology; Series 5, Fascicle 10; American Registry of Pathology: Washington, DC, USA, 2022; pp. 1–59. [Google Scholar]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- MacDonald, G.A.; Greenson, J.K.; DelBuono, E.A.; Grady, W.M.; Merion, R.M.; Frank, T.S.; Lucey, M.R.; Appelman, H.D. Mini-microabscess syndrome in liver transplant recipients. Hepatology 1997, 26, 192–197. [Google Scholar] [CrossRef]
- Lamps, L.W.; Pinson, C.W.; Raiford, D.S.; Shyr, Y.; Scott, M.A.; Washington, M.K. The significance of microabscesses in liver transplant biopsies: A clinicopathological study. Hepatology 1998, 28, 1532–1537. [Google Scholar] [CrossRef]
- Daniels, J.A.; Torbenson, M.; Anders, R.A.; Boitnott, J.K. Immunostaining of plasma cells in primary biliary cirrhosis. Am. J. Clin. Pathol. 2009, 131, 243–249. [Google Scholar] [CrossRef]
- Cabibi, D.; Tarantino, G.; Barbaria, F.; Campione, M.; Craxì, A.; Di Marco, V. Intrahepatic IgG/IgM plasma cells ratio helps in classifying autoimmune liver diseases. Dig. Liver Dis. 2010, 42, 585–592. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994, 20, 15–20. [Google Scholar] [CrossRef]
- Zatloukal, K.; French, S.W.; Stumptner, C.; Strnad, P.; Harada, M.; Toivola, D.M.; Cadrin, M.; Omary, M.B. From Mallory to Mallory-Denk bodies: What, how and why? Exp. Cell Res. 2007, 313, 2033–2049. [Google Scholar] [CrossRef]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef]
- Mathai, A.M.; Alexander, J.; Kuo, F.Y.; Torbenson, M.; Swanson, P.E.; Yeh, M.M. Type II ground-glass hepatocytes as a marker of hepatocellular carcinoma in chronic hepatitis B. Hum. Pathol. 2013, 44, 1665–1671. [Google Scholar] [CrossRef]
- Gouw, A.S.; Clouston, A.D.; Theise, N.D. Ductular reactions in human liver: Diversity at the interface. Hepatology 2011, 54, 1853–1863. [Google Scholar] [CrossRef]
- Saxena, R.; Theise, N. Canals of Hering: Recent insights and current knowledge. Semin. Liver Dis. 2004, 24, 43–48. [Google Scholar] [CrossRef]
- Mavila, N.; Siraganahalli Eshwaraiah, M.; Kennedy, J. Ductular Reactions in Liver Injury, Regeneration, and Disease Progression-An Overview. Cells 2024, 13, 579. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kakar, S. Histological patterns in drug-induced liver disease. J. Clin. Pathol. 2009, 62, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ourfali, M.B.; Hirsch, D.; Scranton, M.; Jabbour, T.E. Post-transplant liver biopsies: A concise and practical approach for beginners. J. Pathol. Transl. Med. 2025, 59, 1–10. [Google Scholar] [CrossRef]
- Park, B.C.; Park, S.M.; Choi, E.Y.; Chae, H.B.; Yoon, S.J.; Sung, R.; Lee, S.K. A case of idiopathic adulthood ductopenia. Korean J. Intern. Med. 2009, 24, 270–273. [Google Scholar] [CrossRef]
- Fiel, M.I.; Schiano, T.D. Idiopathic noncirrhotic portal hypertension. Semin. Diagn. Pathol. 2019, 36, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.; Alves, V.A.F.; Balabaud, C.; Bathal, P.S.; Bioulac-Sage, P.; Colombari, R.; Crawford, J.M.; Dhillon, A.P.; Ferrell, L.D.; Gill, R.M.; et al. Histology of portal vascular changes associated with idiopathic non-cirrhotic portal hypertension: Nomenclature and definition. Histopathology 2019, 74, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Verheij, J.; Schouten, J.N.; Komuta, M.; Nevens, F.; Hansen, B.E.; Janssen, H.L.; Roskams, T. Histological features in western patients with idiopathic non-cirrhotic portal hypertension. Histopathology 2013, 62, 1083–1091. [Google Scholar] [CrossRef]
- Goekkurt, E.; Stoehlmacher, J.; Stueber, C.; Wolschke, C.; Eiermann, T.; Iacobelli, S.; Zander, A.R.; Ehninger, G.; Kröger, N. Pharmacogenetic analysis of liver toxicity after busulfan/cyclophosphamide-based allogeneic hematopoietic stem cell transplantation. Anticancer Res. 2007, 27, 4377–4380. [Google Scholar] [PubMed]
