Circulating Aryl Hydrocarbon Receptor Is Associated with Latent Tuberculosis Infection in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Measurements
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHAS | acetohydroxyacid synthase |
| AhR | aryl hydrocarbon receptor |
| ALT | alanine aminotransferase |
| ARNT | ahR nuclear translocator |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DPP4 | dipeptidyl peptidase-4 |
| eGFR | estimated glomerular filtration rate |
| GLP-1 RAs | glucagon-like peptide-1 receptor agonists |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| IL | interleukin |
| IR | Insulin resistance |
| LTBI | latent tuberculosis infection |
| Mtb | mycobacterium tuberculosis |
| OR | odds ratio |
| QFT | QuantiFERON-TB |
| QFT-GIT | QuantiFERON-TB Gold In-Tube |
| ROC | receiver operating characteristic |
| SGLT2 | sodium–glucose cotransporter 2 |
| TB | tuberculosis |
| TNF | tumor necrosis factor |
| UACR | urine albumin–creatinine ratio |
References
- GBD 2021 Tuberculosis Collaborators. Global, regional, and national age-specific progress towards the 2020 milestones of the WHO End TB Strategy: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 698–725. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef]
- Kahwati, L.C.; Feltner, C.; Halpern, M.; Woodell, C.L.; Boland, E.; Amick, H.R.; Weber, R.P.; Jonas, D.E. Primary Care Screening and Treatment for Latent Tuberculosis Infection in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 316, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Gil, C.; Carrillo-Larco, R.M.; Kirwan, D.E. Latent tuberculosis infection and non-infectious co-morbidities: Diabetes mellitus type 2, chronic kidney disease and rheumatoid arthritis. Int. J. Infect. Dis. 2019, 80S, S29–S31. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, W.; Liu, R.; Bo, E.; Liu, J.; Liu, M. The Association Between Diabetes Mellitus and the Risk of Latent Tuberculosis Infection: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 899821. [Google Scholar] [CrossRef]
- Chang, A.; Wu, C.Z.; Lin, J.D.; Lee, C.N.; Tsai, K.Y.; Wu, P.H.; Hsieh, A.T. Prevalence and risk factors for latent tuberculosis among diabetes patients in Taiwan: A cross-sectional study. J. Infect. Dev. Ctries 2022, 16, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kaur, M.; Singh, L.V.; Aggarwal, D.; Verma, I.; Radotra, B.D.; Sharma, S. Reactivation of latent tuberculosis through modulation of resuscitation promoting factors by diabetes. Sci. Rep. 2021, 11, 19700. [Google Scholar] [CrossRef]
- Sumbal, A.; Sheikh, S.M.; Ikram, A.; Amir, A.; Sumbal, R.; Saeed, A.R. Latent Tuberculosis Infection (LTBI) as a predictor of coronary artery disease: A systematic review and meta-analysis. Heliyon 2023, 9, e15365. [Google Scholar] [CrossRef]
