Deciphering Important Odorants in a Spirulina (Arthrospira platensis) Dietary Supplement by Aroma Extract Dilution Analysis Using Offline and Online Fractionation Approaches
Abstract
1. Introduction
2. Results and Discussion
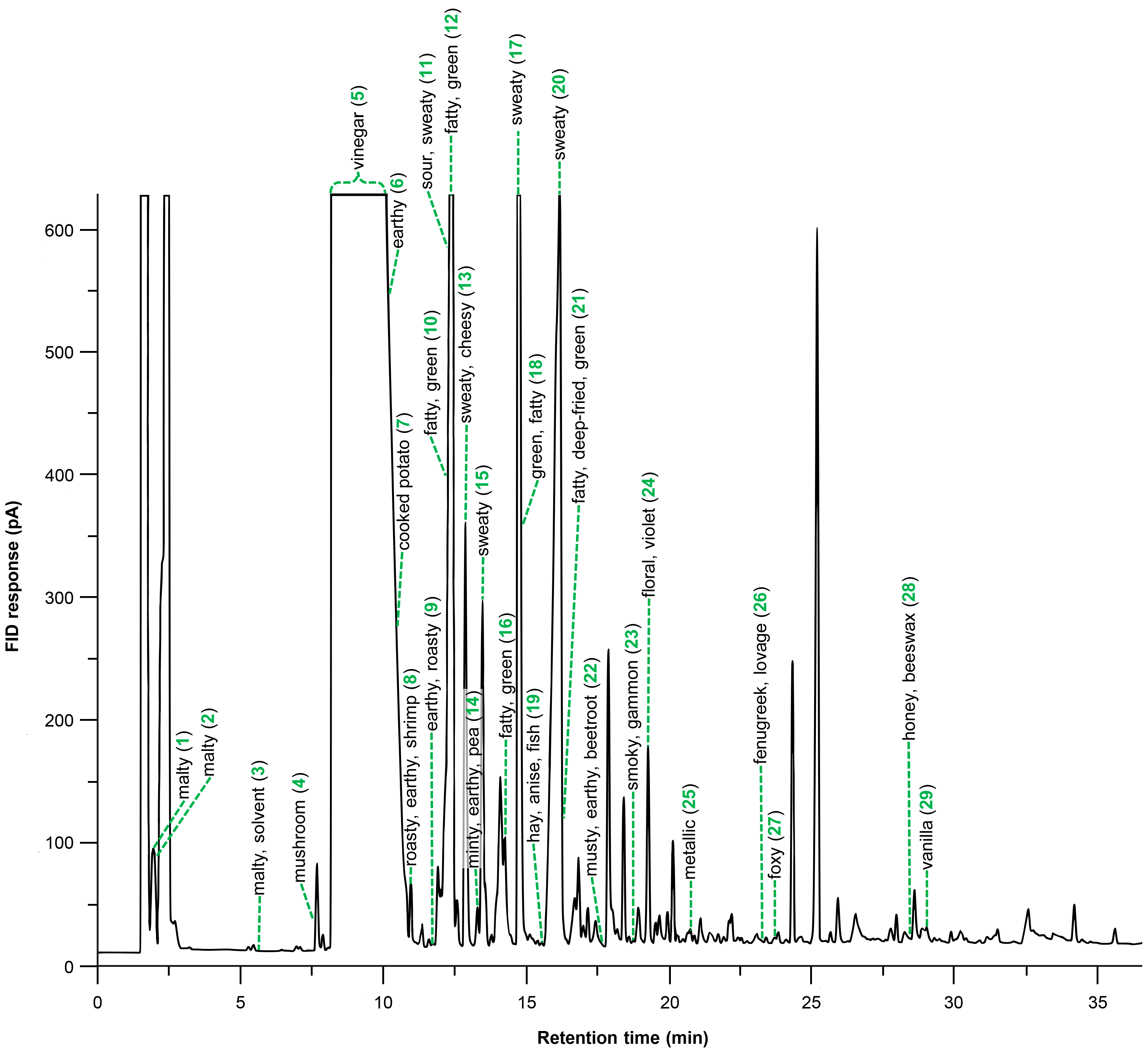
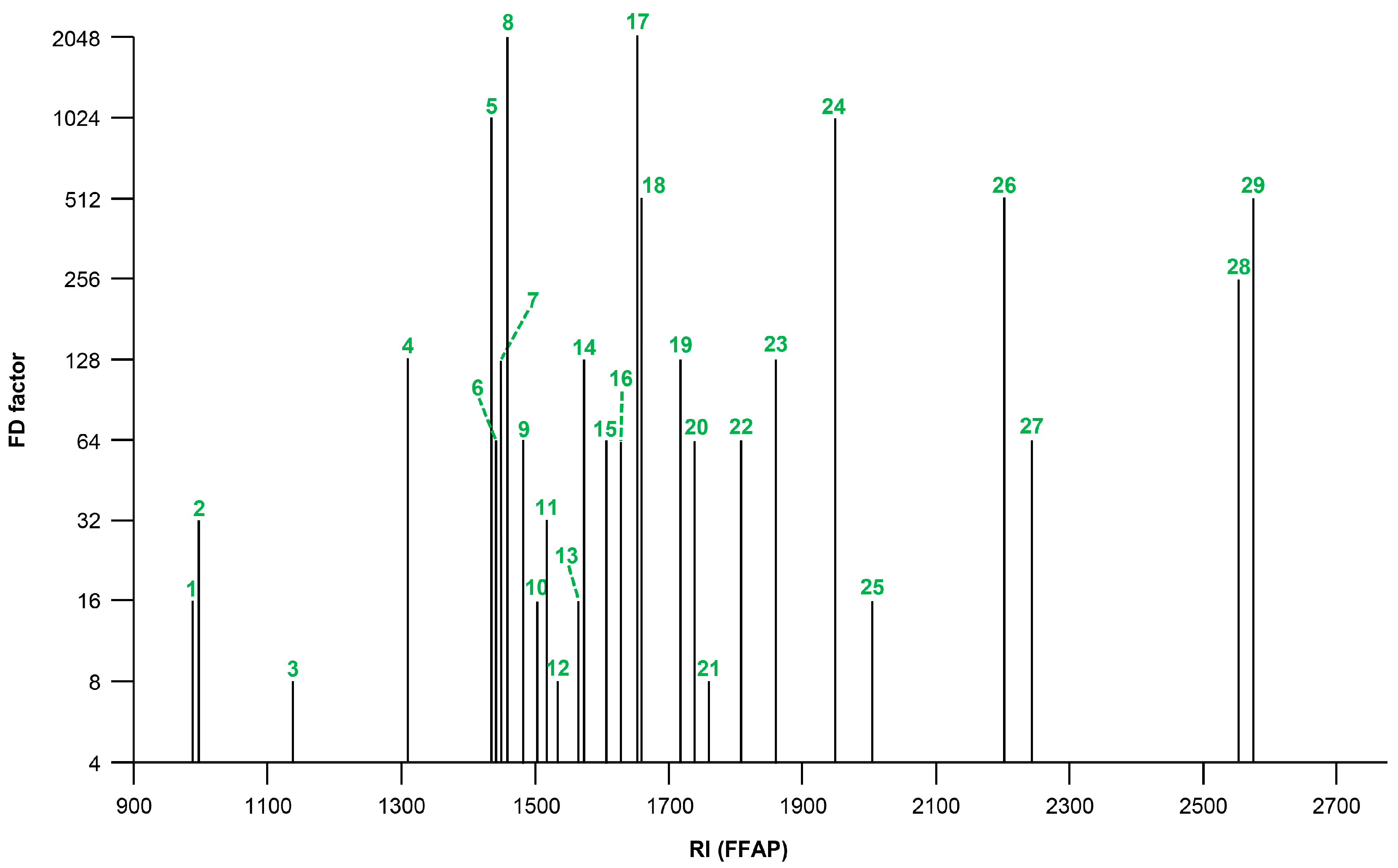
2.1. Odorants in the Spirulina Dietary Supplement
2.2. Discussion
3. Materials and Methods
3.1. Dried Spirulina Flakes
3.2. Chemicals
3.3. Isolation of Spirulina Volatiles
3.4. Offline Fractionation
3.4.1. Separation of Neutral/Basic Volatiles (NBV) and Acidic Volatiles (AV)
3.4.2. Fractionation of NBV by Liquid Chromatography
3.5. Gas Chromatography (GC)
3.6. Aroma Extract Dilution Analysis (AEDA)
3.7. SH Dilution Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEDA | Aroma extract dilution analysis |
| aSAFE | Automated solvent-assisted flavor evaporation |
| AV | Acidic volatiles |
| CI | Chemical ionization |
| EI | Electron ionization |
| FD | Flavor dilution |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC–GC | Two-dimensional heart-cut gas chromatography |
| GC×GC | Comprehensive two-dimensional gas chromatography |
| GC–O | Gas chromatography–olfactometry |
| KFO | Key food odorant |
| MS | Mass spectrometry |
| NBV | Neutral and basic volatiles |
| NCI | Negative chemical ionization |
| RI | Retention index |
| SH | Static headspace |
| SPME | Solid-phase microextraction |
| PTV | Programmable temperature vaporizing |
| TOF | Time-of-flight |
References
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; del Prete, F.; Aquino, R.P.; Campiglia, P. Fast profiling of natural pigments in different spirulina (Arthrospira platensis) dietary supplements by DI-FT-ICR and evaluation of their antioxidant potential by pre-column DPPH-UHPLC assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The true value of spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef]
- Ozdemir, G.; Karabay, N.U.; Dalay, M.C.; Pazarbasi, B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother. Res. 2004, 18, 754–757. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Shahid, A.; Fan, Z.; Su, K.; Zhao, A.; Mehmood, M.A.; Chang, J.-S.; Solovchenko, A.E.; Alam, A.; Xu, J. Sensory chemistry of spirulina: Unveiling trends and innovations in aromatic volatile organic compound biosynthesis in off-flavors and odor mitigation strategies. Trends Food Sci. Technol. 