Potential Role of Inflammasomes in Aging
Abstract
1. Introduction
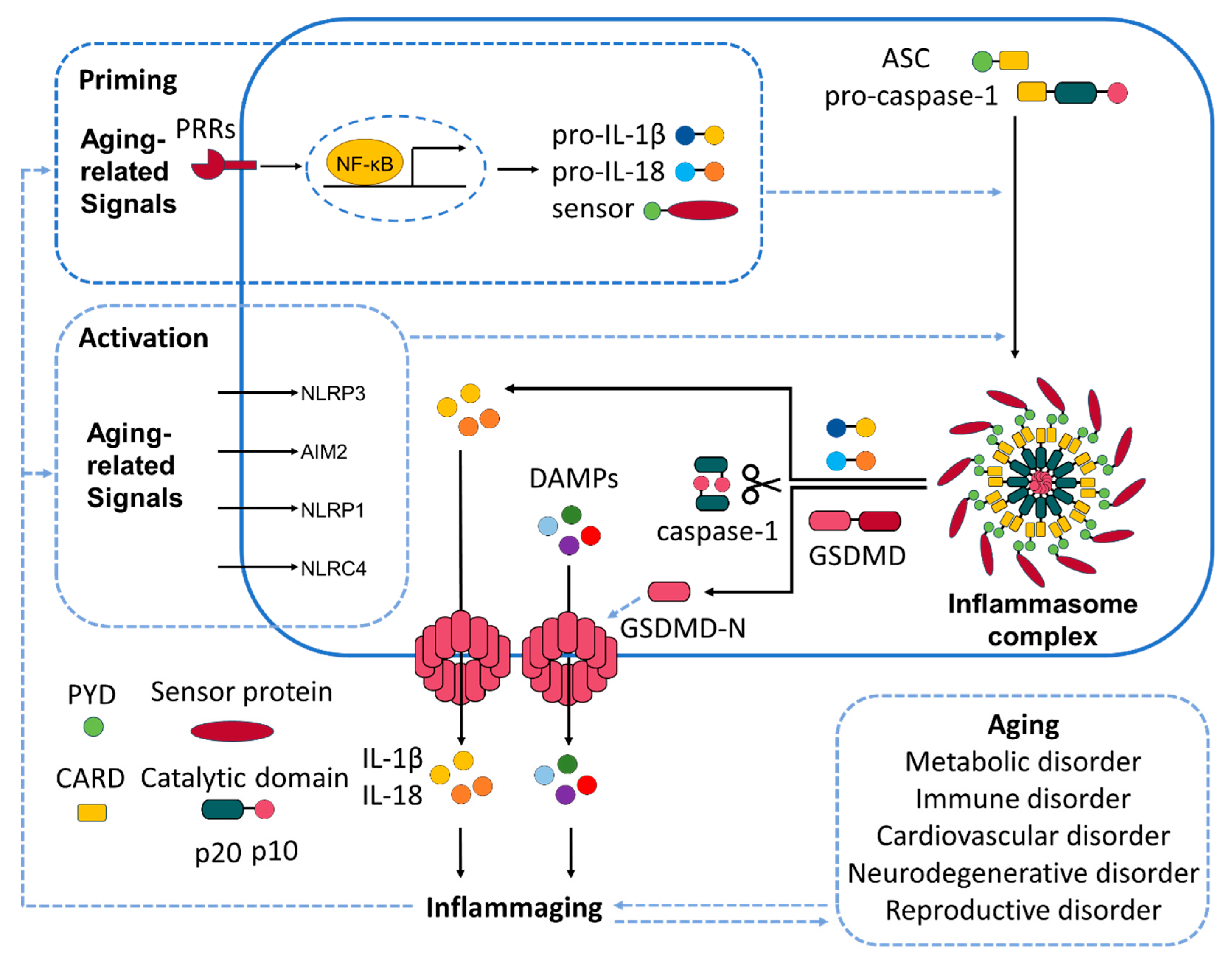
2. Molecular Mechanisms of Inflammasome Activation
3. Role of Inflammasomes in Immunosenescence
3.1. Adaptive Immunity
3.2. Innate Immunity
3.3. Inflammaging
4. Therapeutic Strategies and Inhibitors
4.1. Key Inflammasomes Driving Inflammaging
4.1.1. NLRP3
4.1.2. NLRP1
4.1.3. AIM2
4.1.4. NAIP/NLRC4 Inflammasomes
4.2. Pharmacological Inhibition of Inflammasomes
4.2.1. Limitations of Current Inhibitors
4.2.2. Upstream Inflammasome Inhibition and Anti-Aging Effects
4.2.3. Alternative NLRP3 Inhibitors: Sulfur-Containing Compounds
4.2.4. Natural Compounds and Nutraceuticals for Inflammasome Modulation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Ageing. Available online: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed on 23 September 2024).
