Is Atopic Dermatitis Associated with Systemic Metabolic Disturbances? A Systematic Review
Abstract
1. Introduction
1.1. Epidemiology of Atopic Dermatitis
1.2. Clinical Presentation of Atopic Dermatitis
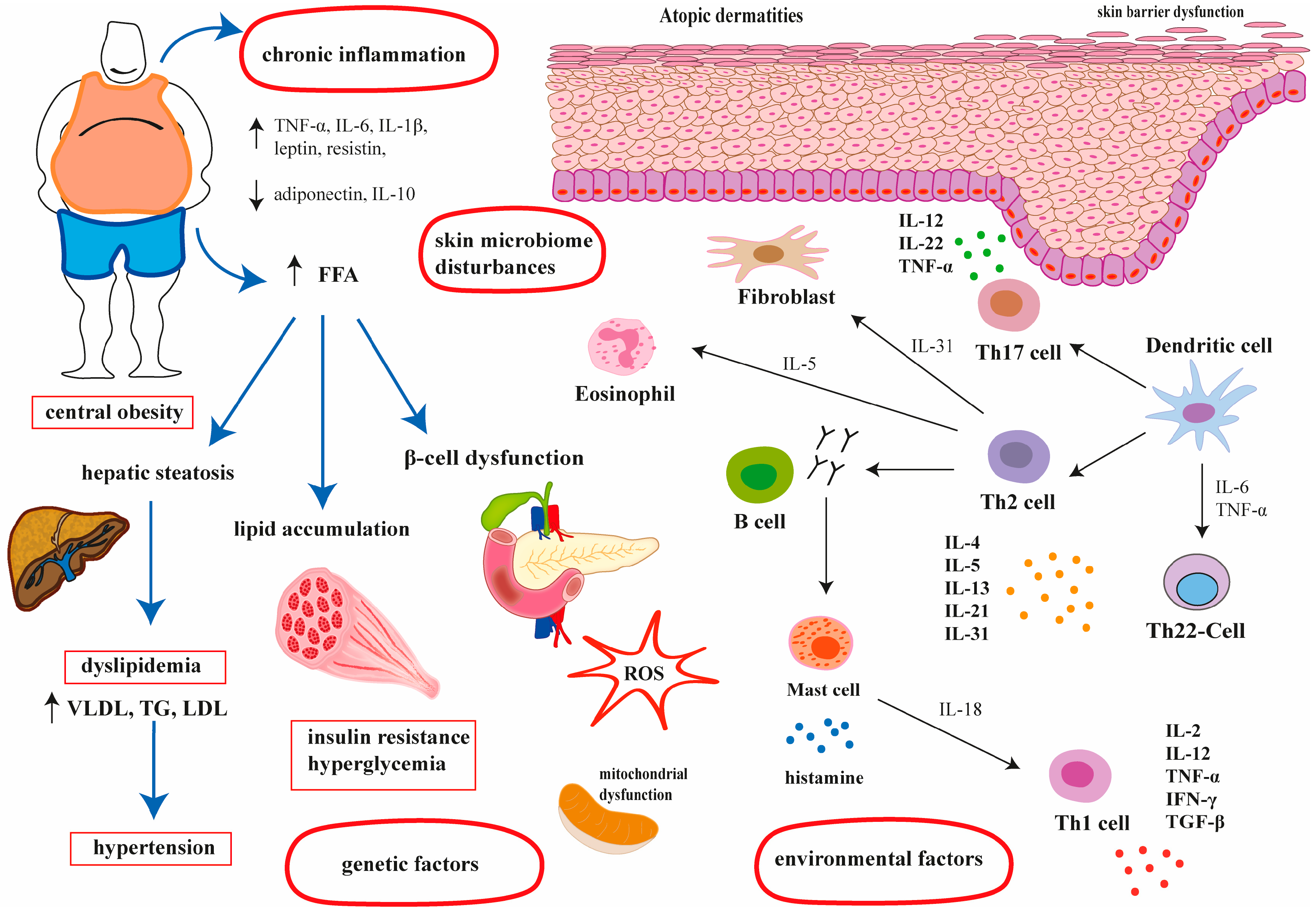
1.3. Pathogenesis of Atopic Dermatitis
1.4. Metabolic Syndrome—Definition and Its Association with AD
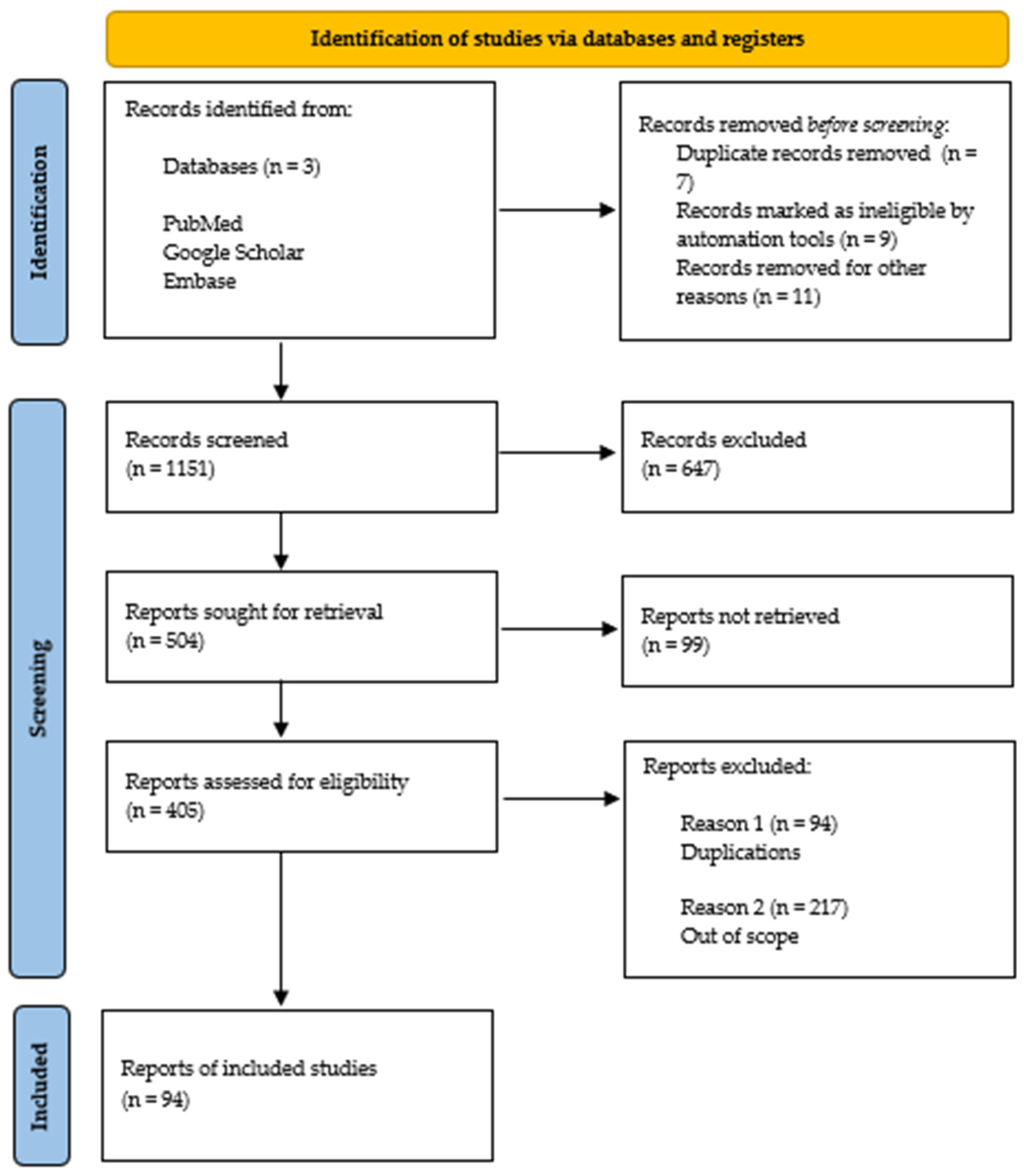
2. Materials and Methods
3. Results and Discussion
3.1. The Association Between Metabolic Syndrome and Atopic Dermatitis
3.2. The Association Between Hypertension and Atopic Dermatitis
3.3. The Association Between Central Obesity and Atopic Dermatitis
3.4. The Association Between Hypertriglyceridaemia and Atopic Dermatitis
3.5. The Association Between HDL Level and Atopic Dermatitis
3.6. The Association Between Hyperglyceamia and Atopic Dermatitis
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682, Erratum in: J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1436. [Google Scholar] [CrossRef] [PubMed]
- Kilic, N.; Kilic, M. Investigation of Quality of Life of Patients with Atopic Dermatitis and Quality of Life, Psychiatric Symptomatology, and Caregiver Burden of Their Mothers. Children 2023, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Duczmal, E.; Bręborowicz, A.; Duczmal, T. Allergic march in childhood. Post Dermatol. Alergol. 2010, 4, 231–237. [Google Scholar]
- Ali, Z.; Ulrik, C.S.; Agner, T.; Thomsen, S.F. Association between Atopic Dermatitis and the Metabolic Syndrome: A Systematic Review. Dermatology 2018, 234, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Illig, T.; Baurecht, H.; Irvine, A.D.; Rodriguez, E.; Diaz-Lacava, A.; Klopp, N.; Wagenpfeil, S.; Zhao, Y.; Liao, H.; et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J. Allergy Clin. Immunol. 2006, 118, 214–219, Erratum in J. Allergy Clin. Immunol. 2006, 118, 724. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandlik, D.S.; Mandlik, S.K. Atopic dermatitis: New insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol. Immunotoxicol. 2021, 43, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Dreiher, J.; Kridin, K.; Horev, A.; Khoury, R.; Battat, E.; Freud, T.; Comaneshter, D.; Cohen, A. Atopic dermatitis and the metabolic syndrome: A cross-sectional study of 116 816 patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1762–1767. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Megna, M.; Patruno, C.; Balato, A.; Rongioletti, F.; Stingeni, L.; Balato, N. An Italian multicentre study on adult atopic dermatitis: Persistent versus adult-onset disease. Arch. Dermatol. Res. 2017, 309, 443–452. [Google Scholar] [CrossRef]
- Schäfer, T.; Ruhdorfer, S.; Weigl, L.; Wessner, D.; Heinrich, J.; Döring, A.; Wichmann, H.; Ring, J. Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin. Exp. Allergy 2003, 33, 1360–1367. [Google Scholar] [CrossRef]
- Uehara, M.; Sugiura, H.; Tanaka, K. Rarity of hypertension in adult patients with atopic dermatitis. Br. J. Dermatol. 2002, 146, 631. [Google Scholar] [CrossRef]
