Enhanced Phytoremediation of Galaxolide Using Lemna minor: Mechanisms, Efficiency, and Environmental Implications
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytoremediation Process Efficacy
2.2. Mechanisms of Removal HHCB by Lemna minor
2.3. Identification of the Primary Metabolite Formed During the Removal of Galaxolide
2.4. Accumulation of HHCB in Lemna minor
2.5. Studies on the Influence of HHCB and Synthetic Wastewater on the Growth, Biochemical Composition, Stress Markers, and Antioxidant Activity of Lemna minor
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Removal Effectiveness and Toxicity Experiments
3.4. Kinetics and Mechanisms of Degradation
3.5. Extraction and Quantification of HHCB in Growth Media
3.6. Extraction and Quantification of HHCB in Plant Tissues
3.7. Analysis of Biochemical Components, Stress Markers, and Antioxidant Activity in Lemna minor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotamraju, A.; Logan, M.; Lens, P.N.L. Coupling Protein Recovery to Methane and Lactic Acid Production to Valorize Duckweed Used in Selenate Remediation. Environ. Technol. Innov. 2024, 33, 103515. [Google Scholar] [CrossRef]
- Calicioglu, O.; Femeena, P.V.; Mutel, C.L.; Sills, D.L.; Richard, T.L.; Brennan, R.A. Techno-Economic Analysis and Life Cycle Assessment of an Integrated Wastewater-Derived Duckweed Biorefinery. ACS Sustain. Chem. Eng. 2021, 9, 9395–9408. [Google Scholar] [CrossRef]
- Petersen, F.; Demann, J.; Restemeyer, D.; Olfs, H.-W.; Westendarp, H.; Appenroth, K.-J.; Ulbrich, A. Influence of Light Intensity and Spectrum on Duckweed Growth and Proteins in a Small-Scale, Re-Circulating Indoor Vertical Farm. Plants 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, S.; Stejskal, V.; Toner, D.; Jansen, M.A.K. Wastewater Valorisation in an Integrated Multitrophic Aquaculture System; Assessing Nutrient Removal and Biomass Production by Duckweed Species. Environ. Pollut. 