Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation
Abstract
1. Introduction
2. Results
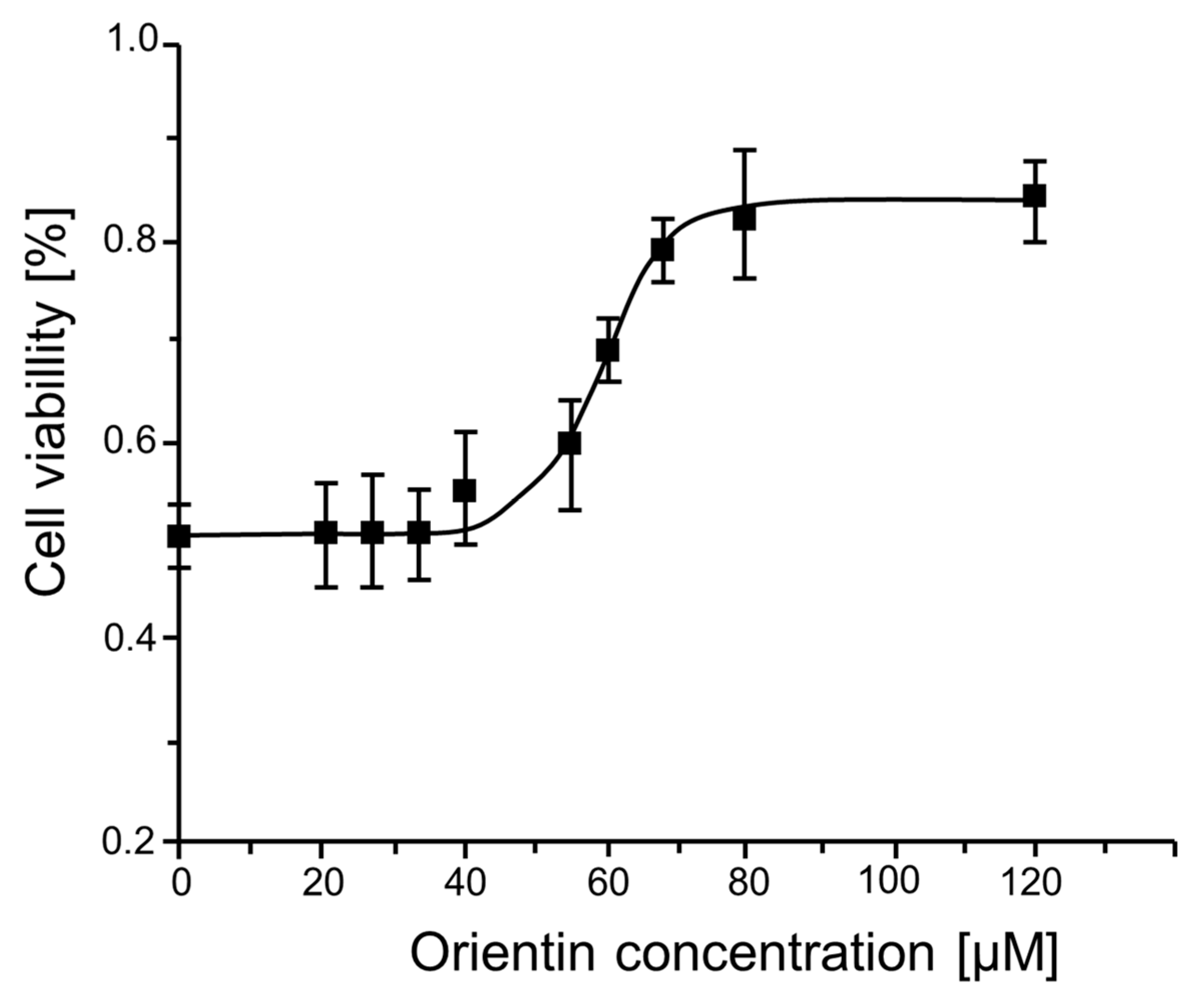
2.1. Evaluation of the Cytotoxicity and Biocompatibility of Orientin and GI50 Determination
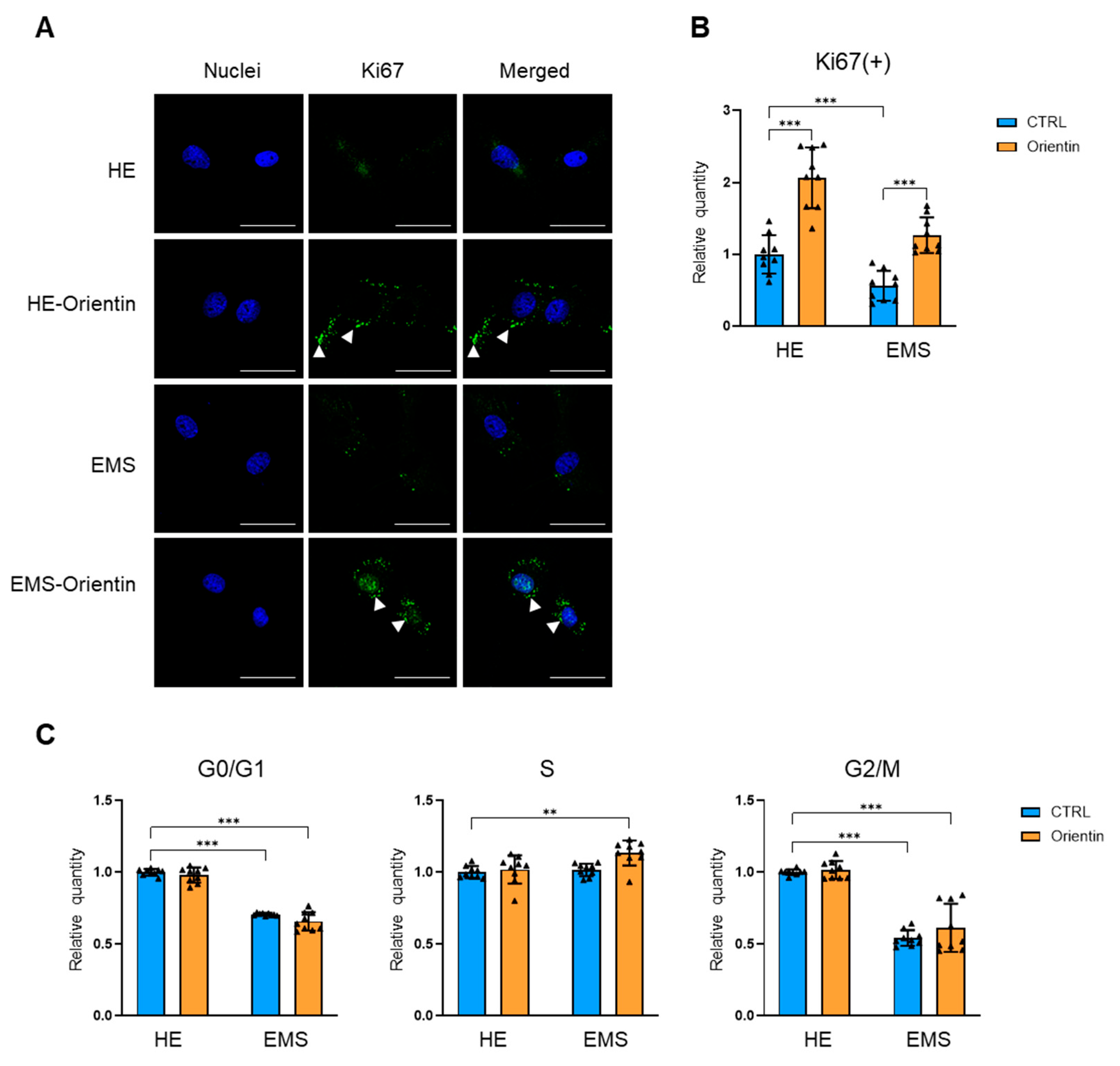
2.2. Orientin Restores Proliferative Capacity and Promotes Cell Cycle Re-Entry in EMS-Derived EqASCs
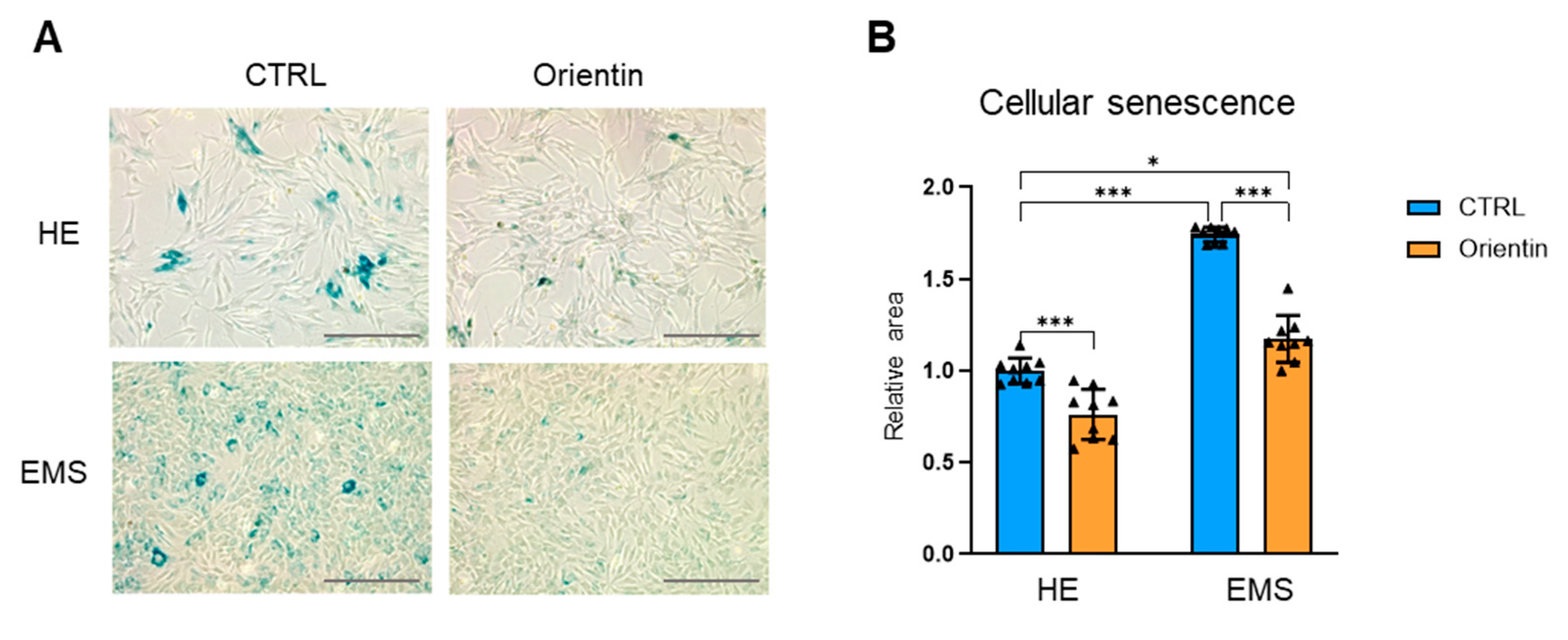
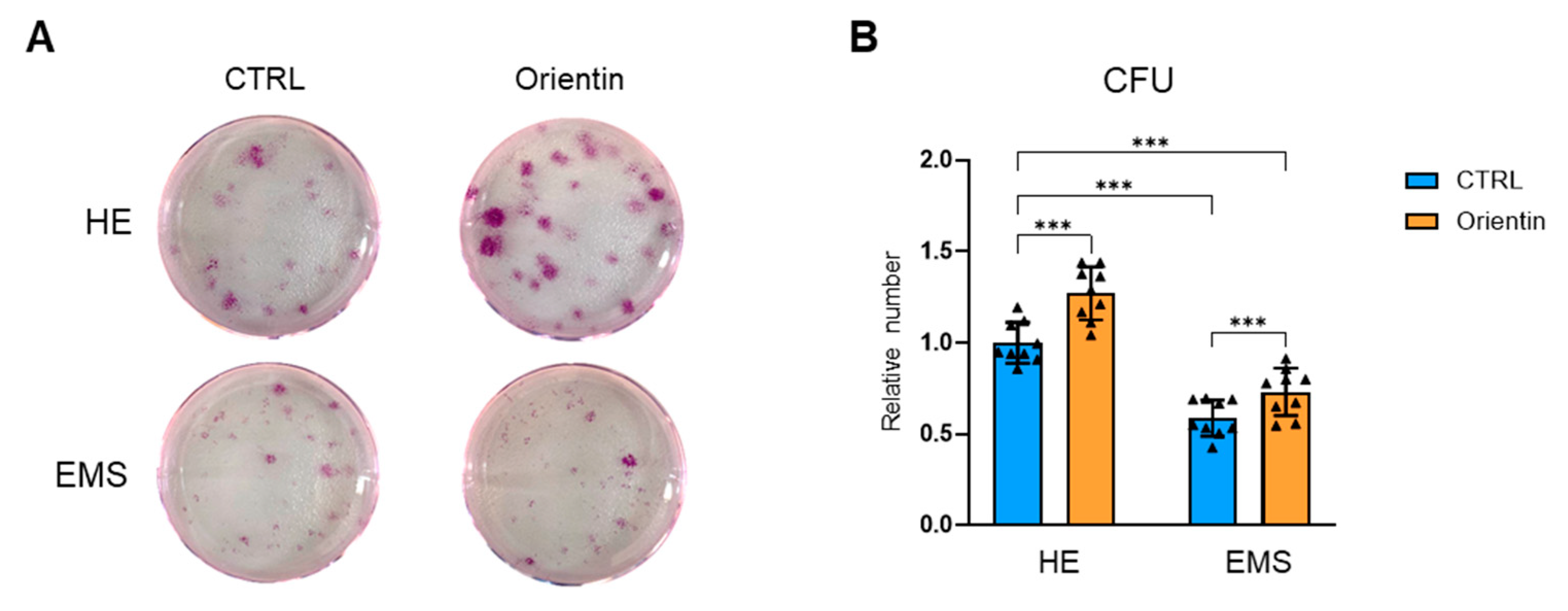
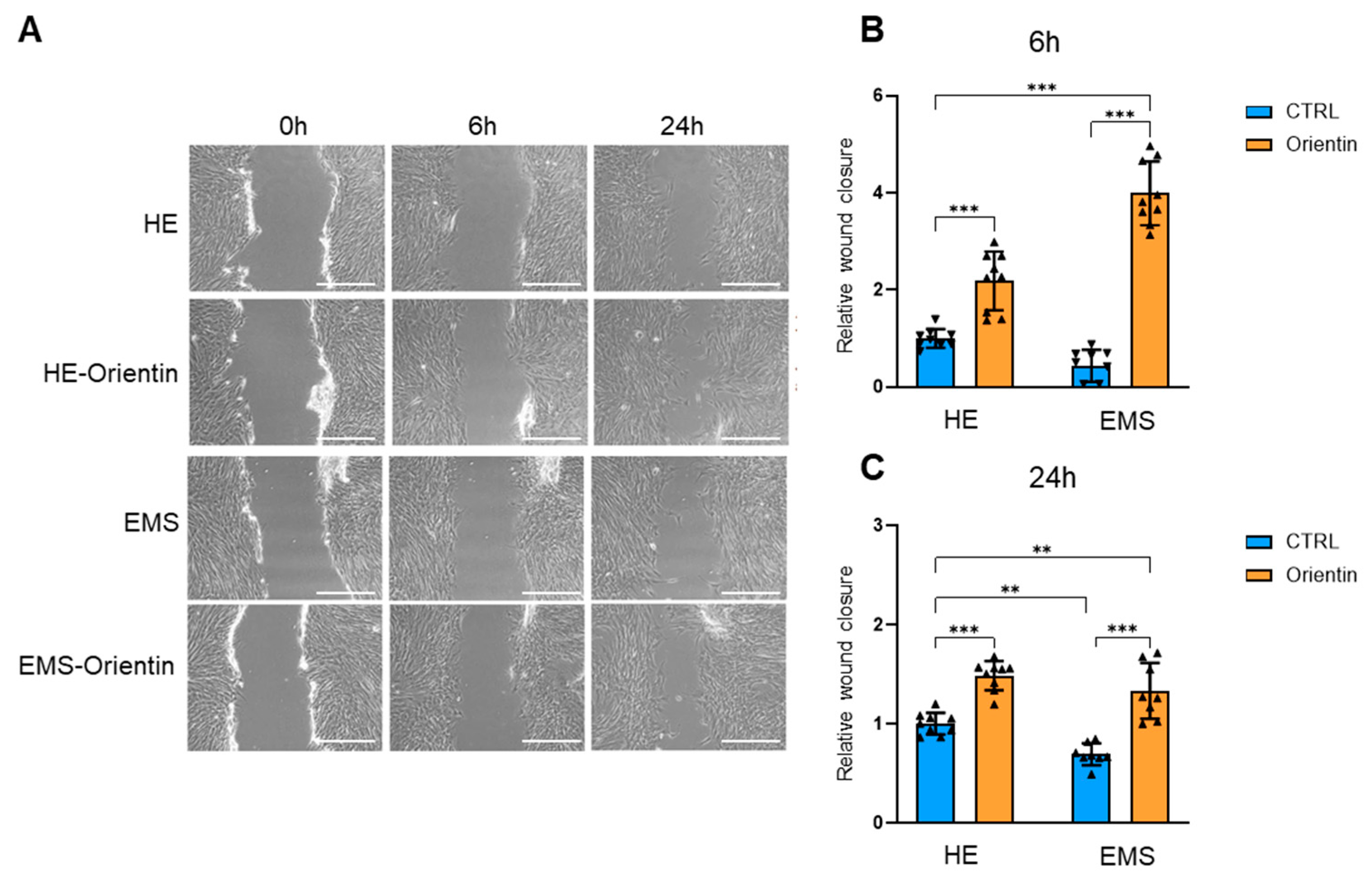
2.3. Orientin Attenuates Cellular Senescence and Enhances Clonogenic and Migratory Potential in EMS-Derived EqASCs
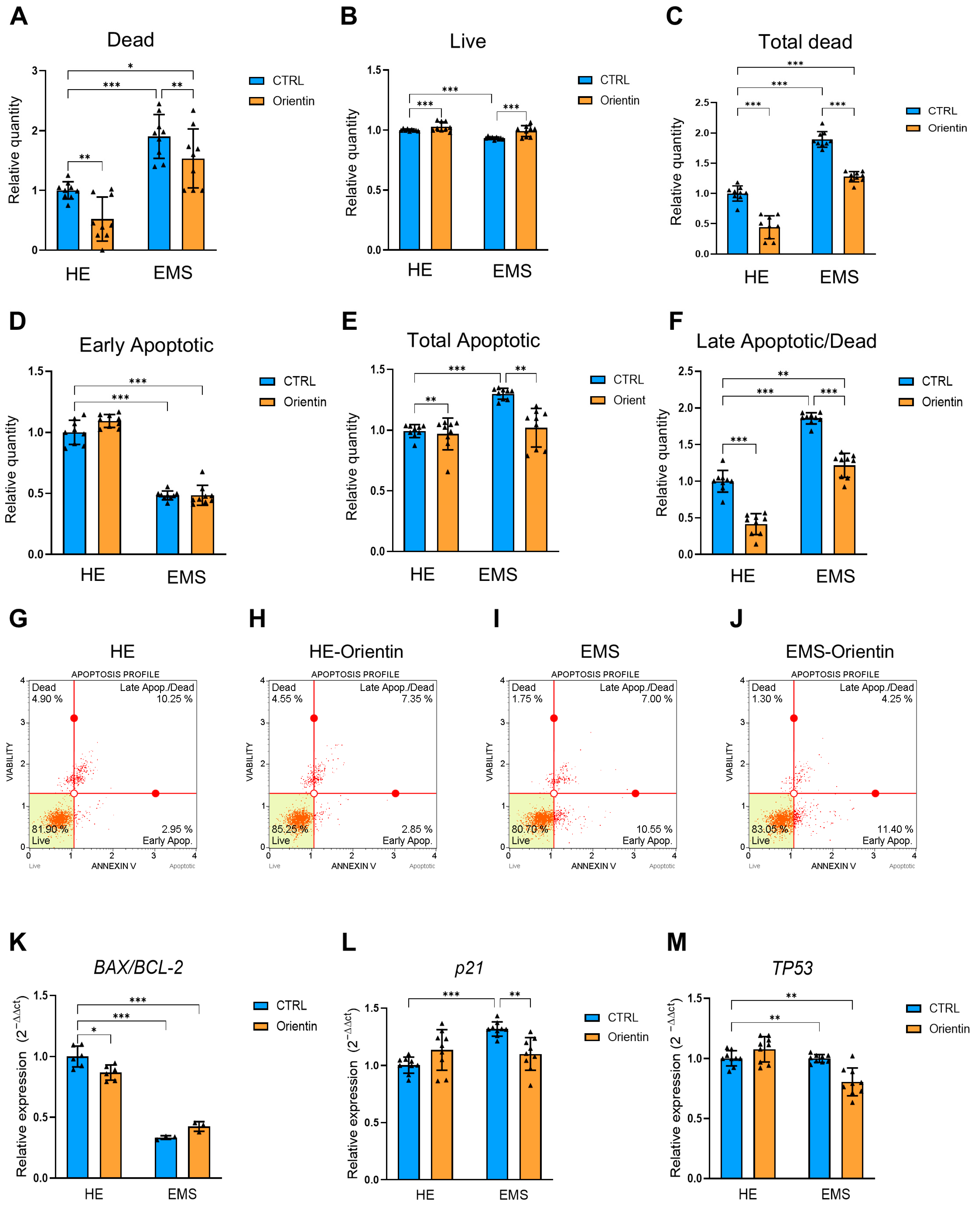
2.4. Orientin Reduces Apoptosis and Modulates the Expression of Key Apoptotic and Cell Cycle Regulatory Genes in EMS-EqASCs
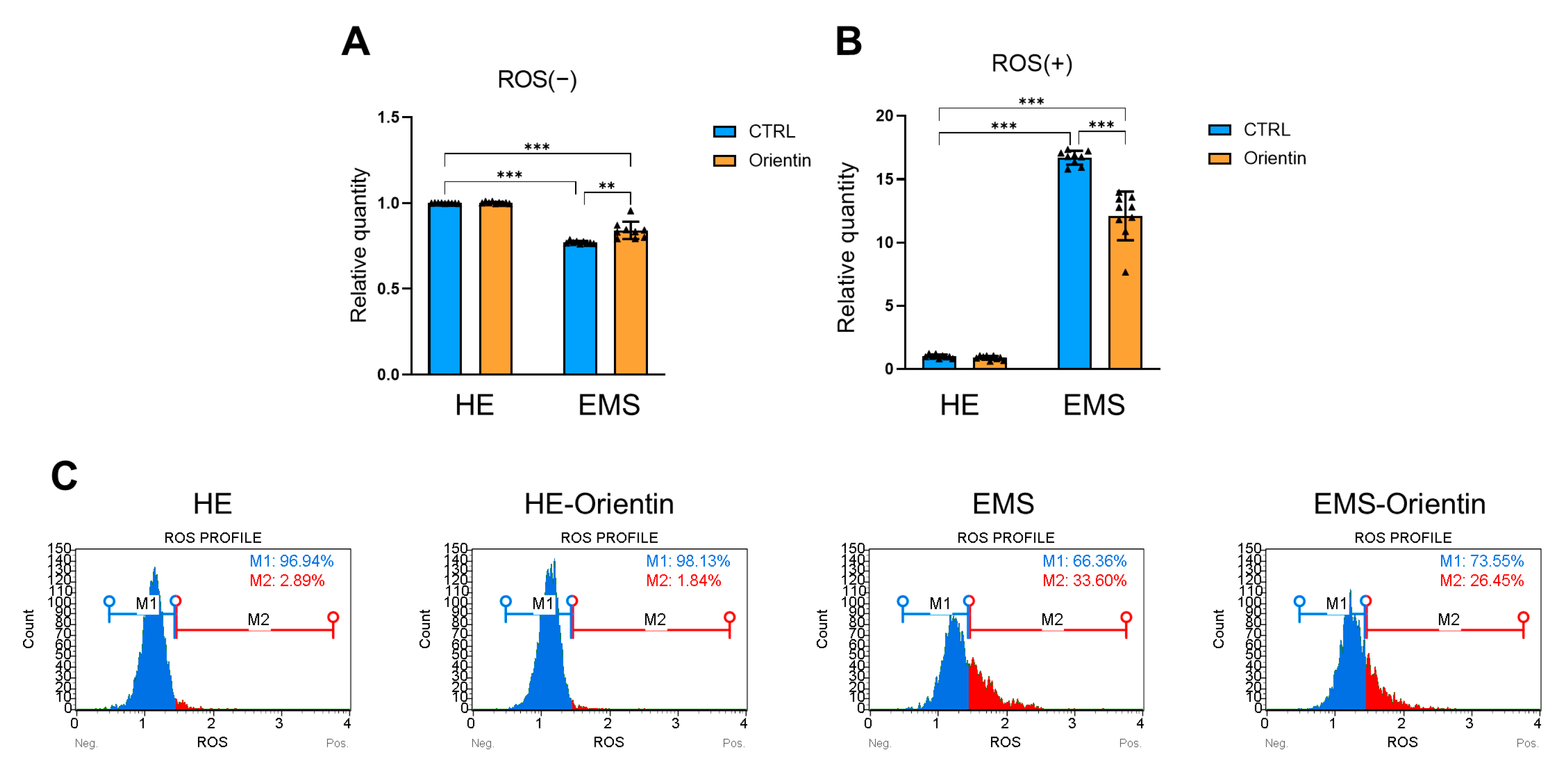
2.5. Orientin Reduces Oxidative Stress in EMS-Derived EqASCs
3. Discussion
4. Materials and Methods
4.1. Equine ASC Cell Culture and Orientin Treatment
4.2. Determination of Cytotoxic Activity of Orientin
4.3. Evaluation of Oxidative Stress
- (a)
- Quantification of ROS accumulation using Muse® Oxidative Stress assay
- (b)
- Fluorescence microscopy-based detection of intracellular ROS with CM-H2DCFDA
- (c)
- Flow cytometric analysis of CM-H2DCFDA-stained cells
4.4. Senescence Analysis
4.5. Evaluation of Viability and Apoptosis
4.6. Evaluation of Cell Proliferation
- (a)
- Immunocytochemical detection of Ki67 expression
- (b)
- Cell cycle distribution analysis by flow cytometry
- (c)
- Clonogenic potential assessed by colony-forming unit (CFU) assay
- (d)