- Standl, M.; Tesch, F.; Baurecht, H.; Rodríguez, E.; Müller-Nurasyid, M.; Gieger, C.; Peters, A.; Wang-Sattler, R.; Prehn, C.; Adamski, J.; et al. Association of Atopic Dermatitis with Cardiovascular Risk Factors and Diseases. J. Investig. Dermatol. 2017, 137, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.; Qureshi, A.; Dummer, T.; Parker, L.; Li, W.-Q. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian Partnership for Tomorrow Project. Br. J. Dermatol. 2017, 177, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, H.M.; Han, K.D.; Lee, S.H.; Lee, J.Y.; Park, Y.G.; Park, Y.M. Association Between Metabolic Syndrome and Atopic Dermatitis in Korean Adults. Acta Derm.-Venereol. 2017, 97, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, K.D.; Jung, H.M.; Youn, Y.H.; Lee, J.Y.; Park, Y.G.; Lee, S.H.; Park, Y.M. Association Between Obesity, Abdominal Obesity, and Adiposity and the Prevalence of Atopic Dermatitis in Young Korean Adults: The Korea National Health and Nutrition Examination Survey 2008-2010. Allergy Asthma Immunol. Res. 2016, 8, 107–114. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Seok, J.; Kim, B. Correlation between socio-economic status and atopic dermatitis in Korean adults: The Korea national health and nutrition examination survey (2007–2014). J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1509–1515. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, E.J.; Yoon, D.J.; Lee, J.K.; Chang, W.S.; Lim, Y.M.; Park, J.W.; Lee, J.S. Prevalence of self-reported allergic diseases and IgE levels: A 2010 KNHANES analysis. Allergy Asthma Immunol. Res. 2017, 9, 329–339. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Becker, L.; Kwasny, M.; Menter, A.; Cordoro, K.M.; Paller, A.S. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015, 151, 144–152. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Greenland, P. Eczema and cardiovascular risk factors in 2 US adult population studies. J. Allergy Clin. Immunol. 2015, 135, 721–728.e6. [Google Scholar] [CrossRef]
- Radtke, M.; Schäfer, I.; Glaeske, G.; Jacobi, A.; Augustin, M. Prevalence and comorbidities in adults with psoriasis compared to atopic eczema. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 151–157. [Google Scholar] [CrossRef]
- Kwa, M.C.; Silverberg, J.I. Association Between Inflammatory Skin Disease and Cardiovascular and Cerebrovascular Co-Morbidities in US Adults: Analysis of Nationwide Inpatient Sample Data. Am. J. Clin. Dermatol. 2017, 18, 813–823. [Google Scholar] [CrossRef]
- Augustin, M.; Radtke, M.A.; Glaeske, G.; Reich, K.; Christophers, E.; Schaefer, I.; Jacobi, A. Epidemiology and Comorbidity in Children with Psoriasis and Atopic Eczema. Dermatology 2015, 231, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.-A.; Sei, J.-F.; Philippe, C.; Taieb, C.; Joly, P.; Ezzedine, K. Prevalence of comorbidities in atopic dermatitis and psoriasis in the French population. Ann. Dermatol. Venereol. 2021, 148, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, H.; Byun, Y.; Baek, Y.S.; Choi, C.U.; Kim, J.H.; Kim, K. Incidence of Cardiovascular Disease After Atopic Dermatitis Development: A Nationwide, Population-Based Study. Allergy Asthma Immunol. Res. 2023, 15, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Choi, E.K.; Han, K.D.; Kwon, S.; Lee, S.Y.; Yang, S.; Lee, E.; Lee, S.R.; Cha, M.J.; Oh, S. Increased Risk of Atrial Fibrillation in Patients with Atopic Triad: A Nationwide Population-Based Study. J Allergy Clin. Immunol. Pract. 2021, 9, 3422–3430.e5. [Google Scholar] [CrossRef]
