Bridging Phytochemistry and Cosmetic Science: Molecular Insights into the Cosmeceutical Promise of Crotalaria juncea L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Appearance and Yield of C. juncea Extracts
2.2. Total Phenolic and Total Flavonoid Contents of C. juncea Extracts
2.3. Antioxidant Activity of C. juncea Extracts
2.4. Anti-Tyrosinase Activity of C. juncea Extracts
2.5. Anti-Aging Activity of C. juncea Extracts
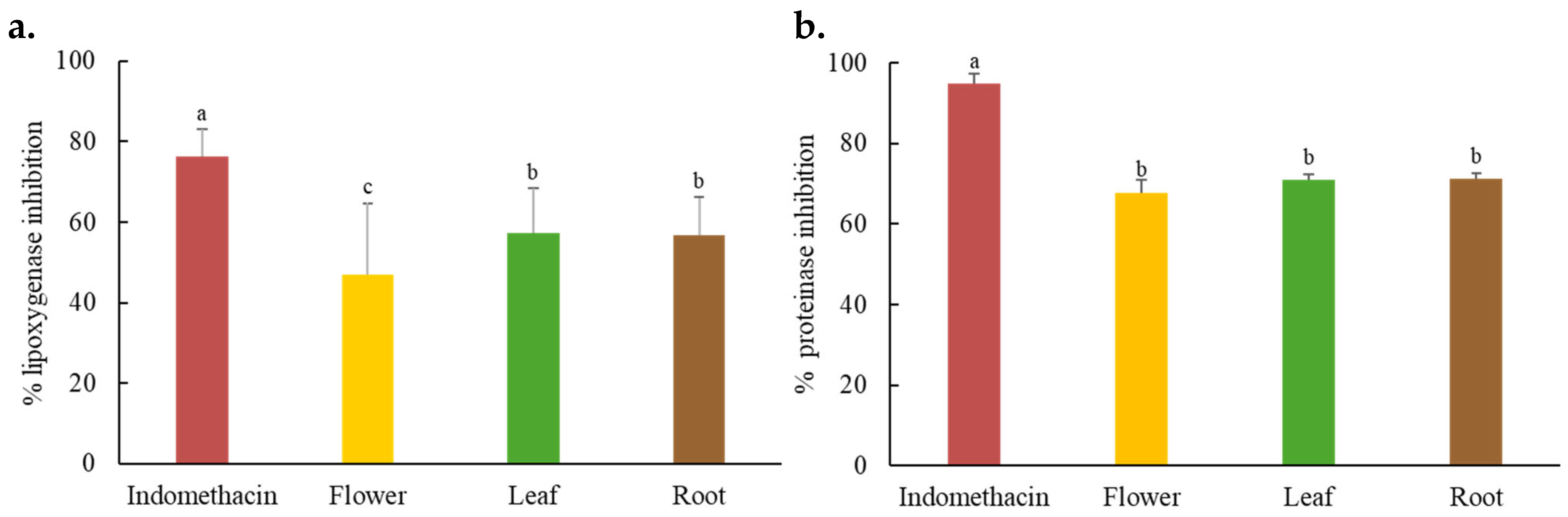
2.6. In Vitro Anti-Inflammatory Activity of C. juncea Extracts
2.7. Anti-Inflammatory Activity of C. juncea Extract in Murine Macrophage
2.7.1. Cytotoxicity Assessment
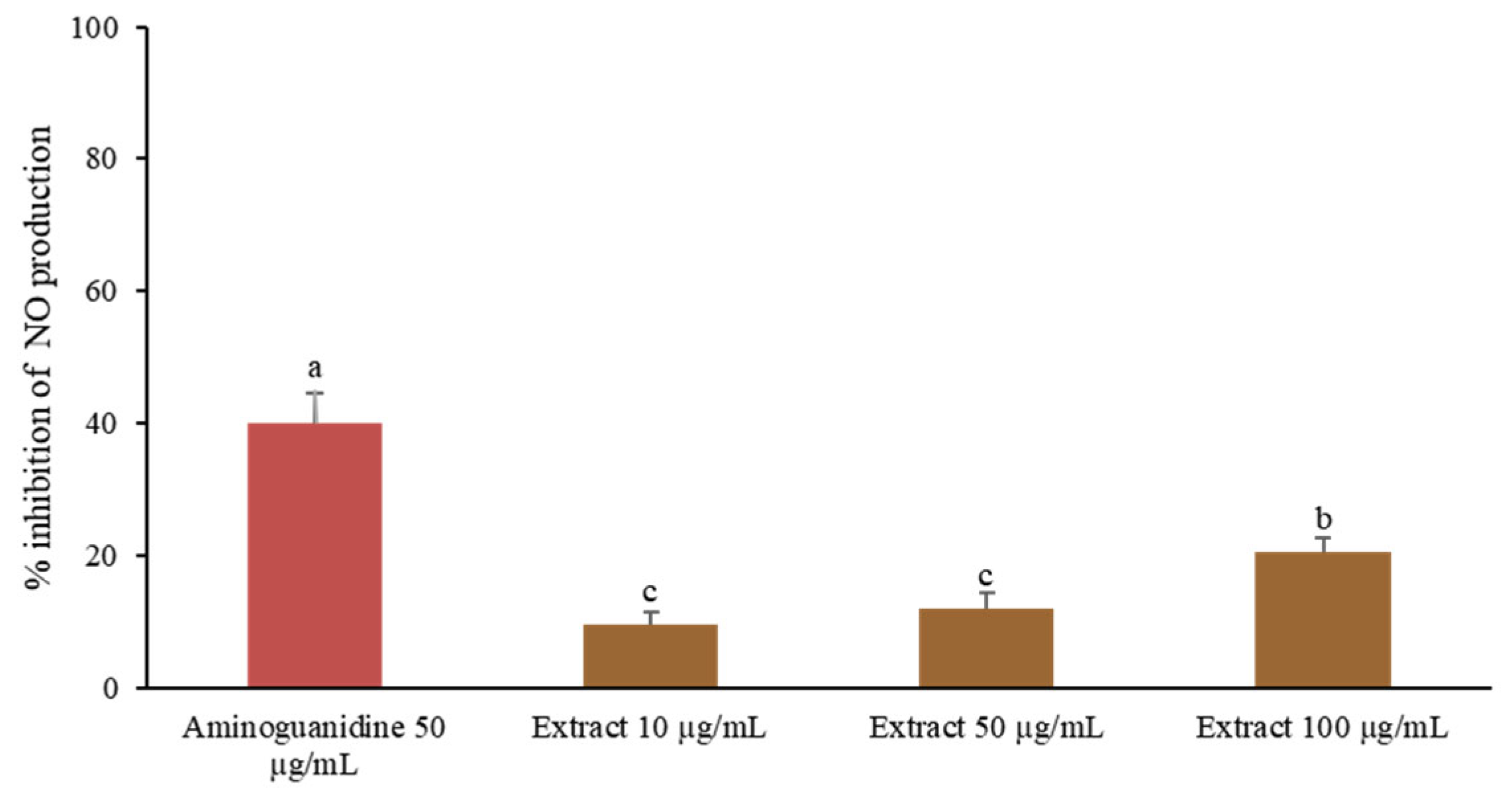
2.7.2. Nitric Oxide Inhibition
2.7.3. Anti-Inflammatory Activity of C. juncea Extract
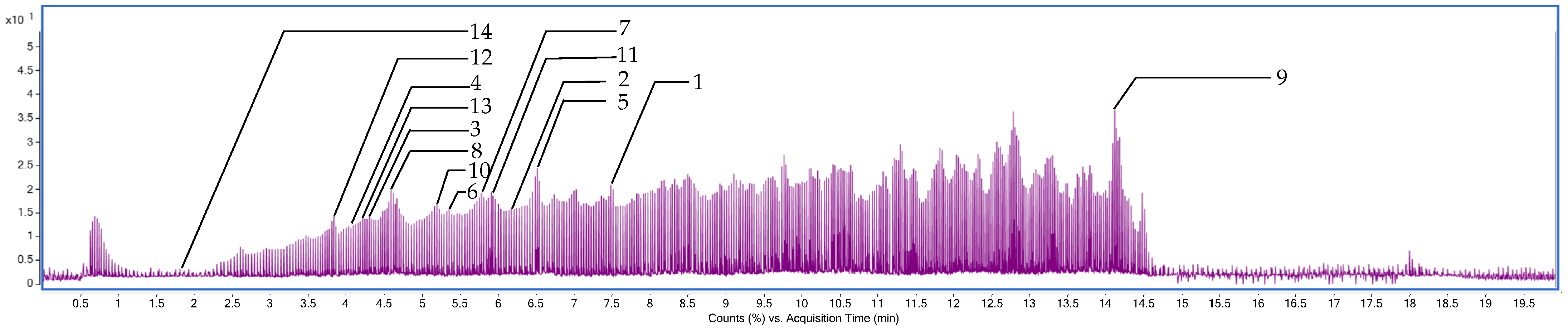
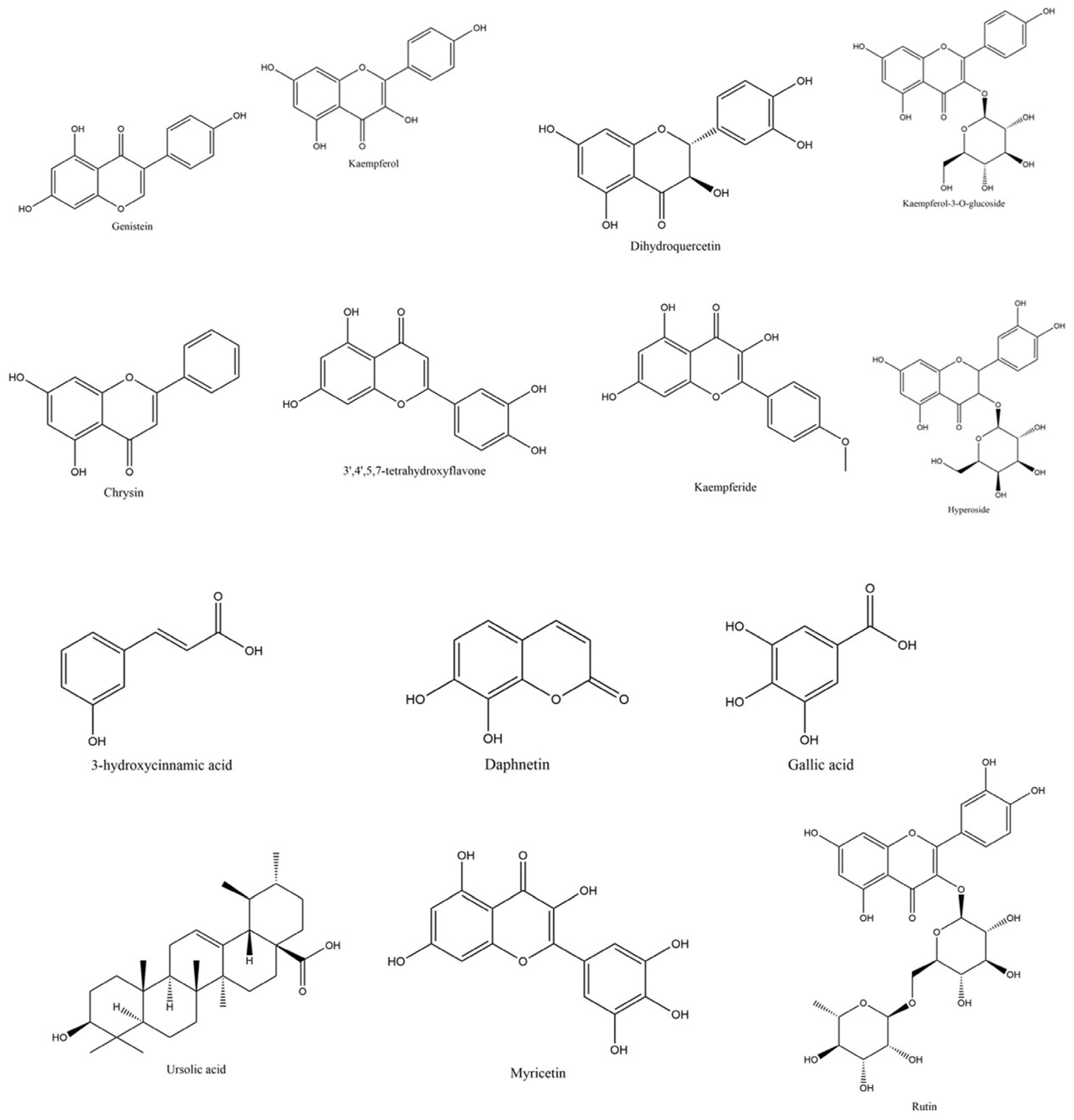
2.8. Characterization of the Phytochemical Composition of C. juncea Extract Using Liquid Chromatography–Mass Spectrometry–Quadrupole Time-of-Flight (LC-MS/QTOF)
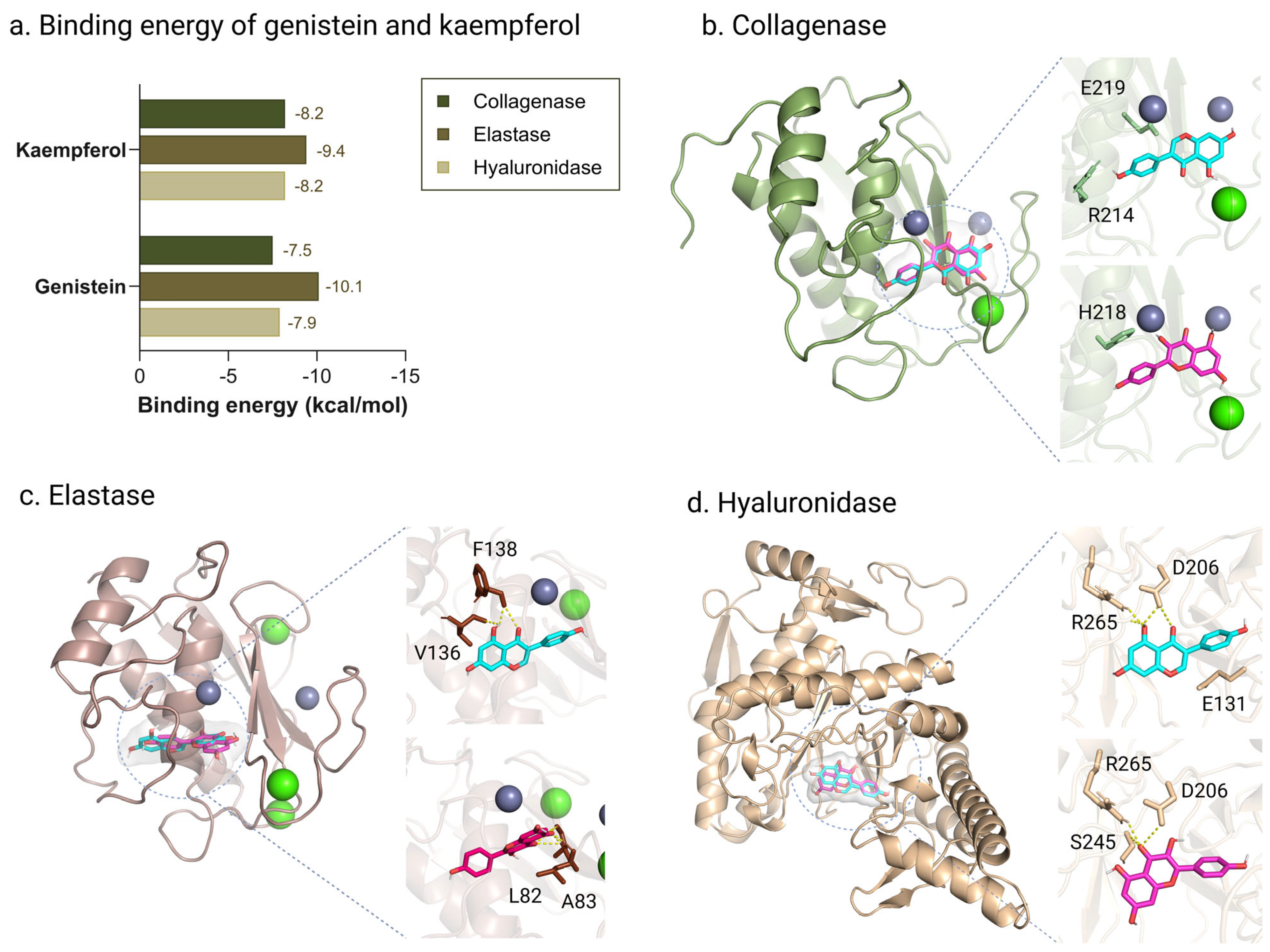
2.9. Molecular Docking of Genistein and Kaempferol Against Anti-Aging Enzymes
3. Materials and Methods
3.1. Reagents
3.2. Preparation of C. juncea Extracts
3.3. Chemical Compounds Analysis
3.3.1. Determination of Total Phenolic Content
3.3.2. Determination of Total Flavonoid Content
3.4. Antioxidant Activity Tests
3.4.1. DPPH Radical Scavenging Assay
3.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4.3. β-Carotene Bleaching Assay
3.4.4. Anti-Tyrosinase Activity
3.5. Determination of Anti-Aging Activity
3.5.1. Collagenase Inhibitory Activity
3.5.2. Elastase Inhibitory Activity
3.5.3. Hyaluronidase Inhibitory Activity
3.6. In Vitro Anti-Inflammatory Activity Tests
3.6.1. Lipoxygenase Inhibitory Activity
3.6.2. Proteinase Inhibitory Activity
3.7. Anti-Inflammatory Activity in Murine Macrophage
3.7.1. Cell Culture
3.7.2. Cytotoxicity Assay
