The First Case Report of a Solitary Pulmonary Metastasis of a Transitional Meningioma and Literature Review
Abstract
1. Introduction
2. Case Report
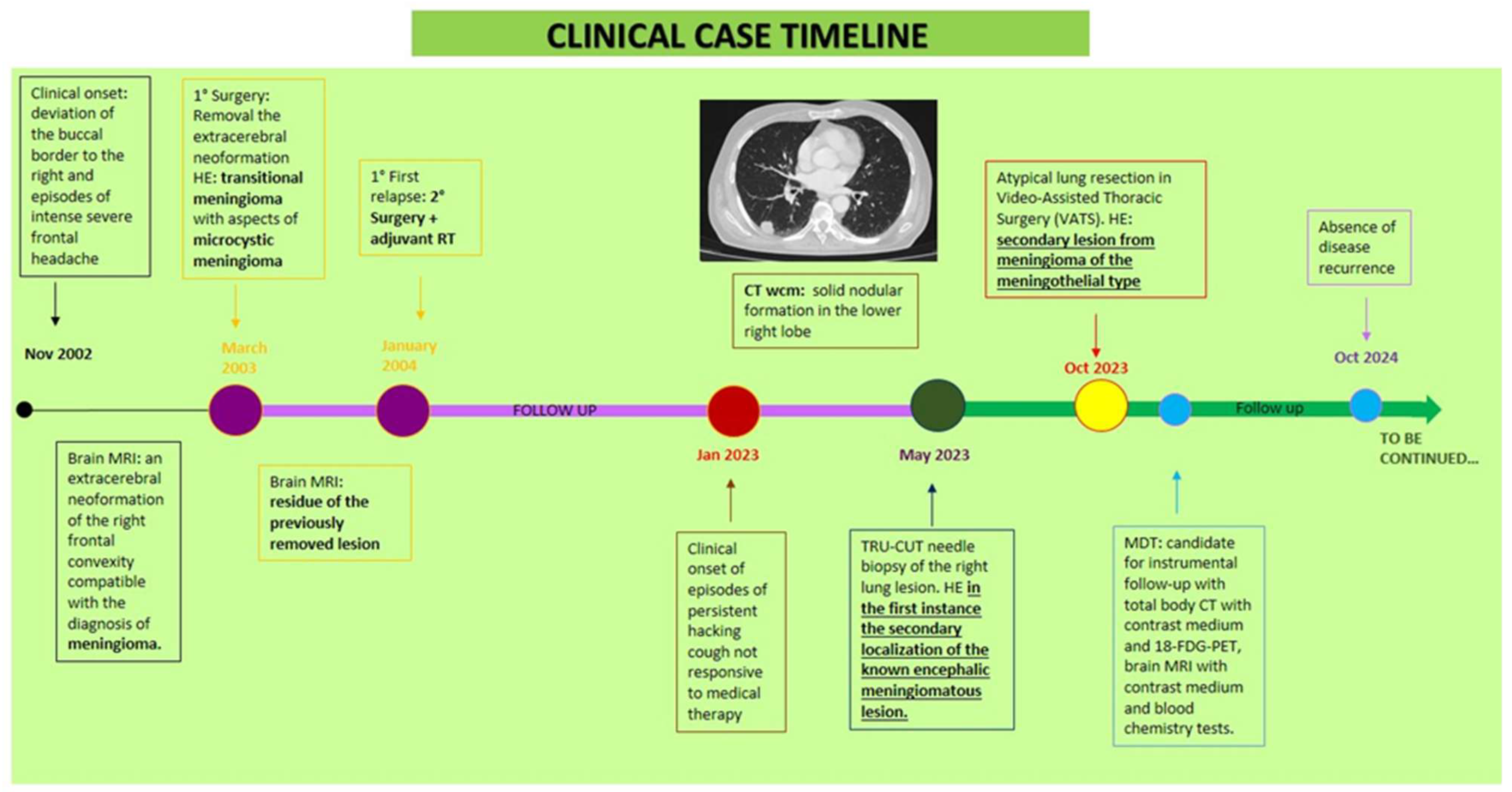
- Clinical history
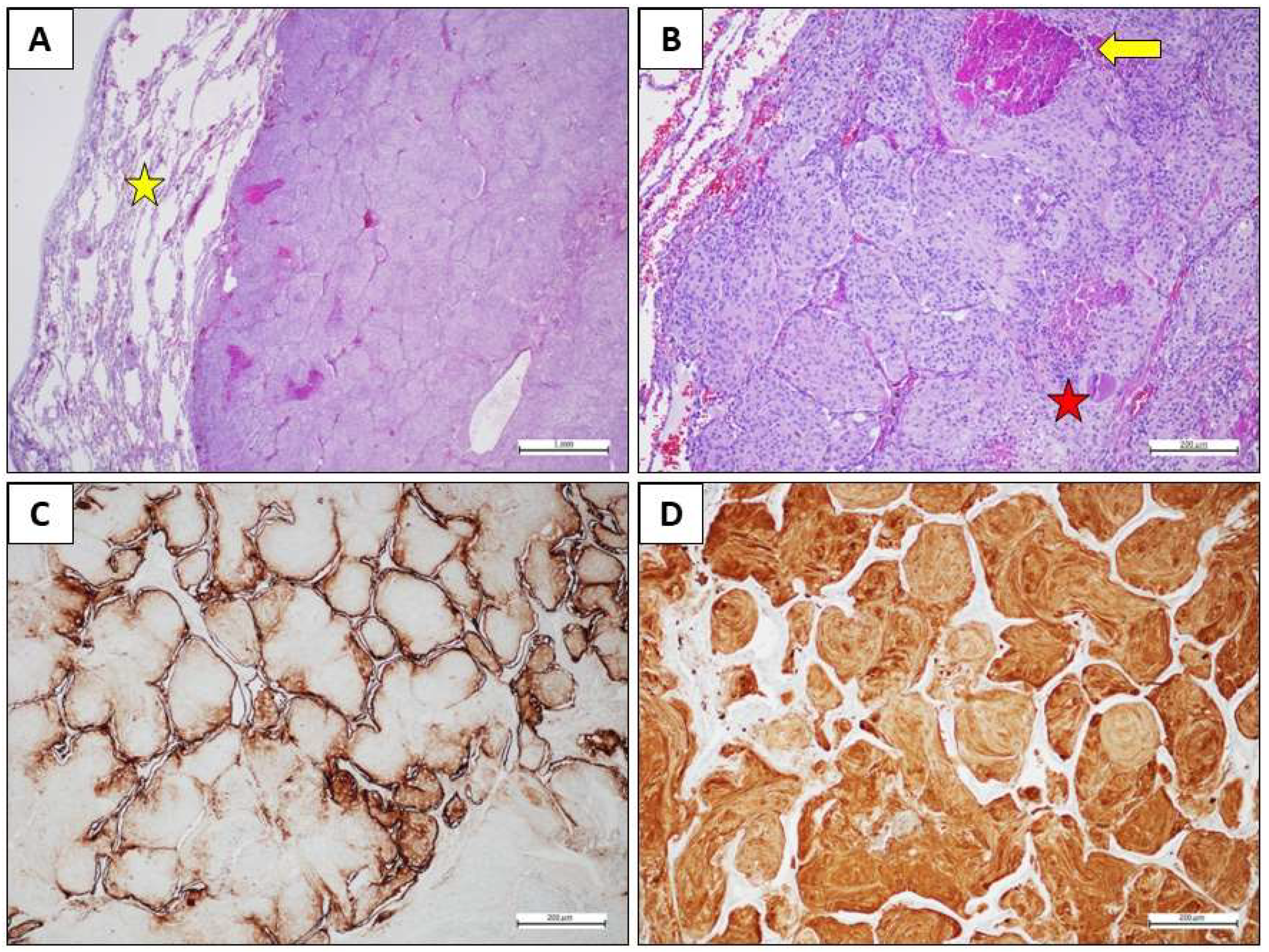
- Clinical presentation, Diagnosis, and Treatment
- Materials and Methods and Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wrange, E.K.M.; Harders, S.M.W. A Rare Case of Metastatic Atypical Meningioma That Highlights the Shortcomings of Treatment Options at Present. Acta Radiol. Open 2022, 12, 20584601221109919. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.E.; Tang, H.; Asemota, A.; Huang, L.; Boling, W.; Bannout, F. Meningioma Related Epilepsy-Pathophysiology, Pre/Post-operative Seizures Predicators and Treatment. Front. Oncol. 2022, 12, 905976. [Google Scholar]
- Clynch, A.; Richardson, G.E.; A Mustafa, M.; Gillespie, C.S.; Rathi, N.; Bakhsh, A.; Zakaria, R.; I Islim, A.; Millward, C.P.; Jenkinson, M.D.; et al. Beyond the WHO Classification of Meningioma: Using Molecular Diagnostics to Guide Management. Adv. Clin. Neurosci. Rehabil. 2023, 22, WVJZ9783. [Google Scholar] [CrossRef]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System Tumours: A Practical Update on What Neurosurgeons Need to Know—A Minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Rim, A.; Alia, M.; Lina, K.; Chiraz, N. Recurrent Intracranial Meningioma with Pulmonary Metastases: A Case Report. Austin Oncol. Case Rep. 2022, 5, 1015. [Google Scholar]
- Yu, S.; Wang, J.; Hou, B.; Sun, J.; Zhang, W. Transitional Meningioma Malignant Transformation and Rib Metastases Following Surgery: A Case Report. Oncol. Lett. 2023, 28, 399. [Google Scholar] [CrossRef]
- Himič, V.; Burman, R.J.; Fountain, D.M.; Hofer, M.; Livermore, L.J.; Jeyaretna, D.S. Metastatic Meningioma: A Case Series and Systematic Review. Acta Neurochir. 2023, 165, 2873–2883. [Google Scholar] [CrossRef]
- Bailey, D.D.; Montgomery, E.Y.; Garzon-Muvdi, T. Metastatic High-Grade Meningioma: A Case Report and Review of Risk Factors for Metastasis. Neuro-Oncol. Adv. 2023, 5, vdad014. [Google Scholar] [CrossRef]