2022, 302, 119059. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.J. Growing Duckweed for Biofuel Production: A Review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, K.; Li, W.; Yan, B.; Yu, Y.; Li, J.; Zhang, Y.; Xia, S.; Cheng, Z.; Lin, F.; et al. A Review on Bioenergy Production from Duckweed. Biomass Bioenergy 2022, 161, 106468. [Google Scholar] [CrossRef]
- Irfan, M.; Mészáros, I.; Szabó, S.; Oláh, V. Comparative Phytotoxicity of Metallic Elements on Duckweed Lemna gibba L. Using Growth- and Chlorophyll Fluorescence Induction-Based Endpoints. Plants 2024, 13, 215. [Google Scholar] [CrossRef]
- Szabó, S.; Zavanyi, G.; Koleszár, G.; del Castillo, D.; Oláh, V.; Braun, M. Phytoremediation, Recovery and Toxic Effects of Ionic Gadolinium Using the Free-Floating Plant Lemna gibba. J. Hazard. Mater. 2023, 458, 131930. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and Nutrient Removal Efficiency of Duckweed (Lemna minor) from Synthetic and Dumpsite Leachate under Artificial and Natural Conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef]
- Golob, A.; Vogel-Mikuš, K.; Brudar, N.; Germ, M. Duckweed (Lemna minor L.) Successfully Accumulates Selenium from Selenium-Impacted Water. Sustainability 2021, 13, 13423. [Google Scholar] [CrossRef]
- Chen, G.; Fang, Y.; Huang, J.; Zhao, Y.; Li, Q.; Lai, F.; Xu, Y.; Tian, X.; He, K.; Jin, Y.; et al. Duckweed Systems for Eutrophic Water Purification through Converting Wastewater Nutrients to High-Starch Biomass: Comparative Evaluation of Three Different Genera (Spirodela Polyrhiza, Lemna minor and Landoltia Punctata) in Monoculture or Polyculture. RSC Adv. 2018, 8, 17927–17937. [Google Scholar] [CrossRef] [PubMed]
