An Italian Study of PM0.5 Toxicity: In Vitro Investigation of Cytotoxicity, Oxidative Stress, Intercellular Communication, and Extracellular Matrix Metalloproteases
Abstract
1. Introduction
2. Results
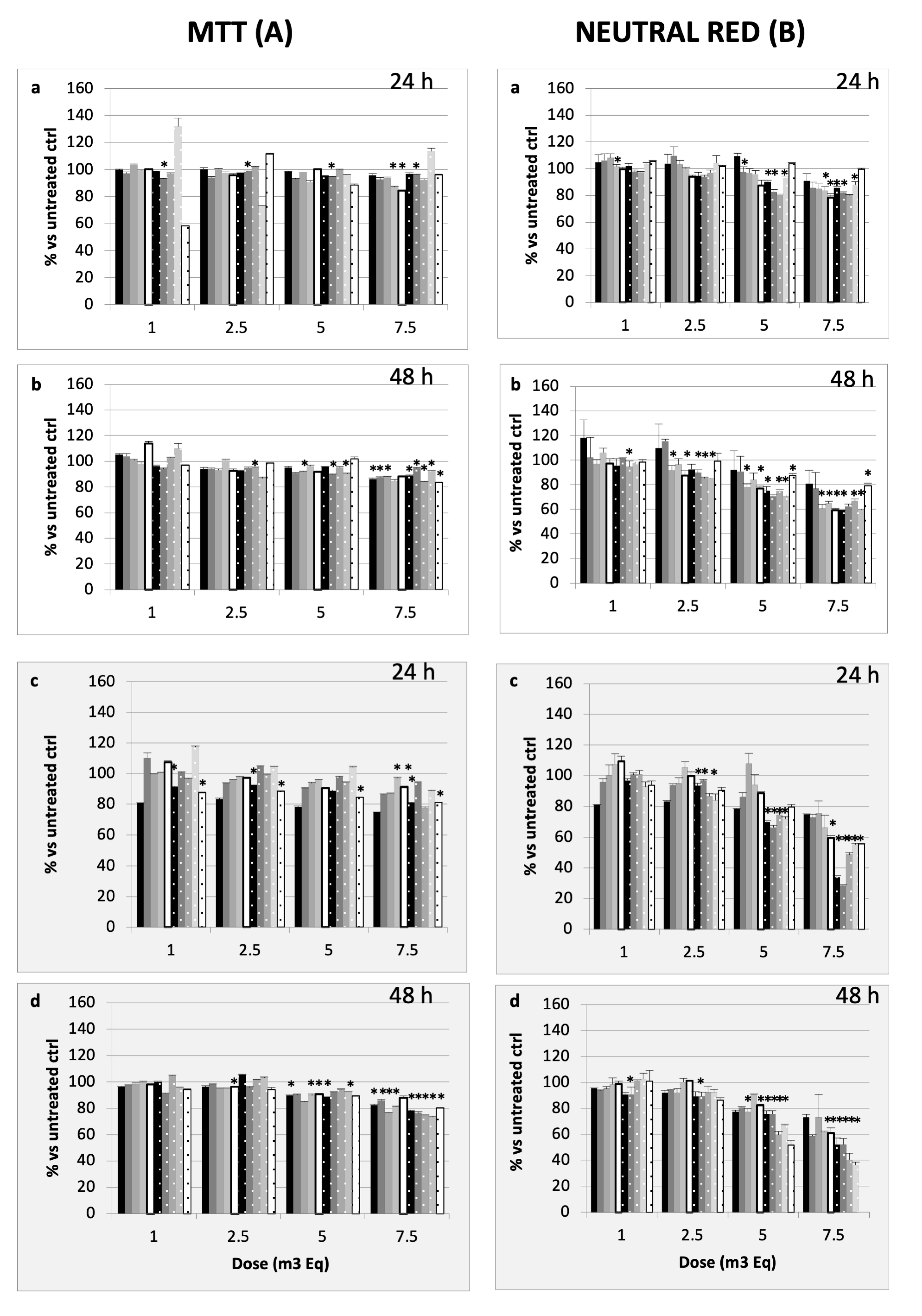
2.1. Baseline Toxicity Assessment
2.1.1. Impact of PM on Cell Growth
2.1.2. Impact of PM on Cell Viability
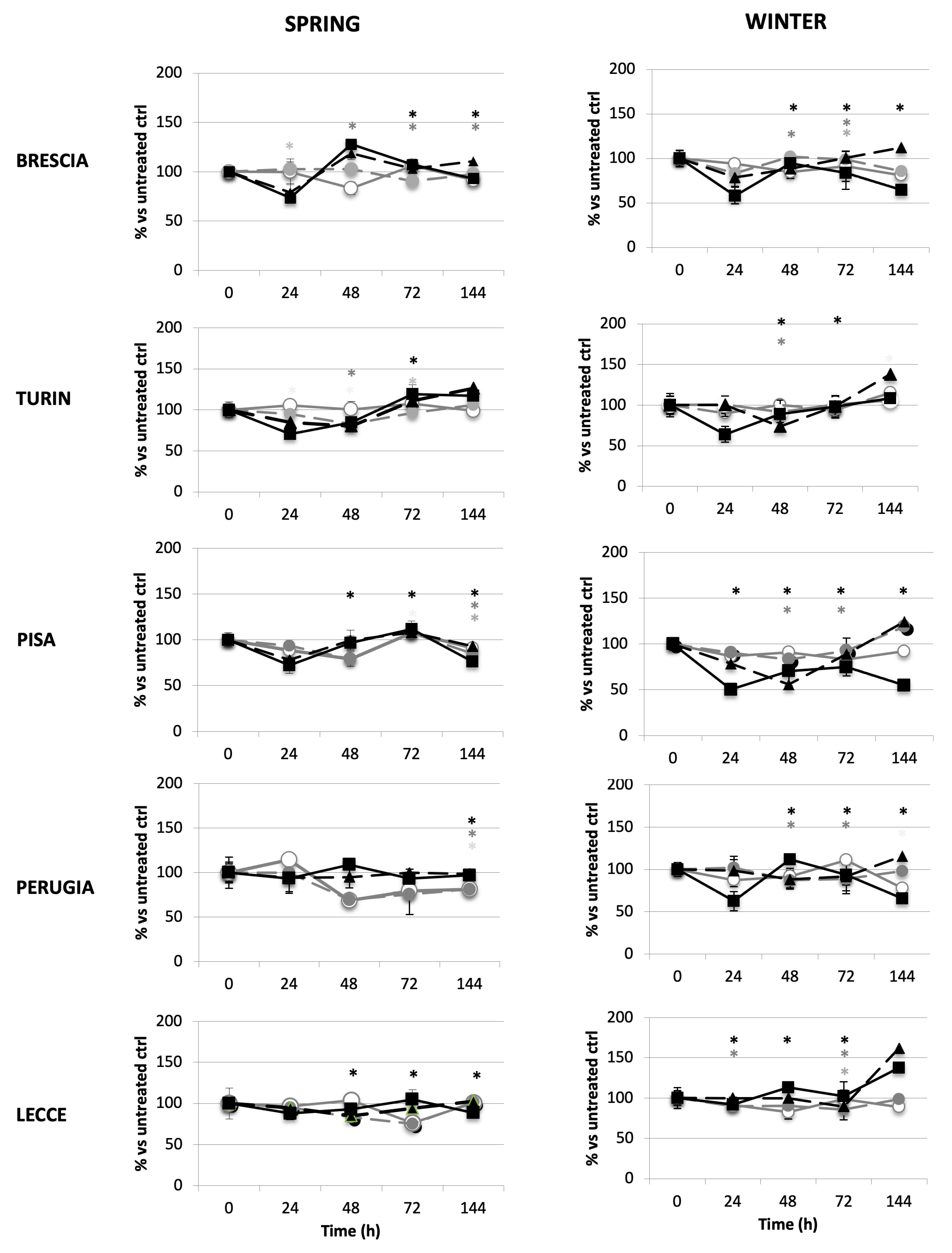
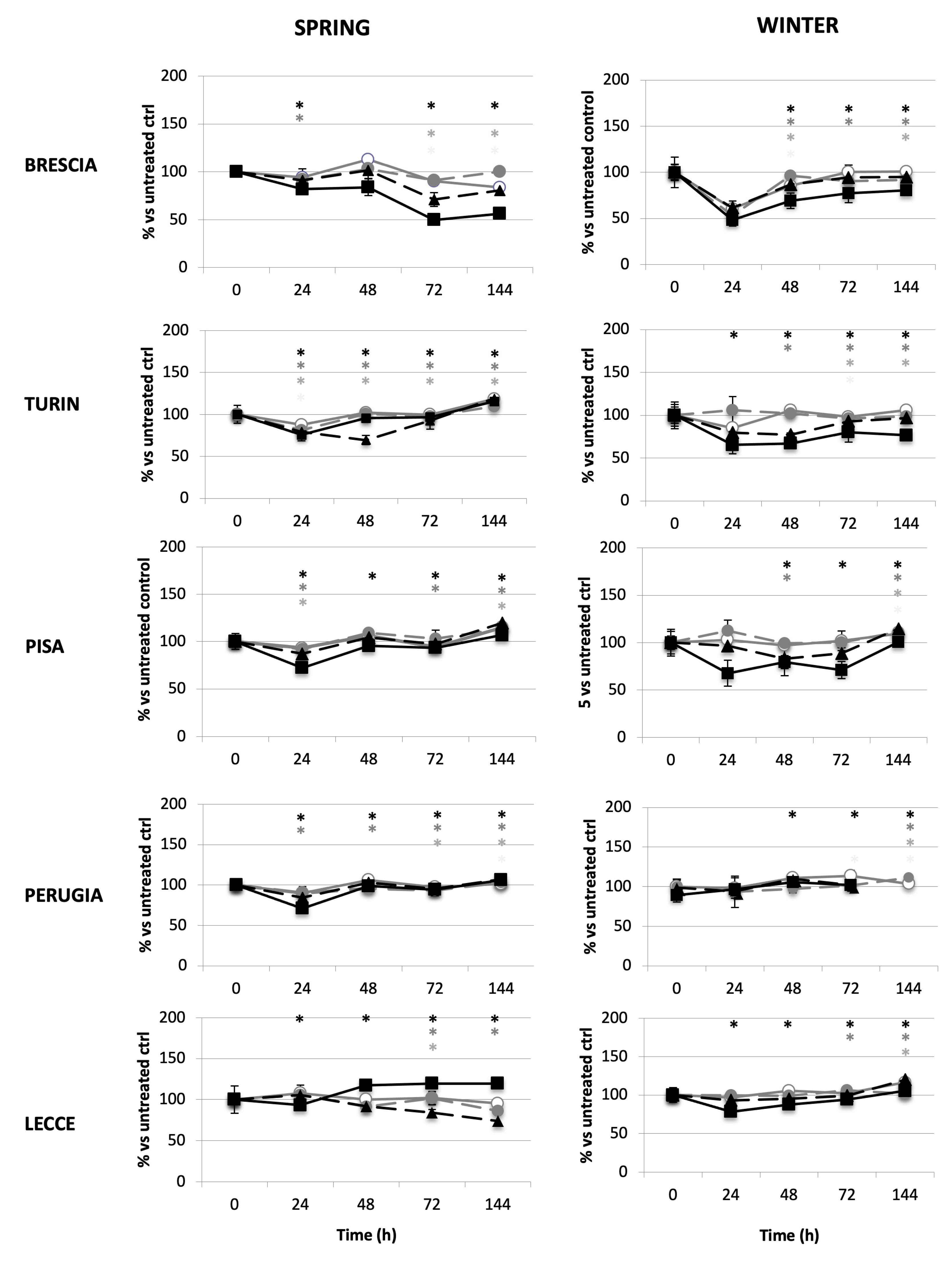
2.2. Specific Targets of PM-Derived Toxicity: Focusing on Brescia’s Airborne Particles
2.2.1. Cell Viability Assessment
2.2.2. Tumour Promotion Evaluation
2.2.3. Oxidative Stress Analysis
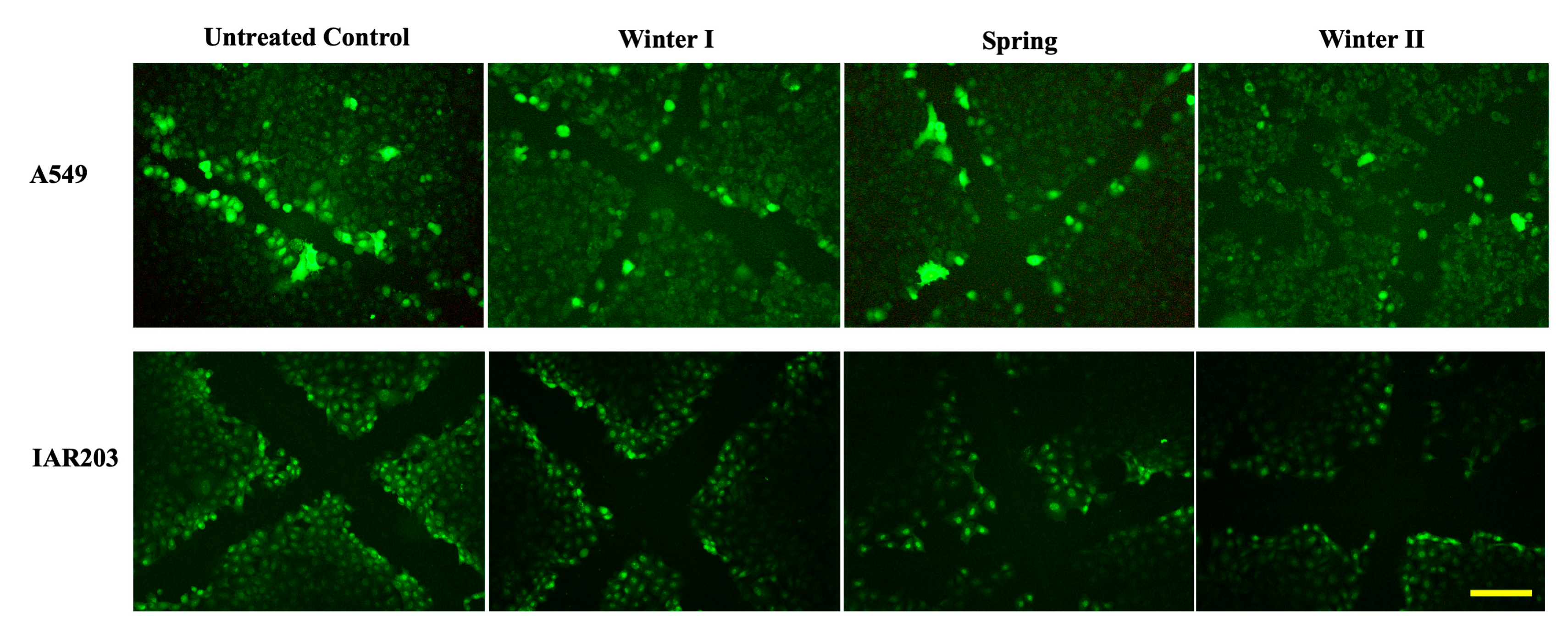
2.2.4. Lung Microenvironment Modulation
3. Discussion
4. Materials and Methods
- Baseline toxicity study.
- “Specific endpoints” for toxicity studies.
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Strak, M.; Weinmayr, G.; Rodopoulou, S.; Chen, J.; De Hoogh, K.