Production of Transgenic Silkworm Using Anti-Serum Against Diapause Hormone in Diapause Strains of Silkworm, Bombyx mori
Abstract
1. Introduction
2. Results
2.1. Sensitivity of Different Diapause Strains to 15 °C-IME-DD on the Non-Diapause Trait
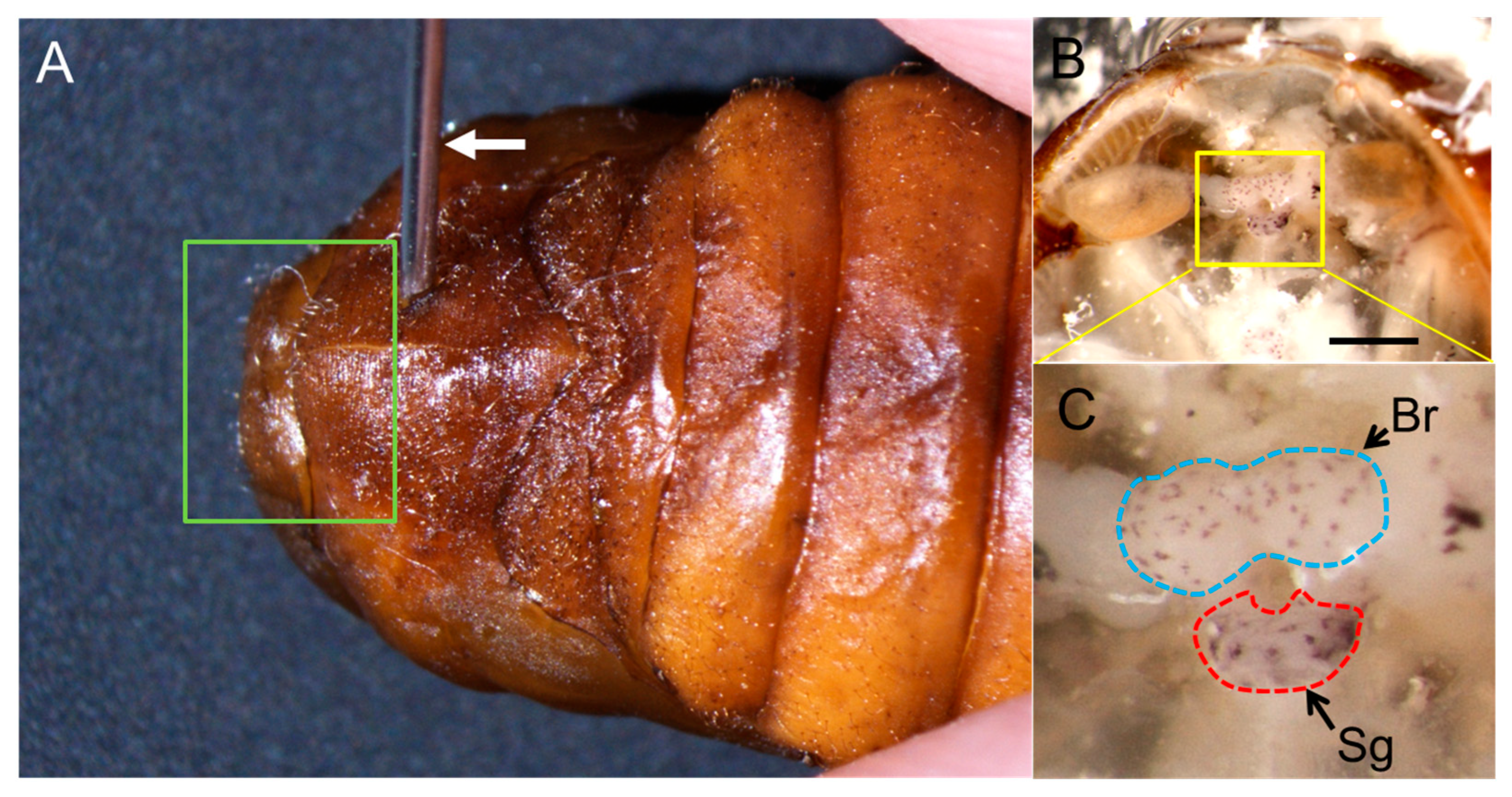
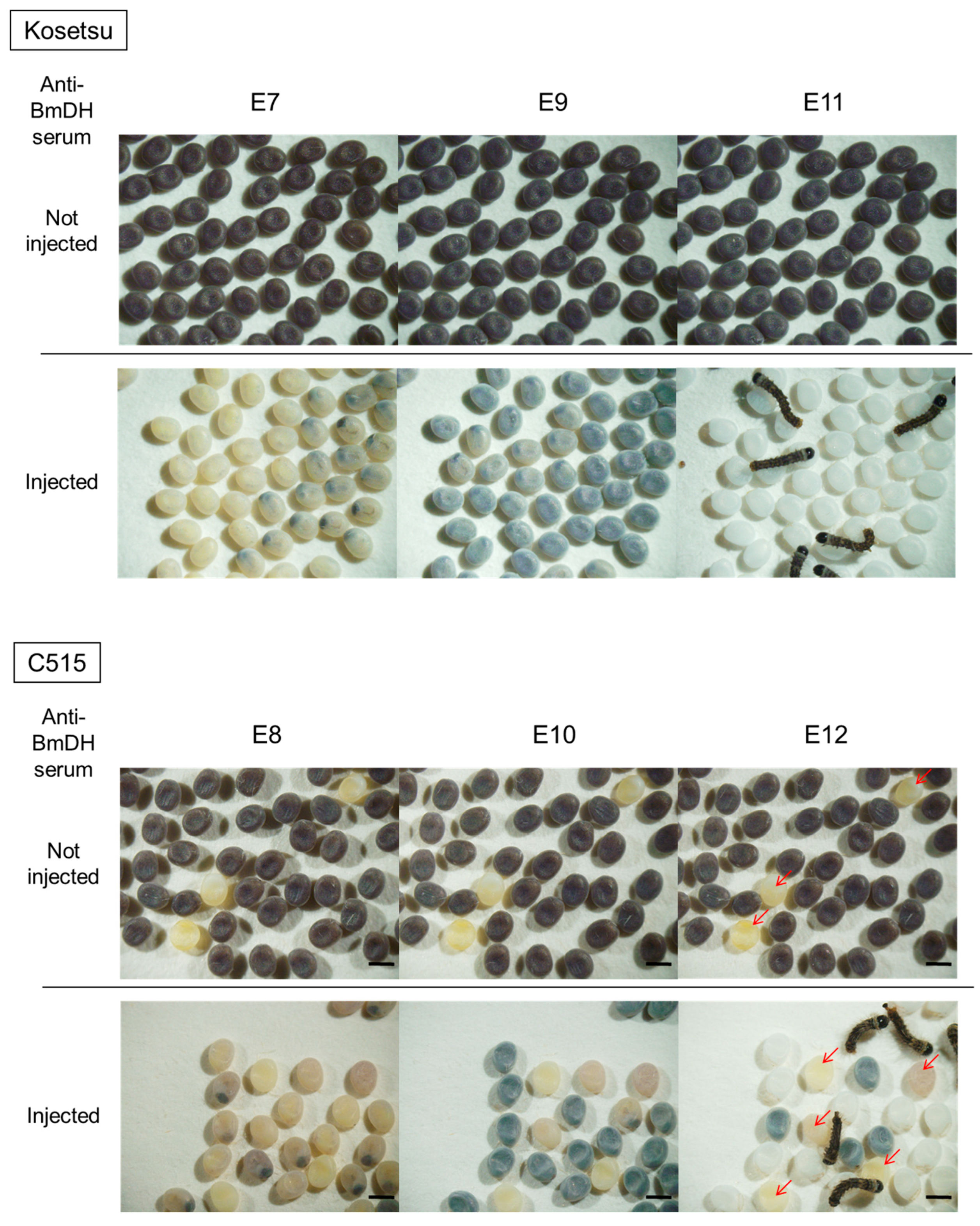
2.2. Injection of Anti-BmDH Serum into Pupa and Production of Non-Diapause Eggs
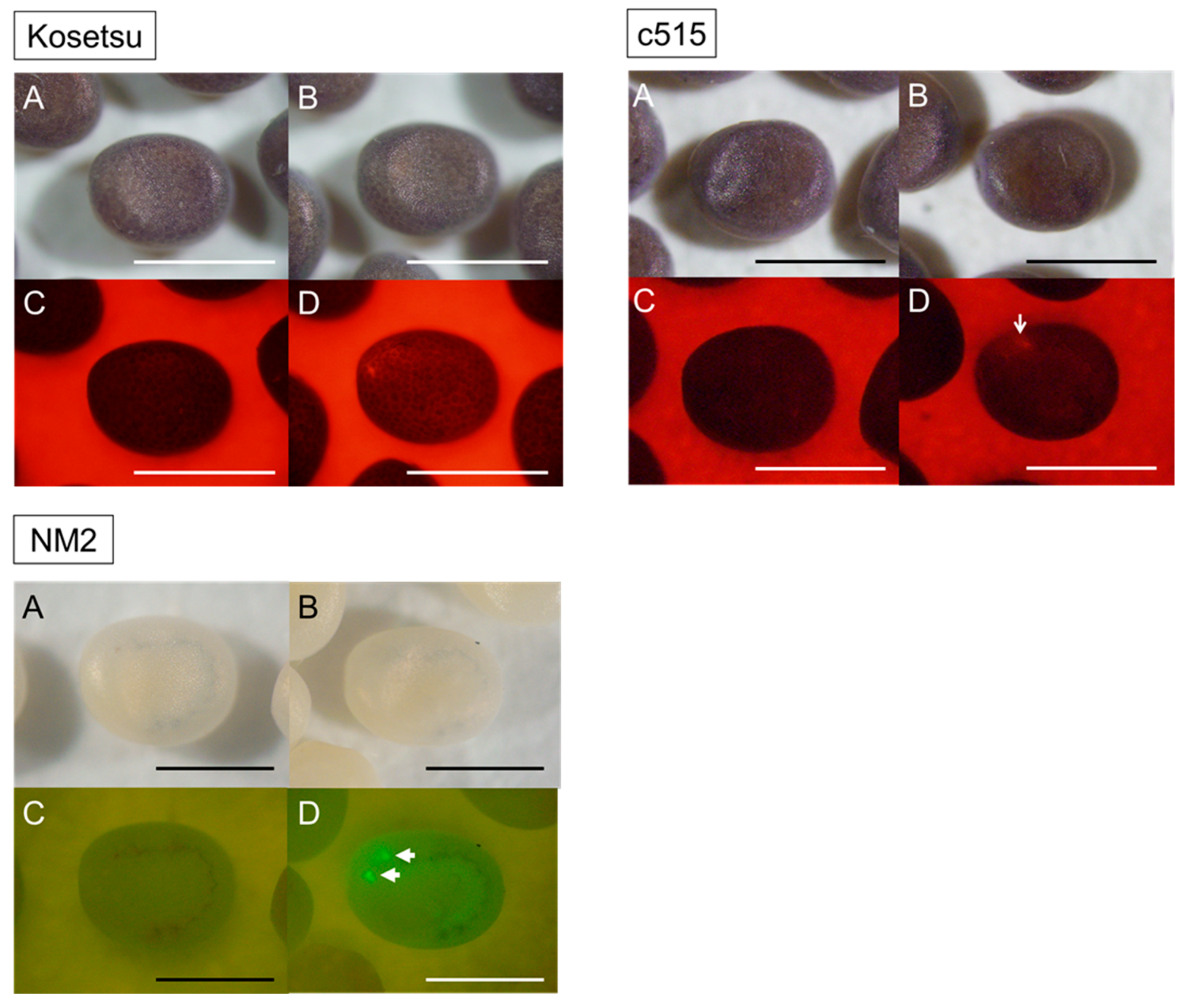
2.3. Production of Transgenic Silkworms
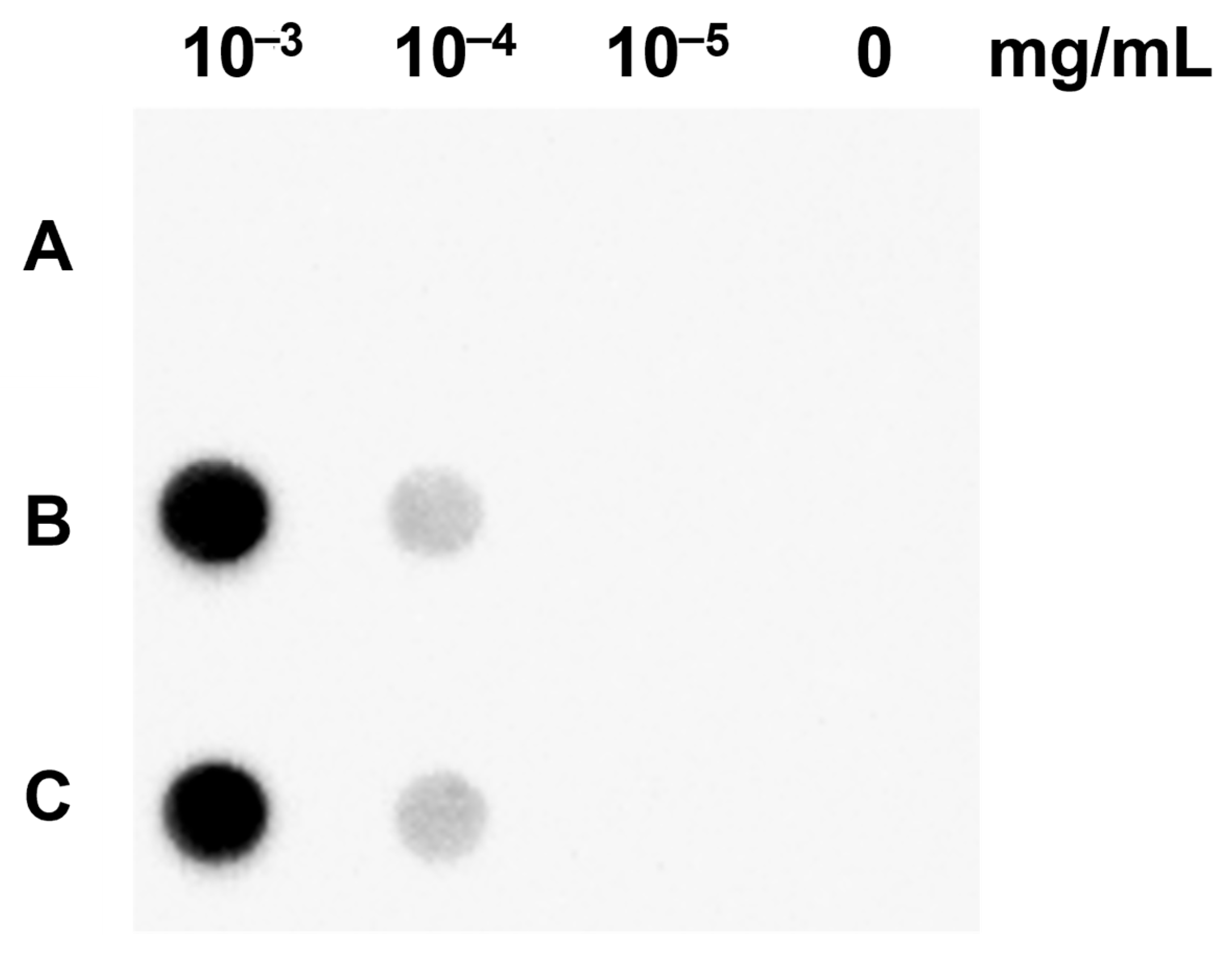
2.4. Genomic Southern Blotting Analysis of Transgenic Lines
3. Discussion
4. Materials and Methods
4.1. Strain and Rearing
4.2. 15 °C-IME-DD Treatment
4.3. Preparation of Plasmid DNA
4.4. Preparation of Non-Diapause Eggs and Embryonic Microinjection
4.5. Screening of Transgenic Silkworms
4.6. Southern Blotting Analysis
4.7. Dot Blotting Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15 °C-IME-DD | incubating maternal eggs at 15 °C in constant darkness |
| aDHST | anti-diapause hormone serum treatment |
| BmDH | diapause hormone of Bombyx mori |
| DMSO | dimethyl sulfoxide |
| HCl | hydrochloric acid |
| HT | HCl treatment |
| ITR | inverted terminal repeat |
| SG | suboesophageal ganglion |
| W3HO-HT | HCl treatment within 3 h of oviposition |
Appendix A
| Strains (Experimental No.) | Treatments in Parents † | No. of Broods Examined in G0 (n=) | Average No. of Diapause Eggs in G0 Broods (Mean ± SD) | Average No. of Eggs Unfertilized in G0 Broods (Mean ± SD) | Average No. of Eggs Died in G0 Broods (Mean ± SD) | Average No. of Eggs Hatched in G0 Broods (Mean ± SD) | Average No. of Eggs Fertilized in G0 Broods (Mean ± SD) | Percentage of Eggs Hatched in Fertilized Eggs |
|---|---|---|---|---|---|---|---|---|
| C510 | Non-treat. | 7 | 386 ± 202 | 33 ± 32 | 0 ± 0 | 0 ± 0 | 419 ± 201 | 0% |
| C510 | Serum 10 μL | 4 | 294 ± 81 | 8 ± 5 | 17 ± 9 | 185 ± 76 | 504 ± 88 | 36.7% |
| C510 | Serum 30 μL | 6 | 37 ± 78 | 74 ± 48 | 15 ± 12 | 430 ± 138 | 556 ± 97 | 77.5% |
| C514 | Non-treat. | 5 | 383 ± 52 | 89 ± 53 | 2 ± 3 | 0 ± 0 | 475 ± 62 | 0% |
| C514 | 15 °C-IME-DD | 12 | 415 ± 107 | 80 ± 34 | 0 ± 0 | 0 ± 0 | 495 ± 99 | 0% |