- Tumová, J.; Šauer, P.; Golovko, O.; Koba Ucun, O.; Grabic, R.; Máchová, J.; Kocour Kroupová, H. Effect of Polycyclic Musk Compounds on Aquatic Organisms: A Critical Literature Review Supplemented by Own Data. Sci. Total Environ. 2019, 651, 2235–2246. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Gómez-Regalado, M.D.C.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical Methods for the Determination of Endocrine Disrupting Chemicals in Cosmetics and Personal Care Products: A Review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef] [PubMed]
- Schmeiser, H.H.; Gminski, R.; Mersch-Sundermann, V. Evaluation of Health Risks Caused by Musk Ketone. Int. J. Hyg. Environ. Health 2001, 203, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Katuri, G.P.; Fan, X.; Siddique, S.; Kubwabo, C.; Kosarac, I.; Harris, S.A.; Foster, W.G. A Selective and Sensitive Gas Chromatography-Tandem Mass Spectrometry Method for Quantitation of Synthetic Musks in Human Serum. J. AOAC Int. 2020, 103, 1461–1468. [Google Scholar] [CrossRef]
- Patel, S.; Homaei, A.; Sharifian, S. Need of the Hour: To Raise Awareness on Vicious Fragrances and Synthetic Musks. Environ. Dev. Sustain. 2021, 23, 4764–4781. [Google Scholar] [CrossRef]
- David, O.R.P. A Chemical History of Polycyclic Musks. Chemistry 2020, 26, 7537–7555. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn, R.; Gatermann, R.; Rimkus, G.G. Synthetic Musks in Environmental Samples: Indicator Compounds with Relevant Properties for Environmental Monitoring. J. Environ. Monit. 1999, 1, 70N–74N. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Z. Determination of Galaxolide (HHCB) and Tonalide (AHTN) by Solid Phase Extraction and Gas Chromatography (SPE-GC). Lett. Appl. NanoBioScience 2022, 11, 3388–3392. [Google Scholar] [CrossRef]