2025, 156, 104886. [Google Scholar] [CrossRef]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermúdez, S.P.; Barba-Davila, B.; Serna-Saldivar, S.O.; Parra-Saldivar, R.; Rodriguez-Rodriguez, J.; Morales-Davila, S.; Goiris, K.; Muylaert, K.; Chuck-Hernández, C. Deodorization of Arthrospira platensis biomass for further scale-up food applications. J. Sci. Food Agric. 2017, 97, 5123–5130. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-state fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.H.; Ren, D.F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Milovanović, I.; Mišan, A.; Simeunović, J.; Kovač, D.; Jambrec, D.; Mandić, A. Determination of volatile organic compounds in selected strains of cyanobacteria. J. Chem. 2015, 2015, 969542. [Google Scholar] [CrossRef]
- Verhoeven, H.; Beuerle, T.; Schwab, W. Solid-phase microextraction: Artefact formation and its avoidance. Chromatographia 1997, 46, 63–66. [Google Scholar] [CrossRef]
- Haberhauer-Troyer, C.; Rosenberg, E.; Grasserbauer, M. Evaluation of solid-phase microextraction for sampling of volatile organic sulfur compounds in air for subsequent gas chromatographic analysis with atomic emission detection. J. Chromatogr. A 1999, 848, 305–315. [Google Scholar] [CrossRef]
- Lestremau, F.; Andersson, F.; Desauziers, V. Investigation of artefact formation during analysis of volatile sulphur compounds using solid phase microextraction (SPME). Chromatographia 2004, 59, 607–613. [Google Scholar] [CrossRef]
- Schlumpberger, P.; Stübner, C.A.; Steinhaus, M. Development and evaluation of an automated solvent-assisted flavour evaporation (aSAFE). Eur. Food Res. Technol. 2022, 248, 2591–2602. [Google Scholar] [CrossRef]
- Reinhardt, J.; Steinhaus, M. Injection artifacts in odorant analysis by gas chromatography. J. Chromatogr. A 2025, 1741, 465624. [Google Scholar] [CrossRef]
- Haag, F.; Krautwurst, D. Olfaction and the complex interaction between odourant ligands and their receptors. Compr. Anal. Chem. 2021, 96, 1–40. [Google Scholar] [CrossRef]
- Steinhaus, M. Gas chromatography–olfactometry: Principles, practical aspects and applications in food analysis. In Advanced Gas Chromatography in Food Analysis; Tranchida, P., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 337–399. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Evaluation of the flavour of wheat and rye bread crusts by aroma extract dilution analysis. Z. Lebensm. Unters. Forsch. 1987, 185, 111–113. [Google Scholar] [CrossRef]
