Mineral Homeostasis and Depression: Implications for Prevention and Therapeutic Support—A Narrative Review
Abstract
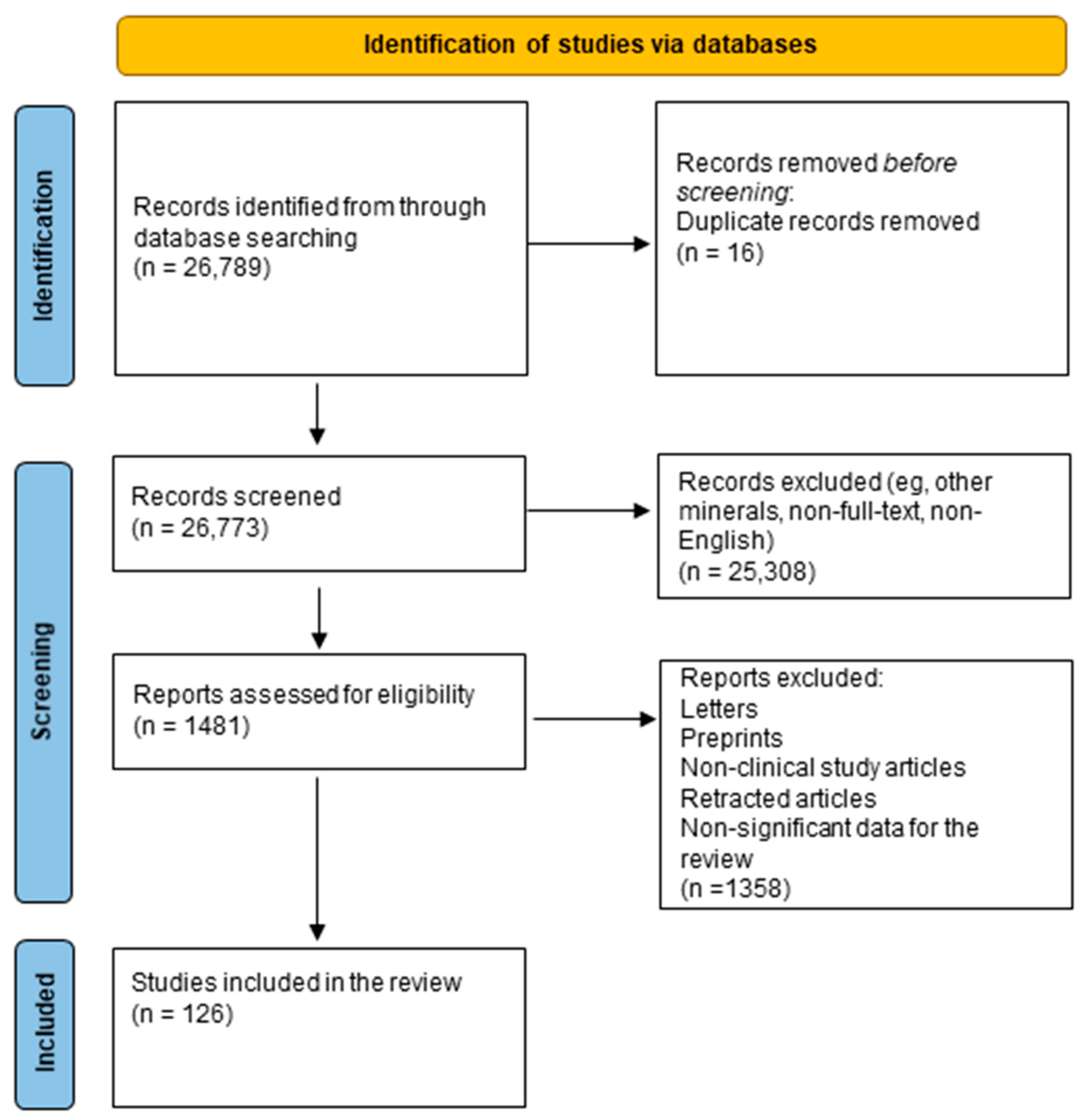
1. Introduction
2. Methods
3. Role of Macroelements in Depression
3.1. Calcium
3.2. Magnesium
4. Role of Microelements in Depression
4.1. Iron
4.2. Zinc
4.3. Copper
4.4. Selenium
4.5. Iodine
| Element | Deficiency | Excess | References |
|---|---|---|---|
| Calcium | Osteopenia, osteoporosis, muscle cramps, rickets in children, impaired nerve transmission, and hypertension | Hypercalcemia, kidney stones, impaired absorption of other minerals (e.g., iron and zinc), and constipation | [15,19,20,26] |
| Magnesium | Muscle weakness, tremors, arrhythmias, fatigue, irritability, and increased risk of depression | Diarrhea, hypotension, nausea, and cardiac arrest | [28,30,31,34,37,38] |
| Iron | Anemia, weakened immunity, and developmental delays | Hemochromatosis, liver damage, oxidative stress, increased risk of infections, and cardiovascular disease | [47,55,56,57,58,59,60,61] |
| Zinc | Impaired immune function, growth retardation, hair loss, diarrhea, delayed wound healing, and taste disturbances | Nausea, vomiting, immune suppression, copper deficiency, and impaired HDL levels | [71,72] |
| Copper | Anemia, neutropenia, bone abnormalities, neurological symptoms (e.g., ataxia), and connective tissue defects | Liver damage, gastrointestinal distress, neurotoxicity, and Wilson’s disease | [11,79,82] |
| Selenium | Hypothyroidism, fatigue, infertility, impaired immunity, and cardiomyopathy (Keshan disease) | Selenosis, gastrointestinal upset, and nervous system abnormalities | [90,91,92] |
| Iodine | Goiter, hypothyroidism, impaired cognitive development, cretinism in infants, and reproductive dysfunction | Hyperthyroidism, thyroid inflammation, iodine-induced goiter, metallic taste, and skin lesions | [50,51,101,102,103,104,105,106,107,108,109,110,111] |
5. Limitations and Future Research Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Center for Complementary and Integrative Health. Depression. 2020. Available online: https://www.nccih.nih.gov/health/depression (accessed on 3 June 2025).