- Hossain, M.B.; Johnston, J.C.; Cook, V.J.; Sadatsafavi, M.; Wong, H.; Romanowski, K.; Karim, M.E. Role of latent tuberculosis infection on elevated risk of cardiovascular disease: A population-based cohort study of immigrants in British Columbia, Canada, 1985–2019. Epidemiol. Infect. 2023, 151, e68. [Google Scholar] [CrossRef]
- Feria, M.G.; Chang, C.; Ticona, E.; Moussa, A.; Zhang, B.; Ballena, I.; Azanero, R.; Ticona, C.; De Cecco, C.N.; Fichtenbaum, C.J.; et al. Pro-Inflammatory Alterations of Circulating Monocytes in Latent Tuberculosis Infection. Open Forum Infect. Dis. 2022, 9, ofac629. [Google Scholar] [CrossRef]
- Djaharuddin, I.; Amir, M.; Qanitha, A. Exploring the link between cardiovascular risk factors and manifestations in latent tuberculosis infection: A comprehensive literature review. Egypt. Heart J. 2023, 75, 43. [Google Scholar] [CrossRef] [PubMed]
- Rejano-Gordillo, C.M.; Marin-Diaz, B.; Ordiales-Talavero, A.; Merino, J.M.; Gonzalez-Rico, F.J.; Fernandez-Salguero, P.M. From Nucleus to Organs: Insights of Aryl Hydrocarbon Receptor Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 14919. [Google Scholar] [CrossRef]
- Falandysz, J.; Fernandes, A.; Gregoraszczuk, E.; Rose, M. The toxicological effects of halogenated naphthalenes: A review of aryl hydrocarbon receptor-mediated (dioxin-like) relative potency factors. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 239–272. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.J.; De Castro, K.P.; Joshi, A.D.; Elferink, C.J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc. Toxicol. 2004, 4, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Tatebe, J.; Namba, S.; Koizumi, M.; Yamazaki, J.; Morita, T. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ. J. 2013, 77, 224–230. [Google Scholar] [CrossRef]
- Memari, B.; Bouttier, M.; Dimitrov, V.; Ouellette, M.; Behr, M.A.; Fritz, J.H.; White, J.H. Engagement of the Aryl Hydrocarbon Receptor in Mycobacterium tuberculosis-Infected Macrophages Has Pleiotropic Effects on Innate Immune Signaling. J. Immunol. 2015, 195, 4479–4491. [Google Scholar] [CrossRef]
- da Silva, J.F.; Bolsoni, J.A.; da Costa, R.M.; Alves, J.V.; Bressan, A.F.M.; Silva, L.E.V.; Costa, T.J.; Oliveira, A.E.R.; Manzato, C.P.; Aguiar, C.A.; et al. Aryl hydrocarbon receptor (AhR) activation contributes to high-fat diet-induced vascular dysfunction. Br. J. Pharmacol. 2022, 179, 2938–2952. [Google Scholar] [CrossRef]
- Andac-Ozturk, S.; Koc, G.; Soyocak, A. Association of aryl hydrocarbon receptor (AhR) serum level and gene rs10247158 polymorphism with anthropometric, biochemical parameters and food consumption in overweight/obese patients. Int. J. Clin. Pract. 2021, 75, e14436. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Ma, W.C.; Li, Y.H.; Wu, J.; Liang, K.W.; Lee, W.J.; Liu, H.C.; Sheu, W.H.; Lee, I.T. Plasma aryl hydrocarbon receptor associated with epicardial adipose tissue in men: A cross-sectional study. Diabetol. Metab. Syndr. 2023, 15, 188. [Google Scholar] [CrossRef]