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bekkevold, T.; Bellander, T.; et al. Long Term Exposure to Low Level Air Pollution and Mortality in Eight European Cohorts within the ELAPSE Project: Pooled Analysis. BMJ 2021, 374, n1904. [Google Scholar] [CrossRef] [PubMed]
- WHO. Evolution of WHO Air Quality Guidelines: Past, Present and Future. 2017. Available online: https://iris.who.int/bitstream/handle/10665/341912/9789289052306-eng.pdf?sequence=1 (accessed on 16 April 2025).
- Brauer, M.; Freedman, G.; Frostad, J.; Van Donkelaar, A.; Martin, R.V.; Dentener, F.; Dingenen, R.V.; Estep, K.; Amini, H.; Apte, J.S.; et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 2016, 50, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Fuller, G.W.; Anderson, H.R.; Harrison, R.M.; Armstrong, B. Urban Ambient Particle Metrics and Health: A Time-Series Analysis. Epidemiology 2010, 21, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Brook, R.; Arden Pope, C. Air Pollution and Cardiovascular Disease. Curr. Probl. Cardiol. 2015, 40, 207–238. [Google Scholar] [CrossRef]
- Guaita, R.; Pichiule, M.; Maté, T.; Linares, C.; Díaz, J. Short-Term Impact of Particulate Matter (PM2.5) on Respiratory Mortality in Madrid. Int. J. Environ. Health Res. 2011, 21, 260–274. [Google Scholar] [CrossRef]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Tiittanen, P.; Kulmala, M.; Pekkanen, J. Particulate Air Pollution and Acute Cardiorespiratory Hospital Admissions and Mortality Among the Elderly. Epidemiology 2009, 20, 143–153. [Google Scholar] [CrossRef]
- Yang, D.; Yang, X.; Deng, F.; Guo, X. Ambient Air Pollution and Biomarkers of Health Effect. In Ambient Air Pollution and Health Impact in China; Dong, G.-H., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1017, pp. 59–102. ISBN 978-981-10-5656-7. [Google Scholar]
- WHO. Burden of Disease from Ambient Air Pollution for 2012. 2012. Available online: https://era.org.mt/wp-content/uploads/2019/05/Burden-of-disease-from-Ambient-Air-Pollution-for-2012.pdf (accessed on 16 April 2025).
