Bioprospecting for Anti-Kinetoplastid Drug Discovery from Aloysia citrodora Essential Oil
Abstract
1. Introduction
2. Results and Discussion
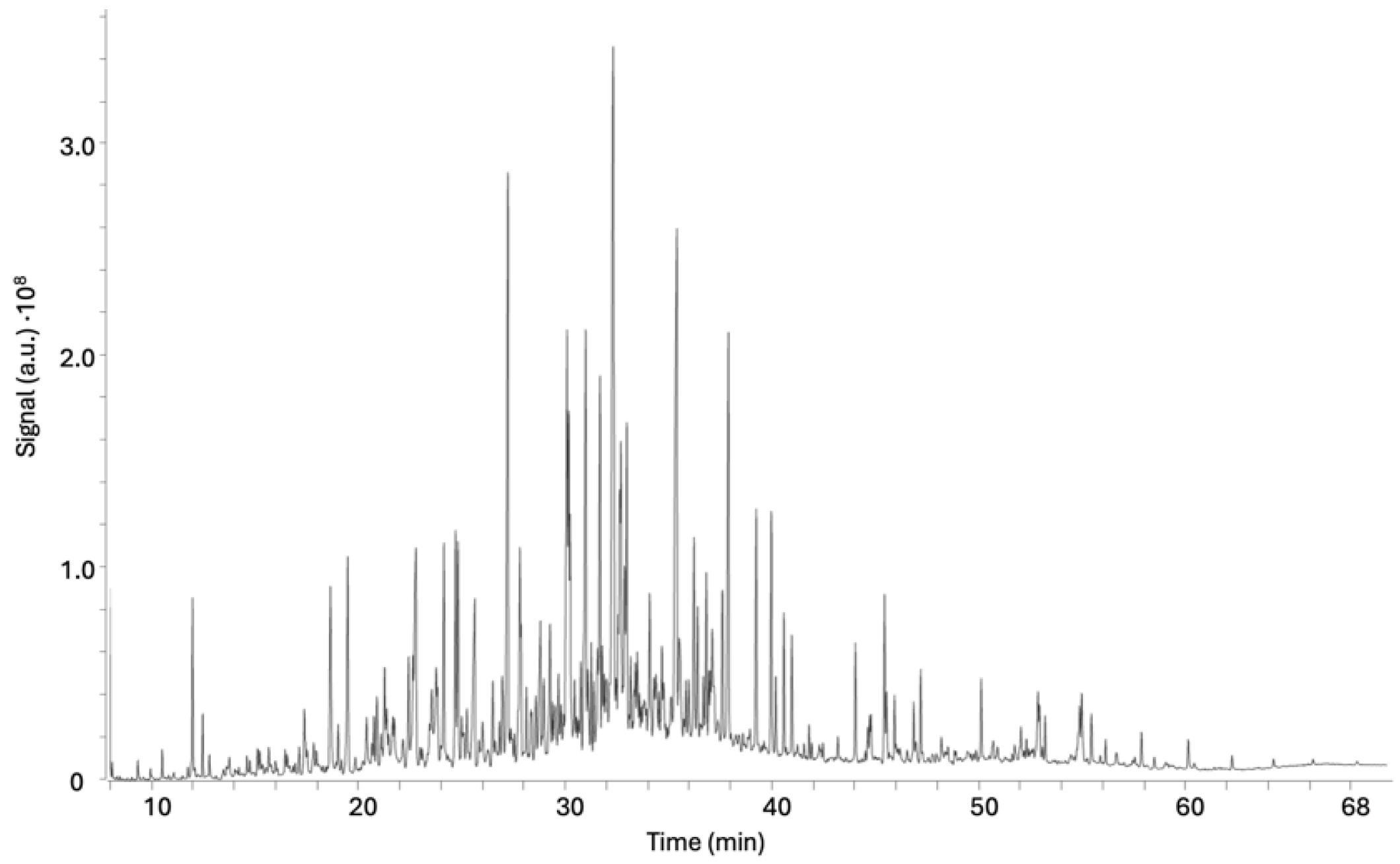
2.1. Bioassay-Guided Fractionation
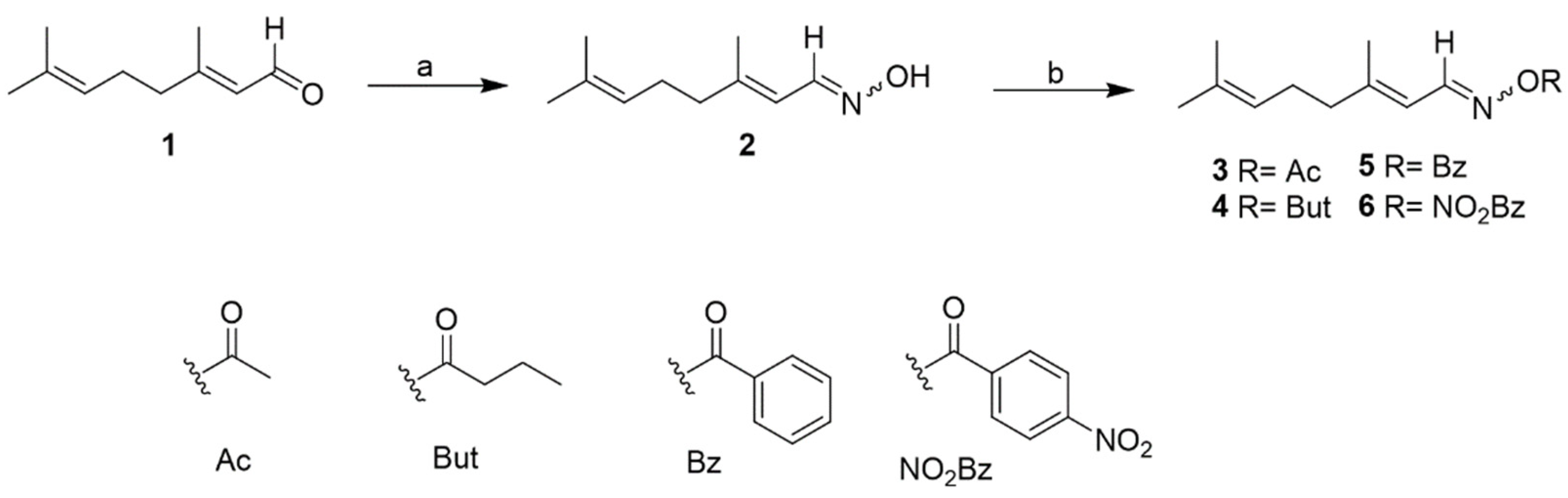
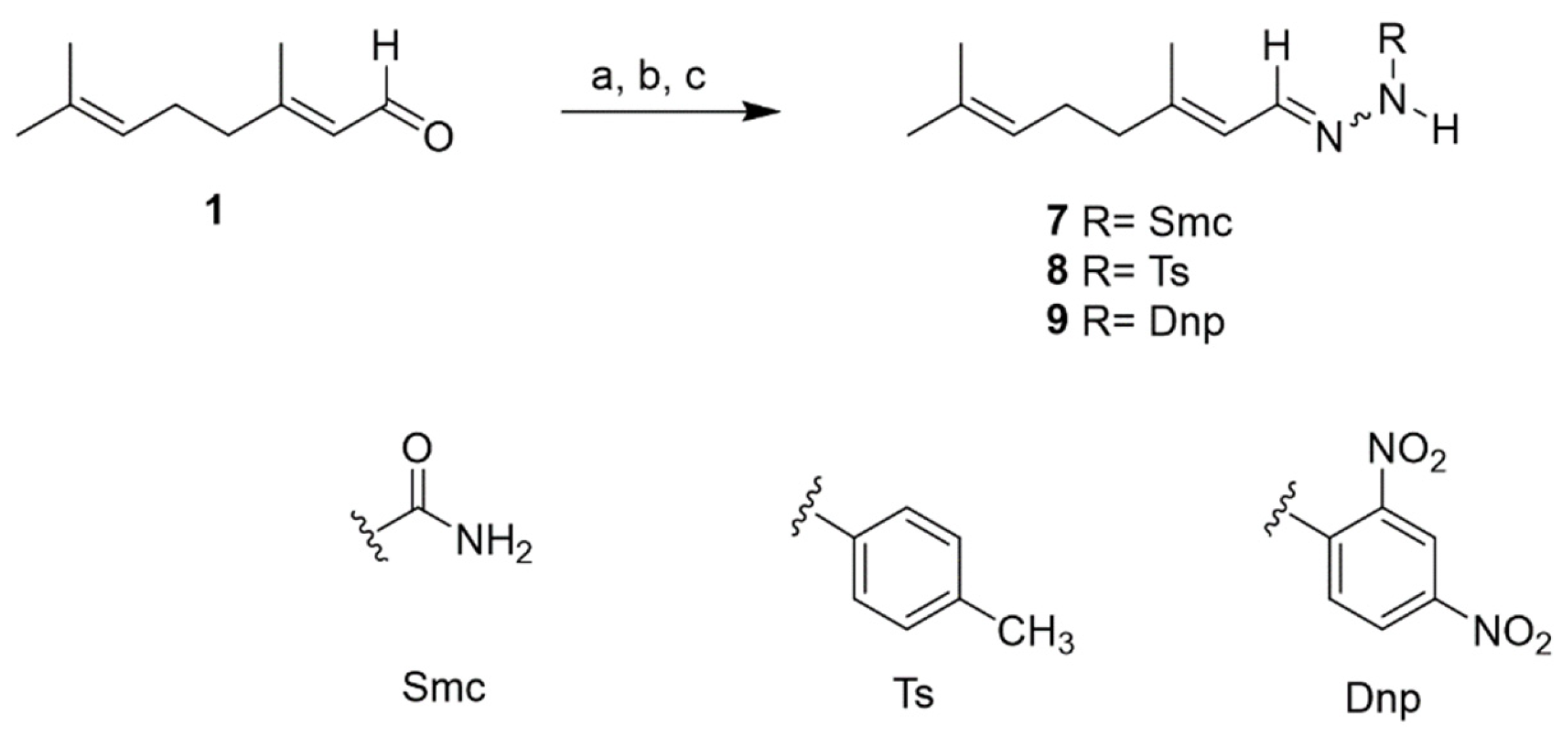
2.2. Chemistry
2.3. In Vitro Kinetoplastid Activity of Citral Derivatives
2.4. In Vitro Leishmanicidal Activity on Intracellular Amastigotes
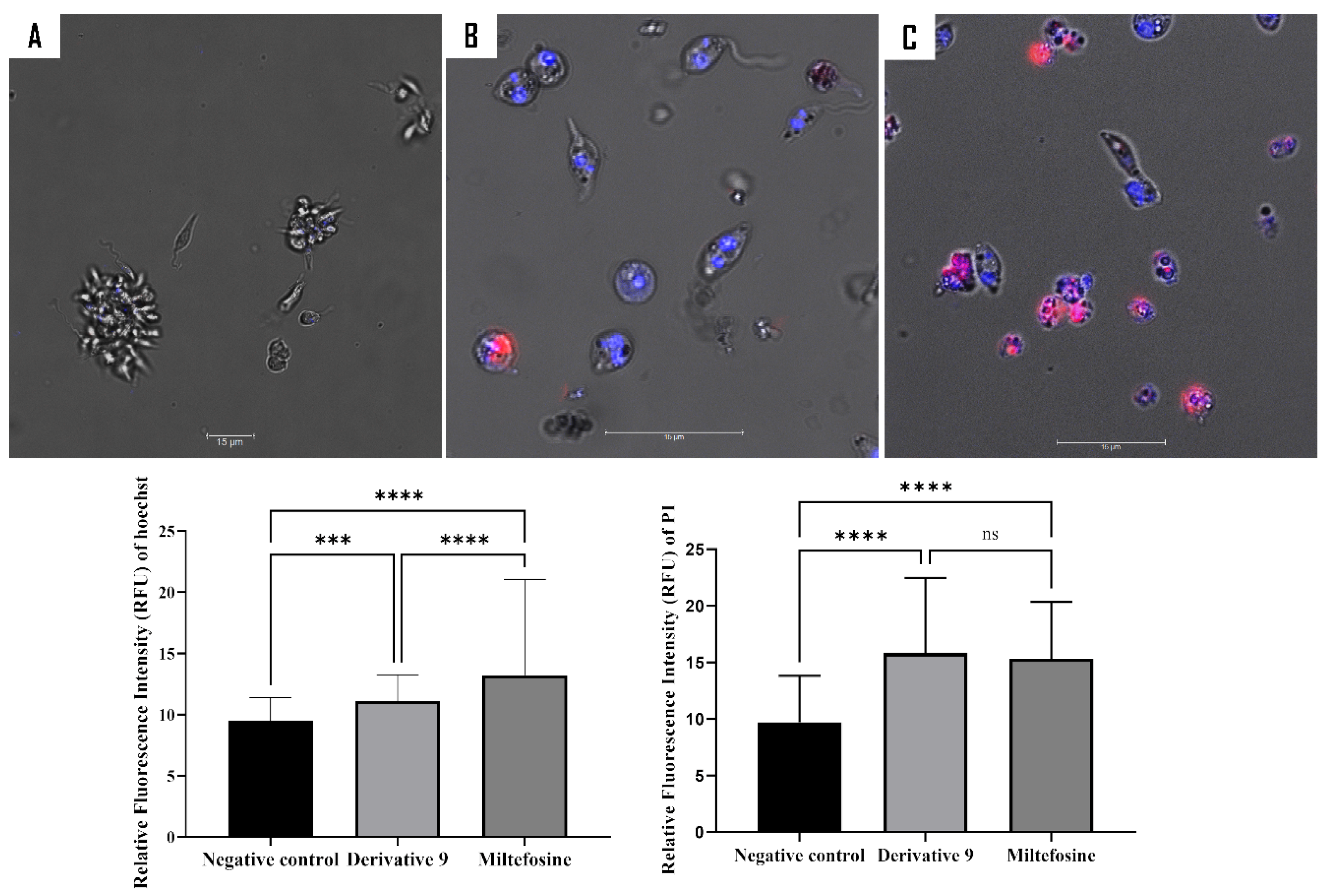
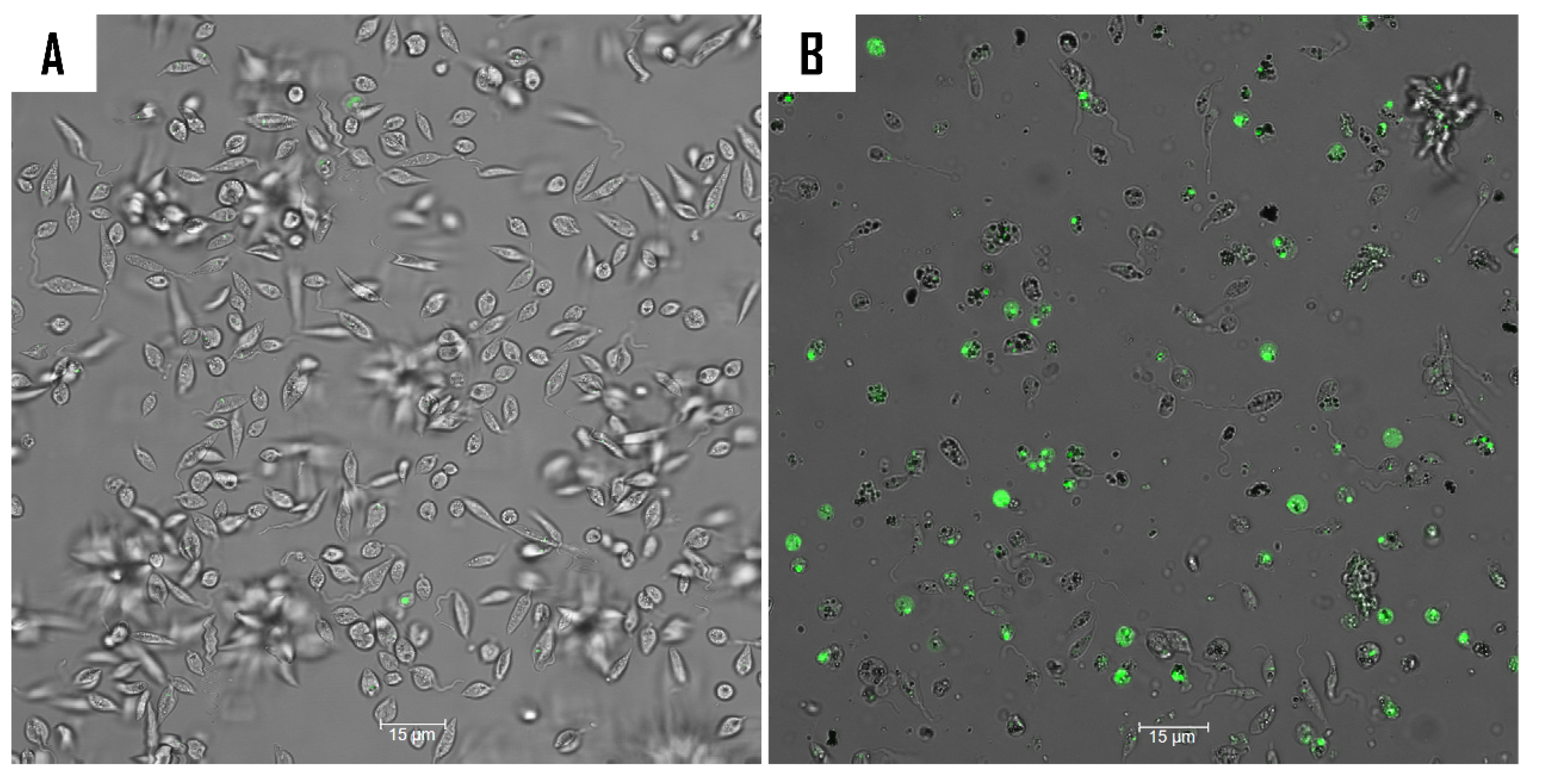
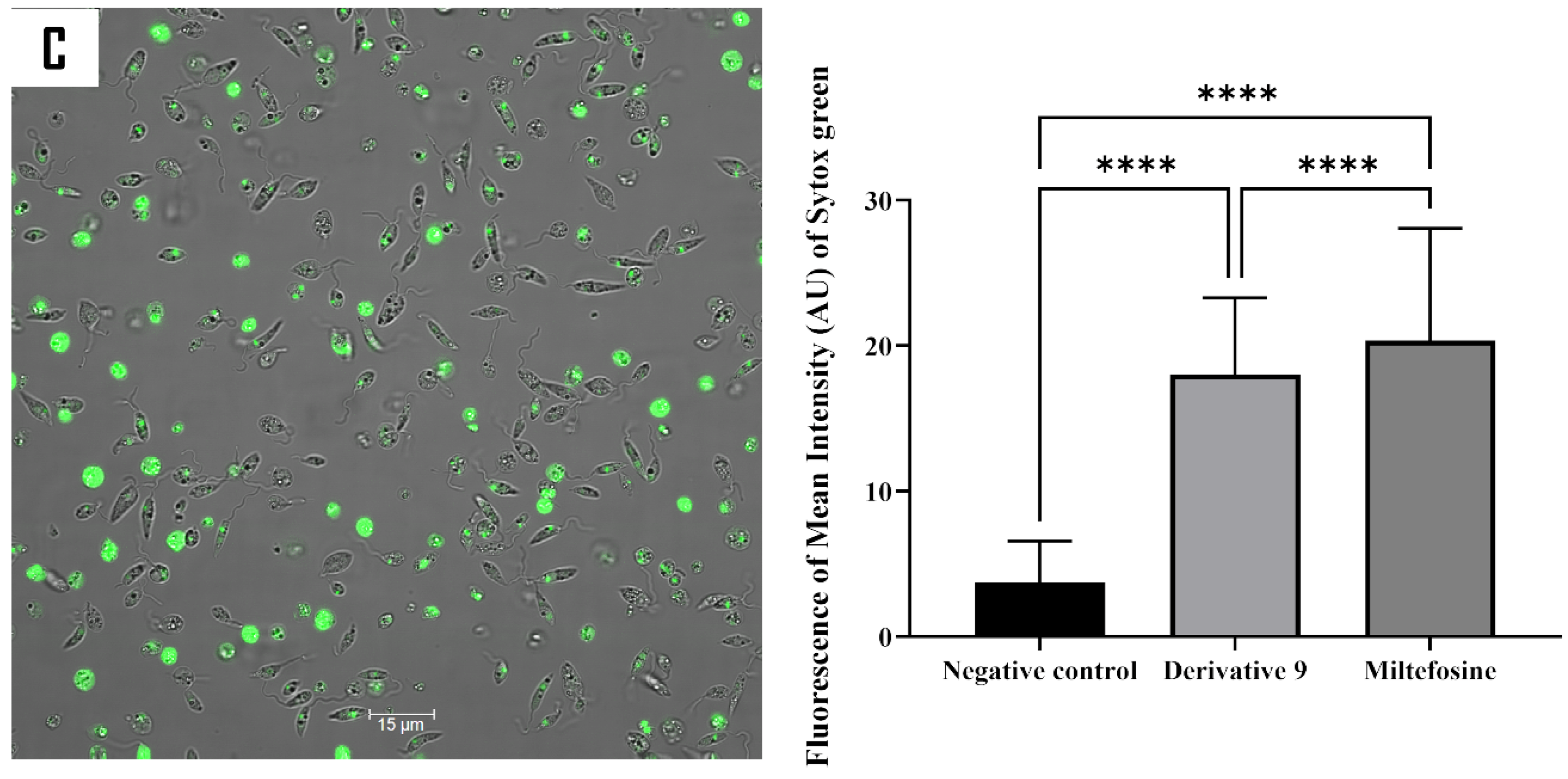
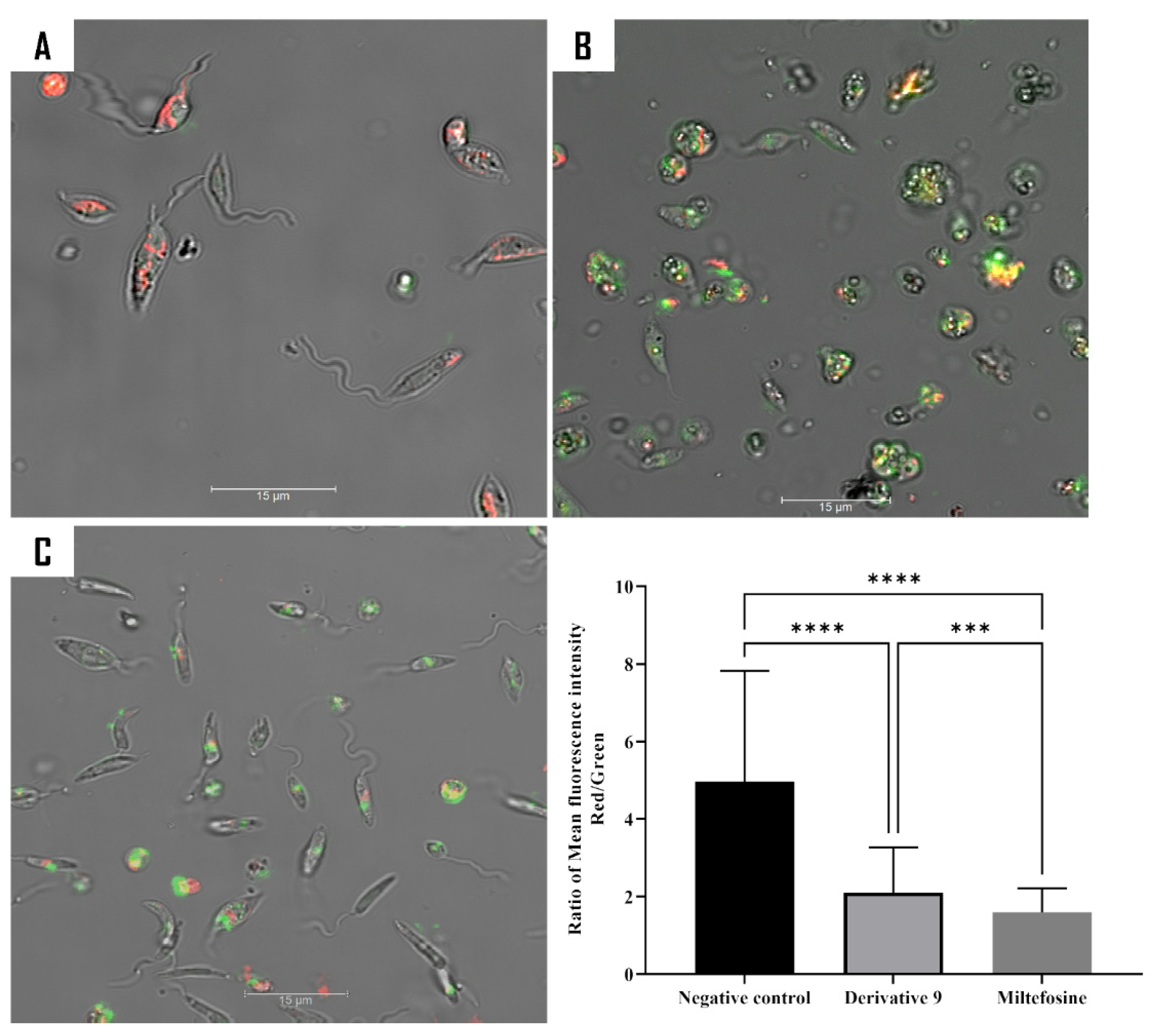
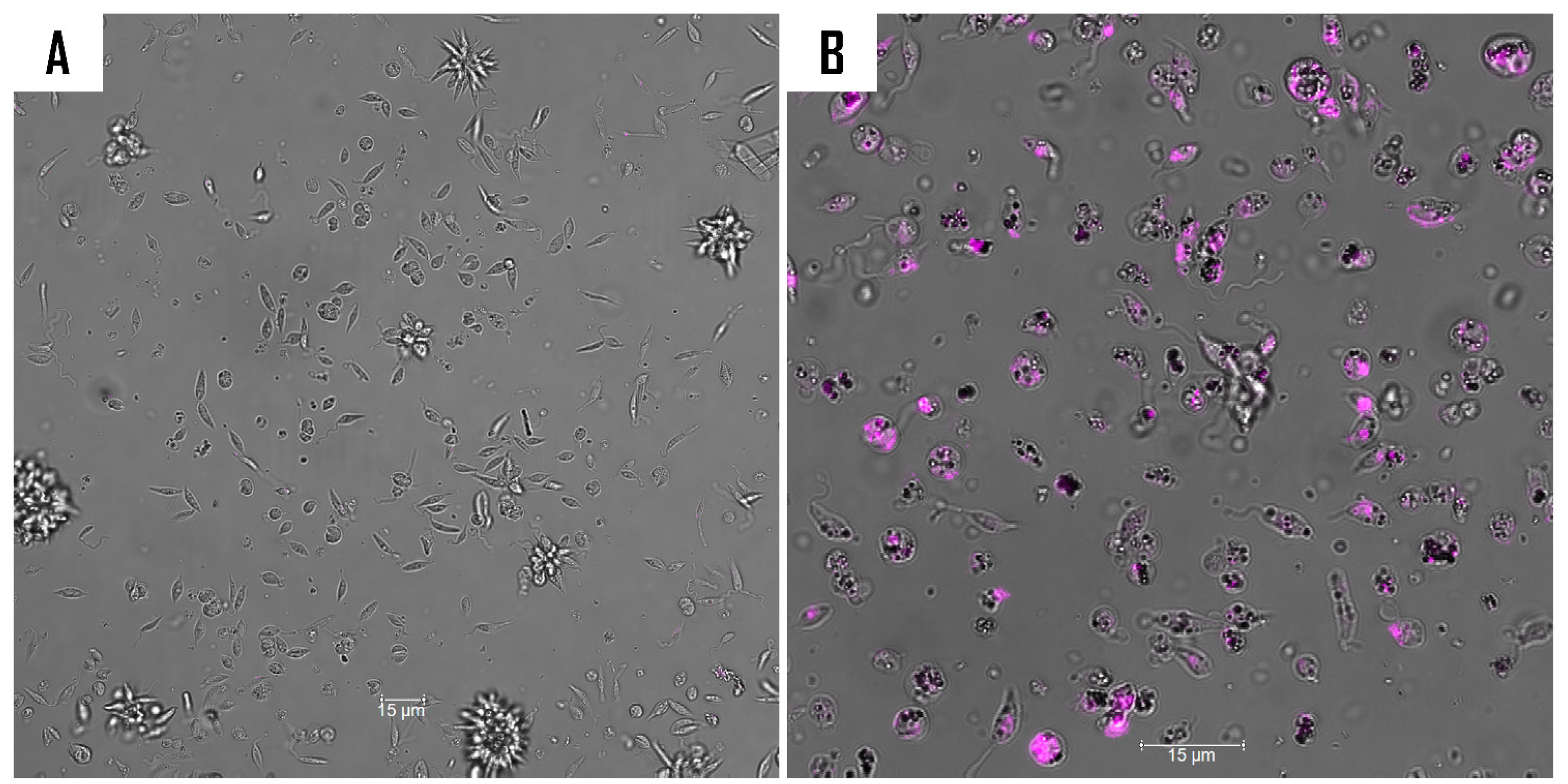
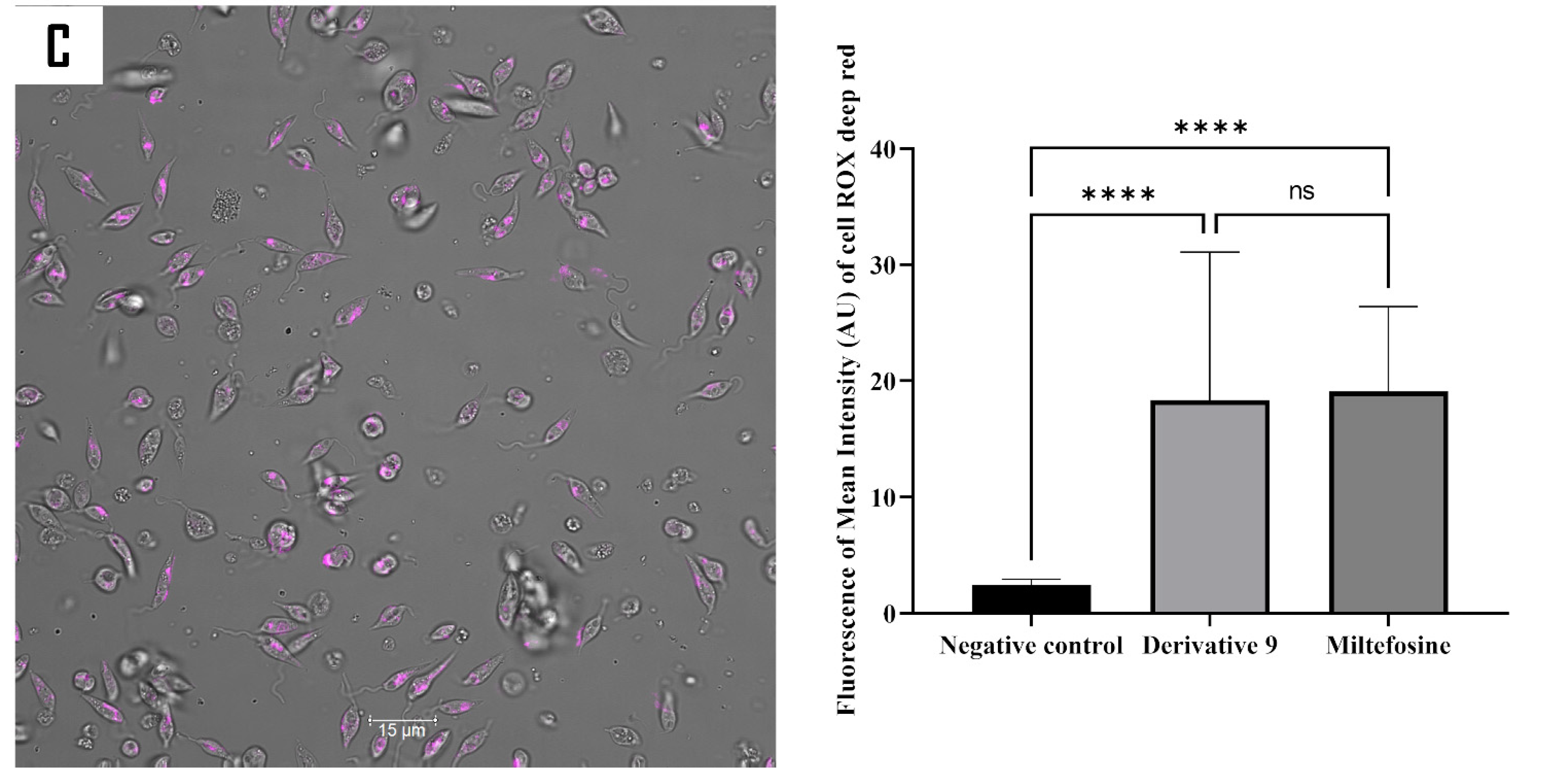
2.5. Mechanism of Action of Compound 9
3. Materials and Methods
3.1. General Procedures
3.2. Plant MaterialCitral
3.3. Preparation of Aloysia citrodora Essential Oil
3.4. Gas Chromatography–Mass Spectrometry Analysis
3.5. Bioactivity-Guided Chromatography Fractionation and Isolation
3.6. Compounds Synthesis
3.7. Biological Assays
3.7.1. In Vitro Leishmanicidal Activity on L. amazonensis Promastigote
3.7.2. In Vitro Trypanocidal Activity on T. cruzi Epimastigotes
3.7.3. In Vitro Leishmanicidal Activity on L. amazonensis Intracellular Amastigotes
3.7.4. Cytotoxicity Activity on Murine Macrophages
3.8. Elucidation of the Mechanism of Action
3.8.1. Double Stain Assay for Detecting Programmed Cell Death (PCD)
3.8.2. Analysis of Plasma Membrane Permeability
3.8.3. Analysis of Mitochondrial Function