| C514 | Serum 30 μL | 3 | 3 ± 3 | 80 ± 70 | 17 ± 3 | 296 ± 148 | 395 ± 85 | 74.9% |
| Kosetsu (Ex1) | Non-treat. | 6 | 414 ± 224 | 113 ± 164 | 4 ± 3 | 36 ± 59 | 566 ± 90 | 6.3% |
| Kosetsu (Ex1) | Serum 10 μL | 5 | 219 ± 207 | 15 ± 15 | 38 ± 18 | 326 ± 218 | 599 ± 73 | 54.5% |
| Kosetsu (Ex2) | Non-treat. | 8 | 363 ± 78 | 31 ± 31 | 1 ± 1 | 4 ± 5 | 398 ± 87 | 0.9% |
| Kosetsu (Ex2) | 15 °C-IME-DD | 4 | 0 ± 0 | 17 ± 8 | 74 ± 56 | 183 ± 95 | 273 ± 145 | 66.9% |
| Kosetsu (Ex2) | Serum 10 μL | 5 | 300 ± 133 | 26 ± 22 | 10 ± 7 | 30 ± 19 | 367 ± 137 | 8.3% |
| Kosetsu (Ex2) | Serum 30 μL | 7 | 4 ± 6 | 47 ± 44 | 40 ± 22 | 339 ± 130 | 430 ± 108 | 78.9% |
| Kosetsu (Ex3) | Non-treat. | 4 | 475 ± 20 | 5 ± 2 | 40 ± 35 | 1 ± 2 | 521 ± 20 | 0.1% |
| C515 (Ex1) | Non-treat. | 3 | 302 ± 150 | 226 ± 123 | 0 ± 0 | 0 ± 0 | 528 ± 44 | 0% |
| C515 (Ex1) | Serum 10 μL | 3 | 164 ± 86 | 156 ± 42 | 29 ± 30 | 51 ± 72 | 400 ± 166 | 12.8% |
| C515 (Ex2) | Non-treat. | 3 | 224 ± 65 | 88 ± 65 | 186 ± 57 | 0 ± 0 | 497 ± 56 | 0% |
| C515 (Ex2) | 15 °C-IME-DD | 3 | 64 ± 43 | 35 ± 18 | 265 ± 61 | 0 ± 1 | 364 ± 111 | 0.1% |
| C515 (Ex2) | Serum 10 μL | 5 | 263 ± 146 | 23 ± 13 | 78 ± 86 | 182 ± 65 | 546 ± 89 | 33.2% |
| C515 (Ex2) | Serum 30 μL | 5 | 50 ± 80 | 30 ± 12 | 85 ± 101 | 398 ± 83 | 563 ± 78 | 70.7% |
| MN2 | Non-treat. | 7 | 358 ± 139 †† | N/A | 0 ± 0 | 0 ± 0 | 358 ± 139 †† | 0% |
| MN2 | Serum 90 μL | 10 | 396 ± 170 †† | N/A | 59 ± 108 | 47 ± 77 | 502 ± 123 †† | 9.3% |
| J137 | Non-treat. | 4 | 131 ± 129 | 81 ± 108 | 1 ± 2 | 0 ± 0 | 212 ± 128 | 0% |
| J137 (Ex1) | 15 °C-IME-DD | 7 | 7 ± 11 | 134 ± 75 | 144 ± 20 | 147 ± 46 | 432 ± 36 | 34.1% |
| J137 (Ex2) | 15 °C-IME-DD | 8 | 3 ± 5 | 329 ± 87 | 47 ± 39 | 47 ± 56 | 426 ± 128 | 11.1% |
| J137 | Serum 30 μL | 7 | 311 ± 98 | 17 ± 13 | 21 ± 16 | 108 ± 79 | 457 ± 130 | 23.7% |
| J603 | Serum 30 μL | 6 | 208 ± 93 | 42 ± 32 | 39 ± 22 | 89 ± 65 | 377 ± 76 | 23.6% |
| J604 | Non-treat. | 8 | 465 ± 55 | 14 ± 6 | 0 ± 0 | 0 ± 0 | 479 ± 50 | 0% |
| J604 | 15 °C-IME-DD | 12 | 443 ± 48 | 58 ± 40 | 7 ± 3 | 2 ± 2 | 510 ± 47 | 0.3% |
| J604 | Serum 30 μL | 13 | 335 ± 152 | 105 ± 75 | 17 ± 10 | 66 ± 124 | 522 ± 94 | 12.6% |
| DH6 | Non-treat. | 6 | 366 ± 46 | 4 ± 2 | 1 ± 1 | 3 ± 3 | 373 ± 46 | 0.8% |