- DeLeve, L.D.; Valla, D.C.; Garcia-Tsao, G. Vascular disorders of the liver. Hepatology 2009, 49, 1729–1764. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019, 25, 1271–1280. [Google Scholar] [CrossRef]
- Kakar, S.; Kamath, P.S.; Burgart, L.J. Sinusoidal dilatation and congestion in liver biopsy: Is it always due to venous outflow impairment? Arch. Pathol. Lab. Med. 2004, 128, 901–904. [Google Scholar] [CrossRef]
- Tsokos, M.; Erbersdobler, A. Pathology of peliosis. Forensic Sci. Int. 2005, 149, 25–33. [Google Scholar] [CrossRef]
- Berzigotti, A.; Magalotti, D.; Zappoli, P.; Rossi, C.; Callea, F.; Zoli, M. Peliosis hepatis as an early histological finding in idiopathic portal hypertension: A case report. World J. Gastroenterol. 2006, 12, 3612–3615. [Google Scholar] [CrossRef]
- Yu, C.Y.; Chang, L.C.; Chen, L.W.; Lee, T.S.; Chien, R.N.; Hsieh, M.F.; Chiang, K.C. Peliosis hepatis complicated by portal hypertension following renal transplantation. World J. Gastroenterol. 2014, 20, 2420–2425. [Google Scholar] [CrossRef]
- Biswas, S.; Gogna, S.; Patel, P. A fatal case of intra-abdominal hemorrhage following diagnostic blind percutaneous liver biopsy in a patient with peliosis hepatis. Gastroenterol. Res. 2017, 10, 318–321. [Google Scholar] [CrossRef]
- Torbenson, M. Iron in the liver: A review for surgical pathologists. Adv. Anat. Pathol. 2011, 18, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Liggi, M.; Mais, C.; Demurtas, M.; Sorbello, O.; Demelia, E.; Civolani, A.; Demelia, L. Uneven distribution of hepatic copper concentration and diagnostic value of double-sample biopsy in Wilson’s disease. Scand. J. Gastroenterol. 2013, 48, 1452–1458. [Google Scholar] [CrossRef]
- Dasari, S.; Theis, J.D.; Vrana, J.A.; Rech, K.L.; Dao, L.N.; Howard, M.T.; Dispenzieri, A.; Gertz, M.A.; Hasadsri, L.; Highsmith, W.E.; et al. Amyloid Typing by Mass Spectrometry in Clinical Practice: A Comprehensive Review of 16,175 Samples. Mayo Clin. Proc. 2020, 95, 1852–1864. [Google Scholar] [CrossRef]
- Taylor, M.S.; Sidiqi, H.; Hare, J.; Kwok, F.; Choi, B.; Lee, D.; Baumwol, J.; Carroll, A.S.; Vucic, S.; Neely, P.; et al. Current approaches to the diagnosis and management of amyloidosis. Intern. Med. J. 2022, 52, 2046–2067. [Google Scholar] [CrossRef] [PubMed]
- Juluri, R.; Vuppalanchi, R.; Olson, J.; Ünalp, A.; Van Natta, M.L.; Cummings, O.W.; Tonascia, J.; Chalasani, N. Generalizability of the NASH CRN histological scoring system for nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2011, 45, 55. [Google Scholar] [CrossRef] [PubMed]
- Hoofring, A.; Boitnott, J.; Torbenson, M. Three-dimensional reconstruction of hepatic bridging fibrosis in chronic hepatitis C viral infection. J. Hepatol. 2003, 39, 738–741. [Google Scholar] [CrossRef]
- Hano, H.; Takasaki, S.; Endo, Y.; Harada, T.; Komine, K.; Koike, Y. Histological reassessment of the role of bridging fibrosis in the angioarchitectural features associated with lobular distortion of the liver in chronic viral hepatitis. Hepatol. Res. 2016, 46, E70–E78. [Google Scholar] [CrossRef]
- Malik, A.H.; Kumar, K.S.; Malet, P.F.; Jain, R.; Prasad, P.; Ostapowicz, G. Correlation of percutaneous liver biopsy fragmentation with the degree of fibrosis. Aliment. Pharmacol. Ther. 2004, 19, 545–549. [Google Scholar] [CrossRef]