- Major, J.; Crotta, S.; Finsterbusch, K.; Chakravarty, P.; Shah, K.; Frederico, B.; D’Antuono, R.; Green, M.; Meader, L.; Suarez-Bonnet, A.; et al. Endothelial AHR activity prevents lung barrier disruption in viral infection. Nature 2023, 621, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alves, P.; Fae, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Wang, J.; Zhu, K.; Tang, Y.; Huang, S.; Shui, X.; Ding, Y.; Chen, C.; Lei, W. Aryl Hydrocarbon Receptor: A New Player of Pathogenesis and Therapy in Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 6058784. [Google Scholar] [CrossRef]
- Humblet, O.; Birnbaum, L.; Rimm, E.; Mittleman, M.A.; Hauser, R. Dioxins and cardiovascular disease mortality. Environ. Health Perspect. 2008, 116, 1443–1448. [Google Scholar] [CrossRef]
- Wu, D.; Nishimura, N.; Kuo, V.; Fiehn, O.; Shahbaz, S.; Van Winkle, L.; Matsumura, F.; Vogel, C.F. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.; Meerarani, P.; Slim, R.; Toborek, M.; Daugherty, A.; Silverstone, A.E.; Robertson, L.W. Proinflammatory properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002, 181, 174–183. [Google Scholar] [CrossRef]
- Huang, S.; Shui, X.; He, Y.; Xue, Y.; Li, J.; Li, G.; Lei, W.; Chen, C. AhR expression and polymorphisms are associated with risk of coronary arterial disease in Chinese population. Sci. Rep. 2015, 5, 8022. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Liu, P.; Mu, Z.L.; Zhang, J.Z. Aryl hydrocarbon receptor expression in serum, peripheral blood mononuclear cells, and skin lesions of patients with atopic dermatitis and its correlation with disease severity. Chin. Med. J. 2020, 133, 148–153. [Google Scholar] [CrossRef]
- Hu, D.; Liu, J.; Yu, W.; Li, C.; Huang, L.; Mao, W.; Lu, Z. Tryptophan intake, not always the more the better. Front. Nutr. 2023, 10, 1140054. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Xin, H.; Liu, J.; Pan, S.; Guan, L.; Shen, F.; Liu, Z.; Wang, D.; Guan, X.; et al. Serum level of IL-1ra was associated with the treatment of latent tuberculosis infection in a Chinese population. BMC Infect. Dis. 2020, 20, 330. [Google Scholar] [CrossRef]
- Sayed, T.S.; Maayah, Z.H.; Zeidan, H.A.; Agouni, A.; Korashy, H.M. Insight into the physiological and pathological roles of the aryl hydrocarbon receptor pathway in glucose homeostasis, insulin resistance, and diabetes development. Cell. Mol. Biol. Lett. 2022, 27, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Lee, W.J.; Lee, I.T.; Lin, S.Y.; Lee, W.L.; Liang, K.W.; Lin, S.J.; Sheu, W.H. Negative association between serum aryl hydrocarbon receptor concentrations and beta-cell function in patients with no history of diabetes undergoing coronary angiography. J. Diabetes 2018, 10, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, V.; Yuvaraj, S. Immune-endocrine network in diabetes-tuberculosis nexus: Does latent tuberculosis infection confer protection against meta-inflammation and insulin resistance? Front. Endocrinol. 