- Patrushev, Y.V. Advantages of two-dimensional gas chromatography. Kinet. Catal. 2015, 56, 386–393. [Google Scholar] [CrossRef]
- Cordero, C.; Kiefl, J.; Reichenbach, S.E.; Bicchi, C. Characterization of odorant patterns by comprehensive two-dimensional gas chromatography: A challenge in omic studies. TrAC Trends Anal. Chem. 2019, 113, 364–378. [Google Scholar] [CrossRef]
- Jia, X.; Cui, H.; Qin, S.; Ren, J.; Zhang, Z.; An, Q.; Zhang, N.; Yang, J.; Yang, Y.; Fan, G.; et al. Characterizing and decoding the key odor compounds of Spirulina platensis at different processing stages by sensomics. Food Chem. 2024, 461, 140944. [Google Scholar] [CrossRef] [PubMed]
- Guth, H.; Grosch, W. Identification of potent odourants in static headspace samples of green and black tea powders on the basis of aroma extract dilution analysis (AEDA). Flavour Fragrance J. 1993, 8, 173–178. [Google Scholar] [CrossRef]
- Buhr, K.; Pammer, C.; Schieberle, P. Influence of water on the generation of Strecker aldehydes from dry processed foods. Eur. Food Res. Technol. 2010, 230, 375–381. [Google Scholar] [CrossRef]
- Ullrich, L.; Neiens, S.; Hühn, T.; Steinhaus, M.; Chetschik, I. Impact of water on odor-active compounds in fermented and dried cocoa beans and chocolates made thereof. J. Agric. Food Chem. 2021, 69, 8504–8510. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Kaloudis, T.; Hiskia, A.; Steinhaus, M.; Dimotikali, D.; Triantis, T.M. Volatile profiling of spirulina food supplements. Foods 2024, 13, 1257. [Google Scholar] [CrossRef]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database, version 1.2.; Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2022; Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 5 May 2025).
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Catalytic pyrolysis-GC/MS of spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Hamad, G.M.; El-Baky, N.A.; Sharaf, M.M.; Amara, A.A. Volatile compounds, fatty acids constituents, and antimicrobial activity of cultured spirulina (Arthrospira fusiformis) isolated from Lake Mariout in Egypt. Sci. World J. 2023, 2023, 9919814. [Google Scholar] [CrossRef]
