The Influence of Exercise and Physical Activity on Autonomic Nervous System Function Measured by Heart Rate Variability in Individuals with Type 1 Diabetes Mellitus—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
2.2. Assessment of Methodological Quality and Synthesis
3. Results
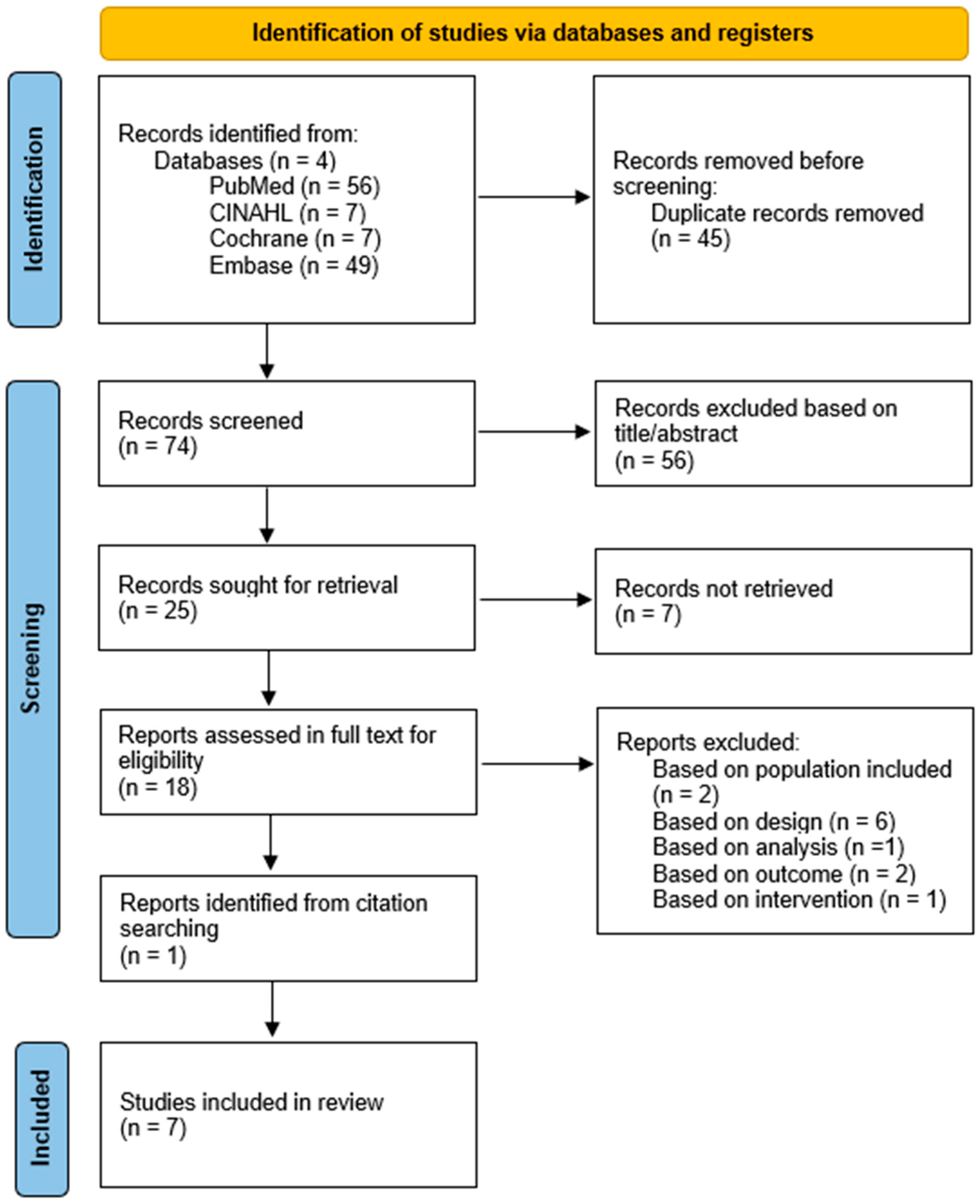
3.1. Study Selection
3.2. Study Characteristics
3.3. Methodological Quality
3.4. Results of Individual Studies
3.5. Synthesis of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HRV | Heart rate variability |
| NN | Normal-to-normal intervals |
| SDNN | Standard deviation of normal-to-normal intervals |
| RMSSD | Sum of squares of differences between adjacent normal-to-normal intervals |
| LF | Low frequency |
| HF | High frequency |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| EPHPP | Effective public health practice project |
| ECG | Electrocardiogram |
| T1DM | Type 1 diabetes mellitus |
| HIIT | High-intensity interval training |
| PPO | Peak power output |
| PAQ | Physical activity questionnaire |
| PA | Physical activity |
| METS | Metabolic equivalents |
| SED | Sedentary |
| LPA | Light physical activity |
| MVPA | Moderate-to-vigorous physical activity |
| VO2max | Maximum oxygen consumption |
Appendix A. Search String
| Database | Search String | Results |
|---|---|---|
| Pubmed | ((((((((((((((((((((((((((Ketosis-Prone Diabetes Mellitus [Title/Abstract]) OR (Diabetes Mellitus, Ketosis Prone [Title/Abstract])) OR (Diabetes Mellitus, Ketosis-Prone [Title/Abstract])) OR (Brittle Diabetes Mellitus [Title/Abstract])) OR (Diabetes Mellitus, Brittle [Title/Abstract])) OR (Autoimmune Diabetes [Title/Abstract])) OR (Diabetes, Autoimmune [Title/Abstract])) OR (Diabetes Mellitus, Type I [Title/Abstract])) OR (Diabetes, Type 1 [Title/Abstract])) OR (Type 1 Diabetes [Title/Abstract])) OR (Insulin Dependent Diabetes Mellitus 1 [Title/Abstract])) OR (Insulin-Dependent Diabetes Mellitus 1 [Title/Abstract])) OR (Diabetes Mellitus, Insulin-Dependent, 1 [Title/Abstract])) OR (Type 1 Diabetes Mellitus [Title/Abstract])) OR (Sudden-Onset Diabetes Mellitus [Title/Abstract])) OR (Diabetes Mellitus, Sudden Onset [Title/Abstract])) OR (Diabetes Mellitus, Sudden-Onset [Title/Abstract])) OR (Juvenile Onset Diabetes [Title/Abstract])) OR (Diabetes, Juvenile-Onset [Title/Abstract])) OR (Juvenile-Onset Diabetes [Title/Abstract])) OR (IDDM [Title/Abstract])) OR (Juvenile-Onset Diabetes Mellitus [Title/Abstract])) OR (Diabetes Mellitus, Juvenile Onset [Title/Abstract])) OR (Diabetes Mellitus, Juvenile-Onset [Title/Abstract])) OR (Insulin-Dependent Diabetes Mellitus [Title/Abstract])) OR (Diabetes Mellitus, Insulin Dependent [Title/Abstract])) OR (Diabetes Mellitus, Insulin-Dependent [Title/Abstract]) OR “Diabetes Mellitus, Type 1” [Mesh] AND (((((((((((((Autonom* Modu*, Cardia* [Title/Abstract]) OR (Cardia* Autonom* Modu* [Title/Abstract])) OR (Interval*, Inter Beat [Title/Abstract])) OR (Interval*, Inter-Beat [Title/Abstract])) OR (Inter Beat Interval* [Title/Abstract])) OR (Inter-Beat Interval* [Title/Abstract])) OR (Heart-Period Variabilit* [Title/Abstract])) OR (Heart Period Variabilit* [Title/Abstract])) OR (Cycle-Length Variabilit* [Title/Abstract])) OR (Cycle Length Variabilit* [Title/Abstract])) OR (Interval, R-R [Title/Abstract])) OR (R-R Interval [Title/Abstract])) OR (Variabilit*, Heart Rate [Title/Abstract])) OR (Heart Rate Variabilit* [Title/Abstract]) AND”Exercise” [Mesh] OR (((((((((((((((((((((((((((((Exercise*, Endurance [Title/Abstract]) OR (Endurance Exercise* [Title/Abstract])) OR (Training*, Endurance [Title/Abstract])) OR (Endurance Training* [Title/Abstract])) OR (Training*, Strength [Title/Abstract])) OR (Strength Training* [Title/Abstract])) OR (Exercise*, Strength [Title/Abstract])) OR (Strength Exercise* [Title/Abstract])) OR (Workout [Title/Abstract])) OR (Interval