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kuan, V.; Fraser, H.C.; Hingorani, M.; Denaxas, S.; Gonzalez-Izquierdo, A.; Direk, K.; Nitsch, D.; Mathur, R.; Parisinos, C.A.; Lumbers, R.T.; et al. Data-driven identification of ageing-related diseases from electronic health records. Sci. Rep. 2021, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Thillainadesan, J. What Is an Aging-Related Disease? An Epidemiological Perspective. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontol. 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Valverde, M.; Pasinetti, G.M. The NLRP3 Inflammasome as a Critical Actor in the Inflammaging Process. Cells 2020, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Lee, G.S. Korean Red Ginseng, a regulator of NLRP3 inflammasome, in the COVID-19 pandemic. J. Ginseng Res. 2022, 46, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, L.J.; de Rivero Vaccari, J.P.; Dale, G.A.; Keane, R.W.; Bramlett, H.M. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Qi, X.; Cai, Q.; Niu, L.; Huang, X.; Zhang, D.; Ling, J.; Wu, Y.; Chen, Y.; Yang, P.; et al. The role of NLRP3 inflammasome in aging and age-related diseases. Immun. Ageing 2024, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 2022, 118, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Kanneganti, T.D.; Vandanmagsar, B.; Zhu, X.; Ravussin, A.; Adijiang, A.; Owen, J.S.; Thomas, M.J.; Francis, J.; Parks, J.S.; et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012, 1, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Fidler, T.P.; Xue, C.; Yalcinkaya, M.; Hardaway, B.; Abramowicz, S.; Xiao, T.; Liu, W.; Thomas, D.G.; Hajebrahimi, M.A.; Pircher, J.; et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 2021, 592, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Ando, F.; Iguchi, A.; Shimokata, H. Effect of aging on serum uric acid levels: Longitudinal changes in a large Japanese population group. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M660–M664. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef] [PubMed]
- Brahadeeswaran, S.; Sivagurunathan, N.; Calivarathan, L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 2288–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2023, 23, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Shanley, D.P.; Aw, D.; Manley, N.R.; Palmer, D.B. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009, 30, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Sahebdel, F.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samini, F.; Samarghandian, S. The Role of Immunosenescence and Inflammaging in the Susceptibility of Older Adults to SARS-CoV-2 Infection. Curr. Pharm. Biotechnol. 2025, 26, e13892010328697. [Google Scholar] [CrossRef] [PubMed]
- Deo, A.S.; Shrijana; Sruthika, S.U.; Karun, S.; Bisaria, K.; Sarkar, K. Participation of T cells in generating immune protection against cancers. Pathol. Res. Pract. 2024, 262, 155534. [Google Scholar] [CrossRef] [PubMed]
- de Mol, J.; Kuiper, J.; Tsiantoulas, D.; Foks, A.C. The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 2021, 12, 733566. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D.; Sander, J.; Spadaro, O.; Lee, A.; Nguyen, K.Y.; Wing, A.; Goldberg, E.L.; Youm, Y.H.; Brown, C.W.; Elsworth, J.; et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017, 550, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D.; Gunther, P.; Lee, A.; Goldberg, E.L.; Spadaro, O.; Youm, Y.H.; Bartke, A.; Hubbard, G.B.; Ikeno, Y.; Ruddle, N.H.; et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019, 30, 1024–1039.E6. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Niepmann, S.; Knolle, P.A.; Hornung, V. Aging-Associated TNF Production Primes Inflammasome Activation and NLRP3-Related Metabolic Disturbances. J. Immunol. 2016, 197, 2900–2908. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Lei, W.; Hou, Y.; Zhang, Y.; Tang, R.; Yang, Z.; Tian, Y.; Zhu, Y.; Wang, C.; et al. The NLRP3 inflammasome in fibrosis and aging: The known unknowns. Ageing Res. Rev. 2022, 79, 101638. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Dupuis, G.; Baehl, S.; Le Page, A.; Bourgade, K.; Frost, E.; Witkowski, J.M.; Pawelec, G.; Larbi, A.; Cunnane, S. From inflamm-aging to immune-paralysis: A slippery slope during aging for immune-adaptation. Biogerontology 2016, 17, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Immunosenescence: The potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell. Mol. Life Sci. 2019, 76, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Heal. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Henriksbo, B.D.; Anhê, F.F.; Schertzer, J.D. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem. J. 2020, 477, 1089–1107. [Google Scholar] [CrossRef] [PubMed]
- Cyr, B.; Hadad, R.; Keane, R.W.; de Rivero Vaccari, J.P. The Role of Non-canonical and Canonical Inflammasomes in Inflammaging. Front. Mol. Neurosci. 2022, 15, 774014. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, J.; Ferrara, F.; Vallese, A.; Guiotto, A.; Colella, S.; Pecorelli, A.; Valacchi, G. Comparison of Pollutant Effects on Cutaneous Inflammasomes Activation. Int. J. Mol. Sci. 2023, 24, 16674. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Tan, L.; Jiang, T.; Zhu, X.C.; Wang, H.F.; Jia, C.D.; Yu, J.T. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014, 5, e1382. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.Y.; Pickard, B.S.; Chan, E.W.L.; Gan, S.Y. The Role of Neuronal NLRP1 Inflammasome in Alzheimer’s Disease: Bringing Neurons into the Neuroinflammation Game. Mol. Neurobiol. 2019, 56, 7741–7753. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Zhou, M.; Feng, S.; Peng, X.; Wang, Y. Microglial TLR4/NLRP3 Inflammasome Signaling in Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 97, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.J.; Hung, Y.F.; Liu, H.Y.; Hsueh, Y.P. Deletion of the Inflammasome Sensor Aim2 Mitigates Abeta Deposition and Microglial Activation but Increases Inflammatory Cytokine Expression in an Alzheimer Disease Mouse Model. Neuroimmunomodulation 2017, 24, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, J.; Ma, D.; Han, Y.; Zhang, Y.; Wang, S.; Wang, Z. Microglial ApoD-induced NLRC4 inflammasome activation promotes Alzheimer’s disease progression. Anim. Models Exp. Med. 2025, 8, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Khilazheva, E.D.; Mosiagina, A.I.; Panina, Y.A.; Belozor, O.S.; Komleva, Y.K. Impact of NLRP3 Depletion on Aging-Related Metaflammation, Cognitive Function, and Social Behavior in Mice. Int. J. Mol. Sci. 2023, 24, 16580. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kwon, H.M.; Lee, E.; Kim, P.H.; Jeung, E.B.; Lee, G.S. Role of inflammasome regulation on immune modulators. J. Biomed. Res. 2018, 32, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Marín-Aguilar, F.; Lechuga-Vieco, A.V.; Alcocer-Gómez, E.; Castejón-Vega, B.; Lucas, J.; Garrido, C.; Peralta-Garcia, A.; Pérez-Pulido, A.J.; Varela-López, A.; Quiles, J.L.; et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 2020, 19, e13050. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pando, J.M.; Alcocer-Gómez, E.; Castejón-Vega, B.; Navarro-Villarán, E.; Condés-Hervás, M.; Mundi-Roldan, M.; Muntané, J.; Pérez-Pulido, A.J.; Bullon, P.; Wang, C.; et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci. Adv. 2021, 7, eabc7409. [Google Scholar] [CrossRef] [PubMed]