- Limarzi, F.; Solaini, L.; Ercolani, G.; Foschini, M.P.; Saragoni, L. Liver Metastasis from a Non-Recurrent Atypical Cranial Meningioma: A Case Report. Pathologica 2020, 112, 46–49. [Google Scholar] [CrossRef]
- Ajler, P.; Davila, E.Z.; Plou, P.; Casto, F.; Christiansen, S.; Boccalatte, L.A.; Larrañaga, J. Multidisciplinary Approach to Anaplastic and Metastatic Meningioma: A Case Report and Review of the Literature. Surg. Neurol. Int. 2023, 14, 58. [Google Scholar] [CrossRef]
- Thomas, R.Z.; Dalal, I. Extracranial Metastases of Anaplastic Meningioma. BJR Case Rep. 2017, 3, 20150092. [Google Scholar] [CrossRef]
- Wu, J.; Dou, Z.; Iranmanesh, Y.; Zhang, B.; Hou, Y.; Wang, J.; Jiang, B.; Wang, Z.; Sun, C. Meningioma with Extracranial Pulmonary Metastasis: A Case Report and Literature Review. Ann. Case Rep. 2020, 14, 494. [Google Scholar]
- Liu, Y.; Li, J.; Duan, Y.; Ye, Y.; Xiao, L.; Mao, R. Subcutaneous Metastasis of Atypical Meningioma: Case Report and Literature Review. World Neurosurg. 2020, 138, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.V.; Sarkar, L.S.; Jagtap, S.S. Transitional Meningioma Benign WHO Grade 1 Tumor—A Case and Review of the Literature. IP Arch. Cytol. Histopathol. Res. 2023, 8, 156–160. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.J.; Kim, H.; Nam, S.H.; Choi, Y.W. A Rare Case of Transitional Meningioma. Arch. Plast. Surg. 2015, 42, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Tansey, P.; Ward, C.; Johnston, A.; Nasoodi, A. Case of Multiple Pulmonary Metastasis from a Benign Intracranial Meningioma. arXiv 2020. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Mady, L.J.; Panara, K.; Andrianus, S.; Cooper, K.; Chen, I.H.; Chalian, A.A.; Brody, R.M. Metastatic Meningioma of the Neck: A Case Report and Systematic Review. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 361–369. [Google Scholar] [CrossRef]