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, J.A. Parasitic diseases. In Historical Diseases from a Modern Perspective; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Deiana, G.; Arghittu, A.; Dettori, M.; Castiglia, P. One world, One health: Zoonotic diseases, parasitic diseases, and infectious diseases. Healthcare 2024, 12, 922. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Teli, G.; Akhtar, M.J.; Matada, G.S.P. The role of natural anti-parasitic guided development of synthetic drugs for leishmaniasis. Eur. J. Med. Chem. 2023, 258, 115609. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Leishmaniasis, Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 10 January 2025).
- Forsyth, C.; Agudelo Higuita, N.I.; Hamer, S.A.; Ibarra-Cerdeña, C.N.; Valdez-Tah, A.; Stigler Granados, P.; Hamer, G.L.; Vingiello, M.; Beatty, N.L. Climate change and Trypanosoma cruzi transmission in North and Central America. Lancet Microbe. 2024, 5, 100946. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Chagas Disease (Also Known as American Trypanosomiasis), Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 10 January 2025).
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. What constitutes a neglected tropical disease? PLOS Negl. Trop. Dis. 2020, 14, e0008001. [Google Scholar] [CrossRef]
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Alvarez-Bardon, M.; Perez-Pertejo, Y.; Ordonez, C.; Sepulveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening marine natural products for new drug leads against trypanosomatids and malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Surede, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nass, J.; Efferth, T. The activity of Artemisia spp. and their constituents against Trypanosomiasis. Phytomedicine 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Gazolla, P.A.R.; da Silva, A.M.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, R.S.