| DH6 | Serum 30 μL | 7 | 1 ± 2 | 4 ± 3 | 12 ± 7 | 396 ± 27 | 413 ± 33 | 95.9% |
| p50T | Non-treat. | 6 | 351 ± 96 | 53 ± 96 | 4 ± 5 | 10 ± 15 | 417 ± 84 | 2.4% |
| p50T | Serum 30 μL | 6 | 0 ± 0 | 9 ± 6 | 37 ± 49 | 331 ± 124 | 376 ± 84 | 88.0% |
| w1 | Non-treat. | 10 | 413 ± 67 | 16 ± 18 | 13 ± 21 | 1 ± 1 | 443 ± 64 | 0.2% |
| w1 | Serum 30 μL | 8 | 119 ± 75 | 87 ± 42 | 84 ± 36 | 200 ± 49 | 490 ± 59 | 40.9% |
References
- Dong, Z.; Qin, Q.; Hu, Z.; Zhang, X.; Miao, J.; Huang, L.; Chen, P.; Lu, C.; Pan, M. CRISPR/Cas12a Mediated Genome Editing Enhances Bombyx mori Resistance to BmNPV. Front. Bioeng. Biotechnol. 2020, 15, 841. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, X.L.; Liu, T.H.; Chen, P.; Jiang, X.; Dong, Z.Q.; Pan, M.H.; Lu, C. A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. Int. J. Mol. Sci. 2021, 22, 5618. [Google Scholar] [CrossRef]
- Yan, H.; Wen, F.; Xiang, H.; Wen, Y.; Shang, D.; Liu, A.; Niu, Y.; Xia, Q.; Wang, G. Biochemical characterization and overexpression of an alpha-amylase (BmAmy) in silkworm, Bombyx mori. Insect Mol. Biol. 2022, 31, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Yashwant, R.S.; Thomas, D.S.; Manoharan, C.; Roy, G.; Kunjupillai, V.; Mishra, R.K.; Nongthomba, U.; Gopalapillai, R. Transgenic Silkworms Overexpressing Relish and Expressing Drosomycin Confer Enhanced Immunity to Multiple Pathogens. Mol. Biotechnol. 2022, 64, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kojima, K.; Iga, M.; Yoshioka, T. Unique Material Properties of Bombyx mori Silk Fiber Incorporated with 3-Azidotyrosine. Biomacromolecules 2023, 24, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Ai, J.; Liu, Y.; He, X.; Xue, H.; Jia, C.; Chen, Z.; Xu, H.; Liu, R.; Yang, Y. Transgenic overexpression of UDP glycosyltransferase gene UGT41A3 induces resistance to nucleopolyhedrovirus in Bombyx mori. Transgenic Res. 2024, 34, 2. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.; He, S.; Hu, Y.; Meng, X.; Wei, J.; Li, T.; Pan, G.; Zhou, Z.; Li, C. Construction of microsporidia-inducible GAL4/UAS-RTA system to generate resistance to Nosema bombycis in Bombyx mori. Insect Sci. 2025, 1–16. [Google Scholar] [CrossRef]