- Poynard, T.; Halfon, P.; Castera, L.; Charlotte, F.; Le Bail, B.; Munteanu, M.; Messous, D.; Ratziu, V.; Benhamou, Y.; Bourlière, M.; et al. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: Impact of biopsy length and fragmentation. Aliment. Pharmacol. Ther. 2007, 25, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Wright, E.C.; Goodman, Z.D.; Dienstag, J.L.; Hoefs, J.C.; Kleiner, D.E.; Ghany, M.G.; Mills, A.S.; Nash, S.R.; Govindarajan, S.; et al. Prognostic value of Ishak fibrosis stage: Findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010, 51, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. A review of liver fibrosis and cirrhosis regression. J. Pathol. Transl. Med. 2023, 57, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J. Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef]
- Desmet, V.J.; Gerber, M.; Hoofnagle, J.H.; Manns, M.; Scheuer, P.J. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology 1994, 19, 1513–1520. [Google Scholar] [CrossRef]
- Batts, K.P.; Ludwig, J. Chronic hepatitis. An update on terminology and reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef]
- Park, Y.N.; Kim, H.G.; Chon, C.Y.; Park, J.B.; Sohn, J.H.; Yang, S.H.; Yu, E.S.; Lee, M.S.; Jang, J.J.; Chang, H.K.; et al. Histological grading and staging of chronic hepatitis—Standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Korean J. Pathol. 1999, 33, 337–346. [Google Scholar]
- Kutami, R.; Girgrah, N.; Wanless, I.R.; Sniderman, K.; Wong, F.; Sherman, M.; Heathcote, J.; Lilly, L. The Laennec grading system for assessment of hepatic fibrosis: Validation by correlation with wedged hepatic vein pressure and clinical features [abstract]. Hepatology 2000, 32, 407a. [Google Scholar]
- Wanless, I.R.; Sweeney, G.; Dhillon, A.P.; Guido, M.; Piga, A.; Galanello, R.; Gamberini, M.R.; Schwartz, E.; Cohen, A.R. Lack of progressive hepatic fibrosis during long-term therapy with deferiprone in subjects with transfusion-dependent beta-thalassemia. Blood 2002, 100, 1566–1569. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, M.Y.; Baik, S.K.; Park, H.J.; Jeon, H.K.; Im, C.K.; Won, C.S.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J. Hepatol. 2011, 55, 1004–1009. [Google Scholar] [CrossRef]
- Choi, J.H.; Thung, S.N. Advances in Histological and Molecular Classification of Hepatocellular Carcinoma. Biomedicines 2023, 11, 2582. [Google Scholar] [CrossRef]
- Choi, J.H.; Thung, S.N. Recent Advances in Pathology of Intrahepatic Cholangiocarcinoma. Cancers 2024, 16, 1537. [Google Scholar] [CrossRef] [PubMed]
- Memon, R.; Saxena, R. Molecular Advances in Cholestatic Liver Diseases. Adv. Anat. Pathol. 2025. [Google Scholar] [CrossRef]
- Kleiner, D.E. Drug-induced liver injury: The hepatic pathologist’s approach. Gastroenterol. Clin. North Am. 2017, 46, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Czeczok, T.W.; Van Arnam, J.S.; Wood, L.D.; Torbenson, M.S.; Mounajjed, T. The Almost-Normal Liver Biopsy: Presentation, Clinical Associations, and Outcome. Am. J. Surg. Pathol. 2017, 41, 1247–1253. [Google Scholar] [CrossRef]
- Torbenson, M.S. Basic patterns in liver pathology. In Atlas of Liver Pathology. A Pattern-Based Approach, 2nd ed.; Torbenson, M.S., Ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020; pp. 1–29. [Google Scholar]
- Dogan, S.; Becker, J.C.; Rekhtman, N.; Tang, L.H.; Nafa, K.; Ladanyi, M.; Klimstra, D.S. Use of touch imprint cytology as a simple method to enrich tumor cells for molecular analysis. Cancer Cytopathol. 2013, 121, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Shackel, N.A.; Gorrell, M.D.; McCaughan, G.W. Gene array analysis and the liver. Hepatology 2002, 36, 1313–1325. [Google Scholar] [CrossRef]
- Smalling, R.V.; Delker, D.A.; Zhang, Y.; Nieto, N.; McGuiness, M.S.; Liu, S.; Friedman, S.L.; Hagedorn, C.H.; Wang, L. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G364–G374. [Google Scholar] [CrossRef]
- Müller, M.; May, S.; Hall, H.; Kendall, T.J.; McGarry, L.; Blukacz, L.; Nuciforo, S.; Georgakopoulou, A.; Jamieson, T.; Phinichkusolchit, N.; et al. Human-correlated genetic models identify precision therapy for liver cancer. Nature 2025, 639, 754–764. [Google Scholar] [CrossRef]