- WHO. Exposure to Ambient Air Pollution from Particulate Matter for 2016 Summary of Results; World Health Organization: Geneva, Switzerland, 2018; Available online: https://cdn.who.int/media/docs/default-source/air-quality-database/aqd-2018/aap_bod_methods_apr2018_final.pdf?sfvrsn=30ac0d62_3 (accessed on 11 July 2025).
- Künzli, N.; Kaiser, R.; Medina, S.; Studnicka, M.; Chanel, O.; Filliger, P.; Herry, M.; Horak, F.; Puybonnieux-Texier, V.; Quénel, P.; et al. Public-Health Impact of Outdoor and Traffic-Related Air Pollution: A European Assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Curtis, L.; Rea, W.; Smith-Willis, P.; Fenyves, E.; Pan, Y. Adverse Health Effects of Outdoor Air Pollutants. Environ. Int. 2006, 32, 815–830. [Google Scholar] [CrossRef]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s Behavior and Physiology and How It Affects Exposure to Environmental Contaminants. Pediatrics 2004, 113, 996–1006. [Google Scholar] [CrossRef]
- WHO. The Effects of Air Pollution on Children’s Health and Development: A Review of Evidence; WHO Regional Office for Europe, European Centre for Environment and Health Bonn Office; WHO Publications: Copenhagen, Denmark, 2005; Available online: https://iris.who.int/bitstream/handle/10665/107652/E86575.pdf?sequence=1&isAllowed=y (accessed on 16 April 2025).
- Makri, A.; Stilianakis, N.I. Vulnerability to Air Pollution Health Effects. Int. J. Hyg. Environ. Health 2008, 211, 326–336. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bárcenas, R.; Chirino, Y.I.; Sánchez-Pérez, Y.; Osornio-Vargas, Á.R.; Melendez-Zajgla, J.; Rosas, I.; García-Cuellar, C.M. Particulate Matter (PM10) Induces Metalloprotease Activity and Invasion in Airway Epithelial Cells. Toxicol. Lett. 2015, 237, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Bonetta, S.; Schilirò, T.; Ceretti, E.; Feretti, D.; Covolo, L.; Vannini, S.; Villarini, M.; Moretti, M.; Verani, M.; et al. Mutagenic and Genotoxic Effects Induced by PM0.5 of Different Italian Towns in Human Cells and Bacteria: The MAPEC_LIFE Study. Environ. Pollut. 2019, 245, 1124–1135. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Chirino, Y.I.; Osornio-Vargas, Á.R.; Morales-Bárcenas, R.; Gutiérrez-Ruíz, C.; Vázquez-López, I.; García-Cuellar, C.M. DNA Damage Response of A549 Cells Treated with Particulate Matter (PM 10 ) of Urban Air Pollutants. Cancer Lett. 2009, 278, 192–200. [Google Scholar] [CrossRef]
- Gualtieri, M.; Øvrevik, J.; Mollerup, S.; Asare, N.; Longhin, E.; Dahlman, H.-J.; Camatini, M.; Holme, J.A. Airborne Urban Particles (Milan Winter-PM2.5) Cause Mitotic Arrest and Cell Death: Effects on DNA, Mitochondria, AhR Binding and Spindle Organization. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 713, 18–31. [Google Scholar] [CrossRef]
- Ebi, K.L.; McGregor, G. Climate Change, Tropospheric Ozone and Particulate Matter, and Health Impacts. Environ. Health Perspect. 2008, 116, 1449–1455. [Google Scholar] [CrossRef]
- Bernardoni, V.; Vecchi, R.; Valli, G.; Piazzalunga, A.; Fermo, P. PM10 Source Apportionment in Milan (Italy) Using Time-Resolved Data. Sci. Total Environ. 2011, 409, 4788–4795. [Google Scholar] [CrossRef]
- Perrone, M.G.; Gualtieri, M.; Ferrero, L.; Porto, C.L.; Udisti, R.; Bolzacchini, E.; Camatini, M. Seasonal Variations in Chemical Composition and in Vitro Biological Effects of Fine PM from Milan. Chemosphere 2010, 78, 1368–1377. [Google Scholar] [CrossRef]
- Perrone, M.G.; Larsen, B.R.; Ferrero, L.; Sangiorgi, G.; De Gennaro, G.; Udisti, R.; Zangrando, R.; Gambaro, A.; Bolzacchini, E. Sources of High PM2.5 Concentrations in Milan, Northern Italy: Molecular Marker Data and CMB Modelling. Sci. Total Environ. 2012, 414, 343–355. [Google Scholar] [CrossRef]
- Perrone, M.G.; Gualtieri, M.; Consonni, V.; Ferrero, L.; Sangiorgi, G.; Longhin, E.; Ballabio, D.; Bolzacchini, E.; Camatini, M. Particle Size, Chemical Composition, Seasons of the Year and Urban, Rural or Remote Site Origins as Determinants of Biological Effects of Particulate Matter on Pulmonary Cells. Environ. Pollut. 2013, 176, 215–227. [Google Scholar] [CrossRef] [PubMed]
- ARPA Puglia. Relazione Annuale Sulla Qualità Dell’aria in Puglia Anno 2015. Available online: https://www.arpa.puglia.it/pagina2873_report-annuali-e-mensili-qualit-dellaria-rrqa.html (accessed on 11 July 2025).