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-trypanosomatidae activity of essential oils and their main components from selected medicinal plants. Molecules 2023, 28, 1467. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Hidmi, A. Chemical composition, anticancer, antimicrobial activity of Aloysia citriodora Palau essential oils from four different locations in Palestine. BMC Complement Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and antiproliferation activities of lemon verbena (Aloysia citrodora): An in vitro and in vivo study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Kiafar, R.; Zadeh, M.A.; Khommami, A.M. Investigation of the effect of some organic fertilizers on the oil of lemon verbena (Lippia citriodora L.) and Its antibacterial effects. IJFAS J. 2013, 2, 866–871. [Google Scholar]
- Shafiee, F.; Moghadamnia, A.A.; Shahandeh, Z.; Sadighian, F.; Khodadadi, E. Evaluation of the antibacterial effects of aqueous and ethanolic leaf extracts of Aloysia citriodora (Lemon verbena) on Streptococcus mutans and Streptococcus sobrinus. Electron Physician 2016, 8, 3363–3368. [Google Scholar] [CrossRef]
- Felgines, C.; Fraisse, D.; Besson, C.; Vasson, M.-P.; Texier, O. Bioavailability of lemon verbena (Aloysia triphylla) polyphenols in rats: Impact of colonic inflammation. Br. J. Nutr. 2014, 111, 1773–1781. [Google Scholar] [CrossRef]
- Sabotič, J.; Bayram, E.; Ezra, D.; Gaudêncio, S.P.; Haznedaroğlu, B.Z.; Janež, N.; Ktari, L.; Luganini, A.; Mandalakis, M.; Safarik, I.; et al. A guide to the use of bioassays in exploration of natural resources. Biotechnol. Adv. 2024, 71, 108307. [Google Scholar] [CrossRef]