3.7.3. Nitric Oxide (NO) Inhibition Assay
3.7.4. Inflammatory-Related Gene Expression
3.8. Characterization of Phytochemical Composition of C. juncea Extracts Using Liquid Chromatography–Mass Spectrometry–Quadrupole Time-of-Flight (LC-MS/QTOF)
3.9. Molecular Docking
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAAPVN | N-succinyl-Ala–Ala–Ala–p-nitroanilide |
| ANOVA | Analysis of Variance |
| BSA | Bovine Serum Albumin |
| DI | Deionized Water |
| DMEM | Dulbecco Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ECM | Extracellular Matrix |
| FALGPA | N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala |
| FOX | Ferrous Oxidation-Xylenol Orange |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalent |
| HCl | Hydrochloric Acid |
| HETEs | Hydroxy-eicosatetraenoic Acids |
| IC50 | Half-Maximal Inhibitory Concentration |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LC-MS/QTOF | Liquid Chromatography–Mass Spectrometry/Quadrupole Time-of-Flight |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NO | Nitric Oxide |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| QE | Quercetin Equivalent |
| RMSD | Root Mean Square Deviation |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| TGF-β | Transforming Growth Factor Beta |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| TNF-α | Tumor Necrosis Factor Alpha |
| TPTZ | 2,4,6-Tripyridyl-s-triazine |
| UV | Ultraviolet |
| VEGF | Vascular Endothelial Growth Factor |
| mRNA | Messenger Ribonucleic Acid |
References
- Cevenini, E.; Invidia, L.; Lescai, F.; Salvioli, S.; Tieri, P.; Castellani, G.; Franceschi, C. Human models of aging and longevity. Expert Opin. Biol. Ther. 2008, 8, 1393–1405. [Google Scholar] [CrossRef]