- Borgie, M.; Dagher, Z.; Ledoux, F.; Verdin, A.; Cazier, F.; Martin, P.; Hachimi, A.; Shirali, P.; Greige-Gerges, H.; Courcot, D. Comparison between Ultrafine and Fine Particulate Matter Collected in Lebanon: Chemical Characterization, in Vitro Cytotoxic Effects and Metabolizing Enzymes Gene Expression in Human Bronchial Epithelial Cells. Environ. Pollut. 2015, 205, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Garcia-Canton, C.; Minet, E.; Anadon, A.; Meredith, C. Metabolic Characterization of Cell Systems Used in in Vitro Toxicology Testing: Lung Cell System BEAS-2B as a Working Example. Toxicol. In Vitro 2013, 27, 1719–1727. [Google Scholar] [CrossRef]
- Mazzoleni, G.; Camplani, A.; Telo, P.; Pozzi, A.; Tanganelli, S.; Elfgang, C.; Willecke, K.; Ragnotti, G. Effect of Tumor-Promoting and Anti-Promoting Chemicals on the Viability and Junctional Coupling of Human Hela Cells Transfected with DNAs Coding for Various Murine Connexin Proteins. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 113, 247–256. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Paul, D.L. Connections with Connexins: The Molecular Basis of Direct Intercellular Signaling. Eur. J. Biochem. 1996, 238, 1–27. [Google Scholar] [CrossRef]
- Swierenga, S.H.; Yamasaki, H. Performance of Tests for Cell Transformation and Gap-Junction Intercellular Communication for Detecting Nongenotoxic Carcinogenic Activity. IARC Sci. Publ. 1992, 116, 165–193. [Google Scholar]
- Bauer, A.K.; Velmurugan, K.; Plöttner, S.; Siegrist, K.J.; Romo, D.; Welge, P.; Brüning, T.; Xiong, K.-N.; Käfferlein, H.U. Environmentally Prevalent Polycyclic Aromatic Hydrocarbons Can Elicit Co-Carcinogenic Properties in an In Vitro Murine Lung Epithelial Cell Model. Arch. Toxicol. 2018, 92, 1311–1322. [Google Scholar] [CrossRef]
- Amara, N.; Bachoual, R.; Desmard, M.; Golda, S.; Guichard, C.; Lanone, S.; Aubier, M.; Ogier-Denis, E.; Boczkowski, J. Diesel Exhaust Particles Induce Matrix Metalloprotease-1 in Human Lung Epithelial Cells via a NADP(H) Oxidase/NOX4 Redox-Dependent Mechanism. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L170–L181. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghio, A.J.; Cho, S.-H.; Brinckerhoff, C.E.; Simon, S.A.; Liedtke, W. Diesel Exhaust Particles Activate the Matrix-Metalloproteinase-1 Gene in Human Bronchial Epithelia in a β-Arrestin–Dependent Manner via Activation of RAS. Environ. Health Perspect. 2009, 117, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, B.; Leblond, V.; Galiacy, S.; Gras, G.; Planus, E.; Laurent, V.; Isabey, D.; Lafuma, C. Negative Impact of DEP Exposure on Human Airway Epithelial Cell Adhesion, Stiffness, and Repair. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 284, L119–L132. [Google Scholar] [CrossRef]
- Su, W.-Y.; Jaskot, R.H.; Richards, J.; Abramson, S.R.; Woessner, J.F.; Yu, W.-H.; Dreher, K.L. Induction of Pulmonary Matrilysin Expression by Combustion and Ambient Air Particles. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L152–L160. [Google Scholar] [CrossRef]
- Corbel, M.; Boichot, E.; Lagente, V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000, 33, 749–754. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]