- Garcia, M.C.F.; Soares, D.C.; Santana, R.C.; Saraiva, E.M.; Siani, A.C.; Ramos, M.F.S.; Danelli, M.D.G.M.; Souto-Padron, T.C.; Pinto-da-Silva, L.H. The in vitro antileishmanial activity of essential oil from Aloysia gratissima and guaiol, its major sesquiterpene against Leishmania amazonensis. Parasitology 2018, 145, 1219–1227. [Google Scholar] [CrossRef]
- Marques, E.M.; Rocha, R.L.; Brandão, C.M.; Xavier, J.K.A.M.; Camara, M.B.P.; Mendonça, C.d.J.S.; de Lima, R.B.; Souza, M.P.; Costa, E.V.; Gonçalves, R.S. Development of an Eco-Friendly Nanogel Incorporating Pectis brevipedunculata Essential Oil as a Larvicidal Agent Against Aedes aegypti. Pharmaceutics 2024, 16, 1337. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.R.; dos Santos, A.O.; Nakamura, C.V.; Dias Filho, B.P.; Ferreira, I.C.; Ueda-Nakamura, T. In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis. Parasitol. Res. 2009, 105, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallah, S.; Cianfaglione, K.; Azzouz, M.; Batiha, G.E.; Alkhuriji, A.F.; Al-Megrin, W.A.I.; Ben-Attia, M.; Eldahshan, O.A. Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils. Pharmaceuticals 2022, 15, 1163. [Google Scholar] [CrossRef]
- Quintero, W.L.; Moreno, E.M.; Pinto, S.M.L.; Sanabria, S.M.; Stashenko, E.; García, L.T. Immunomodulatory, trypanocide, and antioxidant properties of essential oil fractions of Lippia alba (Verbenaceae). BMC Complement Med. Ther. 2021, 21, 187. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Batohi, N.; Lone, S.A.; Marimani, M.; Wani, M.Y.; Al-Bogami, A.S.; Ahmad, A. Citral and its derivatives inhibit quorum sensing and biofilm formation in Chromobacterium violaceum. Arch. Microbiol. 2021, 203, 1451–1459. [Google Scholar] [CrossRef]