2024, 15, 1303338. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, V.; Bobhate, A.; Sathishkumar, K.; Patil, A.; Kumpatla, S.; Viswanathan, V. Unique Reciprocal Association Seen Between Latent Tuberculosis Infection and Diabetes Is Due to Immunoendocrine Modulation (DM-LTB-1). Front. Microbiol. 2022, 13, 884374. [Google Scholar] [CrossRef]
- Meregildo-Rodriguez, E.D.; Asmat-Rubio, M.G.; Zavaleta-Alaya, P.; Vasquez-Tirado, G.A. Effect of Oral Antidiabetic Drugs on Tuberculosis Risk and Treatment Outcomes: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 343. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xia, L.; Feng, X.; Chen, F.; Cao, S.; Wei, X. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: A systematic review. BMC Infect. Dis. 2019, 19, 859. [Google Scholar] [CrossRef]
- Zohar, Y.; Einav, M.; Chipman, D.M.; Barak, Z. Acetohydroxyacid synthase from Mycobacterium avium and its inhibition by sulfonylureas and imidazolinones. Biochim. Biophys. Acta 2003, 1649, 97–105. [Google Scholar] [CrossRef]
- Choi, K.J.; Yu, Y.G.; Hahn, H.G.; Choi, J.D.; Yoon, M.Y. Characterization of acetohydroxyacid synthase from Mycobacterium tuberculosis and the identification of its new inhibitor from the screening of a chemical library. FEBS Lett. 2005, 579, 4903–4910. [Google Scholar] [CrossRef]
- Turco, A.; Scalisi, G.; Gargaro, M.; Pirro, M.; Fallarino, F. Aryl hydrocarbon receptor: A novel target for the anti-inflammatory activity of statin therapy. J. Immunol. 2017, 198 (Suppl. 1), 67.10. [Google Scholar]
- Wang, D.; Pan, L.; Cao, G.; Lei, H.; Meng, X.; He, J.; Dong, M.; Li, Z.; Liu, Z. Evaluation of the in vitro and intracellular efficacy of new monosubstituted sulfonylureas against extensively drug-resistant tuberculosis. Int. J. Antimicrob. Agents 2012, 40, 463–466. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Bahman, F.; Choudhry, K.; Al-Rashed, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Aryl hydrocarbon receptor: Current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases. Front. Immunol. 2024, 15, 1421346. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shih, A.Z.L.; Woo, Y.C.; Fong, C.H.Y.; Yuen, M.M.A.; Chow, W.S.; Lam, K.S.L. Which creatinine-based estimated glomerular filtration rate equation best predicts all-cause mortality in Chinese subjects with type 2 diabetes? Diabetes Res. Clin. Pract. 2017, 126, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Flegal, K.M.; Chang, H.Y.; Yeh, W.T.; Yeh, C.J.; Lee, W.C. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am. J. Clin. Nutr. 2004, 79, 31–39. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S191–S202. [Google Scholar] [CrossRef]