- Mattone, M.; Masci, G.M.; Landini, N.; Zingaropoli, M.A.; Catalano, C.; Ciardi, M.R.; Panebianco, V. MMP-9 metalloproteinase and its regulator are not associated with mid-term CT residual abnormalities in patients with COVID-19 pneumonia. Acta Radiol. Open 2025, 14, 20584601251330563. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Atienza-Mateo, B.; Batista-Liz, J.C.; Sebastián Mora-Gil, M.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Izquierdo Cuervo, S.; Aguirre Portilla, C.; Blanco, R.; López-Mejías, R. Matrix metalloproteinases and their tissue inhibitors as upcoming biomarker signatures of connective tissue diseases-related interstitial lung disease: Towards an earlier and accurate diagnosis. Mol. Med. 2025, 31, 70. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef]
- Elkington, P.T.; Friedland, J.S. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006, 61, 259–266. [Google Scholar] [CrossRef]
- Feretti, D.; Ceretti, E.; De Donno, A.; Moretti, M.; Carducci, A.; Bonetta, S.; Marrese, M.R.; Bonetti, A.; Covolo, L.; Bagordo, F.; et al. Monitoring Air Pollution Effects on Children for Supporting Public Health Policy: The Protocol of the Prospective Cohort MAPEC Study. BMJ Open 2014, 4, e006096. [Google Scholar] [CrossRef] [PubMed]
- MAPEC_LIFE Study Group; Bagordo, F.; De Donno, A.; Grassi, T.; Guido, M.; Devoti, G.; Ceretti, E.; Zani, C.; Feretti, D.; Villarini, M.; et al. Lifestyles and Socio-Cultural Factors among Children Aged 6–8 Years from Five Italian Towns: The MAPEC_LIFE Study Cohort. BMC Public Health 2017, 17, 233. [Google Scholar] [CrossRef]
- De Donno, A.; De Giorgi, M.; Bagordo, F.; Grassi, T.; Idolo, A.; Serio, F.; Ceretti, E.; Feretti, D.; Villarini, M.; Moretti, M.; et al. Health Risk Associated with Exposure to PM10 and Benzene in Three Italian Towns. Int. J. Environ. Res. Public Health 2018, 15, 1672. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Levorato, S.; Salvatori, T.; Ceretti, E.; Bonetta, S.; Carducci, A.; Grassi, T.; Vannini, S.; Donato, F.; Bonetta, S.; et al. Buccal Micronucleus Cytome Assay in Primary School Children: A Descriptive Analysis of the MAPEC_LIFE Multicenter Cohort Study. Int. J. Hyg. Environ. Health 2018, 221, 883–892. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 Cell Line as a Type II Pulmonary Epithelial Cell Model for Drug Metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]


| Brescia | IC50 at 24 h (m3Eq) | IC50 at 48 h (m3Eq) | |
|---|---|---|---|
| A549 | Winter I | nd | nd |
| Spring | nd | nd | |
| Winter II | 6.1 | nd | |
| BEAS-2B | Winter I | 6.3 | nd |
| Spring | nd | nd | |
| Winter II | 6.7 | 6.3 | |
| Communicating Cells | Untreated Control | Winter I | Spring | Winter II |
|---|---|---|---|---|
| A549 | 1.94 ± 0.08 | 1.53 ± 0.07 | 1.56 ± 0.05 | 1.48 ± 0.07 |
| IAR203 | 3.60 ± 0.08 | 2.39 ± 0.07 | 2.28 ± 0.07 | 2.13 ± 0.07 |
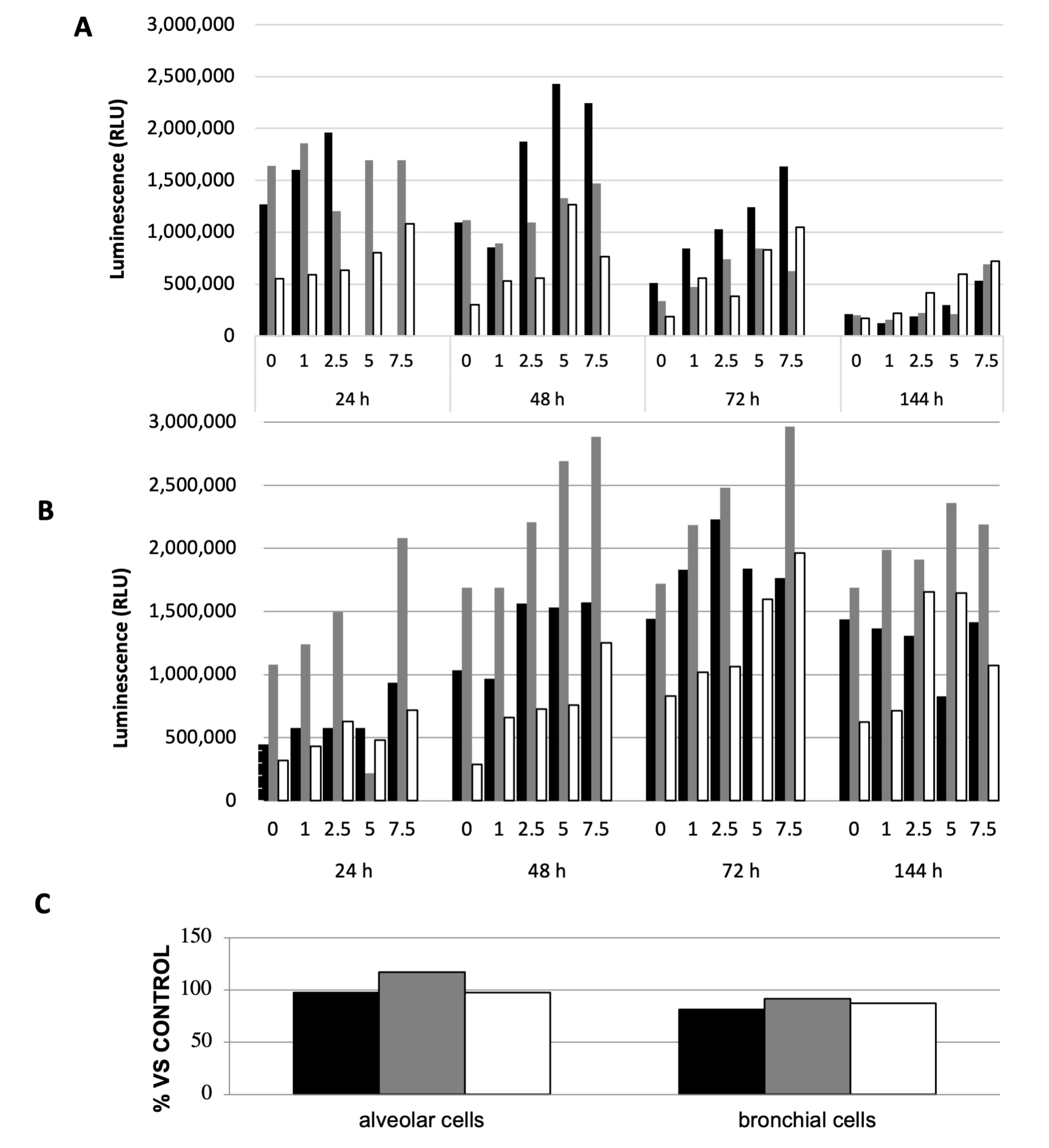
| Alveolar Cells | Winter I | Spring | Winter II | ||||||||||
| MMP9 | SEM | MMP2 | SEM | MMP9 | SEM | MMP2 | SEM | MMP9 | SEM | MMP2 | SEM | ||
| 24 h | 0 | 24,992,882 | 3,478,117 | 22,831,460 | 3,306,950 | 20,504,128 | 2,242,435 | 19,764,231 | 525,768 | 15,411,420 | 217,354 | 14,877,338 | 1,447,950 |
| 1 | 28,849,746 | 4,120,032 | 21,705,531 | 1,593,586 | 21,884,018 | 589,597 | 18,792,141 | 282,707 | 14,770,667 | 91,107 | 12,440,435 | 1,525,147 | |
| 2.5 | 28,062,871 | 1,249,571 | 20,428,692 | 1,091,354 | 20,981,947 | 17,354 | 19,638,256 | 228,500 | 14,565,091 | 332,732 | 14,838,217 | 690,243 | |
| 5 | ND | ND | ND | ND | 20,919,896 | 537,182 | 19,331,493 | 3707 | 13,165,728 | 1,989,440 | 10,706,192 | 3,005,490 | |
| 7.5 | ND | ND | ND | ND | 19,132,629 | 288,329 | 17,396,775 | 378,232 | 10,947,142 | 1,471,511 | 10,604,702 | 117,828 | |
| 48 h | 0 | 27,475,386 | 412,621 | 20,615,409 | 34,707 | 20,918,745 | 2,589,617 | 17,924,616 | 3,272,730 | 13,348,556 | 372,389 | 12,304,267 | 296,414 |