Exercise* [Title/Abstract])) OR (Training, Interval* [Title/Abstract])) OR (Interval Training* [Title/Abstract])) OR (High Intensit* Interval* Training* [Title/Abstract])) OR (HIIT [Title/Abstract])) OR (High-Intensity-Interval-Training* [Title/Abstract])) OR (Training*, Resistance [Title/Abstract])) OR (Resistance Training* [Title/Abstract])) OR (Training*, Exercise* [Title/Abstract])) OR (Exercise* Training* [Title/Abstract])) OR (Aerobic Exercise* [Title/Abstract])) OR (Exercise*, Aerobic [Title/Abstract])) OR (Isometric Exercise* [Title/Abstract])) OR (Exercise*, Isometric [Title/Abstract])) OR (Exercise*, Acute [Title/Abstract])) OR (Acute Exercise* [Title/Abstract])) OR (Physical Exercise* [Title/Abstract])) OR (Exercise*, Physical [Title/Abstract])) OR (Activit*, Physical [Title/Abstract])) OR (Physical Activit* [Title/Abstract])) OR (Exercise* [Title/Abstract]) | 55 |
| Embase | ‘cycle length variabilit*’:ab,ti OR ‘heart period variabilit*’:ab,ti OR ‘r r interval’:ab,ti OR ‘autonom* modu*, cardia*’:ab,ti OR ‘cardia* autonom* modu*’:ab,ti OR ‘interval*, inter beat’:ab,ti OR ‘inter beat interval*’:ab,ti OR ‘variabilit*, heart rate*’:ab,ti OR ‘heart rate variabilit*’:ab,ti AND ‘exercise*’:ab,ti OR ‘physical activit*’:ab,ti OR ‘endurance training*’:ab,ti OR ‘resistance training*’:ab,ti OR ‘acute exercise*’:ab,ti OR ‘aerobic exercise*’:ab,ti OR ‘isometric exercise*’:ab,ti OR ‘interval training*’:ab,ti OR ‘high intensity* interval training*’ab,ti OR ‘high intensity training*’: ab,ti OR ‘high intensity exercise*’:ab,ti OR ‘interval exercise*’: ab,ti OR ‘HIIT’:ab,ti AND ‘ketosis prone diabetes’:ab,ti OR ‘diabetes mellitus, ketosis prone’:ab,ti OR ‘brittle diabetes mellitus’:ab,ti OR ‘diabetes mellitus, brittle’:ab,ti OR ‘autoimmune diabetes mellitus’:ab,ti OR ‘diabetes, autoimmune’:ab,ti OR ‘Type 1 Diabetes Mellitus’:ab,ti OR ‘Type 1 diabetes’:ab,ti OR ‘Diabetes Type 1’:ab,ti OR ‘Diabetes Mellitus, type 1’:ab,ti OR ‘insuline dependent diabetes mellitus’:ab,ti OR ‘diabetes mellitus, insuline dependent’:ab,ti OR ‘sudden onset diabetes mellitus’:ab,ti OR ‘diabetes mellitus, sudden onset’:ab,ti OR ‘juvenile onset diabetes mellitus’:ab,ti OR ‘juvenile diabetes mellitus’:ab,ti OR ‘IDDM’:ab,ti OR ‘DM1’:ab,ti OR ‘T1DM’:ab,ti OR ‘DMT1’:ab,ti | 49 |
| Cochrane |
| |
| CINAHL | (MH “Diabetes Mellitus, Type 1”) OR diabetes mellitus type 1 OR insulin dependent diabetes mellitus OR sudden onset diabetes mellitus OR autoimmune diabetes mellitus OR ketosis prone diabetes OR dm1 OR juvenile onset diabetes mellitus OR IDDM OR Type 1 Diabetes Mellitus OR Brittle Diabetes Mellitus AND (MH “Exercise”) OR (MH “Physical Activity”) OR (MH “Physical Fitness”) OR (MH “Leisure Activities”) OR (MH “Physical Performance”) OR (MH “Sports”) OR exercise* OR physical activit* OR endurance training* OR resistance training* OR acute exercise* OR aerobic exercise* OR isometric exercise* OR interval training* OR high intensit* interval training* OR interval exercise* OR high intensity training* OR HIIT AND(MH “Heart Rate Variability”) OR cycle length variabilit* OR heart period variabilit* OR cardia* autonom* modulation OR inter-beat interval OR inter beat interval | 7 |
Appendix B. Heart Rate Variability Parameters
| Variables | Units | Description | Interpretation |
|---|---|---|---|
| Time-domain analysis | |||
| Entry 2 | data | Data | |
| RR | ms | RR intervals | Basis for analysis |
| SDNN | ms | Standard deviation of all NN intervals | Reflect overall variability |
| RMSSD | ms | Square root of the mean of the sum of squares of differences between adjacent NN intervals | Reflecting parasympathetic modulation |
| MSSD | ms | Mean squared successive differences | |
| pNN50 | % | Percentage difference between adjacent NN intervals that are greater than 50 ms | Reflecting parasympathetic modulation |
| Frequency-domain analysis | |||