- Ji, X.; Li, Y.; Wang, J.; Wang, G.; Ma, B.; Shi, J.; Cui, C.; Wang, R. Silk Protein Gene Engineering and Its Applications: Recent Advances in Biomedicine Driven by Molecular Biotechnology. Drug Des. Devel. Ther. 2025, 19, 599–626. [Google Scholar] [CrossRef]
- Sato, M.; Kojima, K.; Sakuma, C.; Murakami, M.; Tamada, Y.; Kitani, H. Production of scFv-conjugated affinity silk film and its application to a novel enzyme-linked immunosorbent assay. Sci. Rep. 2014, 4, 4080. [Google Scholar] [CrossRef]
- Sato, M.; Kitani, H.; Kojima, K. Development and validation of scFv-conjugated affinity silk protein for specific detection of carcinoembryonic antigen. Sci. Rep. 2017, 7, 16077. [Google Scholar] [CrossRef]
- Minagawa, S.; Nakaso, Y.; Tomita, M.; Igarashi, T.; Miura, Y.; Yasuda, H.; Sekiguchi, S. Novel recombinant feline interferon carrying N-glycans with reduced allergy risk produced by a transgenic silkworm system. BMC Vet. Res. 2018, 14, 260. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kojima, K.; Tamada, Y. Higher Gene Expression Related to Wound Healing by Fibroblasts on Silk Fibroin Biomaterial than on Collagen. Molecules 2020, 25, 1939. [Google Scholar] [CrossRef]
- Minagawa, S.; Sekiguchi, S.; Nakaso, Y.; Igarashi, T.; Tomita, M. Production of a correctly assembled fibrinogen using transgenic silkworms. Transgenic Res. 2020, 29, 339–353. [Google Scholar] [CrossRef]
- Ma, Y.; Canup, B.S.B.; Tong, X.; Dai, F.; Xiao, B. Multi-Responsive Silk Fibroin-Based Nanoparticles for Drug Delivery. Front. Chem. 2020, 8, 585077. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xu, S.; Wang, R.; Tian, C.; Ji, Y.; Yang, Q.; Zhao, P.; Xia, Q. Transdermal peptide conjugated to human connective tissue growth factor with enhanced cell proliferation and hyaluronic acid synthesis activities produced by a silkworm silk gland bioreactor. Appl. Microbiol. Biotechnol. 2020, 104, 9979–9990. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tan, H.; Yang, Q.; Wang, R.; Tian, C.; Ji, Y.; Zhao, P.; Xia, Q.; Wang, F. Fabrication of a Silk Sericin Hydrogel System Delivering Human Lactoferrin Using Genetically Engineered Silk with Improved Bioavailability to Alleviate Chemotherapy-Induced Immunosuppression. ACS Appl. Mater. Interfaces 2021, 13, 45175–45190. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Sato, M.; Kojima, K.; Sato, A.; Maruyama, S.; Nagasawa, T.; Nakao, M.; Somamoto, T. Development of a filter device for the prevention of aquatic bacterial disease using a single-chain variable fragment (scFv)-conjugated affinity silk. Sci. Rep. 2022, 12, 9475. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, R.Y.; Zhong, D.B.; Zhao, P.; Xia, Q.Y. Highly efficient expression of human extracellular superoxide dismutase (rhEcSOD) with ultraviolet-B-induced damage-resistance activity in transgenic silkworm cocoons. Insect Sci. 2024, 31, 1150–1164. [Google Scholar] [CrossRef]
- Tan, H.; Ji, Y.; Lei, H.; Wang, F.; Dong, H.; Yang, S.; Zhou, H.; Deng, H.; Chen, S.; Kaplan, D.L.; et al. Large-scale and cost-effective production of recombinant human serum albumin (rHSA) in transgenic Bombyx mori cocoons. Int. J. Biol. Macromol. 2023, 245, 125527. [Google Scholar] [CrossRef]
- Aramwit, P.; Jiang, Q.; Muppuri, S.; Reddy, N. Transgenic modifications of silkworms as a means to obtain therapeutic biomolecules and protein fibers with exceptional properties. Biotechnol. Bioeng. 2023, 120, 2827–2839. [Google Scholar] [CrossRef]
- Dai, X.; Ye, X.; Shi, L.; Yu, S.; Wang, X.; Zhong, B. High mechanical property silk produced by transgenic silkworms expressing the Drosophila Dumpy. Front. Bioeng. Biotechnol. 2024, 12, 1359587. [Google Scholar] [CrossRef]
- Wang, F.; Ning, A.; Sun, X.; Zhou, Y.; Deng, H.; Zhou, H.; Chen, S.; He, M.; Meng, Z.; Wang, Y.; et al. Fabrication of a transforming growth factor β1 functionalized silk sericin hydrogel through genetical engineering to repair alveolar bone defects in rabbit. Biomaterials 2025, 316, 122986. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Kajiura, Z.; Nakagaki, M.; Yamashita, O. Baculovirus-Mediated Efficient Gene Transfer into the Central Nervous System of the Silkworm, Bombyx mori. J. Insect Biotechnol. Sericol. 2003, 72, 149–155. [Google Scholar] [CrossRef]
- Imai, K.; Konno, T.; Nakazawa, Y.; Komiya, T.; Isobe, M.; Koga, K.; Goto, T.; Yaginuma, T.; Sakakibara, K.; Hasegawa, K.; et al. Isolation and structure of diapause hormone of the silkworm, Bombyx mori. Proc. Jpn. Acad. Ser. B 1991, 67, 98–101. [Google Scholar] [CrossRef]