- Diabetes Association of The Republic of China Taiwan. Executive summary of the DAROC clinical practice guidelines for diabetes care-2018. J. Formos. Med. Assoc. 2020, 119, 577–586. [Google Scholar] [CrossRef]

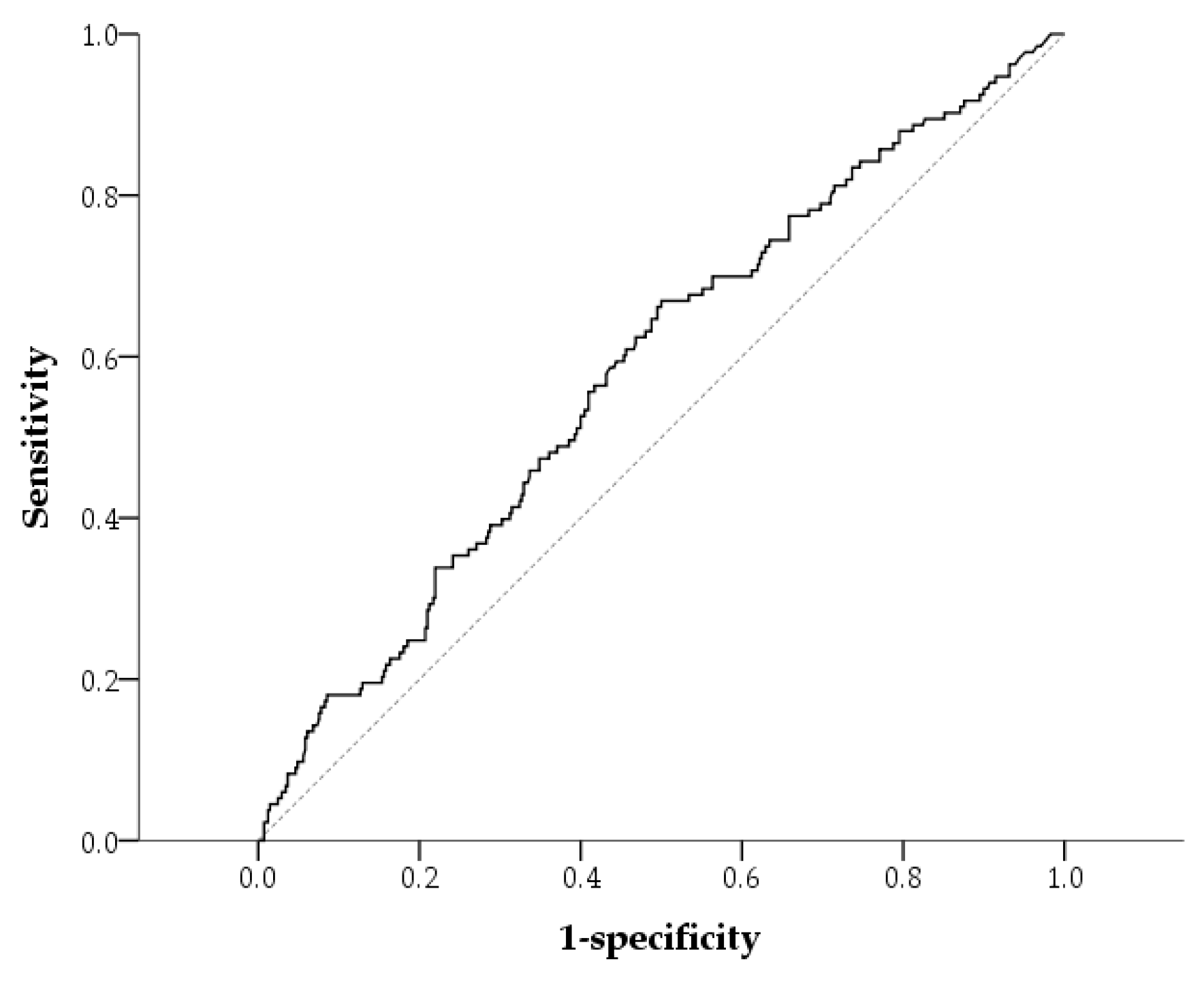
| QFT-Positive (n = 133) | QFT-Negative (n = 410) | p | |
|---|---|---|---|
| Age (year) | 66.0 (60.0, 72.0) | 61.0 (55.0, 68.0) | <0.001 * |
| Male, n (%) | 81 (60.9%) | 228 (55.6%) | 0.332 |
| Hypertension, n (%) | 106 (79.7%) | 308 (75.1%) | 0.337 |
| Current smoker, n (%) | 18 (13.5%) | 39 (9.5%) | 0.249 |
| CAD history, n (%) | 16 (12.0%) | 35 (8.5%) | 0.303 |
| Diabetes duration (years) | 13.0 (8.0, 20.0) | 12.0 (7.0, 17.0) | 0.024 * |
| BMI (kg/m2) | 25.7 (23.3, 28.1) | 25.7 (23.1, 28.5) | 0.902 |
| Systolic BP (mmHg) | 132.0 (123.5, 146.0) | 130.0 (120.0, 141.0) | 0.066 |
| Diastolic BP (mmHg) | 74.0 (65.0, 80.0) | 74.0 (65.0, 80.0) | 0.480 |
| Fasting glucose (mmol/L) | 8.1 (6.5, 9.9) | 8.2 (6.7, 10.2) | 0.361 |
| HbA1c (%) | 8.4 (7.6, 9.7) | 8.4 (7.4, 9.4) | 0.705 |
| Total cholesterol (mmol/L) | 3.9 (3.3, 4.5) | 4.0 (3.5, 4.6) | 0.096 |
| LDL cholesterol (mmol/L) | 2.1 (1.7, 2.6) | 2.2 (1.8, 2.8) | 0.049 * |
| HDL cholesterol (mmol/L) | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.5) | 0.352 |
| Triglycerides (mmol/L) | 1.3 (0.9, 2.1) | 1.4 (0.9, 2.0) | 0.638 |
| ALT (U/L) | 22.0 (14.5, 31.0) | 21.0 (15.0, 32.0) | 0.789 |
| C-reactive protein (mg/L) | 1.0 (0.5, 1.8) | 1.1 (0.5, 2.5) | 0.084 |
| eGFR (mL/min/1.73 m2) | 67.0 (53.6, 81.5) | 71.9 (59.8, 85.8) | 0.001 * |
| Increased UACR | 68 (51.1%) | 195 (47.6%) | 0.538 |
| Use of antiplatelet agent, n (%) | 24 (18.0%) | 54 (13.2%) | 0.211 |
| Use of statins, n (%) | 78 (58.6%) | 251 (61.2%) | 0.670 |
| Use of antihypertensive medications, n (%) | 55 (41.4%) | 159 (38.8%) | 0.670 |
| ACE inhibitors or ARBs, n (%) | 41 (30.8%) | 143 (34.9%) | 0.452 |
| α-blockers, n (%) | 4 (3.0%) | 12 (2.9%) | 0.999 F |
| β-blockers, n (%) | 9 (6.8%) | 25 (6.1%) | 0.943 |
| Calcium channel blockers, n (%) | 25 (18.8%) | 59 (14.4%) | 0.279 |
| Diuretics, n (%) | 12 (9.0%) | 29 (7.1%) | 0.408 |
| Use of insulin therapy, n (%) | 67 (50.4%) | 195 (47.6%) | 0.642 |
| Use of GLP-1 RAs, n (%) | 7 (5.3%) | 32 (7.8%) | 0.428 |
| Use of oral glucose-lowering medications, n (%) | 119 (89.5%) | 368 (89.8%) | 0.999 |
| Sulfonylurea, n (%) | 43 (32.3%) | 178 (43.4%) | 0.031 * |
| Glinides, n (%) | 17 (12.8%) | 29 (7.1%) | 0.061 |
| Metformin, n (%) | 86 (64.7%) | 278 (67.8%) | 0.649 |
| DPP4 inhibitors, n (%) | 76 (57.1%) | 202 (49.3%) | 0.139 |
| SGLT2 inhibitors, n (%) | 12 (9.0%) | 68 (16.6%) | 0.046 * |
| Thiazolidinediones, n (%) | 27 (20.3%) | 100 (24.4%) | 0.395 |
| α-glucosidase inhibitors, n (%) | 10 (7.5%) | 16 (3.9%) | 0.143 |