- Balk, F.; Ford, R.A. Environmental Risk Assessment for the Polycyclic Musks AHTN and HHCB in the EU. Toxicol. Lett. 1999, 111, 57–79. [Google Scholar] [CrossRef]
- Lung, S.C.C.; Liu, C.H. High-Sensitivity Analysis of Six Synthetic Musks by Ultra-Performance Liquid Chromatography-Atmospheric Pressure Photoionization-Tandem Mass Spectrometry. Anal. Chem. 2011, 83, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in the Aquatic Environment: A Review of Recent Research Data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Bester, K. Retention Characteristics and Balance Assessment for Two Polycyclic Musk Fragrances (HHCB and AHTN) in a Typical German Sewage Treatment Plant. Chemosphere 2004, 57, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Tasselli, S.; Guzzella, L. Polycyclic Musk Fragrances (PMFs) in Wastewater and Activated Sludge: Analytical Protocol and Application to a Real Case Study. Environ. Sci. Pollut. Res. 2020, 27, 30977–30986. [Google Scholar] [CrossRef]
- Rimkus, G.G. Polycyclic Musk Fragrances in the Aquatic Environment. Toxicol. Lett. 1999, 111, 37–56. [Google Scholar] [CrossRef]
- Wong, F.; Robson, M.; Melymuk, L.; Shunthirasingham, C.; Alexandrou, N.; Shoeib, M.; Luk, E.; Helm, P.; Diamond, M.L.; Hung, H. Urban Sources of Synthetic Musk Compounds to the Environment. Environ. Sci. Process Impacts 2019, 21, 74–88. [Google Scholar] [CrossRef]
- Ehiguese, F.O.; González-Delgado, M.J.; Garrido-Perez, C.; Araújo, C.V.M.; Martin-Diaz, M.L. Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish. Processes 2021, 9, 371. [Google Scholar] [CrossRef]