- Cell migration and interaction asessed by scratch assay
4.7. Reverse Transcription Quantitative Real-Time PCR Analysis
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMS | Equine Metabolic Syndrome |
| ASCs | Adipose-Derived Mesenchymal Stromal Cells |
| SA-β-Gal | Senescence-Associated β-Galactosidase |
| SASP | Senescence-Associated Secretory Phenotype |
| ROS | Reactive Oxygen Species |
| mTOR | Mammalian Target of Rapamycin |
| FOXO | Forkhead Box O (transcription factors) |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin 6 |
| MMPs | Matrix Metalloproteinases |
| SOD | Superoxide Dismutase |
| GPx | Glutathione Peroxidase |
| PI3K | Phosphoinositide 3-Kinase |
| Akt | Protein Kinase B |
| AMPK | AMP-Activated Protein Kinase |
| p16^INK4a | Cyclin-Dependent Kinase Inhibitor 2A (also known as p16INK4a) |
| p21 | Cyclin-Dependent Kinase Inhibitor 1A (CDKN1A) |
| TP53 | Tumor Protein P53 |
| CM-H2DCFDA | 5-(and-6)-Chloromethyl-2′,7′-Dichlorodihydrofluorescein Diacetate, Acetyl Ester |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
References
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine metabolic syndrome affects viability, senescence, and stress factors of equine adipose-derived mesenchymal stromal stem cells: New insight into EqASCs isolated from EMS horses in the context of their aging. Oxidative Med. Cell Longev. 2016, 2016, 4710326. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J. Practical management of equine metabolic syndrome. Livestock 2014, 19, 186–193. [Google Scholar] [CrossRef]