- Agustini, T.W.; Dewi, E.N.; Amalia, U.; Kurniasih, R.A. Application of basil leaf extracts to decrease Spirulina platensis off-odour in increasing food consumption. Int. Food Res. J. 2019, 26, 1789–1794. [Google Scholar]
- Ould Bellahcen, T.; Cherki, M.; Sánchez, J.A.C.; Cherif, A.; EL Amrani, A. Chemical composition and antibacterial activity of the essential oil of Spirulina platensis from Morocco. J. Essent. Oil Bear. Plants 2019, 22, 1265–1276. [Google Scholar] [CrossRef]
- van Durme, J.; Goiris, K.; de Winne, A.; de Cooman, L.; Muylaert, K. Evaluation of the volatile composition and sensory properties of five species of microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, K.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, H.; Prichard, R.K.; McManus, W.R.; Schofield, P.J. The dissimilation of leucine, isoleucine and valine to volatile fatty acids by adult Fasciola hepatica. Int. J. Parasitol. 1971, 1, 223–233. [Google Scholar] [CrossRef] [PubMed]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies affecting the growth and cultivation of genus spirulina: An investigative review on current trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Hofmann, T.; Münch, P.; Schieberle, P. Quantitative model studies on the formation of aroma-active aldehydes and acids by Strecker-type reactions. J. Agric. Food Chem. 2000, 48, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Bortolini, D.; Maciel, G.M.; de Andrade Arruda Fernandes, I.; Pedro, A.C.; Vieira Rubio, F.T.; Guiherme Branco, I.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Zhang, Y.; Song, H.; Raza, A.; Pan, W.; Gong, L.; Jiang, C. Comparison of different volatile extraction methods for the identification of fishy off-odor in fish by-products. Molecules 2022, 27, 6177. [Google Scholar] [CrossRef]
- Aguero, J.; Lora, J.; Estrada, K.; Concepcion, F.; Nunez, A.; Rodriguez, A.; Pino, J.A. Volatile components of a commercial sample of the blue-green algae Spirulina platensis. J. Essent. Oil Res. 2003, 15, 114–117. [Google Scholar] [CrossRef]
- Bemis-Young, G.L.; Huang, J.; Bernhard, R.A. Effect of pH on pyrazine formation in glucose—glycine model systems. Food Chem. 1993, 46, 383–387. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 563–614. [Google Scholar]
- Mall, V.; Schieberle, P. Characterization of key aroma compounds in raw and thermally processed prawns and thermally processed lobsters by application of aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 6433–6442. [Google Scholar] [CrossRef]