| Group | Case Number | Median | IQR (25%, 75%) | p | |
|---|---|---|---|---|---|
| Age | <63years † | 271 | 37.5 | (17.4, 54.3) | 0.013 * |
| ≥63years † | 272 | 42.6 | (21.5, 58.1) | ||
| Sex | Female | 234 | 41.1 | (18.2, 58.1) | 0.856 |
| Male | 309 | 39.6 | (21.3, 55.7) | ||
| Hypertension | No | 129 | 39.3 | (14.3, 51.5) | 0.117 |
| Yes | 414 | 41.0 | (20.7, 57.3) | ||
| Current smoker | No | 486 | 40.2 | (18.7, 56.8) | 0.769 |
| Yes | 57 | 41.6 | (28.6, 52.9) | ||
| CAD history | No | 492 | 40.4 | (19.1, 57.2) | 0.446 |
| Yes | 51 | 40.9 | (25.7, 48.2) | ||
| Diabetes duration | <13 years † | 265 | 39.3 | (20.7, 55.9) | 0.737 |
| ≥13 years † | 278 | 41.7 | (19.2, 56.5) | ||
| BMI | <27 kg/m2 | 339 | 41.0 | (21.2, 56.2) | 0.634 |
| ≥27 kg/m2 | 204 | 39.2 | (17.6, 56.6) | ||
| Systolic BP | <130 mmHg | 234 | 38.0 | (18.8, 55.4) | 0.134 |
| ≥130 mmHg | 309 | 41.9 | (20.4, 56.9) | ||
| Diastolic BP | <80 mmHg | 369 | 40.7 | (19.5, 56.1) | 0.741 |
| ≥80 mmHg | 174 | 39.5 | (19.9, 56.7) | ||
| Fasting glucose | <7.2 mmol/L | 187 | 38.6 | (18.6, 57.6) | 0.561 |
| ≥7.2 mmol/L | 356 | 41.2 | (20.5, 55.4) | ||
| HbA1c | <8.4% † | 265 | 39.5 | (18.3, 54.4) | 0.178 |
| ≥8.4% † | 278 | 41.4 | (20.8, 57.9) | ||
| Total cholesterol | <4.1 mmol/L | 303 | 41.8 | (20.5, 56.2) | 0.390 |
| ≥4.1 mmol/L | 240 | 39.2 | (17.7, 56.5) | ||
| LDL cholesterol | <2.6 mmol/L | 377 | 40.3 | (19.9, 56.5) | 0.835 |
| ≥2.6 mmol/L | 166 | 40.8 | (18.5, 55.4) | ||
| Low HDL cholesterol | No | 349 | 40.9 | (16.9, 57.1) | 0.823 |
| Yes | 194 | 39.5 | (21.8, 55.2) | ||
| Triglycerides | <1.7 mmol/L | 343 | 41.0 | (17.4, 55.8) | 0.632 |
| ≥1.7 mmol/L | 200 | 38.6 | (22.7, 56.7) | ||
| ALT | <21 U/L † | 224 | 40.6 | (19.2, 55.7) | 0.659 |
| ≥21 U/L † | 319 | 40.2 | (20.3, 56.9) | ||
| C-reactive protein | <1.11 mg/L † | 272 | 39.5 | (20.5, 55.8) | 0.935 |
| ≥1.11 mg/L † | 271 | 41.6 | (18.6, 56.5) | ||
| eGFR (mL/min/1.73 m2) | ≥60 | 392 | 39.4 | (18.0, 55.1) | 0.043 * |
| <60 | 151 | 42.8 | (22.9, 59.2) | ||
| UACR | <30 mg/g | 280 | 39.7 | (16.8, 54.3) | 0.032 * |
| ≥30 mg/g | 263 | 42.1 | (21.9, 58.7) | ||
| Use of antiplatelet | No | 465 | 40.7 | (20.4, 56.6) | 0.555 |
| Yes | 78 | 39.2 | (16.0, 55.0) | ||
| Use of statins | No | 214 | 44.3 | (23.6, 58.1) | 0.013 * |
| Yes | 329 | 38.4 | (17.4, 55.0) | ||
| Use of antihypertensive drugs | No | 329 | 40.5 | (18.2, 55.5) | 0.360 |
| Yes | 214 | 40.4 | (20.8, 57.7) | ||
| ACE inhibitors or ARBs | No | 359 | 40.5 | (18.8, 55.5) | 0.293 |
| Yes | 184 | 40.4 | (20.8, 58.4) | ||
| α-blockers | No | 527 | 40.6 | (20.3, 56.2) | 0.808 |
| Yes | 16 | 38.5 | (12.4, 58.4) | ||
| β-blockers | No | 509 | 40.3 | (19.9, 56.0) | 0.496 |
| Yes | 34 | 46.3 | (17.9, 57.6) | ||
| Calcium channel blockers | No | 459 | 40.6 | (20.3, 55.9) | 0.970 |
| Yes | 84 | 39.4 | (18.7, 56.7) | ||