| Total Power | ms2 | Variance of all NN intervals < 0.4 Hz | Reflects overall ANS function |
| VLF | ms2 | Very low frequency < 0.003–0.04 Hz | Measure of sympathetic activity |
| LF | ms2 | Low-frequency power 0.04–0.15 Hz | Sympathetic and parasympathetic activity |
| HF | ms2 | High-frequency power 0.15–0.4 Hz | |
| LF/HF | |||
| Additional | |||
| PSD | Power spectral density | ||
| CCV | Coefficients of component variance | Power ½ × 100/RR interval mean | |
| SNS Index | Sympathetic Nervous System Index | Global Sympathetic Nervous Activity | |
References
- Ozougwu, J.C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. About Diabetes–Type 1 Diabetes; IDF: Brussels, Belgium, 2023. [Google Scholar]
- Lu, X.; Zhao, C. Exercise and Type 1 Diabetes. Adv. Exp. Med. Biol. 2020, 1228, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S179–S218. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Chacko, E.C.; Pappachan, J.M. The management of diabetes mellitus. Eur. Endocrinol. 2018, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Helleputte, S.; Stautemas, J.; De Craemer, M.; Bogaert, L.; De Backer, T.; Calders, P.; Lapauw, B. Physical activity and sedentary behaviour in relation to body composition, estimated insulin sensitivity and arterial stiffness in adults with type 1 diabetes. Diabetes Res. Clin. Pract. 2024, 217, 111860. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Neves, J.S.; Neves, C.; Carvalho, D. Physical exercise and glycemic management in patients with type 1 diabetes on insulin pump therapy—A cross-sectional study. Acta Diabetol. 2023, 60, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGillet, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. Developing a diabetes mellitus comprehensive care plan. Dep. Health Hum. Serv. USA 2023, 28, 923–949. [Google Scholar]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 106, 126–131. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2023. Diabetes Care 2024, 47 (Suppl. 1), S77–S110. [Google Scholar] [CrossRef] [PubMed]
- Röhling, M.; Strom, A.; Bönhof, G.J.; Roden, M.; Ziegler, D. Cardiorespiratory fitness and cardiac autonomic function in diabetes. Curr. Diab Rep. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Catai, A.M.; Pastre, C.M.; Godoy, M.F.; Silva, E.D.; Takahashi, A.C.; Vanderlei, L.C.M. Heart rate variability: Are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2020, 24, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Morgan, S.; Molina Mora, J.A. Effect of heart rate variability biofeedback on sport performance: A systematic review. Appl. Psychophysiol. Biofeedback 2017, 42, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart rate variability today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 379–393. [Google Scholar]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: A systematic review. Eur. Rev. Aging Phys. Act. 2021, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Estévez-González, A.J.; Pérez-Ruiz, M.; Cobo-Vicente, F.; Donadio, M.V.F.; Larumbe-Zabala, E. Effects of physical training on heart rate variability in children and adolescents with chronic diseases: A systematic review and meta-analysis. Int. J. Sports Med. 2022, 43, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F.; Abdelbasset, W.K. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e025. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, M.L.; Tulppo, M.P.; Kiviniemi, A.M.; Kallio, M.A.; Perkiömäki, J.S.; Salmela, P.I.; Airaksinen, K.J.; Huikuri, H.V. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care 2012, 35, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, M.L.; Salmela, P.I.; Airaksinen, K.E.J.; Tapanainen, J.S.; Ruokonen, A.; Mäkikallio, T.H.; Huikuri, H.V. Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes 2005, 54, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Urbina, E.M.; Wadwa, R.P.; Talton, J.W.; D’Agostino, R.B.; Hamman, R.F.; Fingerlin, T.E.; Daniels, S.; Marcovina, S.M.; Dolan, L.M.; et al. Reduced heart rate variability among youth with type 1 diabetes: The SEARCH CVD study. Diabetes Care 2013, 36, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Lespagnol, E.; Bocock, O.; Heyman, J.; Gamelin, F.-X.; Berthoin, S.; Pereira, B.; Boissièreet, J.; Duclos, M.; Heyman, E. In amateur athletes with type 1 diabetes, a 9-day period of cycling at moderate-to-vigorous intensity unexpectedly increased the time spent in hyperglycemia, which was associated with impairment in heart rate variability. Diabetes Care 2020, 43, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.W.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef] [PubMed]
- El-Malahi, O.; Mohajeri, D.; Mincu, R.; Bäuerle, A.; Rothenaicher, K.; Knuschke, R.; Rammos, C.; Rassaf, T.; Lortz, J.; Constantinou, D. Beneficial impacts of physical activity on heart rate variability: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0299793. [Google Scholar] [CrossRef] [PubMed]
- Lefrandt, J.D.; Smit, A.J.; Zeebregts, C.J.; Gans, R.O.B.; Hoogenberg, K.H. Autonomic dysfunction in diabetes: A consequence of cardiovascular damage. Curr. Diabetes Rev. 2010, 6, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Breenan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.F.; Christofaro, D.G.D.; Vanderlei, F.M.; Barbosa, M.P.C.R.; Garner, D.M.; Vanderlei, L.C.M. Association of cardiac autonomic modulation with physical and clinical features of young people with type 1 diabetes. Cardiol. Young 2017, 27, 37–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colhoun, H.; Francis, D.; Rubens, M.; Underwood, S.; Fuller, J. The association of heart-rate variability with cardiovascular risk factors and. Diabetes Care 2001, 24, 1108–1114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid.-Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Proper, K.I.; Singh, A.S.; van Mechelen, W.; Chinapaw, M.J.M. Sedentary behaviours and health outcomes among adults. Am. J. Prev. Med. 2011, 40, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Laptev, D.; Polyakov, S.; Korneeva, I.; Namazova-Baranova, L.; Kuraeva, T.; Peterkova, V. Impact of regular physical activity on exercise tolerance, blood glucose and heart rate variability in children and adolescents with T1DM. Pediatr. Diabetes 2012, 13, 61–62. [Google Scholar]
- Mohammed, M.A.; Rahmy, A.F.; Mohamed, G.S.; Kaddah, A.F. Effect of exercise training on cardiovascular responses in diabetic autonomic neuropathy. Int. J. PharmTech Res. 2016, 9, 110–118. [Google Scholar]
- Chen, S.R.; Lee, Y.J.; Chiu, H.W.; Jeng, C. Impact of glycemic control, disease duration, and exercise on heart rate variability in children with type 1 diabetes mellitus. J. Formos. Med. Assoc. 2007, 106, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, M.S.; Quinn, L.; Rimmer, J.H.; Rich, B.H. Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol. Res. Nurs. 2005, 7, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.H.; Khachadurian, A.K.; Amorosa, L.F.; Clemow, L.; Ruderman, N.B. Ten-year experience with an exercise-based outpatient lifestyle modification program in the treatment of diabetes mellitus. Diabetes Care 1992, 15, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, M.S.; Quinn, L.; Fritschi, C.; Tripp, N.; Hayat, M.J. Heart rate variability and cardiorespiratory fitness in non-Hispanic Black versus non-Hispanic White adolescents with type 1 diabetes. J. Cardiovasc. Nurs. 2019, 34, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.K.D.; Jester, M.; Tryggestad, J.B.; Short, K.R. A pilot study of the effects of a high-intensity aerobic exercise session on heart rate variability and arterial compliance in adolescents with or without type 1 diabetes. Pediatr. Diabetes 2020, 21, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Anaruma, C.P.; Sponton, C.H.G.; Delbin, M.A.; Zanesco, A. Unbalance of redox state and altered heart rate variability in young type 1 diabetic patients. Diabetes 2014, 63, A187. [Google Scholar]