- Eassa, H.A.; Eltokhy, M.A.; Fayyaz, H.A.; Khalifa, M.K.A.; Shawky, S.; Helal, N.A.; Eassa, H.A.; Youssef, S.F.; Latz, I.K.; Nounou, M.I. Current Topical Strategies for Skin-Aging and Inflammaging Treatment: Science versus Fiction. J. Cosmet. Sci. 2020, 71, 321–350. [Google Scholar]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Chouhan, H.S.; Singh, S.K. Antibacterial activity of seed and flower parts of Crotalaria juncea Linn. Am.-Eurasian J. Sci. Res. 2010, 5, 212–215. [Google Scholar]
- Dzoyem, J.P.; McGaw, L.J.; Eloff, J.N. In vitro antibacterial, antioxidant and cytotoxic activity of acetone leaf extracts of nine under-investigated Fabaceae tree species leads to potentially useful extracts in animal health and productivity. BMC Complement. Altern. Med. 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The pharmacological importance of Aloe vera-A review. Int. J. Phytopharm. Res. 2015, 6, 28–33. [Google Scholar]
- Ateş, D.A.; Turgay, Ö. Antimicrobial activities of various medicinal and commercial plant extracts. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Gao, T.; Yao, H.; Song, J.; Liu, C.; Zhu, Y.; Ma, X.; Pang, X.; Xu, H.; Chen, S. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J. Ethnopharmacol. 2010, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, R.; Sharma, S.K.; Bhandari, D.C. Diversity assessment of useful Crotalaria species in India for plant genetic resources management. Genet. Resour. Crop Evol. 2010, 57, 461–470. [Google Scholar] [CrossRef]
- Kaneko, M.; Kato, N.; Hattori, I.; Matsuoka, M.; Vendramini, J.M.B. Seeding and Harvesting Times and Climate Conditions Are Important for Improving Nitrogen and Fiber Contents of Green Manure Sunn Hemp. Sustainability 2023, 15, 7103. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Ramya, L.B.; Mohana, L.S.; Saravana, K.A. Evaluation of anti-diarrhoeal activity of methanolic extract of Crotalaria juncea Linn. in albino Wistar rats. Int. J. Preclin. Pharm. Res. 2011, 2, 66–70. [Google Scholar]