- Chei, S.; Oh, H.J.; Jang, H.; Lee, K.; Jin, H.; Choi, Y.; Lee, B.Y. Korean Red Ginseng Suppresses the Expression of Oxidative Stress Response and NLRP3 Inflammasome Genes in Aged C57BL/6 Mouse Ovaries. Foods 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.; Murphy, C.; Mansell, A. NLRP1- A CINDERELLA STORY: A perspective of recent advances in NLRP1 and the questions they raise. Commun. Biol. 2023, 6, 1274. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Kanneganti, T.D. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int. J. Mol. Sci. 2021, 22, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mejias, N.H.; Martinez, C.C.; Stephens, M.E.; de Rivero Vaccari, J.P. Contribution of the inflammasome to inflammaging. J. Inflamm. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Dongil, P.; Valencia, I.; Vallejo, S.; Hipolito-Luengo, A.S.; Diaz-Araya, G.; Bartha, J.L.; Gonzalez-Arlanzon, M.M.; Rivilla, F.; de la Cuesta, F.; et al. Pharmacological Blockade of NLRP3 Inflammasome/IL-1beta-Positive Loop Mitigates Endothelial Cell Senescence and Dysfunction. Aging Dis. 2022, 13, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Talley, S.; Nguyen, T.; Van Ye, L.; Valiauga, R.; DeCarlo, J.; Mustafa, J.; Cook, B.; White, F.A.; Campbell, E.M. Characterization of age-associated inflammasome activation reveals tissue specific differences in transcriptional and post-translational inflammatory responses. Immun. Ageing 2024, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.J.; Ma, Y.J.; Feng, J.L.; Li, J.P.; Qiu, S.; Yu, Q.Q.; Liu, R.H.; Wankumbu, S.C.; Wang, X.; Li, X.X.; et al. NLRP3 inflammasome-mediated premature immunosenescence drives diabetic vascular aging dependent on the induction of perivascular adipose tissue dysfunction. Cardiovasc. Res. 2025, 121, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.; Jeung, E.B.; Lee, G.S. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 2014, 219, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.; Lee, M.J.; Kim, Y.J.; Cho, Y.W.; Lee, G.S. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 2015, 71, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, H.; Han, B.C.; Lee, S.H.; Cho, Y.W.; Kim, C.H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol. Lett. 2014, 158, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zang, L.; Yu, J.; Yu, J.; Wang, S.; Zhou, L.; Song, H.; Ma, Y.; Niu, X.; Li, W. Anti-inflammatory effect of proanthocyanidins from blueberry through NF-κβ/NLRP3 signaling pathway in vivo and in vitro. Immunopharmacol. Immunotoxicol. 2024, 46, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.Y.; Kang, M.J.; Jeong, Y.J.; Choi, J.A.; Oh, S.M.; Lee, K.B.; Park, J.H. Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1β in Dendritic Cells by Regulating NF-κB and NLRP3 Inflammasome Activation. Immune Netw. 2015, 15, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, Y.; Jiang, M.; Kuang, L.; Wan, J.; Liu, S.; Zhang, Q.; Yu, K.; Li, N.; Le, A.; et al. Curcumin activates NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by up-regulated ISG3 transcript factor in acute myeloid leukemia cell lines. Cancer Biol. Ther. 2022, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, G.S. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity. Sci. Rep. 2020, 10, 19091. [Google Scholar] [CrossRef] [PubMed]