- Rhee, T.-M.; Choi, E.-K.; Han, K.-D.; Lee, S.-R.; Oh, S. Impact of the Combinations of Allergic Diseases on Myocardial Infarction and Mortality. J. Allergy Clin. Immunol. Pract. 2021, 9, 872–880.e4. [Google Scholar] [CrossRef]
- Silverwood, R.J.; Forbes, H.J.; Abuabara, K.; Ascott, A.; Schmidt, M.; Schmidt, S.A.J.; Smeeth, L.; Langan, S.M. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: Population based cohort study. BMJ 2018, 361, k1786. [Google Scholar] [CrossRef]
- Lundin, S.; Wahlgren, C.F.; Johansson, E.K.; Andersson, N.; Mogensen, I.; Ekstrom, S.; Jonsson, M.; Melen, E.; Ljungman, P.L.S.; Bergstrom, A.; et al. Childhood atopic dermatitis is associated with cardiovascular risk factors in young adulthood-A population-based cohort study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1854–1862. [Google Scholar] [CrossRef]
- Marani, A.; Bianchelli, T.; Gesuita, R.; Faragalli, A.; Foti, C.; Malara, G.; Micali, G.; Amerio, P.; Rongioletti, F.; Corazza, M.; et al. Gender differences in adult atopic dermatitis and clinical implication: Results from a nationwide multicentre study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 375–383. [Google Scholar] [CrossRef]
- Kiiski, V.; Ukkola-Vuoti, L.; Vikkula, J.; Ranta, M.; Lassenius, M.I.; Kopra, J. Effect of Disease Severity on Comorbid Conditions in Atopic Dermatitis: Nationwide Registry-Based Investigation in Finnish Adults. Acta Dermato-Venereol. 2023, 103, adv00882. [Google Scholar] [CrossRef]
- Artime, E.; Serra, E.; Mert, C.; Díaz-Cerezo, S.; Huete, T.; Hernández-Subirá, I.; Lledo-Bryant, L.; Sicras-Mainar, A. Real-World Treatment Patterns, Resource Use and Costs in Adult Patients With Atopic Dermatitis Receiving Systemic Treatment: Derma-Atopic Study in Spain. Actas Dermo-Sifiliogr. 2023, 114, 9–18. [Google Scholar] [CrossRef]
- Yoo, J.; Koo, H.Y.R.; Han, K.; Lee, Y.B. Impairment of Quality of Life and Mental Health Status in Adult-Onset Atopic Dermatitis. Ann. Dermatol. 2022, 34, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.K.; Yang, A.C.; Aoki, V.; Criado, R.F.J.; Pires, M.C.; Lupi, O.; Fabricio, L.H.Z.; Abreu, D.; Silvi, S. Clinical Features and Disease Management in Adult Patients With Atopic Dermatitis Receiving Care at Reference Hospitals in Brazil: The ADAPT Study. J. Investig. Allergol. Clin. Immunol. 2021, 31, 236–245. [Google Scholar] [PubMed]
- Wu, J.J.; Amand, C.; No, D.J.; Mahajan, P.; Gadkari, A.; Ghorayeb, E.; Kaur, M.; Korotzer, A.; Eckert, L. The Use of Real-World Data to Evaluate the Association Between Atopic Dermatitis and Cardiovascular Disease: A Retrospective Claims Analysis. Dermatol. Ther. 2021, 11, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Ivert, L.; Johansson, E.; Dal, H.; Lindelöf, B.; Wahlgren, C.; Bradley, M. Association Between Atopic Dermatitis and Cardiovascular Disease: A Nationwide Register-based Case-control Study from Sweden. Acta Derm.-Venereol. 2020, 100, adv00167. [Google Scholar] [CrossRef]