- Sun, J.; Shi, R.; Wu, Y.; Lou, Y.; Nie, L.; Zhang, C.; Cao, Y.; Yan, Q.; Ye, L.; Zhang, S. Integration of transcriptomic analysis and multiple machine learning approaches identifies NAFLD progression-specific hub genes to reveal distinct genomic patterns and actionable targets. J. Big Data 2024, 11, 40. [Google Scholar] [CrossRef]
- Govaere, O.; Hasoon, M.; Alexander, L.; Cockell, S.; Tiniakos, D.; Ekstedt, M.; Schattenberg, J.M.; Boursier, J.; Bugianesi, E.; Ratziu, V.; et al. A proteo-transcriptomic map of non-alcoholic fatty liver disease signatures. Nat. Metab. 2023, 5, 572–578. [Google Scholar] [CrossRef]
- Ercan, C.; Kordy, K.; Knuuttila, A.; Zhou, X.; Kumar, D.; Koponen, V.; Mesenbrink, P.; Eppenberger-Castori, S.; Amini, P.; Pedrosa, M.C.; et al. A deep-learning-based model for assessment of autoimmune hepatitis from histology: AI(H). Virchows Arch. 2024, 485, 1095–1105. [Google Scholar] [CrossRef]
- Abdurrachim, D.; Lek, S.; Ong, C.Z.L.; Wong, C.K.; Zhou, Y.; Wee, A.; Soon, G.; Kendall, T.J.; Idowu, M.O.; Hendra, C.; et al. Utility of AI digital pathology as an aid for pathologists scoring fibrosis in MASH. J. Hepatol. 2025, 82, 898–908. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Hu, X.; Robert, M.E.; Zhang, X. The evolving role of liver biopsy: Current applications and future prospects. Hepatol. Commun. 2025, 9, e0628. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tacke, F.; Sugimoto, A.; Friedman, S.L. Antifibrotic therapies for metabolic dysfunction-associated steatotic liver disease. JHEP Rep. 2025, 7, 101421. [Google Scholar] [CrossRef]
- Lee, B.P.; Witkiewitz, K.; Mellinger, J.; Anania, F.A.; Bataller, R.; Cotter, T.G.; Curtis, B.; Dasarathy, S.; DeMartini, K.S.; Diamond, I.; et al. Designing clinical trials to address alcohol use and alcohol-associated liver disease: An expert panel Consensus Statement. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 626–645. [Google Scholar] [CrossRef]
- Wernberg, C.W.; Indira Chandran, V.; Lauridsen, M.M.; Skytthe, M.K.; Hansen, C.D.; Hansen, J.K.; Grønkjær, L.L.; Jacobsen, B.G.; Di Caterino, T.; Detlefsen, S.; et al. Ability of soluble TREM2 and PRO-C3 as biomarkers to predict changes in MASLD activity. JHEP Rep. 2025, 7, 101432. [Google Scholar] [CrossRef]
- Torbenson, M.; Washington, K. Pathology of liver disease: Advances in the last 50 years. Hum. Pathol. 2020, 95, 78–98. [Google Scholar] [CrossRef] [PubMed]







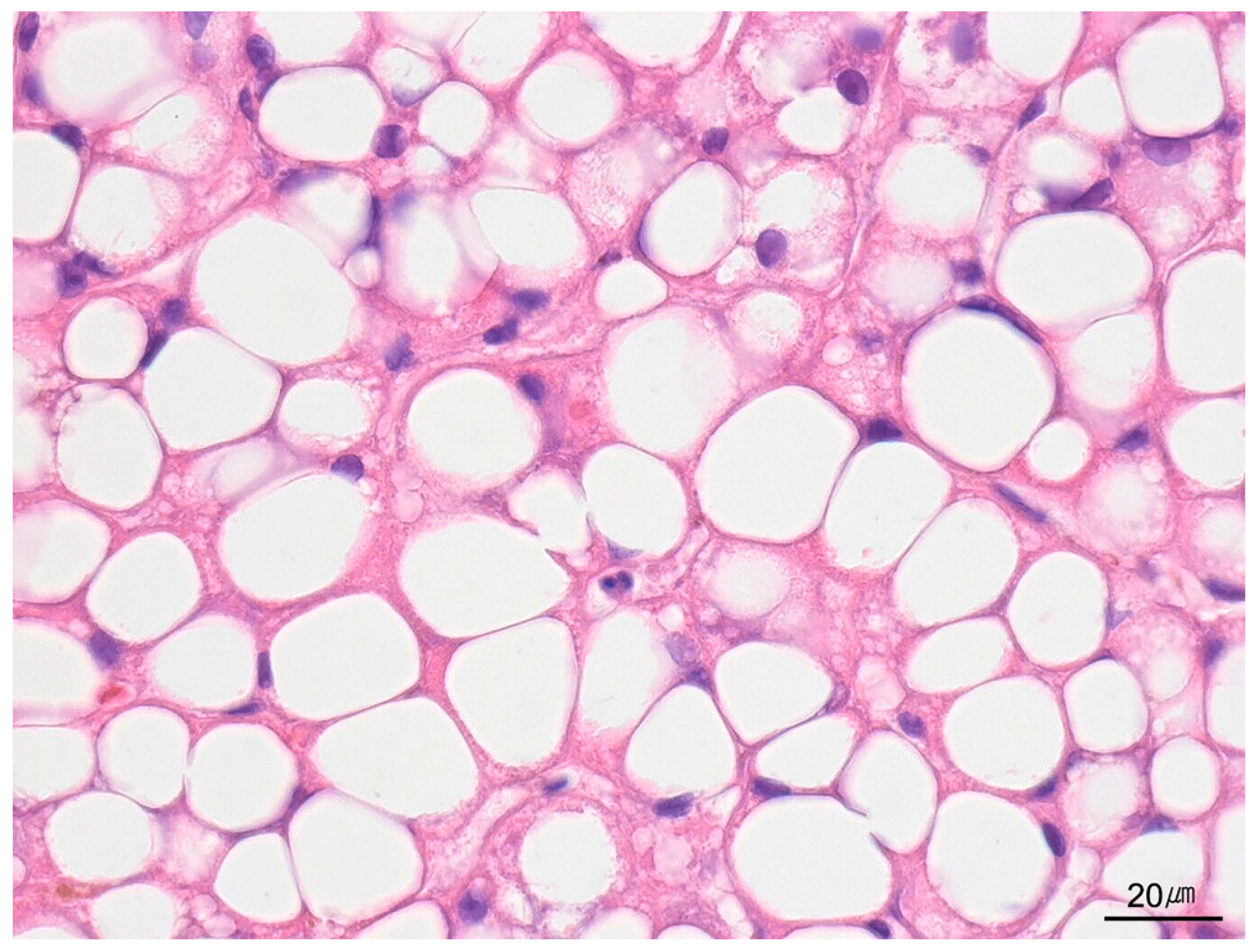

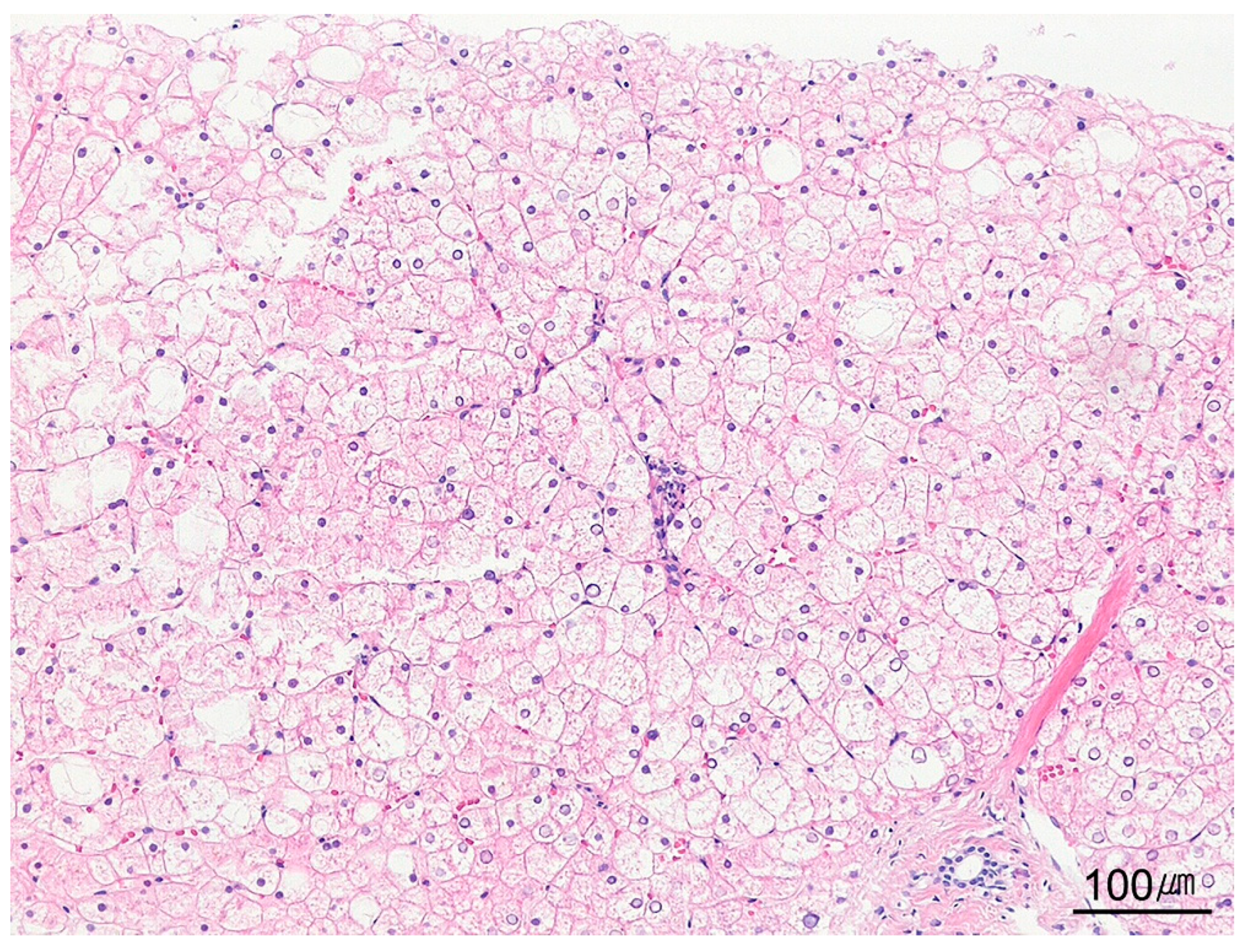
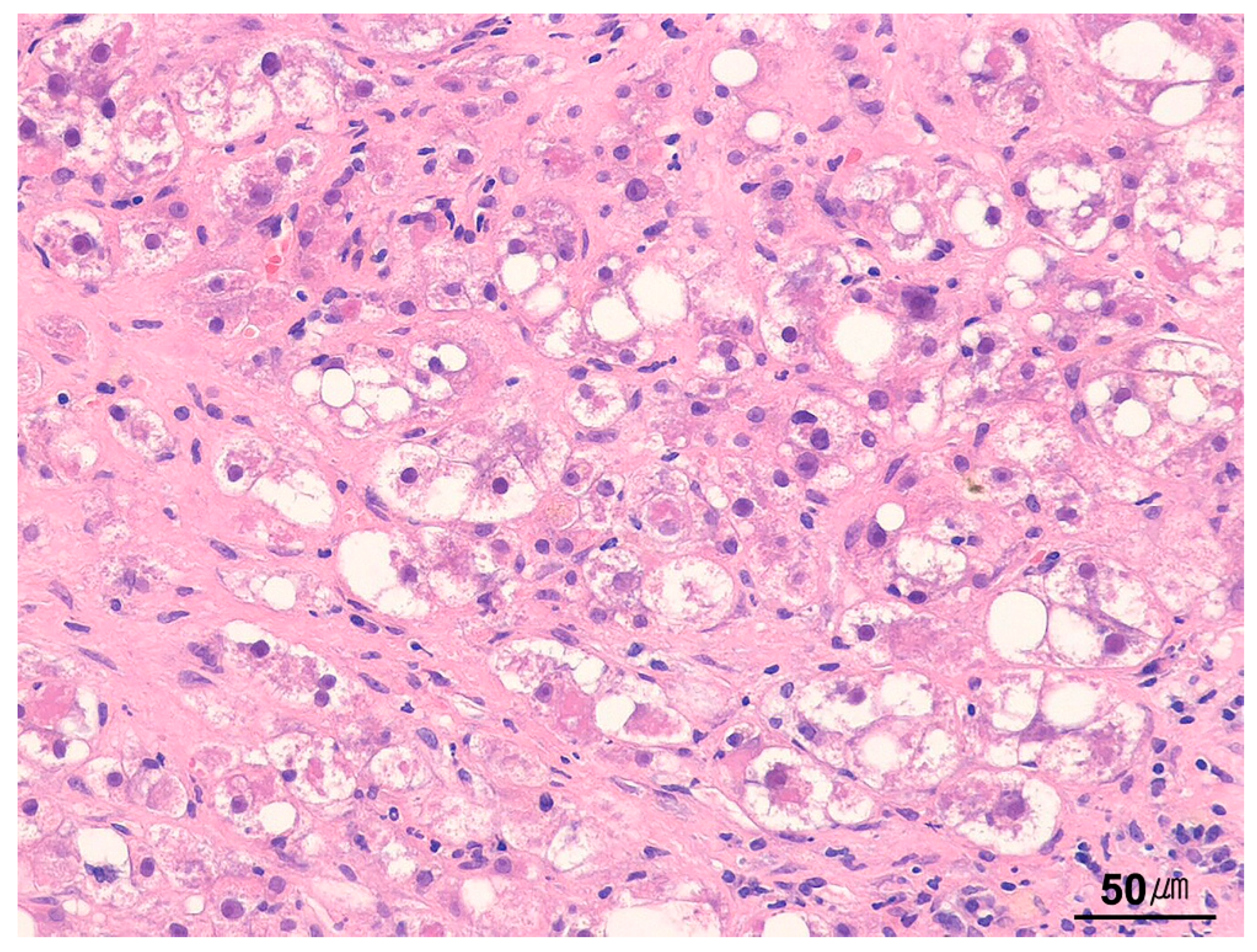
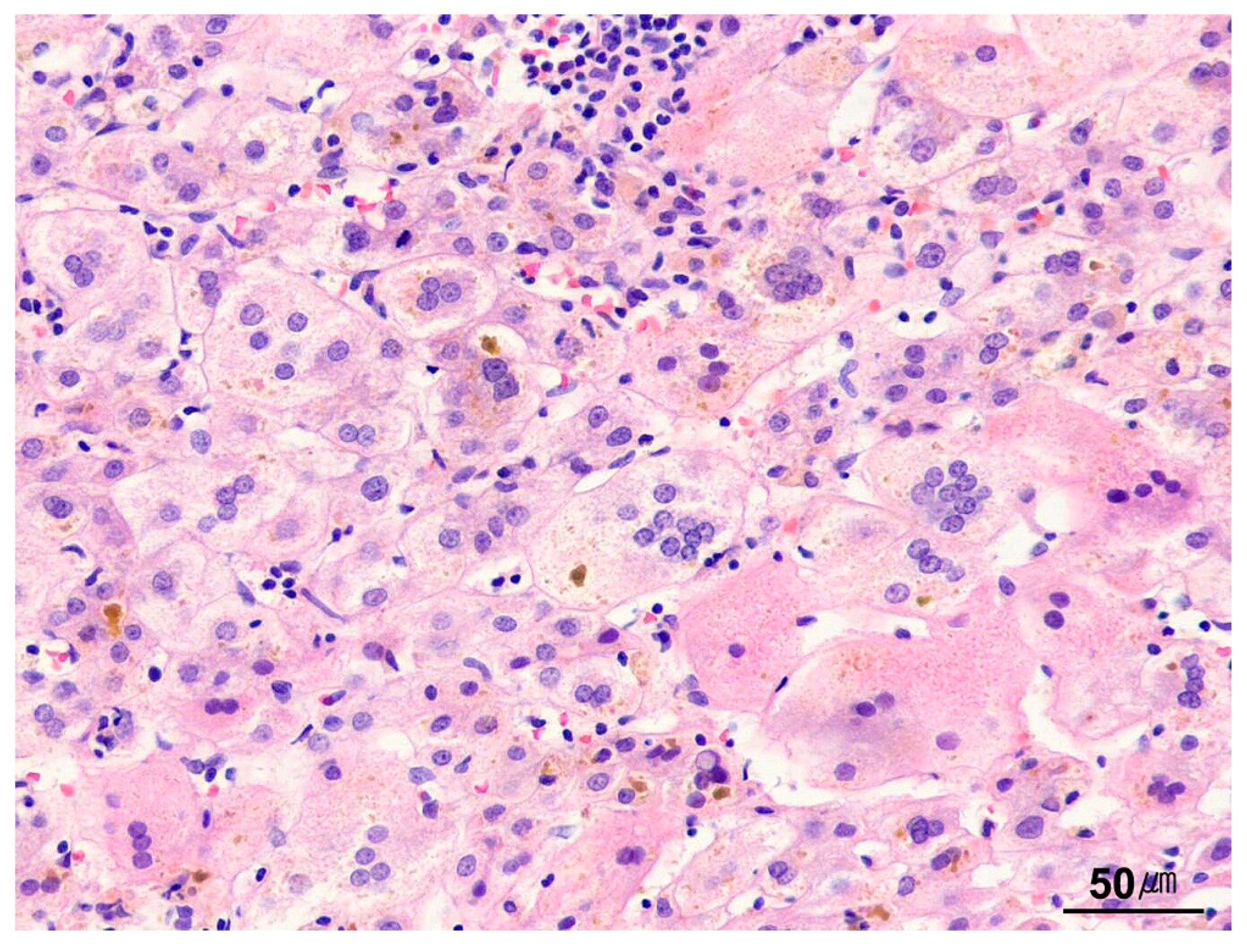

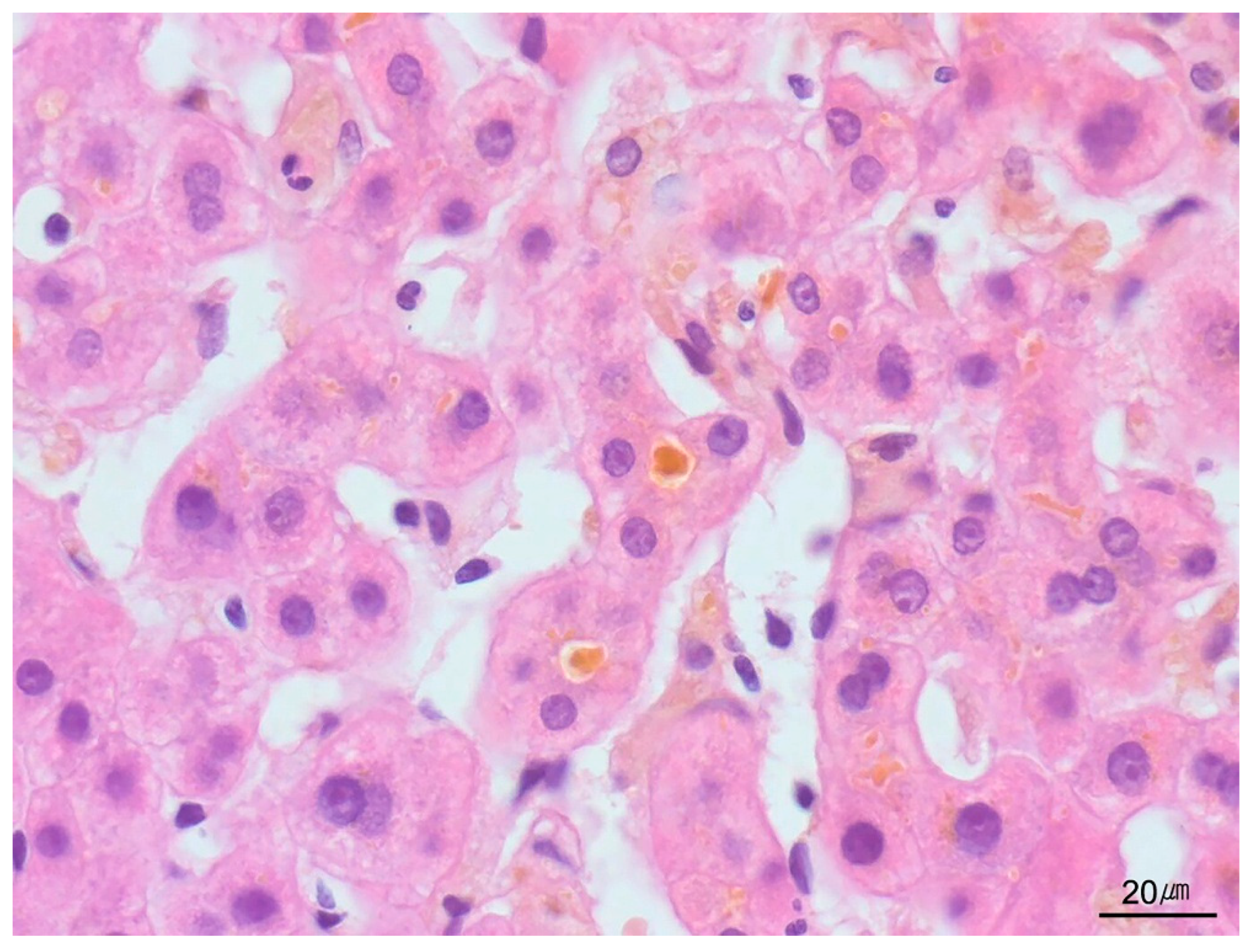
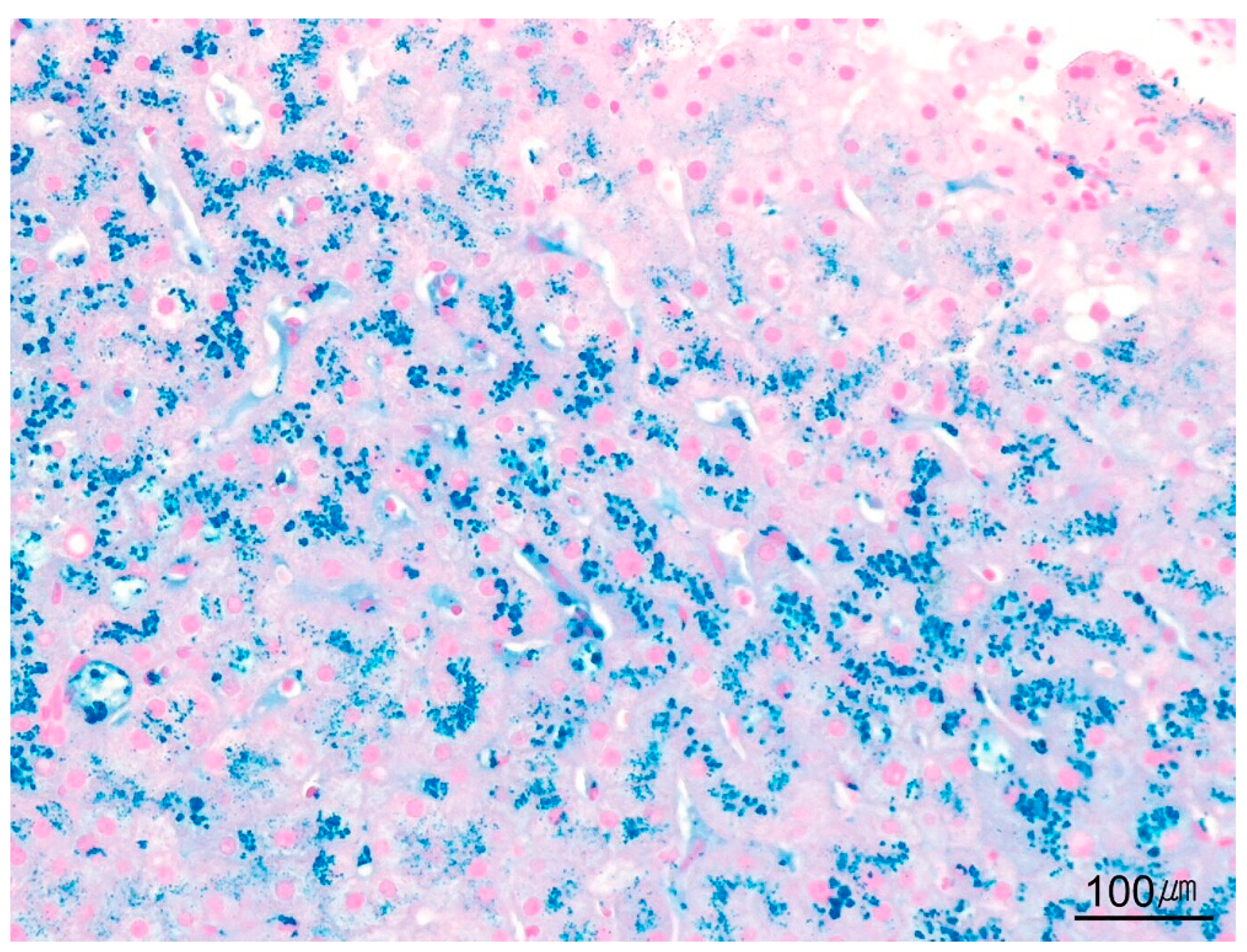
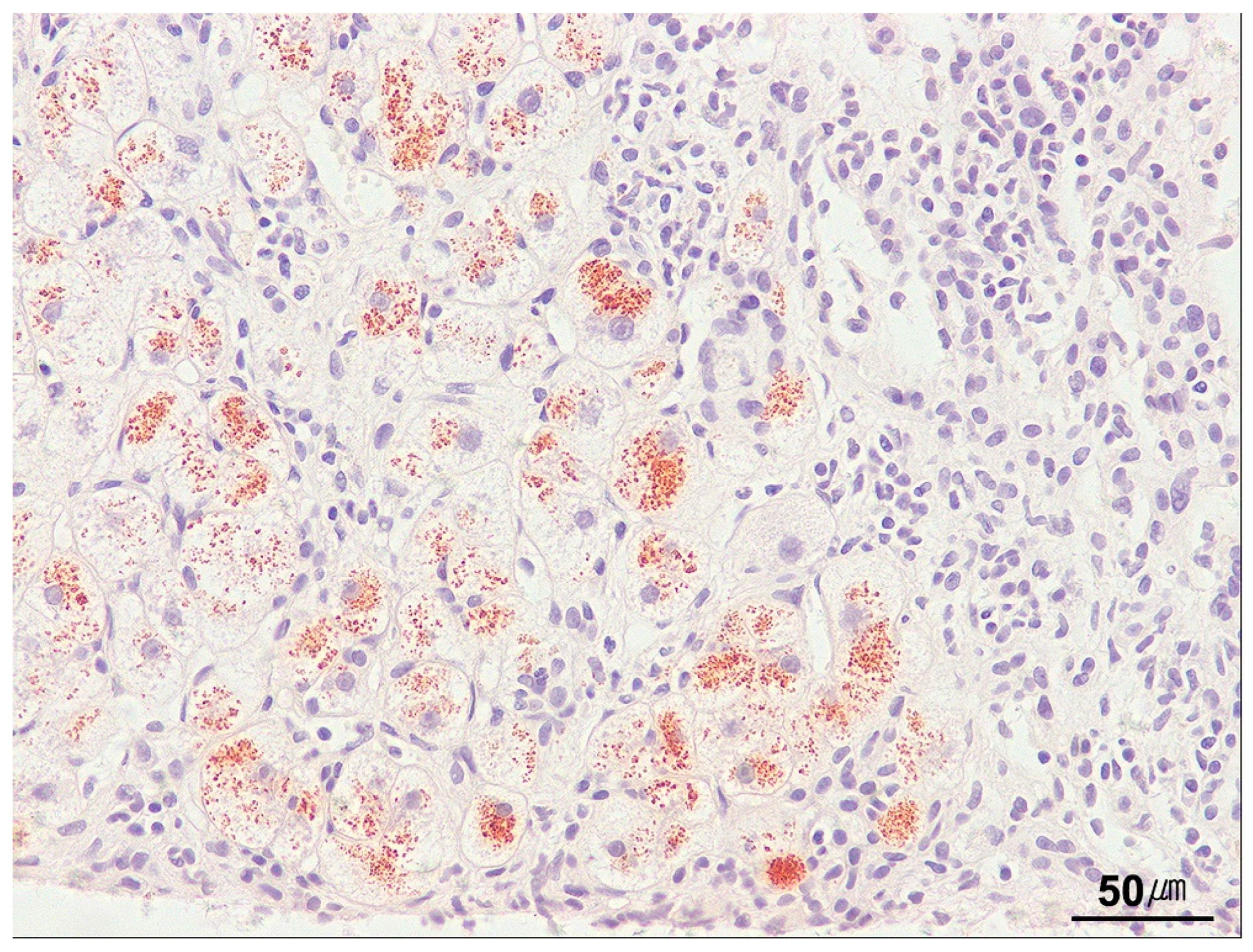
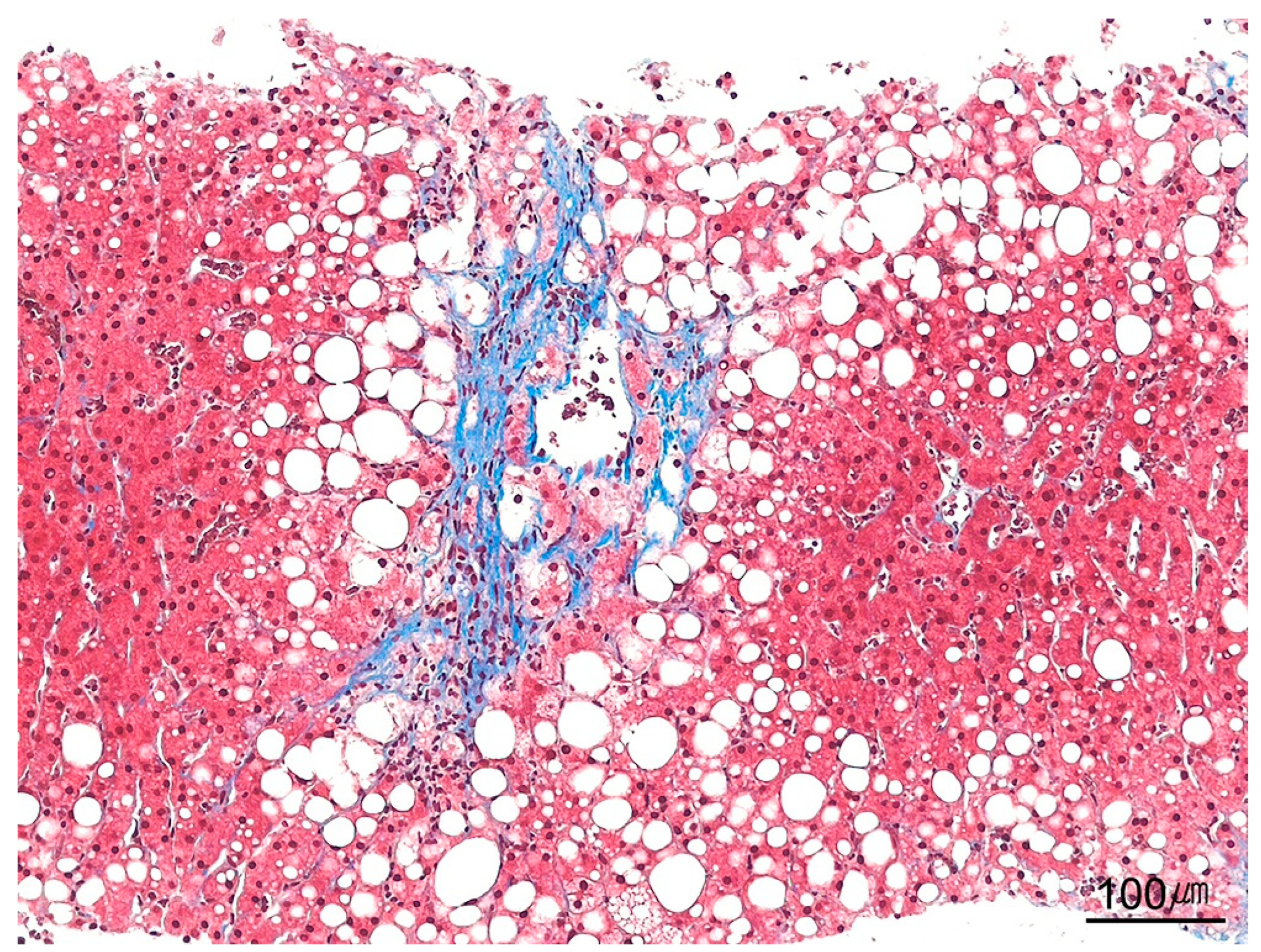

| Category | Indication |
|---|---|
| Diagnostic evaluation and etiologic determination | Unexplained elevation in liver function tests |
| Fever of unknown origin with suspected hepatic involvement | |
| Diagnostic confirmation and characterization of hepatic neoplasms | |
| Persistent jaundice of unknown origin | |
| Suspected inherited metabolic liver disorders | |
| Acute liver failure of unknown etiology | |
| Disease staging and prognostic assessment | Histological grading and staging of chronic hepatitis (viral, autoimmune, drug-induced) |
| Quantification of steatosis and steatohepatitis | |
| Assessment of ascites or portal hypertension | |
| Fibrosis staging in candidates for combined organ transplantation (e.g., heart–liver) | |
| Post-transplant and therapeutic monitoring | Unexplained graft dysfunction after solid-organ or hematopoietic stem cell transplantation |
| Histological monitoring of treatment response or drug-related hepatic injury |