- Dikusar, E.A.; Zhukovskaya, N.A.; Moiseichuk, K.L.; Zalesskaya, E.G.; Kurman, P.V.; Vyglazov, O.G. Preparative Synthesis of Veratraldehyde and Citral Oxime Esters. Russ. J. Appl. Chem. 2008, 81, 643–646. [Google Scholar] [CrossRef]
- Varalaxmi, K.; Pradhan, K.; Begam, H.M.; Polley, A.; Kumar, D.; Jana, R. Transition-metal-free iterative two-fold reductive coupling and 1,3-borotropic shift to form 1,4-skipped dienes. Org. Biomol. Chem. 2024, 22, 8596. [Google Scholar] [CrossRef]
- Mohapatra, S.C.; Tehlan, S.; Hundal, M.S.; Mathur, P. Crystal structure and catalytic activity of a copper (II) complex based on a tetradentate bis-benzimidazole diamide ligand. Inorganica Chim. Acta 2008, 361, 1897–1907. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays Related to Cancer Drug Discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; pp. 71–133. [Google Scholar]
- Zhivotovsky, B.; Orrenius, S. Current concepts in cell death. Curr. Protoc. Cell Biol. 2001, 11, 1–18. [Google Scholar] [CrossRef]
- Núñez, M.J.; Martínez, M.L.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Jiménez, I.A.; Lorenzo-Morales, J.; Piñero, J.E.; Bazzocchi, I.L. In vitro susceptibility of kinetoplastids to celastroloids from Maytenus chiapensis. Antimicrob. Agents. Chemother. 2021, 65, e02236-20. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Selective activity of oleanolic and maslinic acids on the amastigote form of Leishmania spp. Iran J. Pharm. Res. 2017, 16, 1190–1193. [Google Scholar]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. In vitro activity and mechanism of cell death induction of cyanomethyl vinyl ethers derivatives against Trypanosoma cruzi. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Salazar-Villatoro, L.; Omaña-Molina, M.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. Acrylonitrile derivatives: In vitro activity and mechanism of cell death induction against Trypanosoma cruzi and Leishmania amazonensis. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100531. [Google Scholar] [CrossRef]