- Luo, N.; Gao, Y.; Chen, X.; Wang, M.; Niu, X.; Li, G.; An, T. A Critical Review of Environmental Exposure, Metabolic Transformation, and the Human Health Risks of Synthetic Musks. Crit. Rev. Environ. Sci. Technol. 2023, 53, 2132–2149. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Gatidou, G.; Damalas, D.; Thomaidis, N.S.; Stasinakis, A.S. Fate of Antimicrobials in Duckweed Lemna minor Wastewater Treatment Systems. J. Hazard. Mater. 2017, 330, 116–126. [Google Scholar] [CrossRef]
- Imron, M.F.; Ananta, A.R.; Ramadhani, I.S.; Kurniawan, S.B.; Abdullah, S.R.S. Potential of Lemna minor for Removal of Methylene Blue in Aqueous Solution: Kinetics, Adsorption Mechanism, and Degradation Pathway. Environ. Technol. Innov. 2021, 24, 101921. [Google Scholar] [CrossRef]
- Mahdi Al-Nabhan, E.A. Removal Efficiency, Accumulation and Biochemical Response of Lemna minor L. Exposed to Some Heavy Metals. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 012037. [Google Scholar] [CrossRef]
- Rakhshaee, R.; Giahi, M.; Pourahmad, A. Studying Effect of Cell Wall’s Carboxyl–Carboxylate Ratio Change of Lemna minor to Remove Heavy Metals from Aqueous Solution. J. Hazard. Mater. 2009, 163, 165–173. [Google Scholar] [CrossRef]
- Kösesakal, T.; Ünlü, V.S.; Külen, O.; Memon, A.; Yüksel, B. Evaluation of the Phytoremediation Capacity of Lemna minor L. Incrude Oil Spiked Cultures. Turk. J. Biol. 2015, 39, 479–484. [Google Scholar] [CrossRef]
- Polińska, W.; Kotowska, U.; Karpińska, J. The Problem with Benzotriazole Ultraviolet Stabilizers in the Environment—Are the Aquatic Plants the Solution for Them? Ind. Crops Prod. 2024, 210, 118050. [Google Scholar] [CrossRef]
- Dosnon-Olette, R.; Couderchet, M.; Oturan, M.A.; Oturan, N.; Eullaffroy, P. Potential Use of Lemna minor for the Phytoremediation of Isoproturon and Glyphosate. Int. J. Phytoremediat. 2011, 13, 601–612. [Google Scholar] [CrossRef]