| Category | Technique | Note |
|---|---|---|
| Percutaneous | Suction | Widely used; may produce fragmented cores in fibrotic liver (e.g., Menghini, Klatskin, Jamshidi needles) |
| Cutting | Yields more intact cores but increases hemorrhage risk (e.g., Vim–Silverman, Tru-Cut needles) | |
| Spring-loaded | Automated devices delivering uniform core length and rapid acquisition (e.g., ASAP gun) | |
| Image-guided | Thin needle (ultrasound- or CT-guided) | Enables precise targeting of focal lesions; suitable for FNA or core biopsy |
| Transvenous | Transjugular | Introduced through the internal jugular into the hepatic vein; preferred in coagulopathy; cores may include venous wall |
| EUS-guided | Transgastric | Reaches lesions inaccessible to the percutaneous approach; particularly valuable for small or deep masses |
| Laparoscopic or surgical | Laparoscopic | Provides direct visualization and targeted sampling of focal or superficial lesions |
| Operative wedge | Performed during laparotomy; yields large tissue volumes but may overrepresent subcapsular parenchyma | |
| Cytologic | FNA | Diagnoses benign versus malignant neoplasms; accuracy is high when it is image-guided |
| Special Stain | Diagnostic Application | Staining Color and Components |
|---|---|---|
| Collagens and fibrin | ||
| Masson’s trichrome | Detection of type I collagen; assessment of fibrosis stage in chronic hepatitis | Type I collagen: blue; megamitochondria: red; Mallory–Denk bodies: red |
| Sirius red | Detection of type I/III collagen; staging and fibrosis assessment | Type I/III collagen: red (a) |
| Gordon and Sweet’s reticulin | Detection of type III collagen; evaluation of lobular architecture, hepatocyte plate thickness, and discrimination of benign versus malignant nodules (e.g., cirrhosis, NRH, HCC) | Reticulin: black; collagen: rose |
| Phosphotungstic acid hematoxylin | Detection of fibrin in fibrin-ring granuloma (e.g., Q fever, hepatitis A) | Fibrin: blue-purple; collagen: red |
| Pigments and minerals | ||
| Perls’ Prussian blue | Detection of hemosiderin iron in overload disorders, including hemochromatosis and hemosiderosis | Ferric iron: blue |
| Hall’s bile | Detection of bilirubin in cholestatic liver diseases and tumors | Bilirubin: green |
| Rhodanine | Detection of copper in Wilson’s disease and chronic cholestasis | Copper: reddish-orange |
| Fontana–Masson | Detection of melanin, lipofuscin, and Dubin–Johnson pigment | Melanin, lipofuscin, Dubin–Johnson pigment: black |
| Glycogen | ||
| PAS | Detection of glycogen, basement membrane, and fungi | Glycogen, basement membrane, fungi: magenta |
| PAS-D | Highlights intracytoplasmic globules in α1-antitrypsin deficiency and ceroid-laden macrophages | Glycoprotein, basement membrane, α1-antitrypsin globules, ceroid-laden macrophages: magenta; glycogen: cleared |
| Amyloid | ||
| Congo red | Detection of amyloid in vessels, portal tracts, and perisinusoidal spaces | Amyloid: salmon pink to red (apple-green birefringence under polarized light) |
| Lipids | ||
| Oil Red O | Detection of lipid (b) | Fat: red; nuclei: blue |
| Microorganisms | ||
| Ziehl–Neelsen | Detection of acid-fast bacilli | Mycobacterium spp. (e.g., M. tuberculosis, M. leprae): red |
| Shikata orcein | Detection of HBsAg | HBsAg: dark brown |
| Victoria blue | Detection of HBsAg | HBsAg: blue |
| Grocott methenamine silver | Detection of fungi and selected bacteria (c) | Fungi, selected bacteria: black |
| Warthin–Starry | Detection of spirochetes | Spirochetes (e.g., Treponema pallidum): black |
| Immunohistochemical Stain | Diagnostic Application |
|---|---|
| Identification of bile duct and bile ductules | |
| Cytokeratin 7 or 19 | Highlights bile ducts and ductules; aids assessment of ductopenia and ductular reaction |