- Marshall, Z.A.; Mackintosh, K.A.; Lewis, M.J.; Ellins, E.A.; McNarry, M.A. Association of physical activity metrics with indicators of cardiovascular function and control in children with and without type 1 diabetes. Pediatr. Diabetes 2021, 22, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Fornasiero, A.; Rakobowchuk, M.; Skafidas, S.; Brighenti, A.; Stella, F.; Zignoli, A.; Savoldelli, A.; Pellegrini, B.; Danese, E.; et al. Similar cardiovascular and autonomic responses in trained type 1 diabetes mellitus and healthy participants in response to half marathon. Diabetes Res. Clin. Pract. 2020, 160, 107995. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Zuccotti, G.V.; Scaramuzza, A.; Malacarne, M.; Gervasi, F.; Pagani, M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2012, 58, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Javorka, K.; Buchanec, J.; Javorková, J.; Buchancová, J. Heart rate variability and physical fitness in children and adolescents with diabetes mellitus type 1. Int. J. Adolesc. Med. Health 2001, 13, 297–309. [Google Scholar] [CrossRef]
- Marshall, Z.A.; Mackintosh, K.A.; Gregory, J.W.; McNarry, M.A. Using compositional analysis to explore the relationship between physical activity and cardiovascular health in children and adolescents with and without type 1 diabetes. Pediatr. Diabetes 2022, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Lee, Y.J.; Chiu, H.W.; Jeng, C. Impact of physical activity on heart rate variability in children with type 1 diabetes. Child’s Nerv. Syst. 2008, 24, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Saki, H.; Nazem, F.; Fariba, F.; Sheikhsharbafan, R. A high intensity interval training (running and swimming) and resistance training intervention on heart rate variability and the selected biochemical factors in boys with type 1 diabetes. Diabetes Res. Clin. Pract. 2023, 204, 110915. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Gómez, J.; Calatayud, J.; Chulvi-Medrano, I.; Martín-Rivera, F. Effects of a HIIT protocol on cardiovascular risk factors in a type 1 diabetes mellitus population. Int. J. Environ. Res. Public Health 2021, 18, 1262. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.O.; Moritani, T.; Woo, J.; Jang, K.S.; Bae, J.Y.; Yoo, J.; Kang, S. Exercise training improves cardiac autonomic nervous system activity in type 1 diabetic children. J. Phys. Ther. Sci. 2014, 26, 111–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malik, M. Heart rate variability–standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Fisher, J.P. Autonomic control of the heart during exercise in humans: Role of skeletal muscle afferents. Exp. Physiol. 2014, 99, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Grotle, A.K.; Macefield, V.G.; Farquhar, W.B.; O’Leary, D.S.; Stone, A.J. Recent advances in exercise pressor reflex function in health and disease. Auton. Neurosci. 2020, 228, 102698. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Autonomic adjustments to exercise in humans. Compr. Physiol. 2015, 5, 475–512. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.Y.; Bunsawat, K.; Amann, M. Autonomic cardiovascular control during exercise. Am. J. Physiol. Endocrinol. Metab. 2023, 325, H675–H686. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.C. Baroreflex and neurovascular responses to skeletal muscle mechanoreflex activation in humans: An exercise in integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R654–R659. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, C.; Braga, D.C.; Ceroni, A.; Michelini, L.C. Activity-dependent neuroplastic changes in autonomic circuitry modulating cardiovascular control: The essential role of baroreceptors and chemoreceptors signaling. Front. Physiol. 