- Isleten Hosoglu, M. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef]
- Imre Blank, I.; Lin, J.; Fumeaux, R.; Welti, D.H.; Fay, L.B. Formation of 3-hydroxy-4,5-dimethyl-2(5H)-furanone (sotolone) from 4-hydroxy-l-isoleucine and 3-amino-4,5-dimethyl-3,4-dihydro-2(5H)-furanone. J. Agric. Food Chem. 1996, 44, 1851–1856. [Google Scholar] [CrossRef]
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of odor-active compounds, polyphenols, and fatty acids in coffee silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Pueschel, V.A.; Schieberle, P. Changes in the key aroma compounds of matsutake mushroom (Tricholoma matsutake Sing.) from Canada during pan-frying elucidated by application of the sensomics approach. Eur. Food Res. Technol. 2021, 247, 51–65. [Google Scholar] [CrossRef]
- Baudouin, D.; Xiang, H.; Vogel, F. On the selective desulphurization of biomass derivatives in supercritical water. Biomass Bioenergy 2022, 164, 106529. [Google Scholar] [CrossRef]
- Höckelmann, C.; Becher, P.G.; von Reuss, S.H.; Jüttner, F. Sesquiterpenes of the geosmin-producing cyanobacterium Calothrix PCC 7507 and their toxicity to invertebrates. Z. Naturforsch. C 2009, 64, 49–55. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Ellakany, H.F.; Abaza, S.S.; Geneedy, A.M.; Salem, H.M.; Taha, A.E.; Swelum, A.A.; Omer, F.A.; et al. Undesirable odour substances (geosmin and 2-methylisoborneol) in water environment: Sources, impacts and removal strategies. Mar. Pollut. Bull. 2022, 178, 113579. [Google Scholar] [CrossRef]
- Li, J.-X.; Schieberle, P.; Steinhaus, M. Characterization of the major odor-active compounds in Thai durian (Durio zibethinus L. ‘Monthong’) by aroma extract dilution analysis and headspace gas chromatography–olfactometry. J. Agric. Food Chem. 2012, 60, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Milo, C.; Grosch, W. Changes in the odorants of boiled trout (Salmo fario) as affected by the storage of the raw material. J. Agric. Food Chem. 1993, 41, 2076–2081. [Google Scholar] [CrossRef]
- Wagner, R.; Czerny, M.; Bielohradsky, J.; Grosch, W. Structure-odour-activity relationships of alkylpyrazines. Z. Lebensm. Unters. Forsch. A 1999, 208, 308–316. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Potent odorants of the wheat bread crumb. Differences to the crust and effect of a longer dough fermentation. Z. Lebensm. Unters. Forsch. 1991, 192, 130–135. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the aroma-active compounds in pink guava (Psidium guajava L.) by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