- Johnson, P.J. The equine metabolic syndrome: Peripheral Cushing’s syndrome. Vet. Clin. Equine Pract. 2002, 18, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk-Szmukier, M.; Piórkowska, K.; Ropka-Molik, K. Equine metabolic syndrome: A complex disease influenced by multifactorial genetic factors. Genes 2023, 14, 1544. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Geor, R.J.; Bailey, S.; Durham, A.; Johnson, P. Equine metabolic syndrome. J. Vet. Intern. Med. 2010, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Geor, R.J. Metabolic predispositions to laminitis in horses and ponies: Obesity, insulin resistance and metabolic syndromes. J. Equine Vet. Sci. 2008, 28, 753–759. [Google Scholar] [CrossRef]
- McCue, M.E.; Geor, R.J.; Schultz, N. Equine metabolic syndrome: A complex disease influenced by genetics and the environment. J. Equine Vet. Sci. 2015, 35, 367–375. [Google Scholar] [CrossRef]
- Fahmy, M.I.; Sadek, M.A.; Abdou, K.; El-Dessouki, A.M.; El-Shiekh, R.A.; Khalaf, S.S. Orientin: A comprehensive review of a promising bioactive flavonoid. Inflammopharmacology 2025, 33, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Loos, C.M.; McLeod, K.R.; Vanzant, E.S.; Stratton, S.A.; Bohannan, A.D.; Coleman, R.J.; van Doorn, D.A.; Urschel, K.L. Differential effect of two dietary protein sources on time course response of muscle anabolic signaling pathways in normal and insulin dysregulated horses. Front. Vet. Sci. 2022, 9, 896220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar] [PubMed]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Śmieszek, A.; Szłapka-Kosarzewska, J.; Irwin Houston, J.M.; Roecken, M.; Marycz, K. Characterization of apoptosis, autophagy and oxidative stress in pancreatic islets cells and intestinal epithelial cells isolated from equine metabolic syndrome (EMS) horses. Int. J. Mol. Sci. 2018, 19, 3068. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in mesenchymal stem cells: Functional alterations, molecular mechanisms, and rejuvenation strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Control of p53 and NF-κB signaling by WIP1 and MIF: Role in cellular senescence and organismal aging. Cell Signal. 2011, 23, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.-P.; Patil, C.K.; Rodier, F.; Krtolica, A.; Beausejour, C.M.; Parrinello, S.; Hodgson, J.G.; Chin, K.; Desprez, P.-Y.; Campisi, J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE 2010, 5, e9188. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.; Siwinska, N.; Elzinga, S.; Barker, V.; Stefaniak, T.; Schanbacher, B.; Place, N.; Niedzwiedz, A.; Adams, A. Effects of equine metabolic syndrome on inflammation and acute-phase markers in horses. Domest. Anim. Endocrinol. 2020, 72, 106448. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xing, J.; Wen, Y.; Chen, L. Orientin alleviates chondrocyte senescence and osteoarthritis by inhibiting PI3K/AKT pathway. Bone Jt. Res. 2025, 14, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.-H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin induces G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules 2019, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Kilroy, G.E.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Fuerer, C.; Ching, W.; Harnish, K.; Logan, C.; Zeng, A.; Ten Berge, D.; Kalani, Y. Wnt signaling and stem cell control. In Cold Spring Harbor Symposia On Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 59–66. [Google Scholar]