| Aging-Related Signal | Description | Detected by | References |
|---|---|---|---|
| Gut microbiome change | Dysbiosis and altered microbial metabolites influencing inflammation. | NLRP3 inflammasome | [21,22] |
| Free cholesterol, ceramides | Lipid molecules that accumulate in aging tissues, contribute to inflammation. | NLRP3 inflammasome | [23] |
| ROS | Highly reactive molecules are formed by the incomplete reduction of oxygen during cellular respiration. | NLRP3, AIM2 inflammasome | [24,25] |
| MSU (monosodium urate) | Crystals formed due to hyperuricemia, which is associated with gout. | NLRP3 inflammasome | [13] |
| Uric acid | Elevated levels lead to crystal formation associated with gout. | NLRP3 inflammasome | [26,27] |
| Cholesterol crystals | Formed in atherosclerosis, they contribute to plaque formation. | NLRP3 inflammasome | [19] |
| Oxidized LDL | Modified form of low-density lipoprotein, contributes to atherosclerosis. | NLRP3 inflammasome | [19] |
| α-synuclein | Aggregates in Parkinson’s disease, forms Lewy bodies. | NLRP3 inflammasome | [28] |
| Adenine and N4-acetylcytidine | Nucleotide-derived metabolites. | NLRC4 inflammasome | [17] |
| dsDNA | DNA fragments released into the cytoplasm due to cellular damage or stress. | AIM2 and IFI16 | [29] |
| Unknown agent | Associated with cognitive impairment in the hippocampus. | NLRP1 | [18] |
| Inflammasome | Key Cells | Trigger | Pathology | References |
|---|---|---|---|---|
| NLRP1 | Cortical and hippocampal neurons | Amyloid-β (Aβ) aggregates activate neuronal NLRP1 directly | Caspase-1 activation in neurons → IL-1β, IL-18 maturation → neuronal pyroptosis → cognitive impairment and neurodegeneration | [52,53] |
| NLRP3 | Microglia | Aβ plaques and tau tangles induce microglial phagocytosis → lysosomal rupture → cathepsin B release → NLRP3 activation; also primed by TLR/NF-κB signaling | Sustained IL-1β and IL-18 release → chronic neuroinflammation, impaired Aβ clearance, microglial M1 polarization → synaptic dysfunction | [54,55] |
| AIM2 | Microglia; some astrocytes | Cytosolic dsDNA from damaged or stressed neurons/mitochondria accumulates in aging brain → AIM2 binds DNA → ASC recruitment | IL-1β, IL-18 secretion → amplifies Aβ deposition and tau hyperphosphorylation → worsens memory deficits | [56] |
| NLRC4 | Microglia | ApoD induces NLRC4 inflammasome activation | ApoD-induced NLRC4 inflammasome activation in microglia → pro-inflammatory phenotype → impaired neural stem cell self-renewal and neuron apoptosis | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Lee, G.-S. Potential Role of Inflammasomes in Aging. Int. J. Mol. Sci. 2025, 26, 6768. https://doi.org/10.3390/ijms26146768
Lee G, Lee G-S. Potential Role of Inflammasomes in Aging. International Journal of Molecular Sciences. 2025; 26(14):6768. https://doi.org/10.3390/ijms26146768
Chicago/Turabian StyleLee, Gilyoung, and Geun-Shik Lee. 2025. "Potential Role of Inflammasomes in Aging" International Journal of Molecular Sciences 26, no. 14: 6768. https://doi.org/10.3390/ijms26146768
APA StyleLee, G., & Lee, G.-S. (2025). Potential Role of Inflammasomes in Aging. International Journal of Molecular Sciences, 26(14), 6768. https://doi.org/10.3390/ijms26146768