| 1 | 30,128,336 | 363,622 | 20,823,606 | 604,511 | 22,152,185 | 1,955,057 | 18,073,989 | 1,940,718 | 17,506,142 | 11,147 | 16,702,595 | 208,500 | |
| 2.5 | 30,270,033 | 352,561 | 37,323,229 | 16,300,134 | 25,820,342 | 1,467,142 | 20,438,593 | 970,406 | 16,340,081 | 19,207 | 16,667,338 | 1000 | |
| 5 | 29,212,472 | 136,829 | 20,490,181 | 507,157 | 21,486,599 | 1,123,136 | 18,499,771 | 667,742 | 11,038,803 | 2000 | 13,863,581 | 143,707 | |
| 7.5 | 25,294,462 | 324,304 | 19,231,560 | 901,364 | 14,307,704 | 371,889 | 16,556,301 | 223,535 | 6,883,843 | 23,061 | 9,024,393 | 82,561 | |
| 72 h | 0 | 34,045,469 | 910,136 | 50,730,604 | 1,818,718 | 16,997,421 | 1,557,000 | 13,944,951 | 596,106 | 9,801,408 | 498,768 | 16,794,970 | 596,440 |
| 1 | 36,520,070 | 405,415 | 52,627,553 | 1,364,596 | 18,188,704 | 274,647 | 16,525,407 | 836,399 | 13,024,382 | 407,500 | 19,399,495 | 400,500 | |
| 2.5 | 36,543,277 | 342,086 | 56,142,897 | 355,061 | 15,771,608 | 1,340,965 | 16,947,093 | 570,542 | 11,571,499 | 90,232 | 14,378,424 | 3,221,178 | |
| 5 | 33,446,852 | 390,096 | 71,120,793 | 385,540 | 15,665,426 | 954,904 | 16,608,124 | 102,439 | 11,610,343 | 342,097 | 18,744,505 | 517,753 | |
| 7.5 | 32,219,170 | 148,757 | 58,877,792 | 8,099,421 | 14,570,047 | 339,718 | 14,684,022 | 294,914 | 9,539,271 | 315,389 | 15,822,863 | 1,001,253 | |
| 144 h | 0 | 20,897,901 | 2,700,835 | 50,755,629 | 513,743 | 15,476,259 | 207,293 | 22,572,296 | 17,146 | 8,778,232 | 923,036 | 21,473,758 | 782,500 |
| 1 | 23,593,244 | 8,805,785 | 54,543,497 | 490,854 | 18,523,183 | 200,975 | 23,657,382 | 155,403 | 10,378,424 | 836,329 | 23,984,804 | 1,113,546 | |
| 2.5 | 21,394,840 | 5,447,431 | 54,543,497 | 1,144,201 | 18,535,926 | 394,940 | 24,909,796 | 159,596 | 11,160,292 | 123,561 | 22,967,330 | 176,243 | |
| 5 | 22,550,876 | 8,127,396 | 51,753,187 | 2,579,486 | 17,378,194 | 1,044,915 | 23,757,725 | 212,353 | 10,744,818 | 1,207,622 | 23,500,077 | 1,354,597 | |
| 7.5 | 22,375,426 | 10,166,138 | 51,193,987 | 4,498,193 | 15,707,662 | 464,768 | 20,517,386 | 441,571 | 10,081,171 | 865,682 | 19,528,773 | 854,243 | |
| Bronchial Cells | Winter I | Spring | Winter II | ||||||||||
| MMP9 | SEM | MMP2 | SEM | MMP9 | SEM | MMP2 | SEM | MMP9 | SEM | MMP2 | SEM | ||
| 24 h | 0 | 18,810,744 | 1,881,728 | 19,015,284 | 3,469,703 | 19,924,892 | 496,571 | 28,916,771 | 1,015,500 | 15,249,667 | 1,481,622 | 18,072,445 | 1,727,864 |
| 1 | 18,982,997 | 1,557,354 | 17,584,773 | 3,723,678 | 20,217,988 | 1,568,910 | 31,383,777 | 853,293 | 16,890,545 | 246,571 | 20,297,405 | 1,528,339 | |
| 2.5 | 17,988,997 | 1,413,596 | 16,781,284 | 3,237,532 | 19,512,210 | 435,939 | 31,843,863 | 1,472,986 | 17,609,545 | 1,831,278 | 20,636,051 | 2,086,571 | |
| 5 | 16,570,486 | 1,652,157 | 16,512,152 | 2,991,349 | 19,549,443 | 1,811,607 | 33,131,499 | 906,743 | 17,017,278 | 1,498,304 | 20,816,627 | 2,339,631 | |
| 7.5 | 13,326,152 | 441,965 | 15,718,359 | 3,109,556 | 21,151,589 | 556,268 | 30,511,817 | 2,606,375 | 13,624,106 | 1,792,718 | 19,436,713 | 2,285,253 | |
| 48 h | 0 | 14,921,137 | 266,000 | 17,949,759 | 467,258 | 32,853,959 | 695,768 | 33,256,417 | 109,732 | 16,990,637 | 1,834,985 | 17,699,924 | 2,377,293 |
| 1 | 15,688,350 | 23,728 | 19,426,855 | 1,794,546 | 32,075,201 | 154,818 | 35,476,720 | 317,207 | 19,598,212 | 1,340,682 | 19,911,738 | 1,480,449 | |
| 2.5 | 15,127,012 | 213,904 | 17,554,213 | 1,050,975 | 29,968,691 | 1,199,672 | 33,886,453 | 254,354 | 18,743,369 | 1,621,182 | 18,657,945 | 1,949,071 | |