- Merrill, B.J. Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb. Perspect. Biol. 2012, 4, a007971. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Robitaille, A.M.; Berndt, J.D.; Davidson, K.C.; Fischer, K.A.; Mathieu, J.; Potter, J.C.; Ruohola-Baker, H.; Moon, R.T. Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6382–E6390. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, H.; Hao, X. The self-renewal signaling pathways utilized by gastric cancer stem cells. Tumor Biol. 2017, 39, 1010428317697577. [Google Scholar] [CrossRef] [PubMed]
- Semba, T.; Sammons, R.; Wang, X.; Xie, X.; Dalby, K.N.; Ueno, N.T. JNK signaling in stem cell self-renewal and differentiation. Int. J. Mol. Sci. 2020, 21, 2613. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan Sajini, D.; Thaggikuppe Krishnamurthy, P.; Chakkittukandiyil, A.; Mudavath, R.N. Orientin Modulates Nrf2-ARE, PI3K/Akt, JNK-ERK1/2, and TLR4/NF-kB Pathways to Produce Neuroprotective Benefits in Parkinson’s Disease. Neurochem. Res. 2024, 49, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-J.; Xu, X.-H.; Zhou, N.; Yan, G.; Gu, T.-W.; Peng, L.-H. Phytochemicals as new therapeutic candidates simultaneously stimulate proliferation and counteract senescence of stem cells. Biomed. Pharmacother. 2022, 151, 113170. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Xu, W. Orientin inhibits the progression of fibroblast-like synovial cells in rheumatoid arthritis by regulating MAPK-signaling pathway. Allergol. Immunopathol. 2022, 50, 154–162. [Google Scholar]