- Cho, Y.-T.; Hsieh, W.-T.; Chan, T.C.; Tang, C.-H.; Chu, C.-Y. Prevalence of baseline comorbidities in patients with atopic dermatitis: A population-based cohort study in Taiwan. JAAD Int. 2020, 1, 50–58. [Google Scholar] [CrossRef]
- Kok, W.; Yew, Y.; Thng, T. Comorbidities Associated with Severity of Atopic Dermatitis in Young Adult Males: A National Cohort Study. Acta Dermato-Venereol. 2019, 99, 652–656. [Google Scholar] [CrossRef]
- Jung, M.J.; Kang, S.Y.; Kim, H.O.; Chung, B.Y.; Park, C.W. Effect of Weight Reduction on Treatment Outcomes for Patients with Atopic Dermatitis. Ann. Dermatol. 2020, 32, 319–326. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Kleiman, E.; Lev-Tov, H.; Silverberg, N.B.; Durkin, H.G.; Joks, R.; Smith-Norowitz, T.A. Association between obesity and atopic dermatitis in childhood: A case-control study. J. Allergy Clin. Immunol. 2011, 127, 1180–1186.e1. [Google Scholar] [CrossRef]
- Kusunoki, T.; Morimoto, T.; Nishikomori, R.; Heike, T.; Ito, M.; Hosoi, S.; Nakahata, T. Obesity and the prevalence of allergic diseases in schoolchildren. Pediatr. Allergy Immunol. 2008, 19, 527–534. [Google Scholar] [CrossRef]
- Luo, X.; Xiang, J.; Dong, X.; Cai, F.; Suo, J.; Wang, Z.; Liu, M. Association between obesity and atopic disorders in Chinese adults: An individually matched case–control study. BMC Public Health 2013, 13, 12. [Google Scholar] [CrossRef]
- Koutroulis, I.; Magnelli, L.; Gaughan, J.; Weiner, E.; Kratimenos, P. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015, 104, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Body mass index and physical activity in relation to asthma and atopic diseases in young adults. Respir. Med. 2006, 100, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.; Silverberg, N.; Lee-Wong, M. Association between atopic dermatitis and obesity in adulthood. Br. J. Dermatol. 2012, 166, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, Z.; Wang, Y.; Liu, M.; Wang, Y. Associations of obesity with newly diagnosed and previously known atopic diseases in Chinese adults: A case-control study. Sci. Rep. 2017, 7, 43672. [Google Scholar] [CrossRef]
- Drucker, A.M.; Pope, E.I.; Field, A.E.; Qureshi, A.A.; Dumas, O.; Camargo Jr, C.A. Association Between Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain, and Offspring Atopic Dermatitis: A Prospective Cohort Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 96–102.e2. [Google Scholar] [CrossRef]
- Zhang, J.; Loman, L.; Oldhoff, M.; Schuttelaar, M.L.A. Association between moderate to severe atopic dermatitis and lifestyle factors in the Dutch general population. Clin. Exp. Dermatol. 2022, 47, 1523–1535. [Google Scholar] [CrossRef]
- Ascott, A.; Mansfield, K.; Schonmann, Y.; Mulick, A.; Abuabara, K.; Roberts, A.; Smeeth, L.; Langan, S. Atopic eczema and obesity: A population-based study*. Br. J. Dermatol. 2020, 184, 871–879. [Google Scholar] [CrossRef]
- Vehapoglu, A.; Cakın, Z.E.; Kahraman, F.U.; Nursoy, M.A.; Toprak, A. Is overweight/obesity a risk factor for atopic allergic disease in prepubertal children? A case–control study. J. Pediatr. Endocrinol. Metab. 2021, 34, 727–732. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, S.H.; Kim, J.D.; Sol, I.S.; Kim, M.J.; Kim, Y.H.; Jung, Y.-C.; Sohn, M.H.; Kim, K.W. Korean Youth with Comorbid Allergic Disease and Obesity Show Heightened Psychological Distress. J. Pediatr. 2019, 206, 99–104.e4. [Google Scholar] [CrossRef]