- Harikumar, K.; Niveditha, B.; Reddy, P.K.; Monica, K.; Gajendra, P. Anti-hyperlipidemic activity of alcoholic and methonolic extracts of Crotolaria juncea in Triton-WR 1339 induced hyperlipidemia. Int. J. Phytopharm. 2012, 3, 256–262. [Google Scholar]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Madsen, H.L.; Thomsen, M.K.; Dragsted, L.O.; Skibsted, L.H. Regeneration of phenolic antioxidants from phenoxyl radicals: An ESR and electrochemical study of antioxidant hierarchy. Free Radic. Res. 1999, 30, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of β-Carotene as an Antioxidant against Lipid Peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Punchuklang, K.; Bunkrongcheap, R.; Petlamul, W.; Punchuklang, A. Antioxidant activity, total phenolic and tannin contents in Crotalatia juncea tea. Thai Sci. Technol. J. 2020, 29, 653–665. [Google Scholar]
- Hwang, J.-H.; Lee, B.M. Inhibitory Effects of Plant Extracts on Tyrosinase, L-DOPA Oxidation, and Melanin Synthesis. J. Toxicol. Environ. Health A 2007, 70, 393–407. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, Q.; Yang, P.-Y.; Huang, X.-X.; Song, S.-J. Isolation and structure elucidation of anti-tyrosinase compounds from the seeds of Crotalaria pallida. J. Asian Nat. Prod. Res. 2020, 23, 738–744. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Geeta; Widodo, W.S.; Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Armansyah, A.; Girsang, E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019, 6, 6. [Google Scholar] [CrossRef]
- Theansungnoen, T.; Nitthikan, N.; Wilai, M.; Chaiwut, P.; Kiattisin, K.; Intharuksa, A. Phytochemical Analysis and Antioxidant, Antimicrobial, and Antiaging Activities of Ethanolic Seed Extracts of Four Mucuna Species. Cosmetics 2022, 9, 14. [Google Scholar] [CrossRef]
- Hibbetts, K.; Hines, B.; Williams, D. An Overview of Proteinase Inhibitors. J. Vet. Intern. Med. 1999, 13, 302–308. [Google Scholar] [CrossRef]