- Kim, S.-J.; Pham, T.-H.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hora, A.B.; Biano, L.S.; Nascimento, A.C.S.; Camargo, Z.T.; Heiden, G.I.; Albulquerque-Júnior, R.L.; Grespan, R.; Aragão, J.M.; Camargo, E.A. Isoorientin Improves Excisional Skin Wound Healing in Mice. Pharmaceuticals 2024, 17, 1368. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Sarian, M.N.; Fakurazi, S.; Shaari, K. In Vitro wound healing potential of flavonoid c-glycosides from oil palm (Elaeis guineensis Jacq.) leaves on 3t3 fibroblast cells. Antioxidants 2020, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Sharma, G.T. Equine mesenchymal stem cells: Properties, sources, characterization, and potential therapeutic applications. J. Equine Vet. Sci. 2019, 72, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of oxidative stress and mitophagy during adipogenic differentiation of adipose-derived stem cells isolated from equine metabolic syndrome (EMS) horses. Stem Cells Int. 2018, 2018, 5340756. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Piao, R.; Wang, H.; Li, C.; Song, L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 118, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zong, J.; Zhang, H.; Zhang, P.; Xu, L.; Liang, K.; Yang, L.; Yong, H.; Qian, W. Orientin reduces myocardial infarction size via eNOS/NO signaling and thus mitigates adverse cardiac remodeling. Front. Pharmacol. 