- Lin, M.H.; Hsieh, C.; Caffrey, J.L.; Lin, Y.S.; Wang, I.J.; Ho, W.C.; Chen, P.C.; Wu, T.N.; Lin, R.S. Fetal Growth, Obesity, and Atopic Disorders in Adolescence: A Retrospective Birth Cohort Study. Paediatr. Périnat. Epidemiol. 2015, 29, 472–479. [Google Scholar] [CrossRef]
- Harpsøe, M.C.J.; Basit, S.; Bager, P.; Wohlfahrt, J.; Benn, C.S.; Nøhr, E.A.; Linneberg, A.; Jess, T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: A study within the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2013, 131, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Sybilski, A.J.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Lusawa, A.; Furmańczyk, K.; Krzych-Fałta, E.; Komorowski, J.; Samoliński, B. Obesity--a risk factor for asthma, but not for atopic dermatitis, allergic rhinitis and sensitization. Public Health Nutr. 2015, 18, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, D.; Salameh, P.; Caillaud, D.; Charpin, D.; de Blay, F.; Kopferschmitt, C.; Lavaud, F.; Annesi-Maesano, I.; Baldi, I.; Raherison, C. High body mass index and allergies in schoolchildren: The French six cities study. BMJ Open Respir. Res. 2014, 1, e000054. [Google Scholar] [CrossRef] [PubMed]
- Von Kries, R.; Hermann, M.; Grunert, V.P.; Von Mutius, E. Is obesity a risk factor for childhood asthma? Allergy 2001, 56, 318–322. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, H.-B.; Lee, S.-Y.; Kim, B.-S.; Kim, J.-H.; Yu, J.-H.; Kim, B.-J.; Hong, S.-J. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int. Arch. Allergy Immunol. 2011, 154, 42–48. [Google Scholar] [CrossRef]
- Vlaski, E.; Stavric, K.; Isjanovska, R.; Seckova, L.; Kimovska, M. Overweight hypothesis in asthma and eczema in young adolescents. Allergol. Immunopathol. 2006, 34, 199–205. [Google Scholar] [CrossRef]
- Kajbaf, T.Z.; Asar, S.; Alipoor, M.R. Relationship between obesity and asthma symptoms among children in Ahvaz, Iran: A cross sectional study. Ital. J. Pediatr. 2011, 37, 1. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Keown-Stoneman, C.D.G.; Maguire, J.L.; Drucker, A.M. Association Between Atopic Dermatitis and Height, Body Mass Index, and Weight in Children. JAMA Dermatol. 2022, 158, 26–32. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.W.; Yon, D.K.; Ha, E.K.; Jee, H.M.; Sung, M.; Sim, H.J.; Yoon, J.W.; Choi, S.H.; Shin, Y.H.; et al. Association of serum lipid parameters with the SCORAD index and onset of atopic dermatitis in children. Pediatr. Allergy Immunol. 2021, 32, 322–330. [Google Scholar] [CrossRef]
- Baek, H.; Han, M.Y.; Choi, S.H. Serum lipid profiles and atopic dermatitis in children. J. Allergy Clin. Immunol. 2020, 145, AB196. [Google Scholar] [CrossRef]