2020, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Ichige, M.H.A.; Santos, C.R.; Jordão, C.P.; Ceroni, A.; Negrão, C.E.; Michelini, L.C. Exercise training preserves vagal preganglionic neurones and restores parasympathetic tonus in heart failure. J. Physiol. 2016, 594, 6241–6254. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. The effect of exercise on cardiovascular autonomic nervous function in patients with diabetes: A systematic review. Healthcare 2023, 11, 2668. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Sjøholm, H.; Berg, T.J.; Sandvik, L.; Brekke, M.; Hanssen, K.F.; Dahl-Jørgensen, K. Eighteen years of fair glycemic control preserves cardiac autonomic function in type 1 diabetes. Diabetes Care 2004, 27, 963–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwasaki, S.; Kozawa, J.; Fukui, K.; Iwahashi, H.; Imagawa, A.; Shimomura, I. Coefficient of variation of R–R interval closely correlates with glycemic variability assessed by continuous glucose monitoring in insulin-depleted patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2015, 109, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Santos-Magalhães, A.F.; Aires, L.; Martins, C.; Silva, G.; Teixeira, A.M.; Mota, J.; Rama, L. Heart rate variability, adiposity, and physical activity in prepubescent children. Clin. Auton. Res. 2015, 25, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, A.I.; Valenzano, A.; Ciliberti, M.A.P.; Moscatelli, F.; Villani, S.; Monda, M.; Messina, G.; Federici, A.; Babiloni, C.; Cibelli, G. Heart rate variability is reduced in underweight and overweight healthy adult women. Clin. Physiol. Funct. Imaging 2017, 37, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Weiss, S.T.; Roberts, M.; Zbikowski, S.M.; Sparrow, D. The relationship between heart rate variability and measures of body habitus. Clin. Auton. Res. 1995, 5, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Bell, C. Chronic sympathetic activation–consequence and cause of age-associated obesity? J. Natl. Med. Assoc. 2004, 96, 276–284. [Google Scholar]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child. Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.; Alifier, M.; Bochen, D.; Urban, M. Heart rate turbulence in children—Age and heart rate relationships. Pediatr. Res. 2007, 62, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.; Lee, P. Publication bias in meta-analysis: Its causes and consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Parekh, S.; Hooper, L.; Loke, Y.K.; Ryder, J.; Sutton, A.J.; Hing, C.; Kwok, C.; Pang, C.; Harvey, I. Dissemination and publication of research findings: An updated review of related biases. Health Technol. Assess. 2010, 14, 1–220. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef] [PubMed]
| Study Characteristics | Sample | HRV Parameters | Intervention § | Relevant Results | ||||
|---|---|---|---|---|---|---|---|---|
| Reference | Design † | N total (m/f) | Type | Age ± SD | Method | Variables | ||
| Alarcón-Gomez et al. 2021 [49] | Randomized experimental, parallel, open-label trial | 19 (10/9) | I: T1DM C: T1DM | I:38 ± 5.5 C:35 ± 8.2 | Heart Rate Sensor (Polar H10 with Polar Pro strap) | RMSSD LF/HF | Exercise intervention: HIIT training 30 s peak at 85% of PPO 1 min recovery at 40% PPO Duration: 3× per week for 6 weeks C: No exercise | I: RMSSD pre/post conditions 37.8 ± 27.9 vs. 44.3 ± 27.7 (p < 0.05, ES: 0.22) LF/HF pre/post conditions 2.6 ± 1.6 vs. 1.5 ± 0.9 (p < 0.05, ES: 0.23) Significant interaction condition × time in LF/HF (p < 0.05) C: No significant changes in control group RMSSD pre/post conditions 40.0 ± 15.9 vs. 39.3 ± 16.6 LF/HF ratio pre/post conditions 2.1 ± 2.0 vs. 1.9 ± 2.2 |