| Odorant | Odor Quality 1 | RI 2 | MS (EI) 3: m/z (%) | |
|---|---|---|---|---|
| DB-FFAP | DB-5 | |||
| odorant 8 | roasty, earthy, shrimp | 1450 | 1089 | 39 (76), 40 (15), 41 (18), 42 (70), 52 (28), 53 (40), 56 (29), 108 (18), 135 (100), 136 (59) |
| 3-ethyl-2,5-dimethylpyrazine | nutty, earthy | 1442 | 1083 | 39 (83), 40 (29), 41 (24), 42 (100), 53 (25), 56 (41), 107 (29), 108 (24), 135 (96), 136 (64) |
| 2-ethyl-3,5-dimethylpyrazine | roasty, earthy, shrimp | 1450 | 1089 | 39 (95), 40 (32), 41 (28), 42 (75), 52 (20), 53 (24), 56 (88), 108 (23), 135 (100), 136 (69) |
| No. | Odorant 1 | Odor Quality 2 | Fraction 3 | RI 4 | FD Factor 5 | Earlier Identified in Spirulina | |
|---|---|---|---|---|---|---|---|
| DB-FFAP | DB-5 | ||||||
| 1 | 3-methylbutanal | malty | NBV | 945 | 665 | 16 | [26,28] |
| 2 | 2-methylbutanal | malty | NBV | 953 | 675 | 32 | [9] |
| 3 | butan-1-ol | malty, solvent | NBV | 1138 | 667 | 8 | |
| 4 | oct-1-en-3-one | mushroom | NBV | 1305 | 988 | 128 | [22] |
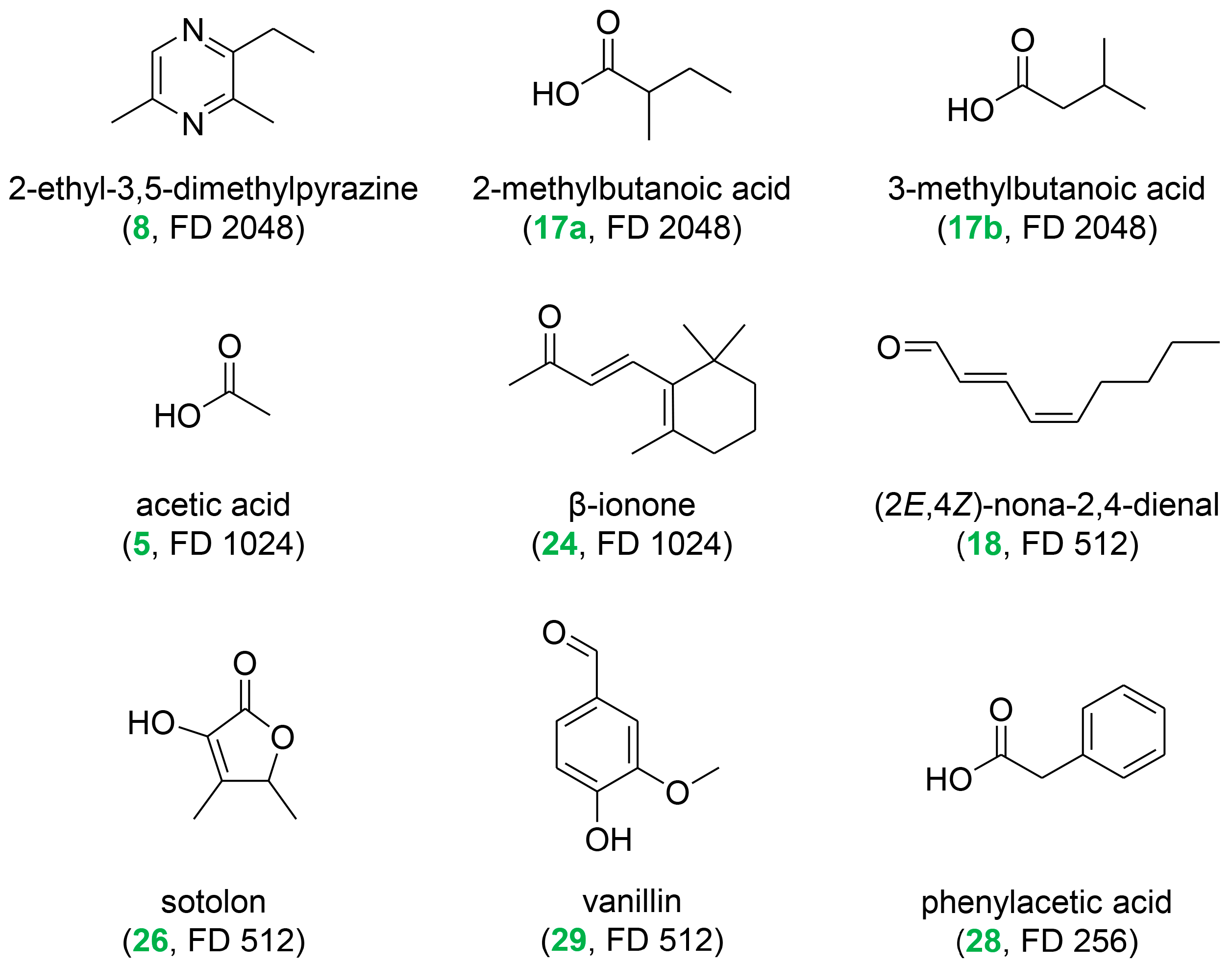
| 5 | acetic acid | vinegar | AV | 1441 | <600 | 1024 | [22,29] |
| 6 | 2,3-diethylpyrazine | earthy | NBV | 1443 | 1085 | 64 | |
| 7 | 3-methylsulfanylpropanal | cooked potato | NBV | 1445 | 1011 | 128 | [9,22] |
| 8 | 2-ethyl-3,5-dimethylpyrazine | roasty, earthy, shrimp | NBV | 1450 | 1089 | 2048 | |
| 9 | 2,3-diethyl-5-methylpyrazine | earthy, roasty | NBV | 1482 | 1154 | 64 | |
| 10 | (3Z,6Z)-nona-3,6-dienal | fatty, green | NBV | 1500 | 1096 | 16 | |
| 11 | propanoic acid | sour, sweaty | AV | 1519 | 750 | 32 | [10] |
| 12 | (2Ε)-non-2-enal | fatty, green | NBV | 1532 | 1169 | 8 | [22] |
| 13 | 2-methylpropanoic acid | sweaty, cheesy | AV | 1559 | 800 | 16 | |
| 14 | 2-butyl-3-methylpyrazine | earthy, pea | NBV | 1571 | 1188 | 128 | |
| 15 | butanoic acid | sweaty | AV | 1616 | 839 | 64 | [22] |
| 16 | (2E)-dec-2-enal | fatty, green | NBV | 1635 | 1284 | 64 | [22] |
| 17 | 2- and 3-methylbutanoic acid 6 | sweaty | AV | 1660 | 887 | 2048 | [22] |
| 18 | (2E,4Z)-nona-2,4-dienal | green, fatty | NBV | 1665 | 1196 | 512 | |
| 19 | 3-methylnonane-2,4-dione | hay, anise, fishy | NBV | 1711 | 1242 | 128 | |