- World Health Organization. Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 3 June 2025).
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major depressive disorder: Advances in neuroscience research and translational applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.R.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Electrolyte and Acid-Base Disturbances in Patients with Diabetes Mellitus. N. Engl. J. Med. 2015, 373, 548–559. [Google Scholar] [CrossRef]
- Díaz, C.; Alonso, J.L.; Bernal, L.M.; Bernal, L.M.; Sánchez, A.; Méndez, M.; Méndez, R.M. Impact of Iron Intake and Reserves on Cognitive Function in Young University Students. Nutrients 2024, 16, 2808. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Shi, M.; Liu, H.; Huang, P.H.U.; Sussman, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef]
- Mei, N.; You, Y.; Chen, J.; Zhang, L. Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2018, 267, 506–515. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Pampaloni, B.; Gronchi, G.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; Brandi, M.L. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Melchers, M.; van Zanten, A.R.H. Management of hypocalcaemia in the critically ill. Curr. Opin. Crit. Care 2023, 29, 330–338. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wu, S.; Zhao, X. Ca2+ Signaling in Oligodendrocyte Development. Cell. Mol. Neurobiol. 2019, 39, 1071–1080. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Calcium: Fact Sheet for Health Professionals. 2024. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/ (accessed on 3 June 2025).
- Tonon, C.R.; Silva, T.A.A.L.; Pereira, F.W.L.; Queiroz, D.A.R.; Favero Junior, E.L.; Martins, D.; Azevedo, P.S.; Okoshi, M.P.; Zornoff, L.A.M.; de Paiva, S.A.R.; et al. A Review of Current Clinical Concepts in the Pathophysiology, Etiology, Diagnosis, and Management of Hypercalcemia. Med. Sci. Monit. 2022, 28, e935821. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- World Health Organization. Calcium Supplementation During Pregnancy to Reduce the Risk of Preeclampsia. 2023. Available online: https://www.who.int/tools/elena/interventions/calcium-pregnancy (accessed on 3 June 2025).