- Rajesh, A.; Doss, A.; Tresina, P.S.; Mohan, V.R. In-vitro anti-inflammatory activity of ethanol extract of Crotalaria longipes. Int. J. Pharmacogn. Phytochem. 2019, 8, 1469–1472. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines. 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Phosri, S.; Kiattisin, K.; Intharuksa, A.; Janon, R.; Na Nongkhai, T.; Theansungnoen, T. Anti-Aging, Anti-Acne, and Cytotoxic Activities of Houttuynia cordata Extracts and Phytochemicals Analysis by LC-MS/MS. Cosmetics 2022, 9, 136. [Google Scholar] [CrossRef]
- Mahasawat, P.; Boukaew, S.; Prasertsan, P. Exploring the potential of Crotalaria juncea flower extracts as a source of antioxidants, antimicrobials, and cytoprotective agents for biomedical applications. BioTechnologia 2023, 104, 359–370. [Google Scholar] [CrossRef]
- Shirai, T.; Hilhorst, M.; Harrison, D.G.; Goronzy, J.J.; Weyand, C.M. Macrophages in vascular inflammation–From atherosclerosis to vasculitis. Autoimmunity 2015, 48, 139–151. [Google Scholar] [CrossRef]
- Chouhan, H.S.; Sahu, A.N.; Singh, S.K. Fatty acid composition, antioxidant, anti-inflammatory and antibacterial activities of seed oil from Crotalaria juncea Linn. J. Med. Plants Res. 2011, 5, 984–991. [Google Scholar]
- Khumalo, G.P.; Nguyen, T.; Van Wyk, B.-E.; Feng, Y.; Cock, I.E. Inhibition of pro-inflammatory cytokines by selected southern African medicinal plants in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2024, 319, 117268. [Google Scholar] [CrossRef] [PubMed]
- Kandilarov, I.; Gardjeva, P.; Georgieva-Kotetarova, M.; Zlatanova, H.; Vilmosh, N.; Kostadinova, I.; Katsarova, M.; Atliev, K.; Dimitrova, S. Effect of Plant Extracts Combinations on TNF-α, IL-6 and IL-10 Levels in Serum of Rats Exposed to Acute and Chronic Stress. Plants 2023, 12, 3049. [Google Scholar] [CrossRef]
- Jang, D.-i.; Lee, A.H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Łuczkiewicz, M.; Głód, D.; Baczek, T.; Buciński, A. LC-DAD UV and LC-MS for the Analysis of Isoflavones and Flavones from In Vitro and In Vivo Biomass of Genista tinctoria L. Chromatographia 2004, 60, 179–185. [Google Scholar] [CrossRef]