- Gatidou, G.; Oursouzidou, M.; Stefanatou, A.; Stasinakis, A.S. Removal Mechanisms of Benzotriazoles in Duckweed Lemna minor Wastewater Treatment Systems. Sci. Total Environ. 2017, 596, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Li, W.; Cai, M.; Jia, X.; Yang, M.; Yang, B.; Li, J. Algal Toxicity, Accumulation and Metabolic Pathways of Galaxolide. J. Hazard. Mater. 2020, 384, 121360. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, F.; Xiao, X.; Li, H.; Wang, D.; You, J. Ecological Risk of Galaxolide and Its Transformation Product Galaxolidone: Evidence from the Literature and a Case Study in Guangzhou Waterways. Environ. Sci. Process Impacts 2023, 25, 1337–1346. [Google Scholar] [CrossRef]
- Tatar, Ş.Y.; Öbek, E. Potential of Lemna gibba L. and Lemna minor L. for Accumulation of Boron from Secondary Effluents. Ecol. Eng. 2014, 70, 332–336. [Google Scholar] [CrossRef]
- Konakci, N. Accumulation Assessment of Mo4+, Pb2+, and Cu2+ in the Acidic Water of Copper Mines with Lemna minor and Lemna gibba. Water 2024, 16, 975. [Google Scholar] [CrossRef]
- Basiglini, E.; Pintore, M.; Forni, C. Effects of Treated Industrial Wastewaters and Temperatures on Growth and Enzymatic Activities of Duckweed (Lemna minor L.). Ecotoxicol. Environ. Saf. 2018, 153, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Walsh, É.; Paolacci, S.; Burnell, G.; Jansen, M.A.K. The Importance of the Calcium-to-Magnesium Ratio for Phytoremediation of Dairy Industry Wastewater Using the Aquatic Plant Lemna minor L. Int. J. Phytoremediat. 2020, 22, 694–702. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Wang, C.; Liao, J.; Ni, J.; Zhang, T.; Wang, R.; Ruan, H. Growth, Physiological Function, and Antioxidant Defense System Responses of Lemna minor L. to Decabromodiphenyl Ether (BDE-209) Induced Phytotoxicity. Plant Physiol. Biochem. 