- Liu, X.-Y.; Mao, L.-M.; Zhang, G.-C.; Papasian, C.J.; Fibuch, E.E.; Lan, H.-X.; Zhou, H.-F.; Xu, M.; Wang, J.Q. Activity-Dependent Modulation of Limbic Dopamine D3 Receptors by CaMKII. Neuron 2009, 61, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Gardner, E.L.; Xi, Z.-X.; Thanos, P.K.; Mugnaini, M.; Hagan, J.J.; Ashby, C.R., Jr. The role of central dopamine D3 receptors in drug addiction: A review of pharmacological evidence. Brain Res. Rev. 2005, 49, 77–105. [Google Scholar] [CrossRef]
- Shen, X.; Gu, X.; Liu, Y.-Y.; Yang, L.; Zheng, M.; Jiang, L. Association between dietary calcium and depression among American adults: National Health and Nutrition Examination Survey. Front. Nutr. 2023, 10, 1042522. [Google Scholar] [CrossRef]
- Mockett, B.G.; Guévremont, D.; Wutte, M.; Hulme, S.R.; Williams, J.M.; Abraham, W.C. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J. Neurosci. 2011, 31, 7380–7391. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Kim, S.-K. Low dietary calcium is associated with self-rated depression in middle-aged Korean women. Nutr. Res. Pract. 2012, 6, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Magnesium: Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 3 June 2025).
- Ghasemi, S.-M.; Ghasemi, S.; Pahlavani, N. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 72, 169–193. [Google Scholar] [CrossRef]
- Uwe, G.; Joachim, S.; Klaus, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Gisèle, P.; André, M.; Marion, T.; Przemyslaw, B.; Natalia, Y.; Mohamed, A.; Lionel, N.; Etienne, P. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Nilank, C.S.; Gatha, J.S.; Zhiqiang, L.; Xian-Cheng, J.; Bella, T.A.; Burton, M.A. Short-term magnesium deficiency downregulates telomerase, upregulates neutral sphingomyelinase and induces oxidative DNA damage in cardiovascular tissues: Relevance to atherogenesis, cardiovascular diseases and aging. Int. J. Clin. Exp. Med. 2014, 7, 497–514. [Google Scholar]
- Luis, E.S.; Amirhossein, S.; Martha, R.; Fernando, G. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol. Res. 2016, 111, 272–282. [Google Scholar]
- Saini, V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J. Diabetes 2010, 1, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- Lynette, J.O.; Steef, K.; Chao, M.; Joost, G.J.H.; Cees, J.T.; Jeroen, H.F.d.B. Magnesium increases insulin-dependent glucose uptake in adipocytes. Front. Endocrinol. 2022, 13, 986616. [Google Scholar] [CrossRef]
- Beibei, L.; Baolong, P.; Guancheng, Z.; Jiefen, L.; Li, S. Association Between Serum Magnesium Levels and Glycemic Control in Type 2 Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 2823–2829. [Google Scholar] [CrossRef]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, W.Y.; Al-Kuraishy, H.M.; Hasan, N.R.; Al-Gareeb, A.I.; Abdulzahra, E.A.; Elshaarawy, O.; Athanasios, A.; Pontikoglou, M.; Jankowski, M.S.; Al-Aubaidy, H.A.; et al. Depression and type 2 diabetes: A causal relationship and mechanistic pathway. Diabet. Med. 2024, 26, 3031–3044. [Google Scholar] [CrossRef]
- Rudez, N.; Mavrinac, M.; Cacic, I.; Bošnjak, A.; Dovhanj, J.K. Platelet serotonin and magnesium concentrations in suicidal and non-suicidal depressed patients. J. Life Environ. 2013, 26, 9–11. [Google Scholar]
- Ryu, Y.; Sugiura, Y.; Otsuka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Ghabriel, M.N.; Vink, R. Magnesium transport across the blood-brain barriers. In Magnesium in the Central Nervous System; Nechifor, M., Vink, R., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 59–74. [Google Scholar]
- Grzebieluch, N.; Cichocki, W.J.; Słupski, J.; Górska, M. Ketamine and magnesium common pathway of antidepressant action. J. Life Environ. 2018, 31, 33–38. [Google Scholar] [CrossRef]
- Pochwat, B.; Szopa, A.; Krzyzanowska, K.; Molasy, M.; Nowak, G.; Szewczyk, B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model associated with the AMPA/BDNF pathway. Psychopharmacology 2015, 232, 355–367. [Google Scholar] [CrossRef]
- Palacios, L.; McLean, D.; Forge, I. Iron deficiency and iron deficiency anaemia in women. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 40, 55–67. [Google Scholar] [CrossRef]
- Zhang, C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 2014, 5, 750–760. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. Iron-Deficiency Anemia. Available online: https://www.nhlbi.nih.gov/health/anemia/iron-deficiency-anemia (accessed on 3 June 2025).