2017, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.; Lin, Q.; Huang, P.; Zhang, L.; Wu, W.; Ma, Y. Research progress on signaling pathway-associated oxidative stress in endothelial cells. Oxidative Med. Cell Longev. 2017, 2017, 7156941. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Cui, Y.; Zhao, Y.; Liu, L.; Wang, H.; Yang, L. Orientin relieves lipopolysaccharide-induced acute lung injury in mice: The involvement of its anti-inflammatory and anti-oxidant properties. Int. Immunopharmacol. 2021, 90, 107189. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic. Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liao, X.; Jiang, L.; Zhao, J.; Wu, S.; Ming, J. Orientin attenuated d-GalN/LPS-induced liver injury through the inhibition of oxidative stress via nrf2/keap1 pathway. J. Agric. Food Chem. 2022, 70, 7953–7967. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Shi, L.; Liu, Y.; Huang, L.; Luo, H.-R.; Wu, G.-S. Orientin prolongs the longevity of caenorhabditis elegans and postpones the development of neurodegenerative diseases via nutrition sensing and cellular protective pathways. Oxidative Med. Cell Longev. 2022, 2022, 8878923. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Zyzak, M.; Sikora, M.; Serwotka-Suszczak, A.; Mularczyk, M.; Al Naem, M.; Marycz, K. Sex hormone-binding globulin (SHBG) maintains proper equine adipose-derived stromal cells (ASCs)’ Metabolic functions and negatively regulates their basal adipogenic potential. Stem Cell Rev. Rep. 2023, 19, 2251–2273. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.P.; Gimble, J.M.; Devay, J.W.; Tucker, H.A.; Chiu, E.S.; Rowan, B.G. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013, 14, 34. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence 5′-3′ | Amplicon Length (bp) | Accession No. |