| 20 | pentanoic acid | sweaty | AV | 1730 | 941 | 64 | [22] |
| 21 | (2E,4Z)-deca-2,4-dienal | fatty, deep-fried, green | NBV | 1745 | 1292 | 8 | [22] |
| 22 | geosmin | musty, earthy, beetroot | NBV | 1816 | 1413 | 64 | [30,31] |
| 23 | 2-methoxyphenol | smoky, gammon | NBV | 1868 | 1096 | 128 | |
| 24 | β-ionone | floral, violet | NBV | 1944 | 1491 | 1024 | [7,8,9,11,22,26,32] |
| 25 | trans-4,5-epoxy-(2E)-dec-2-enal | metallic | NBV | 2012 | 1375 | 16 | [22] |
| 26 | sotolon 7 | fenugreek, lovage | AV | 2206 | 1110 | 512 | |
| 27 | 1-(2-aminophenyl)ethenone 7 | foxy | NBV | 2233 | 1300 | 64 | |
| 28 | phenylacetic acid | honey, beeswax | AV | 2540 | 1256 | 256 | |
| 29 | vanillin | vanilla, sweet | AV | 2567 | 1400 | 512 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, A.; Mall, V.; Triantis, T.M.; Kaloudis, T.; Hiskia, A.; Dimotikali, D.; Steinhaus, M. Deciphering Important Odorants in a Spirulina (Arthrospira platensis) Dietary Supplement by Aroma Extract Dilution Analysis Using Offline and Online Fractionation Approaches. Int. J. Mol. Sci. 2025, 26, 6767. https://doi.org/10.3390/ijms26146767
Paraskevopoulou A, Mall V, Triantis TM, Kaloudis T, Hiskia A, Dimotikali D, Steinhaus M. Deciphering Important Odorants in a Spirulina (Arthrospira platensis) Dietary Supplement by Aroma Extract Dilution Analysis Using Offline and Online Fractionation Approaches. International Journal of Molecular Sciences. 2025; 26(14):6767. https://doi.org/10.3390/ijms26146767
Chicago/Turabian StyleParaskevopoulou, Aikaterina, Veronika Mall, Theodoros M. Triantis, Triantafyllos Kaloudis, Anastasia Hiskia, Dimitra Dimotikali, and Martin Steinhaus. 2025. "Deciphering Important Odorants in a Spirulina (Arthrospira platensis) Dietary Supplement by Aroma Extract Dilution Analysis Using Offline and Online Fractionation Approaches" International Journal of Molecular Sciences 26, no. 14: 6767. https://doi.org/10.3390/ijms26146767
APA StyleParaskevopoulou, A., Mall, V., Triantis, T. M., Kaloudis, T., Hiskia, A., Dimotikali, D., & Steinhaus, M. (2025). Deciphering Important Odorants in a Spirulina (Arthrospira platensis) Dietary Supplement by Aroma Extract Dilution Analysis Using Offline and Online Fractionation Approaches. International Journal of Molecular Sciences, 26(14), 6767. https://doi.org/10.3390/ijms26146767