- National Institutes of Health, Office of Dietary Supplements. Iron: Fact Sheet for Health Professionals. 2024. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 3 June 2025).
- National Institutes of Health, Office of Dietary Supplements, Zinc, Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 3 June 2025).
- National Institutes of Health, Office of Dietary Supplements. Copper: Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/ (accessed on 3 June 2025).
- National Institutes of Health, Office of Dietary Supplements. Selenium: Fact Sheet for Health Professionals. 2024. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 3 June 2025).
- National Institutes of Health, Office of Dietary Supplements. Iodine: Fact Sheet for Health Professionals. 2024. Available online: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/ (accessed on 3 June 2025).
- Albaramin, N.; Hurrell, R.; Khosravi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Kamran, M. Iron Deficiency Anemia; National Center for Biotechnology Information: Bethesda, MD, USA, 2023. [Google Scholar]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39, Erratum in Blood 2023, 141, 682. [Google Scholar] [CrossRef] [PubMed]
- Koduru, P.; Abraham, B.P. The role of ferric carboxymaltose in the treatment of iron deficiency anemia in patients with gastrointestinal disease. Therap. Adv. Gastroenterol. 2016, 9, 76–85. [Google Scholar] [CrossRef]
- Goshtasebi, A.; Alizadeh, M.; Gandevani, S.M. Association between Maternal Anaemia and Postpartum Depression in an Urban Sample of Pregnant Women in Iran. J. Health Popul. Nutr. 2013, 31, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Xue, X. Targeting iron metabolism in cancer therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- Von Brackel, F.N.; Oheim, R. Iron and bones: Effects of iron overload, deficiency and anemia treatments on bone. JBMR Plus 2024, 8, ziae064. [Google Scholar] [CrossRef] [PubMed]
- Lomagno, K.A.; Hu, F.; Riddell, L.J.; Booth, A.O.; Szymlek-Gay, E.A.; Nowson, C.A.; Byrne, L.K. Increasing iron and zinc in pre-menopausal women and its effects on mood and cognition: A systematic review. Nutrients 2014, 6, 5117–5141. [Google Scholar] [CrossRef]
- Murray, N.T.; McNamara, R.; White, J.B.; Wallace, M.J.; Curley, L.; Binchy, E.M.; Sharkey, J.G.; Howley, N.K.; Verhaeghe, A.A.E.; Clark, B.; et al. Investigating the relationship between iron and depression. J. Psychiatr. Res. 2017, 94, 148–155. [Google Scholar] [CrossRef]
- Michalak, S.S.; Sterna, W. Coexistence and clinical implications of anemia and depression in the elderly population. Psychiatr. Pol. 2023, 57, 517–528. [Google Scholar] [CrossRef]
- Leung, C.Y.; Kyung, M. Associations of iron deficiency and depressive symptoms among young adult males and females: NHANES 2017 to 2020. Prev. Med. Rep. 2023, 37, 102549. [Google Scholar] [CrossRef] [PubMed]
- Berthou, C.; Iliou, J.P.; Barba, D. Iron, neuro-bioavailability and depression, One Library Wiley. eJHaem 2021, 3, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya, S.; Nahavandi, A.; Khojasteh, F.; Soleimani, M.; Ahmadi, M.; Barati, M. Iron administration prevents BDNF decrease and depressive-like behavior following chronic stress. Brain Res. 2015, 1596, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Carlson, E.S.; Fretham, S.J.B.; Georgieff, M.K. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J. Nutr. 2008, 138, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef]
- Wang, J.; Ulph, P.; Dick, B.A.; Li, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Muhamed, P.K.; Vadstrup, S. Zinc is the most important trace element. Ugeskr. Laeger 2014, 176, V11120654. (In Danish) [Google Scholar] [PubMed]