| Saki et al. 2023 [48] | N/R Clinical controlled trial | 36 (36/0) | I: T1DM C1: T1DM C2: Healthy | I: 15.6 ± 1.80 C1: 15.25 ± 1.76 C2: 15.08 ± 1.67 | ECG Holter monitor | SDNN RMSSD HF LF LF/HF | Exercise intervention: HIIT running and swimming 10–15 min w/u, stretching, training and 10 min c/u Running: 30–55 min with 3–6 periods of 5 min at 50–75% HRR and 4 min recovery at 10–20% HRR. Swimming: 10–15 mini front crawl leg, 30–55 min with 3–6 periods of 5 min full front crawl at 50–75% and 4 min recovery at 10–20% of HRR. Resistance training Duration: 3× per week for 12 weeks | Pre-test HRV was significantly different in C2 group compared to I and C1 group. Significant ME in the intervention group for SDNN, RMSSD, LF, HF and LF/HF after intervention. Significant between-group differences after intervention (adjusted means ± SE): I vs. C1: SDNN 137.19 ± 3.00 vs. 135.02 ± 2.33 (p < 0.001) RMSSD 41.10 ± 1.68 vs. 40.86 ± 1.38 (p < 0.001) LF 1465.23 ± 40.26 vs. 1544.64 ± 35.14 (p < 0.001), HF 737.71 ± 45.50 vs. 779.56 ± 29.59 (p < 0.001) LF/HF 2.15 ± 0.05 vs. 2.24 ± 0.03 (p < 0.001) I group improved significantly better than C1 group. I vs. C2: SDNN 137.19 ± 3.00 vs. 156.34 ± 2.23 (p < 0.001) RMSSD 41.10 ± 1.68 vs. 48.38 ± 1.22 (p < 0.001) LF 1465.23 ± 40.26 vs. 2042.48 ± 44.26 (p < 0.001) HF 737.71 ± 45.50 vs. 1312.28 ± 29.29 (p < 0.001) LF/HF 2.15 ± 0.05 vs. 1.37 ± 0.04 (p < 0.001) C2 group was significantly better than I group. No significant differences between C1 and C2 after testing |
| Chen et al. 2007 [47] | Retrospective pre/post design | 200 (95/105) | I: T1DM C: Healthy | I:10.3 ± 1.6 C:10.4 ± 1.6 | Three-channel ECG | LF HF LF/HF | I: PAQ-C, in which children were divided into Low (<2), Moderate (>2 and <3) or High (>3) activity levels based on the past 7 days. Stair stepper for 10 min C: Same as I | Significant between-group differences: LnHF (4.9 ± 0.9), LnLF (6.0 ± 0.7) and LnTP (6.7 ± 0.4) significantly ↓ in resting state (p < 0.05). No significant differences in LnHF/LF. In active state, no significant differences between T1DM and controls: LnHF (2.2 ± 1.1 vs. 2.3 ± 1.1), LnLF (4.0 ± 0.9 vs. 4.1 ± 0.8), LnHF/LF (1.7 ± 0.5 vs. 1.7 ± 0.7) and LnTP (5.0 ± 0.8 vs. 5.0 ± 0.7) Significantly decreased within-group changes when going from resting to active state in LnHF, LnLF and LnTP. PA predicted 54% of the variance in HRV parameters (r = −0.21, p < 0.05). |
| Javorka et al. 2001 [45] | N/R Prospective cohort study | 20 (N/R) | I: Trained T1DM C: Non-trained T1DM | 15.5 ± 1.2 | ECG signal through chest belt (Varia Pulse TF3 System) | RR MSSD HF LF VLF TP LF/HF VLF/LF LF/HF | Reconditioning summer camp for 8 days including non-mandatory activities: table tennis, badminton, 3–5 km distance walking, hiking, swimming 2× per day. Self-reported weekly physical activity levels for the last 6 months. C: Same as I | Trained subgroup compared to non-trained group had significantly longer RR intervals, ↑MSSD, VLF, HF and LF↑ (p < 0.05). Abnormal HRV parameters in 14.6 ± 6% of trained individuals vs. 54 ± 9% in untrained. Significant changes before and after with n total. No relevant differences between trained and untrained group (p < 0.05): RR↑ (715 ± 23 vs. 786 ± 22) MSDD↑ (2103 ± 714 vs. 6072 ± 1603) HF↑ (1024 ± 411 vs. 2195 ± 547) Rel.P.VLF↓ (47.6 ± 3.6 vs. 33.5 ± 4.1) Rel.P.HF↑ (30.4 ± 4.1 vs. 47.9 ± 5.1) VLF/HF↓ (2.8 ± 0.6 vs. 1.1 ± 0.3) |
| Lucini et al. 2012 [44] | N/R Prospective cohort study | 77 (50/27) | T1DM | 15.0 ± 0.6 | Two-way radio telemetry system (Finapres) | RR VARRR LFRR HFRR LF/HFRR Units used: ms and nu | METS calculation through assessment of time spent walking (>10 min) and/or exercise (structured or leisure time). At T1, subgroups were designated according to increased (Group 1), unchanged (Group 2) or diminished (Group 3) total weekly METS. | Significant interaction of between-group results in RR (p = 0.04), LFRR [nu] (p = 0.03) and HFRR [nu] (p = 0.01). Group 1: RR↑ (835. 1 ± 24.7 vs. 885.6 ± 23.2), VAR↑ (4443 ± 703 vs. 5042 ± 755), LF(ms)↓ (1176 ± 220 vs. 1152 ± 170), LF(nu)↓ (44.2 ± 3.2 vs. 41.1 ± 3.2) HF(ms)↑ (1918 ± 436 vs. 2425 ± 533), HF(nu)↑ (47.8 ± 2.9 vs. 52.6 ± 3.0) LF/HF↓(1.5 ± 0.3 vs. 1.1 ± 0.2) Exercise amount ↑ (METS/p.m/p.w.) is associated with a small reduction in LFRR and increase in HFRR. |