| 5 | 17,564,576 | 1,089,247 | 16,611,556 | 1,154,682 | 25,090,408 | 2,255,318 | 32,135,750 | 849,743 | 13,918,167 | 1,376,486 | 16,810,313 | 1,842,581 | |
| 7.5 | 14,646,405 | 106,975 | 15,427,303 | 1,483,400 | 25,126,310 | 2,450,190 | 29,179,715 | 399,172 | 11,961,389 | 1,208,950 | 17,414,202 | 1,853,157 | |
| 72 h | 0 | 13,203,662 | 217,525 | 22,668,800 | 1,075,219 | 19,270,714 | 327,000 | 25,596,746 | 473,940 | 25,111,394 | 2,241,132 | 22,128,269 | 164,647 |
| 1 | 15,153,648 | 256,147 | 22,868,786 | 1,567,356 | 19,330,778 | 772,350 | 24,647,940 | 3,715,711 | 25,699,394 | 968,718 | 25,954,725 | 222,404 | |
| 2.5 | 13,574,123 | 514,036 | 22,411,189 | 1,280,002 | 22,334,382 | 515,061 | 23,030,229 | 305,121 | 24,868,066 | 785,218 | 23,944,982 | 192,611 | |
| 5 | 13,060,112 | 131,854 | 19,537,947 | 893,124 | 22,122,271 | 380,829 | 23,289,972 | 720,622 | 26,827,526 | 689,708 | 22,858,396 | 257,025 | |
| 7.5 | 13,938,187 | 587,000 | 17,195,401 | 1,023,356 | 19,650,978 | 1,011,707 | 23,316,422 | 720,536 | 21,779,398 | 1,358,207 | 19,895,760 | 427,318 | |
| 144 h | 0 | 22,888,453 | 1,150,940 | 27,375,486 | 594,011 | 20,855,139 | 2,517,596 | 26,585,240 | 60,526 | 18,904,266 | 780,389 | 25,883,645 | 2,231,546 |
| 1 | 24,609,231 | 198,475 | 27,375,486 | 584,339 | 19,792,032 | 985,096 | 24,026,936 | 388,121 | 19,820,869 | 813,071 | 24,238,080 | 712,525 | |
| 2.5 | 25,143,574 | 930,718 | 28,499,078 | 107,389 | 21,461,361 | 83,354 | 23,041,754 | 419,768 | 18,924,327 | 722,036 | 22,640,620 | 522,965 | |
| 5 | 22,357,871 | 327,914 | 26,807,228 | 71,654 | 23,391,346 | 1,082,975 | 21,337,073 | 23,329 | 18,036,093 | 1,090,400 | 21,006,474 | 884,597 | |
| 7.5 | 19,929,033 | 1,311,440 | 22,993,322 | 515,157 | 23,359,589 | 669,389 | 18,750,551 | 39,293 | 16,966,351 | 240,536 | 16,467,538 | 1,190,117 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steimberg, N.; Mazzoleni, G.; Boniotti, J.; Villarini, M.; Moretti, M.; Carducci, A.; Verani, M.; Grassi, T.; Serio, F.; Bonetta, S.; et al. An Italian Study of PM0.5 Toxicity: In Vitro Investigation of Cytotoxicity, Oxidative Stress, Intercellular Communication, and Extracellular Matrix Metalloproteases. Int. J. Mol. Sci. 2025, 26, 6769. https://doi.org/10.3390/ijms26146769
Steimberg N, Mazzoleni G, Boniotti J, Villarini M, Moretti M, Carducci A, Verani M, Grassi T, Serio F, Bonetta S, et al. An Italian Study of PM0.5 Toxicity: In Vitro Investigation of Cytotoxicity, Oxidative Stress, Intercellular Communication, and Extracellular Matrix Metalloproteases. International Journal of Molecular Sciences. 2025; 26(14):6769. https://doi.org/10.3390/ijms26146769
Chicago/Turabian StyleSteimberg, Nathalie, Giovanna Mazzoleni, Jennifer Boniotti, Milena Villarini, Massimo Moretti, Annalaura Carducci, Marco Verani, Tiziana Grassi, Francesca Serio, Sara Bonetta, and et al. 2025. "An Italian Study of PM0.5 Toxicity: In Vitro Investigation of Cytotoxicity, Oxidative Stress, Intercellular Communication, and Extracellular Matrix Metalloproteases" International Journal of Molecular Sciences 26, no. 14: 6769. https://doi.org/10.3390/ijms26146769
APA StyleSteimberg, N., Mazzoleni, G., Boniotti, J., Villarini, M., Moretti, M., Carducci, A., Verani, M., Grassi, T., Serio, F., Bonetta, S., Carraro, E., Bonetti, A., Bonizzoni, S., Gelatti, U., & the MAPEC_LIFE Study Group. (2025). An Italian Study of PM0.5 Toxicity: In Vitro Investigation of Cytotoxicity, Oxidative Stress, Intercellular Communication, and Extracellular Matrix Metalloproteases. International Journal of Molecular Sciences, 26(14), 6769. https://doi.org/10.3390/ijms26146769