- Kang, S.; Chung, J.H.; Lee, J.H.; Fisher, G.J.; Wan, Y.S.; Duell, E.A.; Voorhees, J.J. Topical N-Acetyl Cysteine and Genistein Prevent Ultraviolet-Light-Induced Signaling That Leads to Photoaging in Human Skin In Vivo. J. Investig. Dermatol. 2003, 120, 835–841. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally occurring flavonoids. Arch. Pharmacal Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Kanuchova, M.; Urban, L.; Melegova, N.; Coma, M.; Dvorankova, B.; Smetana, K., Jr.; Gal, P. Genistein does not inhibit TGF-β1-induced conversion of human dermal fibroblasts to myofibroblasts. Physiol. Res. 2021, 70, 815–820. [Google Scholar] [CrossRef]
- Tang, S.-C.; Hsiao, Y.-P.; Ko, J.-L. Genistein protects against ultraviolet B–induced wrinkling and photoinflammation in in vitro and in vivo models. Genes Nutr. 2022, 17, 4. [Google Scholar] [CrossRef]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M. Anti-elastase, anti-tyrosinase and matrix metalloproteinase-1 inhibitory activity of earthworm extracts as potential new anti-aging agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Yang, C.; Yang, W.; He, Z.; Guo, J.; Yang, X.; Wang, R.; Li, H. Kaempferol Alleviates Oxidative Stress and Apoptosis Through Mitochondria-dependent Pathway During Lung Ischemia-Reperfusion Injury. Front. Pharmacol. 2021, 12, 624402. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Cîrîc, A.-I.; Begea, M. Anti-Aging Effects of Flavonoids from Plant Extracts. Foods 2024, 13, 2441. [Google Scholar] [CrossRef] [PubMed]