| N° | Compound a | Composition b (%) | Formula | RT c (min) | KIexp d | KItheo e |
|---|---|---|---|---|---|---|
| 1 | Sabinene | 2.59 | C10H16 | 9.44 | 969 | 974 |
| 2 | Sulcatone | 1.85 | C8H14O | 9.95 | - | - |
| 3 | D-Limonene | 16.0 | C10H16 | 11.4 | 1089 | 1018 |
| 4 | Eucalyptol | 6.91 | C10H18O | 11.5 | 1093 | 1059 |
| 5 | α-Terpineol | 1.92 | C10H18O | 17.2 | 1387 | 1190 |
| 6 | Neral | 11.1 | C10H16O | 19.0 | 1377 | 1228 |
| 7 | α-Citral | 11.8 | C10H16O | 20.0 | 1413 | 1174 |
| 8 | Nerolidyl acetate | 2.08 | C17H28O | 23.7 | 1701 | 1805 |
| 9 | Caryophyllene | 2.87 | C15H24 | 24.8 | 1756 | 1423 |
| 10 | α-Curcumene | 8.71 | C15H22 | 26.8 | 1586 | 1524 |
| 11 | Spathulenol | 4.21 | C15H24O | 29.6 | 1658 | 1576 |
| 12 | Caryophyllene oxide | 6.34 | C15H24O | 29.7 | 1663 | 1581 |
| Samples | L. amazonensis IC50 µg/mL a | T. cruzi IC50 µg/mL | Samples | L. amazonensis IC50 µg/mL | T. cruzi IC50 µg/mL |
|---|---|---|---|---|---|
| EO | 10.67 ± 2.42 | 22.60 ± 7.84 | EO2A | ˃100 | ˃100 |
| EO1 | 17.38 ± 5.77 | 24.35 ± 4.82 | EO2B | ˃100 | ˃100 |
| EO2 | 36.71 ± 4.07 | 59.11 ± 4.97 | EO2C | 12.62 ± 2.30 | 26.27 ± 2.07 |
| EO3 | ˃100 | ˃100 | EO2D | 61.91 ± 6.32 | 34.41 ± 4.86 |
| EO4 | ˃100 | ˃100 | Citral (1) | 8.47 ± 4.59 | 12.90 ± 1.92 |
| EO5 | ˃100 | ˃100 | Miltefosine b | 2.64 ± 0.09 | |
| Benznidazole c | 1.81 ± 0.50 |
| N° | Compound a | Composition b (%) | Formula | RT c (min) | KIexp d | KItheo e |
|---|---|---|---|---|---|---|
| 1 | Eucalyptol | 10.6 | C10H18O | 11.9 | 1030 | 1032 |