2019, 139, 113–120. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Y.; Gao, X.; Du, G.; Ma, X.; Liu, M.; Guo, J.; Chen, Y. Ammonium-Induced Oxidative Stress on Plant Growth and Antioxidative Response of Duckweed (Lemna minor L.). Ecol. Eng. 2013, 58, 355–362. [Google Scholar] [CrossRef]
- Steinberg, R.A. Mineral requirements of Lemna minor. Plant Physiol. 1946, 21, 42–48. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of Endocrine Disrupters from the Contaminated Environment: Public Health Concerns, Treatment Strategies and Future Perspectives—A Review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. A photometric adaptation of the somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.-K.; Choi, J.; Kim, J.O.; Jeon, B.-H. Biodegradation of Carbamazepine Using Freshwater Microalgae Chlamydomonas Mexicana and Scenedesmus Obliquus and the Determination of Its Metabolic Fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Schaedlea, M.; Basshamb, J.A. Chloroplast glutathione reductase. Plant Physiol 1977, 59, 1011–1012. [Google Scholar] [CrossRef]
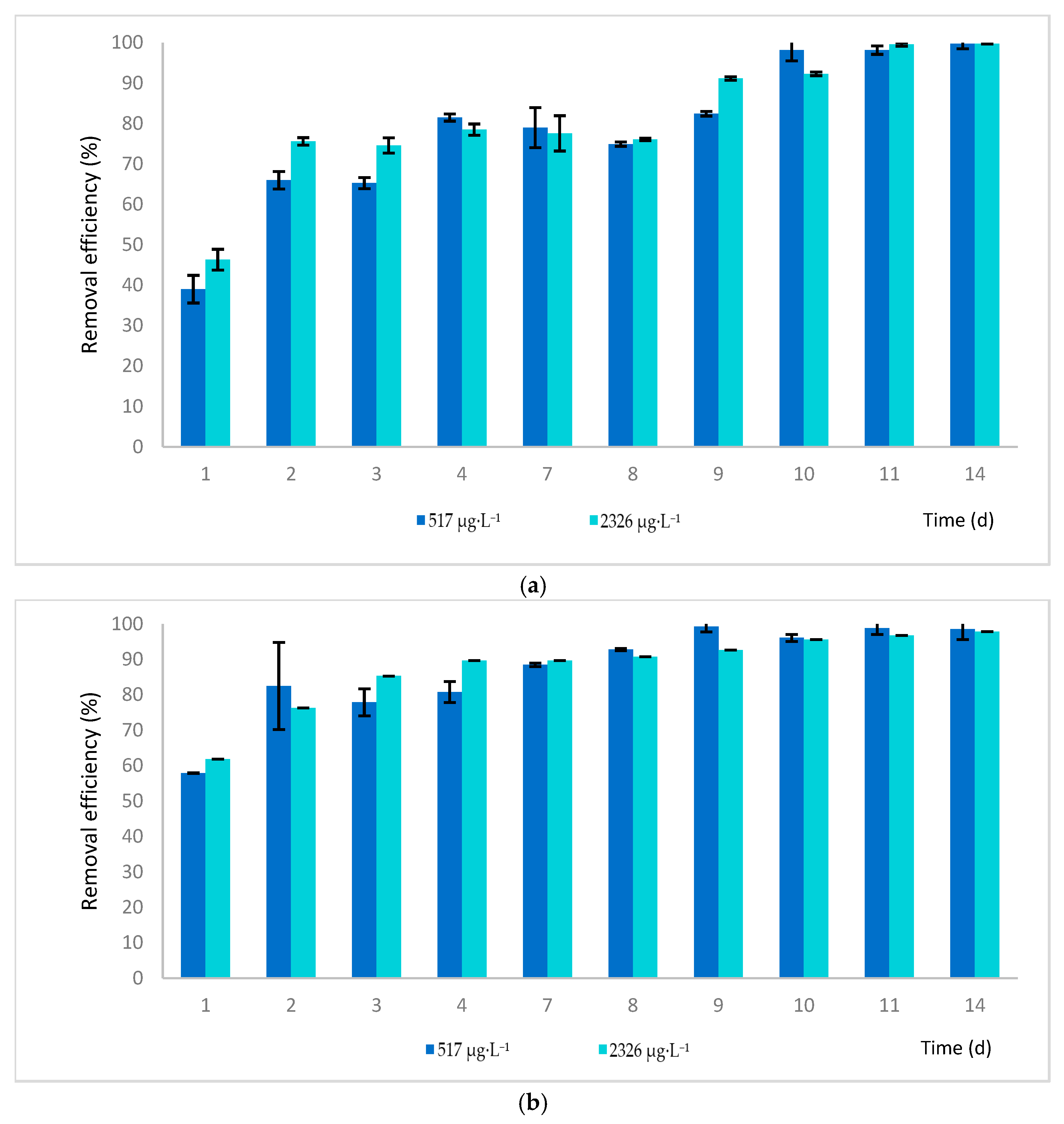
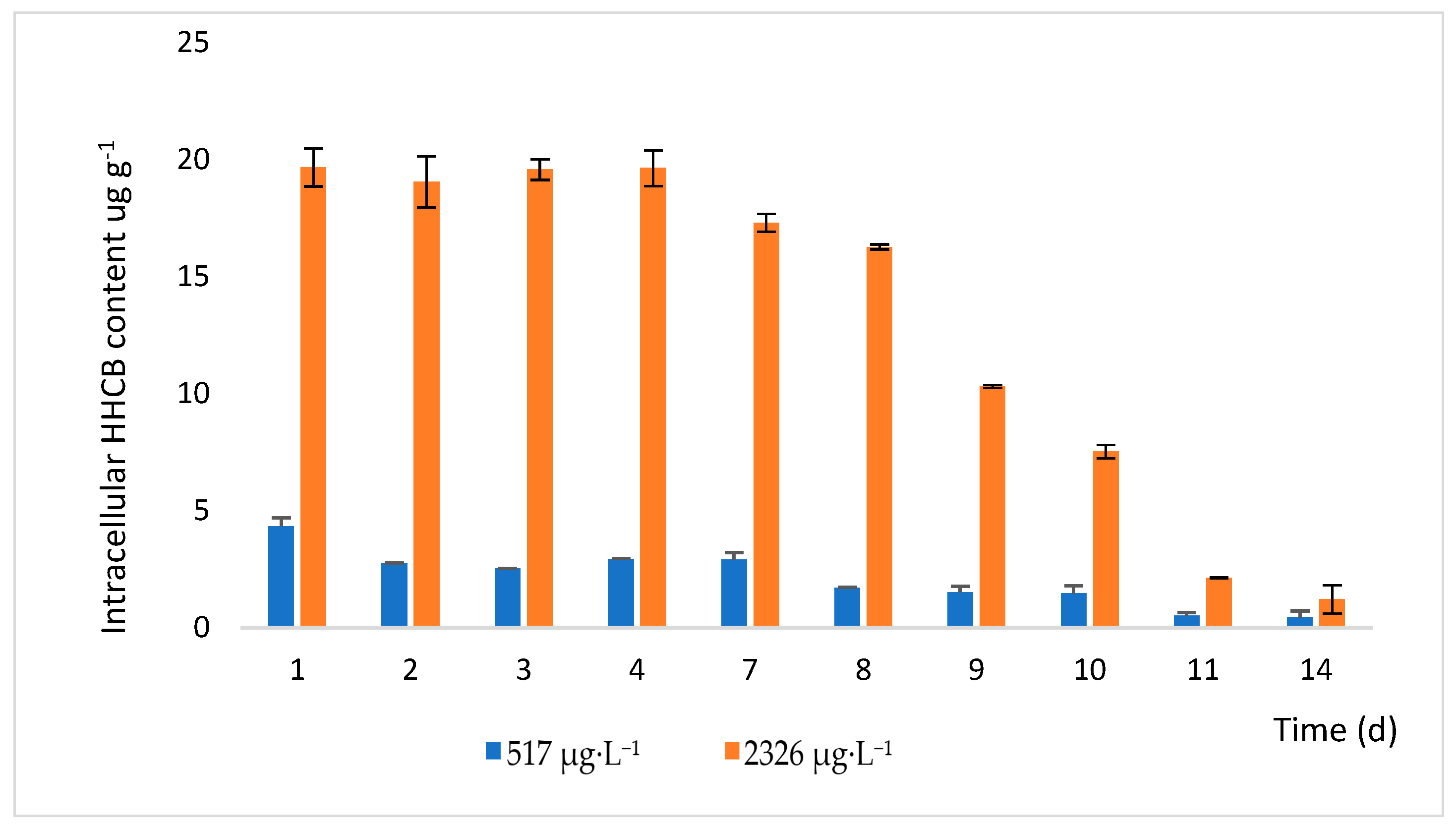
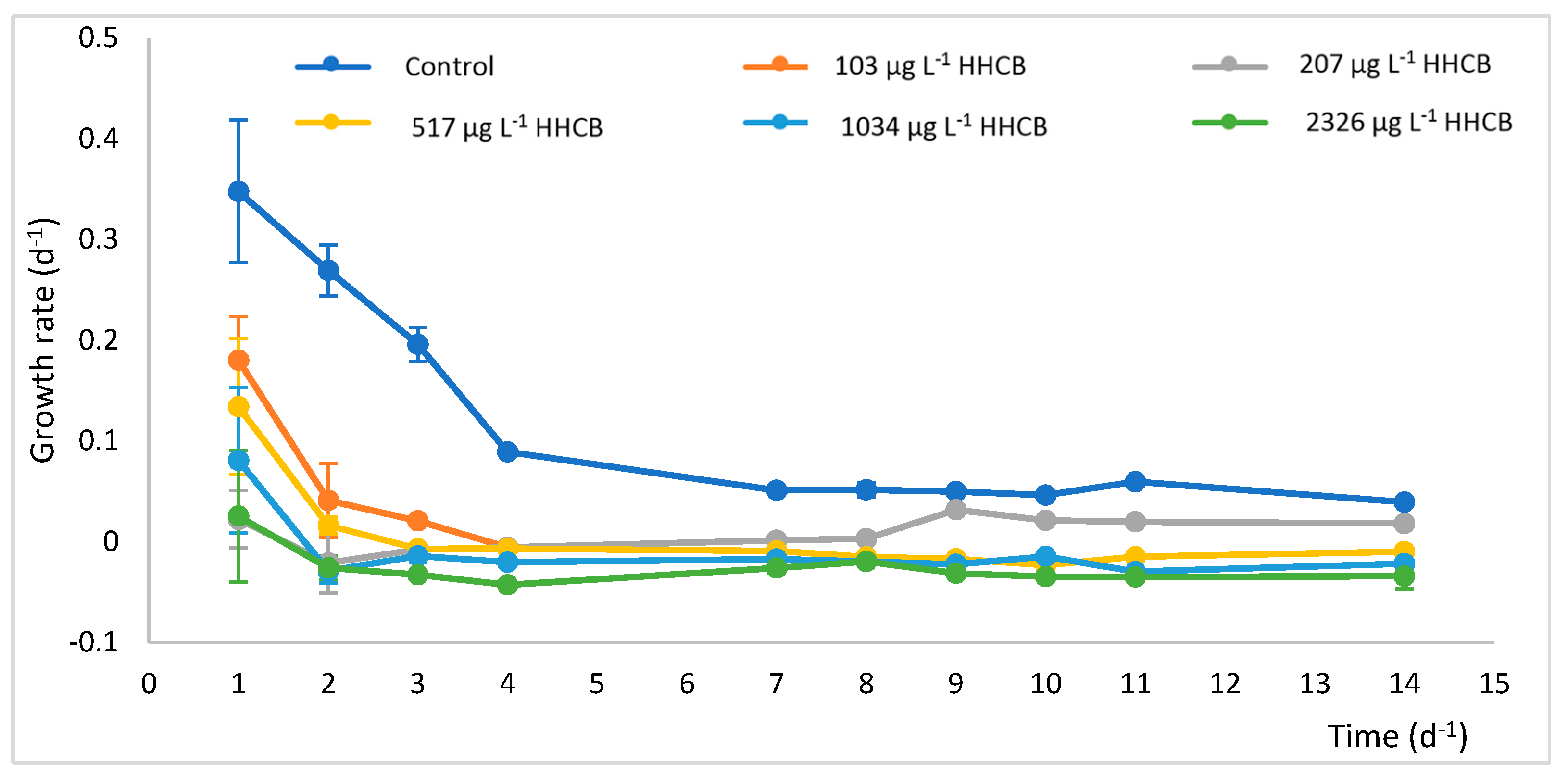
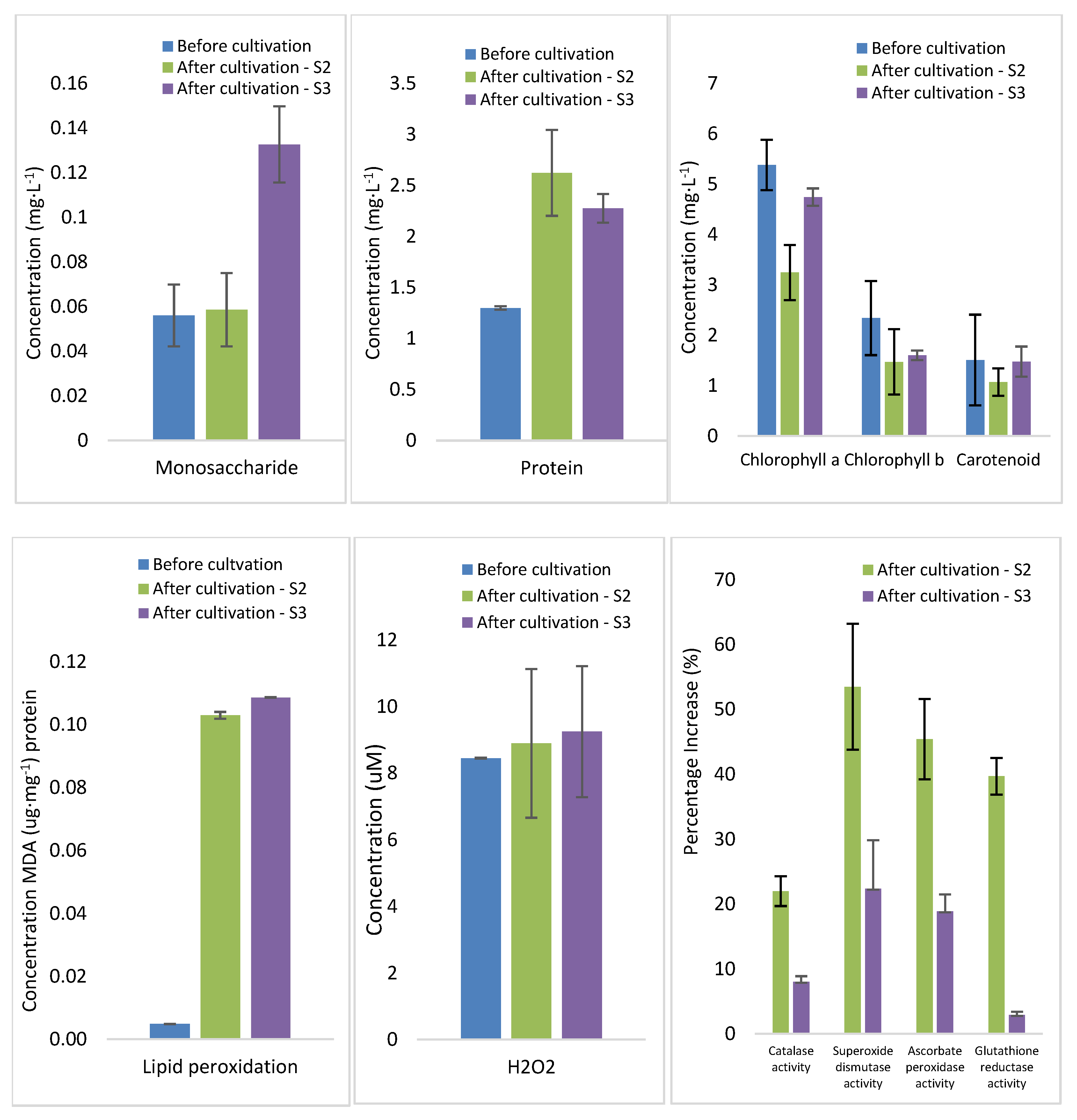
| Test Solution | HHCB Concentration (µg·L−1) | k (d−1) ± SD; (n = 3) | t1/2(d) ± SD; (n = 3) | R2 |
|---|---|---|---|---|
| Laboratory nutrient solution | 517 | 0.359 ± 0.012 | 1.93 ± 0.61 | 0.935 |
| 2326 | 0.345 ± 0.08 | 2.21 ± 0.332 | 0.950 | |
| Synthetic wastewater | 517 | 0.37 ± 0.011 | 1.87 ± 0.006 | 0.981 |
| 2326 | 0.312 ± 0.368 | 12.22 ± 0.028 | 0.864 |
| Test Solution | HHCB Concentration (µg·L−1) | khydrolysis (d−1); (n = 3) R2 | kphotodegradation (d−1); (n = 3) R2 | ksorption (d−1); (n = 3) R2 | kuptake (d−1); (n = 3) |