- Agón-Banzo, P.J.; Sanmartin, R.; García-Malinis, A.J.; Hernández-Martín, Á.; Puzo, J.; Doste, D.; Pardos, C.; Gilaberte, Y. Body mass index and serum lipid profile: Association with atopic dermatitis in a paediatric population. Australas. J. Dermatol. 2020, 61, E60–E64. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.K.; Shin, M. Low-Density Lipoprotein Cholesterol Is Associated with Atopic Dermatitis in Korean Adolescents. Int. Arch. Allergy Immunol. 2023, 184, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M.; et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br. J. Dermatol. 2017, 177, e125–e127. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Wolf, P.; Knuplez, E.; Weger, W.; Schuster, C.; Peinhaupt, M.; Holzer, M.; Trakaki, A.; Eichmann, T.; Lass, A.; et al. Abnormal composition and function of high-density lipoproteins in atopic dermatitis patients. Allergy 2018, 74, 398–402. [Google Scholar] [CrossRef]
- Kusunoki, T.; Morimoto, T.; Sakuma, M.; Mukaida, K.; Yasumi, T.; Nishikomori, R.; Fujii, T.; Heike, T. Total and low-density lipoprotein cholesterol levels are associated with atopy in schoolchildren. J. Pediatr. 2011, 158, 334–336. [Google Scholar] [CrossRef]
- Leigh, J.-H.; Park, H.J.; Chun, S.-M.; Min, Y.-S.; Choi, M. Association of Atopic Dermatitis with Dyslipidemia in Adolescents: A Cross-Sectional Study. Ann. Dermatol. 2021, 33, 483–485. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, J.; Kim, H.; Jang, J.; Choi, K.M.; Baek, Y.S.; Kim, M.G.; Kim, K. Risk of type 2 diabetes mellitus in adult patients with atopic dermatitis. Diabetes Res. Clin. Pract. 2023, 204, 110883. [Google Scholar] [CrossRef]
- Hung, Y.H.; Liu, H.Y.; Chang, R.; Huang, J.Y.; Wu, C.D.; Yen, M.S.; Hung, Y.M.; Wei, J.C.; Wang, P.Y. Association between parental autoimmune disease and childhood atopic dermatitis varied by sex: A nationwide case-control study. Arch Dermatol. Res. 2023, 315, 2011–2021. [Google Scholar] [CrossRef]
- Lu, F.; Wu, B.; Wang, Y. Mendelian randomization indicates that atopic dermatitis contributes to the occurrence of diabetes. BMC Med. Genom. 2023, 16, 132. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, M.; Han, K.D.; Cho, S.H.; Lee, J.H. Increased Risk of Dementia in Patients with Atopic Dermatitis: A Nationwide Population-Based Cohort Study. Acta Dermato-Venereol. 2023, 103, adv4557. [Google Scholar] [CrossRef]
- Olesen, A.B.; Juul, S.; Birkebæk, N.; Thestrup-Pedersen, K. Association between atopic dermatitis and insulin-dependent diabetes mellitus: A case-control study. Lancet 2001, 357, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Wu, C.-Y.; Chang, Y.-T.; Lyu, Y.-S.; Wu, C.-Y. Risk of Type 1 Diabetes mellitus in patients with atopic dermatitis: A nationwide population-based cohort study. Dermatology 2023, 240, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ouyang, F.; Story, R.E.; Pongracic, J.A.; Hong, X.; Wang, G.; Pearson, C.; Ortiz, K.; Bauchner, H.; Wang, X. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J. Allergy Clin. Immunol. 2009, 124, 1031–1038.e4. [Google Scholar] [CrossRef] [PubMed]