| Identification of liver zonation | |
| Glutamine synthetase | Marks metabolic zonation, with characteristic peri-terminal venular hepatocyte staining |
| Identification of viral antigens | |
| HBsAg and HBcAg | Confirms hepatitis B infection and indicates HBV replication activity (a) |
| Hepatitis D virus | Detects hepatitis D coinfection or superinfection in HBV-infected patients |
| Non-hepatotropic viruses | Detects cytomegalovirus, herpes simplex virus, EBV, adenovirus, and others, particularly in immunocompromised patients |
| Hereditary and storage diseases | |
| α1-antitrypsin | Highlights eosinophilic intracytoplasmic globules in α1-antitrypsin deficiency |
| Fibrinogen | Demonstrates hepatocytic fibrinogen accumulation in fibrinogen storage disease |
| Identification and classification of primary liver tumors | |
| Hep Par-1 | Positive in 70–85% of HCC (b) |
| Arginase-1 | Positive in 45–95% of HCC (c) |
| α-fetoprotein | Positive in 30–40% of HCC and >90% of hepatoblastomas, particularly fetal and embryonal subtypes |
| Polyclonal CEA | Positive in 45–80% of HCC (d) |
| CD10 | Positive in 50–75% of HCC (d) |
| Glypican-3 | Positive in 50–80% of HCC (e) |
| CD34 | Shows diffuse sinusoidal staining in HCC |
| Cytokeratin 7 and 19 (f) | Positive in 70–90% of ICCA |
| Monoclonal CEA | Positive in ~60% of ICCA |
| CA19-9 | Positive in ~60% of ICCA |
| Glutamine synthetase | Displays a map-like pattern in focal nodular hyperplasia |
| LFABP | Loss of expression in H-HCA |
| Serum amyloid A | Positive in inflammatory HCA |
| C-reactive protein | Positive in inflammatory HCA |
| β-catenin | Displays nuclear positivity in B-HCA exon 3 |
| CD31 and ERG | Positive in angiosarcoma |
| SMA and HMB45 | Positive in PEComa (angiomyolipoma) |
| Technique | Diagnostic Applications |
|---|---|
| In situ hybridization | Detection of hepatotropic and non-hepatotropic viruses (e.g., HBV, HCV, EBV, CMV) |
| Detection of albumin mRNA in hepatocellular neoplasms | |
| Polymerase chain reaction | Quantification and genotyping of HBV and HCV |
| Detection of infectious agents (e.g., viruses, bacteria, parasites) | |
| Detection of genetic mutations (e.g., HFE in hereditary hemochromatosis, ATP8B1 in progressive familial intrahepatic cholestasis) | |
| Characterization of hereditary and metabolic liver disorders | |
| Microarray analysis | Gene expression profiling across liver disease subtypes |
| Comparative genomic hybridization for chromosomal aberrations | |
| Detection of single-nucleotide polymorphisms linked to disease susceptibility and prognosis | |
| Next-generation sequencing | Detection of cholestasis-related genes Identification of mutations in HCC (e.g., TP53, CTNNB1, TERT promoter) Comprehensive genomic profiling of biliary tract cancers for targeted therapy Detection of inherited liver diseases (e.g., Wilson’s disease, α1-antitrypsin deficiency) |
| System, Year of Publication | Grading (Necroinflammatory Activity) | Staging (Fibrosis) | Note | Reference |
|---|---|---|---|---|
| Knodell (HAI), 1981 | Periportal/bridging necrosis (score 0–10); intralobular degeneration and focal necrosis (score 0–4); portal inflammation (score 0–4) | Score 0–4 (no fibrosis to cirrhosis) | Introduced HAI; fibrosis included in total score (0–22); composite scoring can obscure the distinction between inflammation and fibrosis | [72] |
| Scheuer, 1991 | Portal/periportal necroinflammatory activity (grade 0–4); lobular necroinflammatory activity (grade 0–4) | Grade 0–4 (no fibrosis to cirrhosis) | Simplified semi-quantitative assessment; independently scores necroinflammation and fibrosis | [106] |