|---|---|---|---|---|---|
| Laboratory nutrient solution | 517 | 0.025 0.934 | 0.105 0.949 | 0.153 0.861 | 0.076 |
| 2326 | 0.051 0.975 | 0.083 0.964 | 0.148 0.858 | 0.063 | |
| Synthetic wastewater | 517 | 0.036 0.983 | 0.105 0.904 | 0.159 0.898 | 0.070 |
| 2326 | 0.053 0.977 | 0.075 0.921 | 0.151 0.858 | 0.033 |
| Experiment | T (°C) | Mechanism of Degradation | Light/Presence of Lemna minor |
|---|---|---|---|
| 1 | 22 ± 2 | Hydrolysis | Dark/Not |
| 2 | Hydrolysis + Photodegradation | Fluorescent lamp/Not | |
| 3 | Fitodegradation (Hydrolysis + Photodegradation + Sorption + Plant uptake) | Fluorescent lamp/Lemna minor | |
| 4 | Hydrolysis + Sorption | Dark/dead Lemna minor (1 g·L−1 NaN3, five days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokół, A.; Karpińska, J. Enhanced Phytoremediation of Galaxolide Using Lemna minor: Mechanisms, Efficiency, and Environmental Implications. Int. J. Mol. Sci. 2025, 26, 6636. https://doi.org/10.3390/ijms26146636
Sokół A, Karpińska J. Enhanced Phytoremediation of Galaxolide Using Lemna minor: Mechanisms, Efficiency, and Environmental Implications. International Journal of Molecular Sciences. 2025; 26(14):6636. https://doi.org/10.3390/ijms26146636
Chicago/Turabian StyleSokół, Aneta, and Joanna Karpińska. 2025. "Enhanced Phytoremediation of Galaxolide Using Lemna minor: Mechanisms, Efficiency, and Environmental Implications" International Journal of Molecular Sciences 26, no. 14: 6636. https://doi.org/10.3390/ijms26146636
APA StyleSokół, A., & Karpińska, J. (2025). Enhanced Phytoremediation of Galaxolide Using Lemna minor: Mechanisms, Efficiency, and Environmental Implications. International Journal of Molecular Sciences, 26(14), 6636. https://doi.org/10.3390/ijms26146636