- Karavanaki, K.; Tsoka, E.; Karayianni, C.; Petrou, V.; Pippidou, E.; Brisimitzi, M.; Mavrikiou, M.; Kakleas, K.; Konstantopoulos, I.; Manoussakis, M.; et al. Prevalence of allergic symptoms among children with diabetes mellitus type 1 of different socioeconomic status. Pediatr. Diabetes 2008, 9, 407–416. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Liu, Y.; Zhu, Y.; Li, X. Associations between type 1 diabetes and autoimmune skin diseases: Mendelian randomization analysis. Heliyon 2024, 10, e32781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berg, A.K.; Svensson, J.; Thyssen, J.P.; Chawes, B.; Zachariae, C.; Egeberg, A.; Thorsen, S.U. No associations between type 1 diabetes and atopic dermatitis, allergic rhinitis, or asthma in childhood: A nationwide Danish case-cohort study. Sci. Rep. 2023, 13, 19933. [Google Scholar] [CrossRef]
- Stromberg, L.; Ludvigsson, G.; Bjorksten, B. Atopic allergy and delayed hypersensitivity in children with diabetes. J. Allergy Clin. Immunol. 1995, 96, 188–192. [Google Scholar] [CrossRef]
- Campanati, A.; Bianchelli, T.; Gesuita, R.; Foti, C.; Malara, G.; Micali, G.; Amerio, P.; Rongioletti, F.; Corazza, M.; Patrizi, A.; et al. Comorbidities and treatment patterns in adult patients with atopic dermatitis: Results from a nationwide multicenter study. Arch Dermatol. Res. 2022, 314, 593–603. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Herzig, P.; Giani, G. Atopic eczema in early childhood could be protective against Type 1 diabetes. Diabetologia 2003, 46, 784. [Google Scholar] [CrossRef]
- Stene, L.C.; Joner, G. Norwegian Childhood Diabetes Study Group; Atopic disorders and risk of childhood-onset type 1 diabetes in individuals. Clin. Exp. Allergy 2004, 34, 201–206. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Duffy, D.L.; Kyvik, K.O.; Skytthe, A.; Backer, V. Relationship between type 1 diabetes and atopic diseases in a twin population. Allergy 2011, 66, 645. [Google Scholar] [CrossRef]
- Cakir, M.; Akcay, S.; Karakas, T.; Gedik, Y.; Okten, A.; Orhan, F. Prevalence of atopy in children with type 1 diabetes mellitus, hepatitis B virus carriers, and healthy children: Role of T helper 1 (Th1)-type immune response. Allergy Asthma Proc. 2008, 29, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Odink, R.J.; Landaeta, R.; Aarts, F.; Brunekreef, B.; Gerritsen, J.; Van Aalderen, W.M.C.; Hoekstra, M.O. A lower prevalence of atopy symptoms in children with type 1 diabetes mellitus. Clin. Exp. Allergy 2002, 32, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Jung, J.-A.; Lee, J.S. Association between social economic status and atopic dermatitis in Korean adult: An analysis of the Fifth Korea National Health and Nutrition Examination Survey (2010–2012). Allergy Asthma Respir. Dis. 2015, 3, 128–133. [Google Scholar] [CrossRef]

| Author | Year | Population | Key Observation |
|---|---|---|---|
| Metabolic Syndrome in Atopic Dermatitis | |||
| Shalom et al. [15] | 2016 | N0—116,812 healthy people N1—116,816 patients with AD | Moderate and severe AD were associated with a higher prevalence of MetS. |
| Megna et al. [18] | 2017 | 151 patients with persistent AD 102 patients with adult-onset AD | A higher prevalence of HTN in patients with adult-onset AD compared to those with persistent AD was found. |
| Schafer et al. [19] | 2003 | N0—1491 healthy people N1—46 patients with AD | HDL-cholesterol level was associated with a higher prevalence of AD. |