| Desmet, 1994 | Piecemeal necrosis and lobular activity (grade: minimal to severe) (a) | Score 0–4 (no fibrosis to cirrhosis) | Chronic hepatitis is diagnosed based on etiology, grade, and stage | [107] |
| Ishak (modified HAI), 1995 | Interface hepatitis (piecemeal necrosis) score 0–4); confluent necrosis (score 0–6); focal lytic necrosis/apoptosis/focal inflammation (score 0–4); portal inflammation (score 0–4); total score 0–18 | Score 0–6 (no fibrosis to cirrhosis) | Improves Knodell HAI by separating grade/stage and adding detailed fibrosis scoring; standardized and reproducible histological assessment | [66] |
| Batts–Ludwig, 1995 | Interface hepatitis (piecemeal necrosis) (grade 0–4); lobular inflammation and necrosis (grade 0–4) | Score 0–4 (no fibrosis to cirrhosis) | Provides a simplified, practical framework for grading and staging chronic hepatitis | [108] |
| METAVIR, 1996 | Interface hepatitis (piecemeal necrosis) (score 0–3); lobular necrosis (score 0–2); overall histological activity (A0–A3) (b) | Score F0–F4 (no fibrosis to cirrhosis) | Simplified scoring system; fibrosis evaluation is reliable | [73,74] |
| Park et al., 1999 | Porto-periportal activity (score 0–4); lobular activity (score 0–4) (c) | Score 0–4 (no fibrosis to cirrhosis) | Practical and reproducible; tailored for Korean clinical and pathological practice | [109] |
| Laennec, 2000 | Not graded | Score F0–F4 (no fibrosis to cirrhosis); subdividing cirrhosis (F4) into F4A, F4B, or F4C (d) | Correlated strongly with both the clinical stage of cirrhosis and the grade of portal hypertension | [110,111,112] |
| Category | Histological Pattern | Representative Causes |
|---|---|---|
| Hepatitic pattern | Lobular hepatitis Portal tract inflammation Interface activity (hepatitis) | Viral hepatitis (HBV or HCV) Autoimmune hepatitis Drug-induced liver injury (DILI) |
| Necrotic pattern | Spotty necrosis Confluent necrosis Zonal necrosis Panacinar necrosis Submassive necrosis Massive necrosis | Acute viral hepatitis DILI Ischemia |
| Steatotic pattern | Macrovesicular steatosis Microvesicular steatosis | Alcohol-associated liver disease NAFLD/NASH (metabolic syndrome) DILI |
| Cytoplasmic changes | Glycogen accumulation Ballooned hepatocyte Giant-cell transformation | Glycogen storage disease Steatohepatitis Cholestatic liver diseases |
| Hepatocyte inclusions | Eosinophilic globules Megamitochondria Ground-glass inclusion Pseudoground-glass inclusion | α1-antitrypsin deficiency Fatty liver disease Chronic hepatitis BDILI |
| Biliary/cholestatic pattern | Biliary obstructive pattern Ductular reaction Ductopenia Bland lobular cholestasis Ascending cholangitis | Biliary stones or stricture PBC, PSC, DILI Biliary obstruction, PBC, PSC, DILI DILI or sepsis Bacterial infection of the biliary tract |
| Vascular pattern | Portal vein disease Sinusoidal/central vein disease Vascular outflow disease Peliosis hepatis | Idiopathic noncirrhotic portal hypertension Veno-occlusive disease Budd–Chiari syndrome Right heart failure |
| Depositional/storage pattern | Iron Copper Amyloid | Primary or secondary hemochromatosis Wilson’s disease Amyloidosis |
| Granuloma | Lipogranuloma Fibrin-ring granuloma Non-caseating granuloma Caseating granuloma Necrotic granuloma Foreign-body granuloma | Steatotic liver disease Q fever Sarcoidosis, PBC, DILI Tuberculosis Bacterial, fungal, or parasitic infections, DILI Drug injection or prior surgery |
| Fibrosis pattern | Pericellular fibrosis Central vein fibrosis Portal/periportal fibrosis Bridging fibrosis Cirrhosis | Steatotic liver disease Steatotic liver disease; vascular outflow disorders Chronic liver diseases (viral hepatitis, DILI, steatotic, biliary, or vascular disorders) |
| Infiltrative/hematologic pattern | Infiltration of sinusoids or portal tracts by atypical cells | Malignant lymphoma Leukemia Histiocytic disorders |