|---|---|---|---|---|
| GAPDH | F: R: | GATGCCCCAATGTTTGTGA AAGCAGGGATGATGTTCTGG | 250 | NM_001163856.1 |
| p21 | F: R: | GAAGAGAAACCCCCAGCTCC TGACTGCATCAAACCCCACA | 241 | XM_023633878.1 |
| SOD1 | F: R: | CATTCCATCATTGGCCGCAC GAGCGATCCCAATCACACCA | 130 | NM_001081826.3 |
| SOD2 | F: R: | GGACAAACCTGAGCCCCAAT TTGGACACCAGCCGATACAG | 125 | NM_001082517.2 |
| TP53 | F: R: | TTTCGACATAGCGTGGTGGT CTCAAAGCTGTTCCGTCCCA | 180 | NM_001202405.1 |
| BAX | F: R: | CGAGTGGCAGCTGAGATGTT AAGGAAGTCCAGTGTCCAGC | 153 | XM_023650076.1 |
| BCL2 | F: R: | TTCTTTGAGTTCGGTGGGGT GGGCCGTACAGTTCCACAA | 164 | XM_001490436.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzoł, D.; Kępska, M.; Zyzak, M. Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation. Int. J. Mol. Sci. 2025, 26, 6867. https://doi.org/10.3390/ijms26146867
Orzoł D, Kępska M, Zyzak M. Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation. International Journal of Molecular Sciences. 2025; 26(14):6867. https://doi.org/10.3390/ijms26146867
Chicago/Turabian StyleOrzoł, Dominika, Martyna Kępska, and Magdalena Zyzak. 2025. "Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation" International Journal of Molecular Sciences 26, no. 14: 6867. https://doi.org/10.3390/ijms26146867
APA StyleOrzoł, D., Kępska, M., & Zyzak, M. (2025). Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation. International Journal of Molecular Sciences, 26(14), 6867. https://doi.org/10.3390/ijms26146867