- Costarelli, L.; Muti, E.; Malavolta, M.; Cipriano, C.; Giacconi, R.; Tesei, S.; Piacenza, F.; Pierpaoli, S.; Gasparini, N.; Faloia, E.; et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J. Nutr. Biochem. 2010, 21, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Mlyniec, K.; Sowa-Kucma, M.; Hebda-Bauer, E.; Nowak, G. GPR39 Zn2+-sensing receptor: A new target in antidepressant development? J. Affect. Disord. 2015, 174, 89–100. [Google Scholar] [CrossRef]
- Zięba, M.; Łuszczki, E.; Dziaman, T. Dietary Nutrient Deficiencies and Risk of Depression (Review Article 2018–2023). Nutrients 2023, 15, 2433. [Google Scholar] [CrossRef]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Progress. Neurobiol. 2009, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, U.; Wójcik, P.; Nowak, G.; Rogóż, M.; Choudhury, R.; Maes, K. Zinc in the Monoaminergic Theory of Depression: Its Relationship to Neural Plasticity. Neural Plast. Mood Disord. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Shakiba, Z.; Jowkar, S.; Tehrani, M.; Mokhber, S.; Rezaei, M. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: A double-blind, randomized, placebo-controlled trial. Int. J. Nutr. Diet. Nerv. Syst. 2014, 18, 162–168. [Google Scholar] [CrossRef]
- Hershfinkel, M.; Lambert, J.C.; Silverman, W.F.; Boag, D.; Sekler, I. GPR39 Signaling Is Stimulated by Zinc Ions But Not by Obestatin. Endocrinology 2007, 148, 13–20. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.-Y.; Lin, X. Copper homeostasis and copper-induced cell death: Novel targeting for intervention in the pathogenesis of vascular aging. Biomed. Pharmacother. 2023, 169, 115839. [Google Scholar] [CrossRef]
- Ersöz, G.N.; Şanlıer, N. The relationship between nutrition and depression in the life process: A mini-review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef]
- Lutsenko, S.; Ralle, M.; Thiele, D.J. Mammalian copper homeostasis: Physiological roles and molecular mechanisms. Physiol. Rev. 2024, 105, 441–491. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, S.; Yang, H.; Cui, H.; Guo, H.; Deng, J.; Ren, Z.; Geng, Y.; Ouyang, P.; Xu, Z.; et al. The Dysregulation of Inflammatory Pathways Triggered by Copper Exposure. Biol. Trace Elem. Res. 2023, 201, 539–548. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Attwooll, N.; Schwarz, A.J.; Nettis, M.A.; Cousins, O.; Dima, D.; Mondelli, V.; Bullmore, E.T.; Pariante, C.; Veronese, M. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: Implications for inflammation and depression. Brain Behav. Immun. 2021, 91, 487–497. [Google Scholar] [CrossRef]
- Bartels, S.; Reiss, S.; Zill, K.M.; Eberhagen, C.; Einer, C.; Weber, E.; Müller, S.M.; Michalke, B.; Lichtmannegger, J.; Wieser, A.; et al. Bis-choline tetrathiomolybdate prevents copper-induced blood–brain barrier damage. Life Sci. Alliance 2021, 5, e202101164. [Google Scholar] [CrossRef]
- Dong, H.; Liu, S.; Zhang, S.; Jin, Y. Association between serum copper, zinc, and selenium concentrations and depressive symptoms in the US adult population, NHANES (2011–2016). BMC Psychiatry 2023, 23, 498. [Google Scholar]
- Mattie, M.D.; Freedman, J.H. Copper-inducible transcription: Regulation by metal- and oxidative stress-responsive pathways. Am. J. Physiol.-Cell Physiol. 2004, 286, C293–C301. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in Depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.; Forbes, I.; Lask, B. Anorexia nervosa—A noradrenergic dysregulation hypothesis. Med. Hypotheses 2012, 78, 580–584. [Google Scholar] [CrossRef]