| Neoplastic pattern | Tumor infiltration with distortion of architecture | Primary liver tumors (HCC, ICCA) Metastatic carcinoma |
| Liver Lesions | Molecular Features |
|---|---|
| Cholestatic liver diseases | |
| Alagille syndrome | JAG1 and NOTCH2 mutations |
| αl-antitrypsin deficiency | SERPINA1 mutations |
| Cystic fibrosis | CFTR mutations |
| PFIC-1 | ATP8B1 mutations |
| PFIC-2 | ABCB11 mutations |
| PFIC-3 | ABCB4 mutations |
| PFIC-4 | TJP2 mutations |
| PFIC-5 | NR1H4 mutations |
| PFIC-6 | MYO5B mutations |
| BRIC | ATP8B1 mutations (BRIC-1) and ABCB11 mutations (BRIC-2) |
| ICP | ABCB4, ABCB11, ABCC2, ATP8B1, and NR1H4 mutations |
| HCA | |
| H-HCA | HNF1A biallelic inactivating mutations |
| I-HCA | IL6ST, FRK, STAT3, GNAS, and JAK1 mutations |
| B-HCA exon 3 | CTNNB1 exon 3 activating mutations |
| B-HCA exon 7/8 | CTNNB1 exon 7 or 8 activating mutations |
| BI-HCA | Share features of both B-HCAs and I-HCAs |
| SH-HCA | Somatic deletions of INHBE |
| HCC | |
| Steatohepatitic HCC | TP53 mutations |
| MM HCC | TP53 mutations and FGF19 amplification |
| Scirrhous HCC | TSC1/2 mutations |
| CH HCC | Alternative lengthening of telomeres |
| FL carcinoma | DNAJB1::PRKACA fusion gene |
| Intrahepatic CCA | |
| Small-duct type | BAP1 and IDH1/2 mutations, FGFR2 fusions, and SMAD4, BAP1, BRAF, ARIDA1A, KRAS, TP53, and SMAD4 mutations |
| Large-duct type | KRAS, TP53, and SMAD4 mutations and MDM2 amplification |
| Low-power inspection Specimen adequacy: length, fragmentation, number of portal tracts Overall architecture Distortion Irregularity Parenchymal nodularity Fibrosis: presence and pattern General overview Variegation Focal lesions Anatomic distribution of abnormalities High-power examination Lobules Inflammation: type, distribution, severity Cholestasis: hepatocellular, canalicular Fibrosis: location, pattern Hepatocytes Injury and necrosis: type, distribution, extent Cytoplasmic changes: steatosis, glycogen, inclusions, pigments Hepatic plates: hyperplasia, regeneration, atrophy Central veins Inflammation: perivenular, venular wall Occlusion: thrombosis, fibrosis Mural thickening: sclerosis Portal tract in general Size and profile: enlargement, irregularity Inflammation: type, distribution, extent Stromal change: edema, fibrosis Periportal alterations: interface activity, fibrosis Bile ducts Number: loss, paucity of bile ducts Epithelial injury: damage, necrosis, degeneration Inflammation: periductal or intraepithelial Periductal fibrosis: fibrous expansion, concentric onionskin Ductal bile plugs: inspissated bile Bile ductules Proliferation: ductular reaction Inflammation: neutrophils, mononuclear cells Ductular bile plugs: luminal bile accumulation Hepatic arteries and portal veins Caliber abnormalities: reduced diameter, dilatation, duplication Inflammation: vasculitis, perivascular infiltrates Thrombosis: vascular occlusion, recanalization Fibrosis: perivascular, mural |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H. Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review. Int. J. Mol. Sci. 2025, 26, 7729. https://doi.org/10.3390/ijms26167729
Choi JH. Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review. International Journal of Molecular Sciences. 2025; 26(16):7729. https://doi.org/10.3390/ijms26167729
Chicago/Turabian StyleChoi, Joon Hyuk. 2025. "Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review" International Journal of Molecular Sciences 26, no. 16: 7729. https://doi.org/10.3390/ijms26167729
APA StyleChoi, J. H. (2025). Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review. International Journal of Molecular Sciences, 26(16), 7729. https://doi.org/10.3390/ijms26167729