- Dalle Ore, C.L.; Magill, S.T.; Yen, A.J.; Shahin, M.N.; Lee, D.S.; Lucas, C.-H.G.; Chen, W.C.; Viner, J.A.; Aghi, M.K.; Theodosopoulos, P.V.; et al. Meningioma Metastases: Incidence and Proposed Screening Paradigm. J. Neurosurg. 2019, 132, 1447–1455. [Google Scholar] [CrossRef]
- Enomoto, T.; Aoki, M.; Kouzaki, Y.; Abe, H.; Imamura, N.; Iwasaki, A.; Inoue, T.; Nabeshima, K. WHO Grade I Meningioma Metastasis to the Lung 26 Years After Initial Surgery: A Case Report and Literature Review. NMC Case Rep. J. 2019, 6, 125–129. [Google Scholar] [CrossRef]
- Imoumby, F.; Mandour, C.; Gazzaz, M. Extensive Pulmonary Metastases of Intracranial Anaplastic Meningioma: Case Report. Open J. Mod. Neurosurg. 2021, 11, 102–106. [Google Scholar] [CrossRef]
- Woo, P.Y.M.; Hung, R.S.L.; Takemura, S.; Chan, K.; Kwok, J.C. Pulmonary Metastasis from a World Health Organization Grade I Intracranial Parasagittal Meningioma: A Case Report. Hong Kong Med. J. 2019, 25, 326–328. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, R.; Zhang, C.; Zhou, Y. Multiple Pulmonary Metastases in Recurrent Intracranial Meningioma: Case Report and Literature Review. J. Int. Med. Res. 2016, 44, 742–752. [Google Scholar] [CrossRef]
- Cao, X.; He, Q.; Ding, M.; Kong, W.; Yin, C.; Zhao, W.; Wang, Y. Hemorrhagic Meningioma with Pulmonary Metastasis: Case Report and Literature Review. Open Life Sci. 2023, 18, 20220745. [Google Scholar] [CrossRef]
- Wang, J.Z.; Landry, A.P.; Raleigh, D.R.; Sahm, F.; Walsh, K.M.; Goldbrunner, R.; Yefet, L.S.; Tonn, J.C.; Gui, C.; Ostrom, Q.T.; et al. Meningioma: International Consortium on Meningiomas Consensus Review on Scientific Advances and Treatment Paradigms for Clinicians, Researchers, and Patients. Neuro-Oncol. 2024, 26, 1742–1780. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Yao, T.; Zhou, J.; Wang, Z. The Immune-Related Role of Beta-2-Microglobulin in Melanoma. Front. Oncol. 2022, 12, 944722. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, F.; Wu, H.; Che, K.; Shi, J.; Wu, N.; Fu, Y.; Wang, Y.; Hu, J.; Qian, X.; et al. Prevalence and Associations of Beta2-Microglobulin Mutations in MSI-H/dMMR Cancers. Oncologist 2023, 28, e136–e144. [Google Scholar] [CrossRef] [PubMed]