- Crayton, J.W.; Walsh, W.J. Elevated serum copper levels in women with a history of post-partum depression. J. Trace Elem. Med. Biol. 2007, 21, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Metry, A.; Alqahtani, A.; Shafqat, W.; Wilson, P.; Aslam, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Kocyła, N.; Gromadzka, E.; Kołodziej, J.; Białecka, A.; Gromadzińska, K.; Krajewska, E. Selenium Intake and Postnatal Depression—A Short Review. Nutrients 2024, 16, 1926. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Han, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. Selenium intake and multiple health-related outcomes: An umbrella review of meta-analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef]
- Roth, J.S.; Awad, H. The contribution of an imbalanced redox signalling to neurological and neurodegenerative conditions. Free Radic. Biol. Med. 2023, 194, 71–83. [Google Scholar]
- Salas-Lucia, F. Mapping Thyroid Hormone Action in the Human Brain. Thyroid. 2024, 34, 1234–1247. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, M.; Zhang, Z. The Association between Depression and Thyroid Function. Front. Endocrinol. 2024, 15, 1454744. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Marini, M.; Foppiani, T.; Ghezzi, E.; Pelusi, C.; Guglielmi, D.; Iervasi, A.M.; Cesareo, A. Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach. Nutrients 2022, 14, 2234. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.-H.; Lee, B.-H.; Kim, Y.-K. Plasma Level of Brain-Derived Neurotrophic Factor (BDNF) in Patients with Postpartum Depression. Biol. Psychiatry 2021, 109, 110245. [Google Scholar] [CrossRef] [PubMed]
- Hatch-McChesney, A.; Lieberman, H.R. Iodine and Iodine Deficiency: A Comprehensive Review of a Re-Emerging Issue. Nutrients 2022, 14, 3474. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Harding, K.B.; Peña-Rosas, J.P.; Webster, A.C.; Yap, C.M.; Payne, B.A.; Ota, E.; De-Regil, L.M. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst. Rev. 2017, 3, CD011761. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Boelaert, K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Song, B.; Wang, Y.; Dong, J.; Min, H.; Chen, J. Developmental Hypothyroxinemia Caused by Mild Iodine Deficiency Leads to HFS-Induced LTD in Rat Hippocampal CA1 Region: Involvement of AMPA Receptor. Mol. Neurobiol. 2014, 50, 348–357. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Garthus-Niegel, S.; Brandlistuen, E.R.; Caspersen, I.H.; Meltzer, H.M.; Abel, M.H. Mild-to-Moderate Iodine Deficiency and Symptoms of Emotional Distress and Depression in Pregnancy and Six Months Postpartum—Results from a Large Pregnancy Cohort. J. Affect. Disord. 2022, 318, 347–356. [Google Scholar] [CrossRef]
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, D.Y.; Rowley, K.D.; Sweeney, L.B. Hypothyroidism: An Update. Am. Fam. Physician 2012, 86, 244–251. [Google Scholar] [CrossRef]
- Osuna, E.; Baumgartner, J.; Walther, A.; Emery, S.; Albermann, M.; Baumgartner, N.; Schmeck, K.; Walitza, S.; Strumberger, M.; Hersberger, M.; et al. Investigating Thyroid Function and Iodine Status in Adolescents with and without Paediatric Major Depressive Disorder. Br. J. Nutr. 2024, 132, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Talhada, D.; Alves Santos, C.R.; Gonçalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms after Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Sacchet, M.D.; Gotlib, I.H. Myelination of the Brain in Major Depressive Disorder: An In Vivo Quantitative Magnetic Resonance Imaging Study. Sci. Rep. 2017, 7, 2200. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid Hormone Receptors in Brain Development and Function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Teng, Y.; Guan, Y.; Zhang, L.; Jia, X.; Cui, D.; Li, J.; Guan, H. The Effect of Iodine-Containing Vitamin Supplementation during Pregnancy on Thyroid Function in Late Pregnancy and Postpartum Depression in an Iodine-Sufficient Area. Biol. Trace Elem. Res. 2020, 198, 1–7. [Google Scholar] [CrossRef]