| Diuretics | No | 501 | 40.2 | (19.4, 55.7) | 0.259 |
| Yes | 42 | 43.2 | (23.3, 58.9) | ||
| Use of insulin therapy | No | 281 | 39.9 | (18.9, 55.0) | 0.542 |
| Yes | 262 | 41.0 | (20.4, 58.3) | ||
| Use of GLP-1 RAs | No | 504 | 40.8 | (20.8, 56.6) | 0.251 |
| Yes | 39 | 35.8 | (12.3, 55.4) | ||
| Use of oral glucose-lowering medications | No | 56 | 33.6 | (13.7, 53.2) | 0.209 |
| Yes | 487 | 40.9 | (20.3, 56.6) | ||
| Sulfonylurea | No | 322 | 38.7 | (18.8, 55.4) | 0.130 |
| Yes | 221 | 42.5 | (20.3, 58.4) | ||
| Glinides | No | 497 | 40.2 | (19.5, 55.8) | 0.242 |
| Yes | 46 | 45.2 | (20.6, 60.4) | ||
| Metformin | No | 173 | 44.4 | (21.2, 59.2) | 0.126 |
| Yes | 370 | 39.3 | (18.7, 55.4) | ||
| DPP4 inhibitors | No | 265 | 37.5 | (17.4, 54.3) | 0.017 * |
| Yes | 278 | 42.3 | (23.6, 58.3) | ||
| SGLT2 inhibitors | No | 463 | 41.0 | (20.8, 57.0) | 0.130 |
| Yes | 80 | 38.0 | (14.5, 50.6) | ||
| Thiazolidinediones | No | 416 | 41.2 | (19.3, 57.6) | 0.580 |
| Yes | 127 | 37.6 | (20.8, 54.4) | ||
| α-Glucosidase inhibitors | No | 517 | 40.5 | (19.9, 56.3) | 0.994 |
| Yes | 26 | 36.7 | (17.9, 58.6) |
| Crude Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| AhR ≥ 37.7 pg/mL | 2.023 | (1.343, 3.047) | <0.001 | 1.902 | (1.254, 2.886) | 0.003 |
| Age ≥ 63 years | 2.258 | (1.454, 3.507) | <0.001 | |||
| CKD | 1.209 | (0.768, 1.904) | 0.412 | |||
| Age < 63 Years (n = 271) | Age ≥ 63 Years (n = 272) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| AhR ≥ 37.7 pg/mL | 2.218 | (1.126, 4.371) | 0.021 | 1.717 | (1.010, 2.918) | 0.046 |
| CKD | 1.910 | (0.783, 4.655) | 0.155 | 1.048 | (0.627, 1.752) | 0.857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Huang, W.-C.; Li, Y.-H.; Liu, S.-S.; Sheu, M.-L.; Lee, I.-T. Circulating Aryl Hydrocarbon Receptor Is Associated with Latent Tuberculosis Infection in Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 5384. https://doi.org/10.3390/ijms26115384
Cheng Y-C, Huang W-C, Li Y-H, Liu S-S, Sheu M-L, Lee I-T. Circulating Aryl Hydrocarbon Receptor Is Associated with Latent Tuberculosis Infection in Patients with Type 2 Diabetes. International Journal of Molecular Sciences. 2025; 26(11):5384. https://doi.org/10.3390/ijms26115384
Chicago/Turabian StyleCheng, Yu-Cheng, Wei-Chang Huang, Yu-Hsuan Li, Shin-Shin Liu, Meei-Ling Sheu, and I-Te Lee. 2025. "Circulating Aryl Hydrocarbon Receptor Is Associated with Latent Tuberculosis Infection in Patients with Type 2 Diabetes" International Journal of Molecular Sciences 26, no. 11: 5384. https://doi.org/10.3390/ijms26115384
APA StyleCheng, Y.-C., Huang, W.-C., Li, Y.-H., Liu, S.-S., Sheu, M.-L., & Lee, I.-T. (2025). Circulating Aryl Hydrocarbon Receptor Is Associated with Latent Tuberculosis Infection in Patients with Type 2 Diabetes. International Journal of Molecular Sciences, 26(11), 5384. https://doi.org/10.3390/ijms26115384