- Inozume, T.; Yaguchi, T.; Ariyasu, R.; Togashi, Y.; Ohnuma, T.; Honobe, A.; Nishikawa, H.; Kawakami, Y.; Kawamura, T. Analysis of the tumor reactivity of tumor-infiltrating lymphocytes in a metastatic melanoma lesion that lost major histocompatibility complex class I expression after anti-PD-1 therapy. J. Investig. Dermatol. 2019, 139, 1490–1496. [Google Scholar] [CrossRef]
- Renne, S.L.; Sama’, L.; Kumar, S.; Mintemur, O.; Ruspi, L.; Santori, I.; Sicoli, F.; Bertuzzi, A.; Laffi, A.; Bonometti, A.; et al. Disruptions in antigen processing and presentation machinery on sarcoma. Cancer Immunol. Immunother. 2024, 73, 228. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.V.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The Function of Programmed Cell Death 1 and Its Ligands in Regulating Autoimmunity and Infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. Cellular Functions of the Protein Kinase ATM and Their Relevance to Human Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 110–128. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Wang, Q.; Bao, Z.; Wang, Z.; Zhang, C.; Jiang, T. PD-L2 Expression Is Correlated with the Molecular and Clinical Features of Glioma, and Acts as an Unfavorable Prognostic Factor. Oncoimmunology 2018, 8, e1541535. [Google Scholar] [CrossRef]
- Machado-Rugolo, J.; Fabro, A.T.; Ascheri, D.; Farhat, C.; Ab’Saber, A.M.; de Sá, V.K.; Nagai, M.A.; Takagaki, T.; Terra, R.; Parra, E.R.; et al. Usefulness of complementary next-generation sequencing and quantitative immunohistochemistry panels for predicting brain metastases and selecting treatment outcomes of non-small cell lung cancer. Hum. Pathol. 2019, 83, 177–191. [Google Scholar] [CrossRef]
- Heesters, B.A.; van Megesen, K.; Tomris, I.; de Vries, R.P.; Magri, G.; Spits, H. Characterization of human FDCs reveals regulation of T cells and antigen presentation to B cells. J. Exp. Med. 2021, 218, e20210790. [Google Scholar] [CrossRef]