- Sato, Y.; Nakazawa, Y.; Menjo, N.; Imai, K.; Komiya, T.; Saito, H.; Shin, M.; Ikeda, M.; Sakakibara, K.; Isobe, M.; et al. A New Diapause Hormone Molecule of the Silkworm, Bombyx mori. Proc. Jpn. Acad. Ser. B 1992, 68, 75–79. [Google Scholar] [CrossRef][Green Version]
- Sato, Y.; Shiomi, K.; Saito, H.; Imai, K.; Yamashita, O. Phe-X-Pro-Arg-Leu-NH2 peptide producing cells in the central nervous system of the silkworm, Bombyx mori. J. Insect Physiol. 1998, 44, 333–342. [Google Scholar] [CrossRef]
- Homma, T.; Watanabe, K.; Tsurumaru, S.; Kataoka, H.; Imai, K.; Kamba, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem. Biophys. Res. Commun. 2006, 344, 386–393. [Google Scholar] [CrossRef]
- Zhao, A.C.; Long, D.P.; Ma, S.Y.; Xu, L.X.; Zhang, M.R.; Dai, F.Y.; Xia, Q.Y.; Lu, C.; Xiang, Z.H. Efficient strategies for changing the diapause character of silkworm eggs and for the germline transformation of diapause silkworm strains. Insect Sci. 2012, 19, 172–182. [Google Scholar] [CrossRef]
- Kosegawa, E.; Vemananda Reddy, G.; Shimizu, K.; Okajima, T. Induction of non-diapause egg by dark and low temperature incubation in local variety of the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 2000, 69, 369–375. (In Japanese) [Google Scholar] [CrossRef]
- Yamamoto, T.; Mase, K.; Sawada, H. Diapause Prevention Effect of Bombyx mori by Dimethyl Sulfoxide. PLoS ONE 2013, 8, e64124. [Google Scholar] [CrossRef]
- Sonobe, H.; Matsumoto, A.; Fukuzaki, Y.; Fujiwara, S. Carbohydrate metabolism and restricted oxygen supply in the eggs of the silkworm, Bombyx mori. J. Insect Physiol. 1979, 25, 381–388. [Google Scholar] [CrossRef]
- Xian, X.; Kobayashi, M. Effect of corona discharge on the termination of diapause in silkworm egg. J. Seric. Sci. Jpn. 1993, 62, 175–177. (In Japanese) [Google Scholar] [CrossRef]
- Zabelina, V.Y.; Klymenko, V.V. Ovary transplantation in the silkworm Bombyx mori L.: Parthenocloning by eggs produced in male recipient. Séricologia 2008, 48, 123–128. Available online: https://www.inserco.org/en/sites/default/files/journal/166.%20SERICOLOGIA%20VOLUME%2048%20(2)2008.pdf#page=3 (accessed on 20 June 2025).