- Chang, X.L.; Shang, Y.; Liu, Y.J.; Li, P.; Wang, Y.Y.; Liang, A.M.; Qi, K.M. Effects of calcium supplementation during the pregnancy and early infancy stage on the body mass index and gut microbiota in the infants. Zhonghua Yu Fang Yi Xue Za Zhi 2018, 52, 642–646. [Google Scholar] [CrossRef]
- Yatsonsky, I.I.D.; Pan, K.; Shendge, V.B.; Liu, J.; Ebraheim, N.A. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 2019, 10, 123–127. [Google Scholar] [CrossRef]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and thyroid interaction in health and disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Sex differences in requirements for micronutrients across the lifecourse. Proc. Nutr. Soc. 2021, 80, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yin, X.; Meng, H.; Fang, X.; Min, J.; Wang, F. Progress on epigenetic regulation of iron homeostasis. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of Magnesium Supplementation in the Treatment of Depression: A Randomized Clinical Trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef]
- Afsharfar, M.; Shahraki, M.; Shakiba, M.; Asbaghi, O.; Dashipour, A. The Effects of Magnesium Supplementation on Serum Level of Brain Derived Neurotrophic Factor (BDNF) and Depression Status in Patients with Depression. Clin. Nutr. ESPEN 2021, 42, 381–386. [Google Scholar] [CrossRef]
- Abiri, B.; Sarbakhsh, P.; Vafa, M. Randomized Study of the Effects of Vitamin D and/or Magnesium Supplementation on Mood, Serum Levels of BDNF, Inflammation, and SIRT1 in Obese Women with Mild to Moderate Depressive Symptoms. Nutr. Neurosci. 2022, 25, 2123–2135. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Sugita, M.; Kawaguchi, M.; Timm, D.; Kawamura, A.; Abe, A.; Okubo, T. Influence of iron supplementation on fatigue, mood states and sweating profiles of healthy non-anemic athletes during a training exercise: A double-blind, randomized, placebo-controlled, parallel-group study. Contemp. Clin. Trials Commun. 2023, 32, 101084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. The efficacy of zinc supplementation in depression: Systematic review of randomised controlled trials. J. Affect. Disord. 2012, 136, e31–e39. [Google Scholar] [CrossRef]
- Ranjbar, E.; Kasaei, M.S.; Mohammad-Shirazi, M.; Nasrollahzadeh, J.; Rashidkhani, B.; Shams, J.; Mostafavi, S.A.; Mohammadi, M.R. Effects of zinc supplementation in patients with major depression: A randomized clinical trial. Iran. J. Psychiatry 2013, 8, 73–79. [Google Scholar] [PubMed] [PubMed Central]
- Afzali, A.; Vakili, Z.; Goli, S.; Bagheri, H.; Mirhosseini, S.; Ebrahimi, H. A Randomized Clinical Trial of the Effect of Zinc Supplement on Depression and Anxiety in the Elderly. Open Public Health J. 2021, 14, 537–544. [Google Scholar] [CrossRef]
- Benton, D.; Cook, R. The impact of selenium supplementation on mood. Biol. Psychiatry 1991, 29, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Ferns, G.A. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. 2011, 24, 104–108. [Google Scholar] [CrossRef] [PubMed]
| Minerals | Symbol | Recommended Daily Intake | Dietary Sources | References | ||
|---|---|---|---|---|---|---|
| Age | Male | Female | ||||
| Calcium | Ca | 1–3 years | 700 mg | 700 mg | milk, yogurt, cheese, sardines, salmon, kale, and broccoli | [17] |
| 9–13 years | 1300 mg | 1300 mg | ||||
| 19–50 years | 1000 mg | 1000 mg | ||||