- Baralle, D.; Baralle, M. Splicing in Action: Assessing Disease-Causing Sequence Changes. J. Med. Genet. 2005, 42, 737–748. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. ClinVar; [VCV000646077.7]. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000646077.7 (accessed on 29 December 2024).
- Varadhan, V.; Manikandan, M.S.; Nagarajan, A.; Palaniyandi, T.; Ravi, M.; Sankareswaran, S.K.; Baskar, G.; Wahab, M.R.A.; Surendran, H. Ataxia-Telangiectasia Mutated (ATM) gene signaling pathways in human cancers and their therapeutic implications. Pathol. Res. Pract. 2024, 260, 155447. [Google Scholar] [CrossRef] [PubMed]
- Casciello, F.; Al-Ejeh, F.; Miranda, M.; Kelly, G.; Baxter, E.; Windloch, K.; Gannon, F.; Lee, J.S. G9a-Mediated Repression of CDH10 in Hypoxia Enhances Breast Tumor Cell Motility and Associates with Poor Survival Outcome. Theranostics 2020, 10, 4515–4529. [Google Scholar] [CrossRef] [PubMed]
- Jinawath, N.; Shiao, M.S.; Norris, A.; Murphy, K.; Klein, A.P.; Yonescu, R.; Iacobuzio-Donahue, C.; Meeker, A.; Jinawath, A.; Yeo, C.J.; et al. Alterations of Type II Classical Cadherin, Cadherin-10 (CDH10), Is Associated with Pancreatic Ductal Adenocarcinomas. Genes Chromosomes Cancer 2017, 56, 427–435. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z.; Li, F.; Li, X.; Sun, Y.; Wang, M.; Li, D.; Wang, R.; Li, F.; Fang, R.; et al. Whole exome sequencing identifies frequent somatic mutations in cell-cell adhesion genes in Chinese patients with lung squamous cell carcinoma. Sci. Rep. 2015, 5, 14237. [Google Scholar] [CrossRef]
- Sim, J.C.; White, S.M.; Lockhart, P.J. ARID1B-Mediated Disorders: Mutations and Possible Mechanisms. Intractable Rare Dis. Res. 2015, 4, 17–23. [Google Scholar] [CrossRef]
- Hanna, C.; Willman, M.; Cole, D.; Mehkri, Y.; Liu, S.; Willman, J.; Lucke-Wold, B. Review of Meningioma Diagnosis and Management. Egypt. J. Neurosurg. 2023, 42, 16. [Google Scholar] [CrossRef]
| GENE | CODING | PROTEIN | dbSNP | VAF % |
|---|---|---|---|---|
| CDH10 | c.23T>A | p.Leu8Gln | 24.53 | |