| 2 | Carveol | 18.1 | C10H16O | 18.6 | 1220 | 1219 |
| 3 | D-Verbenone | 3.31 | C10H14O | 18.9 | 1231 | 1324 |
| 4 | Citral | 1.13 | C10H16O | 20.3 | 1272 | 1276 |
| 5 | Myrtenyl acetate | 4.49 | C12H18O2 | 20.8 | 1287 | 1327 |
| 6 | Acetomesitylene | 2.29 | C9H12 | 21.7 | 1312 | 1369 |
| 7 | Limonene-1,2-diol | 26.8 | C10H18O2 | 22.7 | 1344 | 1321 |
| 8 | 2-(Phenylcyclobutyl) benzene | 0.99 | C16H16 | 23.0 | 1354 | 1749 |
| 9 | Lavandulol acetate | 16.9 | C12H20O2 | 24.1 | 1386 | 1270 |
| 10 | 4-(4-Methylphenyl) pentanal | 17.5 | C12H16O | 24.7 | 1404 | 1429 |
| 11 | 2-epi-α-Funebrene | 3.08 | C15H24 | 25.0 | 1413 | 1403 |
| 12 | β-Copaene | 5.25 | C15H24 | 25.2 | 1422 | 1432 |
| 13 | Neryl (S)-2-methylbutanoate | 7.33 | C15H26O2 | 26.9 | 1477 | 1576 |
| 14 | Isoledene | 2.03 | C15H24 | 27.0 | 1478 | 1375 |
| 15 | α-Curcumene | 57.7 | C15H22 | 27.2 | 1465 | 1483 |
| 16 | (±)-γ-Muurolene | 3.89 | C15H24 | 28.1 | 1539 | 1477 |
| 17 | cis-Calamenene | 2.11 | C15H22O | 28.4 | 1561 | 1531 |
| 18 | β-Calacorene | 1.00 | C15H22 | 28.9 | 1601 | 1563 |
| 19 | α-Calacorene | 6.62 | C15H22 | 28.9 | 1601 | 1542 |
| 20 | Caryophyllene oxide | 10.7 | C15H24O | 29.2 | 1624 | 1581 |
| 21 | Norbourbonone | 3.21 | C9H12O | 29.5 | 1639 | 1563 |
| 22 | α-Dehydro-ar-himachalene | 1.61 | C15H22 | 29.9 | 1663 | 1601 |
| Cmpds | L. amazonensis Promastigotes | Selectivity | L. amazonensis Amastigotes | Selectivity | Cytotoxicity |
|---|---|---|---|---|---|
| IC50 a | SI c | IC50 a | SI c | CC50 b | |
| 1 | 55.64 ± 30.02 | 6.97 | >300 | ||
| 2 | 40.84 ± 19.43 | 12.61 | 56.86 ± 19.13 | 9.39 | >300 |
| 5 | 29.30 ± 8.07 | 10.41 | >300 | ||
| 6 | 34.17 ± 14.44 | 9.25 | >300 | ||
| 7 | 43.75 ± 12.05 | 9.29 | >300 | ||
| 8 | 40.17 ± 22.43 | 7.78 | 34.45 ± 6.56 | 9.07 | >300 |