| Marshall et al. 2021 [46] | N/R Prospective cohort study | 37 (21/16) | I: T1DM C: Non-diabetic subjects | I: 11.9 ± 1.5 C:11.6 ± 2.2 | ECG | RMSSD LF HF | Triaxial accelerometer measurement for 28 days, 24 h p.d. SED, LPA, MVPA and sleep time were determined. C: Same as I | RMSSD is significantly negatively associated with SED (γ = −28.94, p < 005) and non-significantly negatively associated with MVPA (γ = −3.06, p > 0.05). Positive association with LPA (γ = 18.13) and sleep (γ = 13.87), both non-significant (p > 0.05). LF was negatively associated with SED (γ = −2.25), MVPA (γ = −1.76) and LPA (γ = −8.09) (non-significant). Positive association with sleep (γ = 12.10) HF was only negatively associated with sleep (γ = −12.04) but positively associated with SED (γ = 2.24), LPA (γ = 8.05) and MVPA (γ = 1.75) (non-significant). |
| Shin et al. 2014 [50] | N/R Prospective cohort study | 15 (15/0) | T1DM | 13.0 ± 1.0 | ECG monitor | TP VLF LF HF SNS index | Walking exercise program 3× p.w. for 12 weeks. Exercise intensity was set at 60% of VO2max. Duration requirement was at least 250 kcal per exercise session. | Pre/post HRV power analysis parameters all increased significantly (p < 0.05): TP (998.46 ± 232.2 vs. 1587.47 ± 449.25) LF (541.26 ± 187.59 vs. 942.22 ± 397.84) VLF (128.11 ± 58.66 vs. 389.44 ± 198.55) SNS index and HF power were not significantly different before and after testing. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | Global Rating |
|---|---|---|---|---|---|---|---|
| Alarcón-Gómez et al. [49] | ~ | − | + | − | + | ~ | Weak |
| Chen et al. [47] | ~ | − | + | − | + | − | Weak |
| Javorka et al. [45] | − | − | + | − | − | − | Weak |
| Lucini et al. [44] | ~ | − | − | − | + | − | Weak |
| Marshall et al. [46] | ~ | − | + | − | + | ~ | Weak |
| Saki et al. [48] | − | − | + | ~ | + | − | Weak |
| Shin et al. [50] | − | − | + | − | + | − | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekker, I.; Kooistra, A.; van Dijk, P.R.; Lefrandt, J.D.; Veeger, N.J.G.M.; van Beek, A.P. The Influence of Exercise and Physical Activity on Autonomic Nervous System Function Measured by Heart Rate Variability in Individuals with Type 1 Diabetes Mellitus—A Systematic Review. Int. J. Mol. Sci. 2025, 26, 7096. https://doi.org/10.3390/ijms26157096
Bekker I, Kooistra A, van Dijk PR, Lefrandt JD, Veeger NJGM, van Beek AP. The Influence of Exercise and Physical Activity on Autonomic Nervous System Function Measured by Heart Rate Variability in Individuals with Type 1 Diabetes Mellitus—A Systematic Review. International Journal of Molecular Sciences. 2025; 26(15):7096. https://doi.org/10.3390/ijms26157096
Chicago/Turabian StyleBekker, Isabel, Arne Kooistra, Peter R. van Dijk, Joop D. Lefrandt, Nic J. G. M. Veeger, and André P. van Beek. 2025. "The Influence of Exercise and Physical Activity on Autonomic Nervous System Function Measured by Heart Rate Variability in Individuals with Type 1 Diabetes Mellitus—A Systematic Review" International Journal of Molecular Sciences 26, no. 15: 7096. https://doi.org/10.3390/ijms26157096
APA StyleBekker, I., Kooistra, A., van Dijk, P. R., Lefrandt, J. D., Veeger, N. J. G. M., & van Beek, A. P. (2025). The Influence of Exercise and Physical Activity on Autonomic Nervous System Function Measured by Heart Rate Variability in Individuals with Type 1 Diabetes Mellitus—A Systematic Review. International Journal of Molecular Sciences, 26(15), 7096. https://doi.org/10.3390/ijms26157096