| ARID1BꟾMIR4466 | c.1592_1593insACCꟾ | p.Pro533dupl | rs572236007:rs1405399488 | 8.11 |
| PDCD1LG2 | c.789_791delCAC | p.Thr265del | rs1816600126:rs890047189 | 92.97 |
| ATM | c.4777-1G>C | p.? | rs1591684268 | 85.74 |
| B2M | c.41_42delCTinsAA | p.Ser14Ter | rs986783978:rs1341857550 | 80.30 |
| B2M | c.43_45delCTTinsTAG | p.Leu15Ter | rs1329285364 | 19.46 |
| B2M | c.47_48delCTinsAA | p.Ser16Ter | rs1435611656 | 21.48 |
| CREBBP | c.2890delG | p.Ala964GlnfsTer34 | rs760906604 | 23.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, S.; Farese, S.; Balbo, C.; Melisi, F.; Scrima, M.; Pasquale, L.S.; Laudato, M.P.; Peluso, T.; Solari, D.; Ronchi, A.; et al. The First Case Report of a Solitary Pulmonary Metastasis of a Transitional Meningioma and Literature Review. Int. J. Mol. Sci. 2025, 26, 6868. https://doi.org/10.3390/ijms26146868
Di Lorenzo S, Farese S, Balbo C, Melisi F, Scrima M, Pasquale LS, Laudato MP, Peluso T, Solari D, Ronchi A, et al. The First Case Report of a Solitary Pulmonary Metastasis of a Transitional Meningioma and Literature Review. International Journal of Molecular Sciences. 2025; 26(14):6868. https://doi.org/10.3390/ijms26146868
Chicago/Turabian StyleDi Lorenzo, Sara, Stefano Farese, Ciro Balbo, Federica Melisi, Marianna Scrima, Lucia Stefania Pasquale, Maria Pasqualina Laudato, Teresa Peluso, Domenico Solari, Andrea Ronchi, and et al. 2025. "The First Case Report of a Solitary Pulmonary Metastasis of a Transitional Meningioma and Literature Review" International Journal of Molecular Sciences 26, no. 14: 6868. https://doi.org/10.3390/ijms26146868
APA StyleDi Lorenzo, S., Farese, S., Balbo, C., Melisi, F., Scrima, M., Pasquale, L. S., Laudato, M. P., Peluso, T., Solari, D., Ronchi, A., Accardo, M., Franco, R., Addeo, R., Somma, T., Pirozzi, M., Ciardiello, F., Caraglia, M., & Fasano, M. (2025). The First Case Report of a Solitary Pulmonary Metastasis of a Transitional Meningioma and Literature Review. International Journal of Molecular Sciences, 26(14), 6868. https://doi.org/10.3390/ijms26146868