| 9 | 10.62 ± 4.78 | 2.98 | 21.45 ± 0.65 | 1.47 | 31.62 ± 1.96 |
| M d | 6.47 ± 0.22 | 11.16 | 3.12 ± 0.30 | 23.13 | 72.18 ± 8.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omrani, A.; Ben Youssef, M.; Sifaoui, I.; Hernández-Álvarez, E.; Trujillo-Rodríguez, M.J.; Saura-Cayuela, M.; Pino, V.; Sebai, H.; Bazzocchi, I.L.; Lorenzo-Morales, J.; et al. Bioprospecting for Anti-Kinetoplastid Drug Discovery from Aloysia citrodora Essential Oil. Int. J. Mol. Sci. 2025, 26, 5697. https://doi.org/10.3390/ijms26125697
Omrani A, Ben Youssef M, Sifaoui I, Hernández-Álvarez E, Trujillo-Rodríguez MJ, Saura-Cayuela M, Pino V, Sebai H, Bazzocchi IL, Lorenzo-Morales J, et al. Bioprospecting for Anti-Kinetoplastid Drug Discovery from Aloysia citrodora Essential Oil. International Journal of Molecular Sciences. 2025; 26(12):5697. https://doi.org/10.3390/ijms26125697
Chicago/Turabian StyleOmrani, Amani, Meriam Ben Youssef, Ines Sifaoui, Eduardo Hernández-Álvarez, María J. Trujillo-Rodríguez, Montse Saura-Cayuela, Verónica Pino, Hichem Sebai, Isabel L. Bazzocchi, Jacob Lorenzo-Morales, and et al. 2025. "Bioprospecting for Anti-Kinetoplastid Drug Discovery from Aloysia citrodora Essential Oil" International Journal of Molecular Sciences 26, no. 12: 5697. https://doi.org/10.3390/ijms26125697
APA StyleOmrani, A., Ben Youssef, M., Sifaoui, I., Hernández-Álvarez, E., Trujillo-Rodríguez, M. J., Saura-Cayuela, M., Pino, V., Sebai, H., Bazzocchi, I. L., Lorenzo-Morales, J., Piñero, J. E., & Jiménez, I. A. (2025). Bioprospecting for Anti-Kinetoplastid Drug Discovery from Aloysia citrodora Essential Oil. International Journal of Molecular Sciences, 26(12), 5697. https://doi.org/10.3390/ijms26125697