- Shiomi, K.; Ishida, Y.; Ikeda, M.; Sato, Y.; Saito, H.; Imai, K.; Isobe, M.; Yamashita, O. Induction of non-diapause eggs by injection of anti-diapause hormone rabbit serum into the diapause type of the silkworm, Bombyx mori. J. Insect Physiol. 1994, 40, 693–699. [Google Scholar] [CrossRef]
- Quan, G.-X.; Kim, I.; Kômoto, N.; Sezutsu, H.; Ote, M.; Shimada, T.; Kanda, T.; Mita, K.; Kobayashi, M.; Tamura, T. Characterization of the kynurenine 3-monooxygenase gene corresponding to the white egg 1 mutant in the silkworm Bombyx mori. Mol. Genet. Genom. 2002, 267, 1–9. [Google Scholar] [CrossRef]
- Inoue, S.; Kanda, T.; Imamura, M.; Quan, G.X.; Kojima, K.; Tanaka, H.; Tomita, M.; Hino, R.; Yoshizato, K.; Mizuno, S.; et al. A fibroin secretion-deficient silkworm mutant, Nd-sD, provides an efficient system for producing recombinant proteins. Insect Biochem. Mol. Biol. 2005, 35, 51–59. [Google Scholar] [CrossRef]
- Osanai-Futahashi, M.; Ohde, T.; Hirata, J.; Uchino, K.; Futahashi, R.; Tamura, T.; Niimi, T.; Sezutsu, H. A visible dominant marker for insect transgenesis. Nat. Commun. 2012, 3, 1295. [Google Scholar] [CrossRef]
- Horn, C.; Wimmer, E.A. A versatile vector set for animal transgenesis. Dev. Genes Evol. 2000, 210, 630–637. [Google Scholar] [CrossRef]
- Uchino, K.; Sumitani, M.; Waizumi, R.; Sakai, H.; Yamada, N.; Kojima, K.; Yonemura, N.; Tatematsu, K.; Iizuka, I.; Sezutsu, H.; et al. Egg Cooling After Oviposition Extends the Permissive Period for Microinjection-Mediated Genome Modification in Bombyx mori. Int. J. Mol. Sci. 2024, 25, 12642. [Google Scholar] [CrossRef]
- Tsuchida, K.; Yoshitake, N. Effect of different artificial diets on diapause induction under controlled temperature and photoperiod in the silkworm, Bombyx mori L. Physiol. Entomol. 1983, 8, 333–338. [Google Scholar] [CrossRef]
- Yamada, N.; Mise, Y.; Yonemura, N.; Sakai, H.; Uchino, K.; Sezutsu, H.; Tamura, T.; Iizuka, T. Development of an Injection Method for the Genetic Engineering of Diapause Silkworm Egg Using Dimethyl Sulfoxide. Jpn. Agric. Res. Q. 2023, 57, 63–72. [Google Scholar] [CrossRef]
- Yamada, N.; Kojima, K.; Mise, Y.; Yonemura, N.; Sakai, H.; Sezutsu, H.; Uchino, K.; Tamura, T.; Iizuka, T. Experimental study of corona discharge in the production of transgenic silkworms from diapause strains. J. Insect Biotechnol. Sericol. 2022, 91, 27–32. [Google Scholar] [CrossRef]
- Yamada, N.; Mise, Y.; Yonemura, N.; Uchino, K.; Zabelina, V.; Sezutsu, H.; Iizuka, T.; Tamura, T. Abolition of egg diapause by ablation of suboesophageal ganglion in parental females is compatible with genetic engineering methods. J. Insect Physiol. 2022, 142, 104438. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Iga, M.; Okada, E. Breeding of commercial diapausing races with white eggs and eyes for creation of transgenic silkworm, Bombyx mori. Sanshi-Konchu Biotec, 2023; in press. (In Japanese) [Google Scholar]
- Hirayama, C.; Mase, K.; Iizuka, T.; Takasu, Y.; Okada, E.; Yamamoto, K. Deficiency of a pyrroline-5-carboxylate reductase produces the yellowish green cocoon ‘Ryokuken’ of the silkworm, Bombyx mori. Heredity 2018, 120, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Kasahara, M.; Sasaki, S.; Nagayasu, Y.; Yamada, T.; Kanamori, H.; Namiki, N.; Kitagawa, M.; Yamashita, H.; Yasukochi, Y.; et al. The genome sequence of silkworm, Bombyx mori. DNA Res. 2004, 11, 27–35. [Google Scholar] [CrossRef]
- Ohshima, Y.; Suzuki, Y. Cloning of the silk fibroin gene and its flanking sequences. Proc. Natl. Acad. Sci. USA 1977, 74, 5363–5367. [Google Scholar] [CrossRef]