- Polito, F.; Marini, H.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D. Genistein aglycone, a soy-derived isoflavone, improves skin changes induced by ovariectomy in rats. Br. J. Pharmacol. 2012, 165, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, N.; Yan, Y.-Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.-M.; Zhang, H.; Liu, Z.-D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Kiattisin, K.; Nitthikan, N.; Poomanee, W.; Leelapornpisid, P.; Viernstein, H.; Mueller, M. Anti-inflammatory, antioxidant activities and safety of Coffea arabica leaf extract for alternative cosmetic ingredient. Chiang Mai J. Sci. 2019, 46, 284–294. [Google Scholar]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of Stability and Transdermal Delivery of Bioactive Compounds in Green Robusta Coffee Beans Extract Loaded Nanostructured Lipid Carriers. J. Nanotechnol. 2018, 2018, 7865024. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef]
- Kiattisin, K.; Nantarat, N.; Leelapornpisid, P. Evaluation of antioxidant and anti-tyrosinase activities as well as stability of green and roasted coffee bean extracts from Coffea arabica and Coffea canephora grown in Thailand. J. Pharmacogn. Phytother. 2016, 8, 182–192. [Google Scholar] [CrossRef]
- Phumat, P.; Chaichit, S.; Potprommanee, S.; Preedalikit, W.; Sainakham, M.; Poomanee, W.; Chaiyana, W.; Kiattisin, K. Influence of Benincasa hispida Peel Extracts on Antioxidant and Anti-Aging Activities, including Molecular Docking Simulation. Foods 2023, 12, 3555. [Google Scholar] [CrossRef] [PubMed]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods 2023, 12, 4292. [Google Scholar] [CrossRef] [PubMed]
- Uğur, D.; Güneş, H.; Güneş, F.; Mammadov, R. Cytotoxic activities of certain medicinal plants on different cancer cell lines. Turk. J. Pharm. Sci. 2017, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Rev. B.01 Release Notes; Gaussian Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/citation/ (accessed on 4 August 2025).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 1.8. 2015. Available online: https://www.pymol.org/support.html (accessed on 4 August 2025).