| Magnesium | Mg | 1–3 years | 80 mg | 80 mg | spinach, legumes, nuts, seeds, and whole grains | [12] |
| 9–13 years | 240 mg | 240 mg | ||||
| 31–50 years | 420 mg | 320 mg | ||||
| Iron | Fe | 1–3 years | 7 mg | 7 mg | nuts, beans, vegetables, and fortified grain products | [48] |
| 9–13 years | 8 mg | 8 mg | ||||
| 19–50 years | 8 mg | 18 mg | ||||
| Zinc | Zn | 1–3 years | 3 mg | 3 mg | meat, fish, seafood, and eggs | [49] |
| 9–13 years | 8 mg | 8 mg | ||||
| 19+ years | 11 mg | 8 mg | ||||
| Copper | Cu | 1–3 years | 340 µg | 340 µg | shellfish, seeds, nuts, organ meats, wheat-bran cereals, whole-grain products, and chocolate | [50] |
| 9–13 years | 700 µg | 700 µg | ||||
| 19+ years | 900 µg | 900 µg | ||||
| Selenium | Se | 1–3 years 9–13 years 19–50 years | 20 µg 40 µg 55 µg | 20 µg 40 µg 55 µg | Brazil nuts, seafood, meat, poultry, organ meats cereals, and eggs | [51] |
| 9–13 years | 40 µg | 40 µg | ||||
| 19–50 years | 55 µg | 55 µg | ||||
| Iodine | I | 1–3 years | 90 µg | 90 µg | seaweed (kelp, nori, kombu, and wakame), fish, and eggs | [52] |
| 9–13 years | 120 µg | 120 µg | ||||
| 19+ years | 150 µg | 150 µg | ||||
| Mineral | Key Findings | Reference |
|---|---|---|
| Magnesium (Mg) | A magnesium dose of 248 mg/day improved depression symptoms in 6 weeks, comparable to selective serotonin reuptake inhibitors in mild–moderate cases. | [118] |
| Administration of 500 mg of magnesium per day can improve the depression status in adults. | [119] | |
| Vitamin D plus magnesium supplementation in obese women with mild to moderate depressive symptoms has beneficial influences on mood, serum levels of brain-derived neurotrophic factor, inflammation, and sirtuin 1. | [120] | |
| Iron (Fe) | Supplementation improved fatigue and mood in women with iron deficiency. | [121] |
| Zinc (Zn) | Supplementation reduced depressive symptoms, may enhance antidepressant efficacy, and is associated with increased brain-derived neurotrophic factor levels. | [122] |
| Zinc supplementation, together with selective serotonin reuptake inhibitors antidepressant drugs, improves major depressive disorders more effectively in patients with placebo plus antidepressants (selective serotonin reuptake inhibitors). | [123] | |
| The use of zinc supplements improved depression and anxiety in the elderly. | [124] | |
| Selenium (Se) | Potential protective effect against depression—the lower the level of selenium in the diet, the more reports of anxiety, depression, and tiredness decreased following 5 weeks of selenium therapy. | [125] |
| Supplementation with selenium during pregnancy might be an effective approach for the prevention of postpartum depression. | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, Z.; Orywal, K. Mineral Homeostasis and Depression: Implications for Prevention and Therapeutic Support—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 6637. https://doi.org/10.3390/ijms26146637
Majewska Z, Orywal K. Mineral Homeostasis and Depression: Implications for Prevention and Therapeutic Support—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(14):6637. https://doi.org/10.3390/ijms26146637
Chicago/Turabian StyleMajewska, Zuzanna, and Karolina Orywal. 2025. "Mineral Homeostasis and Depression: Implications for Prevention and Therapeutic Support—A Narrative Review" International Journal of Molecular Sciences 26, no. 14: 6637. https://doi.org/10.3390/ijms26146637
APA StyleMajewska, Z., & Orywal, K. (2025). Mineral Homeostasis and Depression: Implications for Prevention and Therapeutic Support—A Narrative Review. International Journal of Molecular Sciences, 26(14), 6637. https://doi.org/10.3390/ijms26146637