- Kojima, K.; Kuwana, Y.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Tamada, Y. A new method for the modification of fibroin heavy chain protein in the transgenic silkworm. Biosci. Biotechnol. Biochem. 2007, 71, 2943–2951. [Google Scholar] [CrossRef]


| Strains | Temperature in Parental Embryonic Development | No. of Broods Examined in G0 (n=) | (a) No. of Eggs Fertilized in G0 (Mean ± SE) | (b) No. of Eggs Hatched in G0 Broods (Mean ± SE) | No. of Diapause Eggs in G0 (Mean ± SE) | No. of Eggs Died in G0 (Mean ± SE) | Percentage of Eggs Hatched in Fertilized Eggs in G0 (b/a × 100) |
|---|---|---|---|---|---|---|---|
| Kosetsu | 15 °C | 5 | 1073 (215 ± 133) | 743 (149 ± 88) | 0 (0 ± 0) | 330 (66 ± 52) | 69.2% |
| 25 °C | 4 | 2066 (517 ± 19) | 2 (1 ± 1) | 2050 (513 ± 20) | 14 (4 ± 3) | 0.1% | |
| C515 | 15 °C | 5 | 1376 (275 ± 142) | 0 (0 ± 0) | 1339 (268 ± 142) | 37 (7 ± 4) | 0% |
| 25 °C | 5 | 1628 (326 ± 177) | 0 (0 ± 0) | 1569 (314 ± 169) | 59 (12 ± 20) | 0% |
| Strains | Anti-BmDH Serum Injection | No. of Broods Examined in G0 (n=) | No. of Diapause Eggs in G0 Broods (Mean ± SE) | No. of Eggs Died in G0 Broods (Mean ± SE) | (a) No. of Eggs Hatched in G0 Broods (Mean ± SE) | (b) No. of Eggs Fertilized in G0 Broods (Mean ± SE) | Percentage of Eggs Hatched in Fertilized Eggs (a/b × 100) |
|---|---|---|---|---|---|---|---|
| Kosetsu | Not injected | 9 | 4005 (445 ± 87) | 73 (8 ± 10) | 2 (0 ± 1) | 4080 (453 ± 82) | 0.05% |
| Injected † | 13 | 7 (1 ± 0) | 492 (38 ± 35) | 4449 (342 ± 94) | 4948 (381 ± 103) | 89.9% | |
| C515 | Not injected | 10 | 4017 (402 ± 121) | 173 (17 ± 28) | 0 (0 ± 0) | 4190 (419 ± 119) | 0% |
| Injected † | 12 | 230 (19 ± 33) | 711 (59 ± 26) | 2248 (187 ± 53) | 3189 (266 ± 60) | 70.5% |
| Host Strain | Vector (Final Consentration) | G0 | G1 | ||
|---|---|---|---|---|---|
| (a) No. of Eggs Injected | (b) No. of Eggs Hatched (b/a × 100) | (c) No. of Broods Examined | (d) No. of Broods with Transgene (d/c × 100) | ||
| Kosetsu | pBac(3xP3DsRed2) (0.2 μg/μL) | 190 | 145 (76.3%) | 59 | 6 (10.2%) |
| C515 | pBac(3xP3DsRed2) (0.1 μg/μL), pBacIE1-Nat3xP3EGFP (0.1 μg/μL) | 280 | 115 (41.1%) | 35 | 2 (5.7%), 0 (0%) † |
| MN2 | pBac{3xP3-EGFPafm} (0.2 μg/μL) | 194 | 121 (62.4%) | 21 | 15 (71.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchino, K.; Sumitani, M.; Iizuka, T.; Sezutsu, H. Production of Transgenic Silkworm Using Anti-Serum Against Diapause Hormone in Diapause Strains of Silkworm, Bombyx mori. Int. J. Mol. Sci. 2025, 26, 7604. https://doi.org/10.3390/ijms26157604
Uchino K, Sumitani M, Iizuka T, Sezutsu H. Production of Transgenic Silkworm Using Anti-Serum Against Diapause Hormone in Diapause Strains of Silkworm, Bombyx mori. International Journal of Molecular Sciences. 2025; 26(15):7604. https://doi.org/10.3390/ijms26157604
Chicago/Turabian StyleUchino, Keiro, Megumi Sumitani, Tetsuya Iizuka, and Hideki Sezutsu. 2025. "Production of Transgenic Silkworm Using Anti-Serum Against Diapause Hormone in Diapause Strains of Silkworm, Bombyx mori" International Journal of Molecular Sciences 26, no. 15: 7604. https://doi.org/10.3390/ijms26157604
APA StyleUchino, K., Sumitani, M., Iizuka, T., & Sezutsu, H. (2025). Production of Transgenic Silkworm Using Anti-Serum Against Diapause Hormone in Diapause Strains of Silkworm, Bombyx mori. International Journal of Molecular Sciences, 26(15), 7604. https://doi.org/10.3390/ijms26157604