| Samples | DPPH Radical Scavenging Activity (IC50 (mg/mL)) | FRAP Value (mg FeSO4/g Extract) | β-Carotene Bleaching (% Inhibition) |
|---|---|---|---|
| Trolox | 0.007 ± 0.00 a | 134.35 ± 1.18 a | 89.86 ± 6.72 a |
| Flower extract | 0.78 ± 0.01 d | 6.77 ± 0.10 c | 90.99 ± 1.27 a |
| Leaf extract | 0.64 ± 0.01 c | 7.17 ± 0.49 c | 89.19 ± 5.84 a |
| Root extract | 0.52 ± 0.06 b | 15.21 ± 1.46 b | 90.99 ± 7.09 a |
| Samples | L-Tyrosine as Substrate (% Inhibition) | L-DOPA as Substrate (% Inhibition) |
|---|---|---|
| Kojic acid | 98.47 ± 0.24 a | 98.58 ± 0.15 a |
| Flower extract | 26.62 ± 2.03 bc | 18.94 ± 2.84 c |
| Leaf extract | 15.45 ± 12.15 c | 43.91 ± 9.17 bc |
| Root extract | 33.56 ± 4.21 b | 44.15 ± 4.03 b |
| Samples | Collagenase Inhibitory Activity (% Inhibition) | Elastase Inhibitory Activity (% Inhibition) | Hyaluronidase Inhibitory Activity (% Inhibition) |
|---|---|---|---|
| Ascorbic acid | 85.44 ± 4.12 a | 86.51 ± 11.70 a | ND |
| Tannic acid | ND | ND | 97.59 ± 2.89 a |
| Flower extract | 15.28 ± 0.78 b | 30.34 ± 2.67 c | 1.72 ± 4.91 b |
| Leaf extract | 19.95 ± 10.10 b | 65.04 ± 13.61 b | 4.48 ± 3.16 b |
| Root extract | 91.60 ± 0.66 a | 67.17 ± 9.34 b | 8.50 ± 2.53 b |
| No | Retention Time (min) | m/z | Tentative Identification | Formula | Mass Error (ppm) | Ion Species |
|---|---|---|---|---|---|---|
| 1 | 7.569 | 269.04825 | Genistein | C15H10O5 | 2.38 | [M−H]− |
| 2 | 6.299 | 285.04547 | Kaempferol | C15H10O6 | −16.07 | [M−H]− |
| 3 | 4.249 | 303.05389 | Dihydroquercetin | C15H12O7 | −9.57 | [M−H]− |
| 4 | 4.102 | 447.09470 | Kaempferol-3-O-glucoside | C21H20O11 | −2.89 | [M−H]− |
| 5 | 6.540 | 253.05211 | Chrysin | C15H10O4 | −5.85 | [M−H]− |
| 6 | 5.307 | 285.04224 | 3′,4′,5,7-tetrahydroxyflavone | C15H10O6 | −6.21 | [M−H]− |
| 7 | 5.823 | 299.05591 | Kaempferide | C16H12O6 | 8.66 | [M−H]− |
| 8 | 4.671 | 463.08795 | Hyperoside | C21H20O12 | 0.54 | [M−H]− |
| 9 | 14.277 | 455.35516 | Ursolic acid | C30H48O3 | −6.50 | [M−H]− |
| 10 | 5.171 | 177.01897 | Daphnetin | C9H6O4 | −0.90 | [M−H]− |
| 11 | 5.902 | 317.02997 | Myricetin | C15H10O8 | 1.04 | [M−H]− |
| 12 | 3.617 | 163.03993 | 3-hydroxycinnamic acid | C9H8O3 | 0.86 | [M−H]− |
| 13 | 4.208 | 609.14624 | Rutin | C27H30O16 | −0.20 | [M−H]− |
| 14 | 1.866 | 169.01414 | Gallic acid | C7H6O5 | −0.77 | [M−H]− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aree, T.; Chaichit, S.; Junlatat, J.; Kiattisin, K.; Intharuksa, A. Bridging Phytochemistry and Cosmetic Science: Molecular Insights into the Cosmeceutical Promise of Crotalaria juncea L. Int. J. Mol. Sci. 2025, 26, 7716. https://doi.org/10.3390/ijms26167716
Aree T, Chaichit S, Junlatat J, Kiattisin K, Intharuksa A. Bridging Phytochemistry and Cosmetic Science: Molecular Insights into the Cosmeceutical Promise of Crotalaria juncea L. International Journal of Molecular Sciences. 2025; 26(16):7716. https://doi.org/10.3390/ijms26167716
Chicago/Turabian StyleAree, Tanatchaporn, Siripat Chaichit, Jintana Junlatat, Kanokwan Kiattisin, and Aekkhaluck Intharuksa. 2025. "Bridging Phytochemistry and Cosmetic Science: Molecular Insights into the Cosmeceutical Promise of Crotalaria juncea L." International Journal of Molecular Sciences 26, no. 16: 7716. https://doi.org/10.3390/ijms26167716
APA StyleAree, T., Chaichit, S., Junlatat, J., Kiattisin, K., & Intharuksa, A. (2025). Bridging Phytochemistry and Cosmetic Science: Molecular Insights into the Cosmeceutical Promise of Crotalaria juncea L. International Journal of Molecular Sciences, 26(16), 7716. https://doi.org/10.3390/ijms26167716