| Uehara et al. [20] | 2002 | 521 patients with active AD 87 adults with “healed” AD | The incidence of HTN was lower in patients with AD compared to the general population. |
| Standl et al. [21] | 2017 | N0—30,047 healthy people N1—10,788 patients with AD | In patients with AD, the risk of developing hypertension was only slightly higher compared to the general population. |
| Drucker et al. [22] | 2017 | N0—237,740 healthy patients N1—21,379 patients with AD | AD patients were not found to be at increased risk of developing HTN or T2DM compared to the general population. |
| Lee et al. [23] | 2017 | 5007 subjects (2142 men and 2865 women) | MetS and its individual components like central obesity and hypertriglyceridaemia were positively associated with the presence of AD in women. |
| Lee et al. [24] | 2016 | 5202 Korean adults aged 19–40 years | High waist circumference (≥80 cm) was associated with an increased risk of AD in women. |
| Lee et al. [25] | 2014 | 47,351 participants | An inverse association has been demonstrated between T2DM and AD. |
| Park et al. [26] | 2017 | 2342 subjects | There was no association between IFG and AD nor between T2DM and AD. |
| Silverberg et al. [27] | 2015 | N0—143 healthy controls N1—132 children with AD | AD was linked with a family history of both HTN and T2DM, but not with obesity or hyperlipidemia. |
| Silverberg et al. [28] | 2015 | 34,525 participants | High cholesterol and HTN were associated with an increased risk of developing AD. |
| Radtke et al. [29] | 2013 | 37,456 patients with psoriasis, 48,140 patients with AD | Obese individuals were 17% more likely to develop AD compared to those with normal weight. |
| Kwa et al. [30] | 2017 | 164,868 patients with AD | HTN was a significant risk factor for AD, particularly in women. |
| Augustin et al. [31] | 2015 | 30,354 patients with AD | Hyperlipidemia was linked with AD while HTN is not associated with AD. |
| Richard et al. [32] | 2019 | 931 patients with AD, 190,891 patients with psoriasis | Obesity, dyslipidemia, HTN, and T2DM were more prevalent among patients with psoriasis than patients with AD. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| High Blood Pressure in Atopic Dermatitis | |||
| Lee et al. [33] | 2023 | N0–40,512 control subjects without AD N1—40,512 individuals with AD | Patients with AD had a significantly increased risk of developing HTN. |
| Choi et al. [34] | 2021 | N0—136,253 subjects with AF N1—30,300 patients with AD in the atopic group N2—105,953 patients with AD in the nonatopic group | Individuals with the atopic triad, which includes asthma, allergic rhinitis, and AD, had a significantly higher prevalence of HTN and AF. |
| Rhee et al. [35] | 2020 | 9,548,939 individuals older than 20 years | Patients suffering from asthma, allergic rhinitis, and AD were at increased risk of developing HTN. |
| Silverwood et al. [36] | 2018 | N0—1,528,477 patients without atopic eczema; N1—387,439 patients with atopic eczema | AD was associated with an increased risk of cardiovascular outcomes, such as HTN. |
| Lundin et al. [37] | 2023 | N0—1850 patients without AD; N1—420 patients with AD | Men with AD had higher SP levels compared to men without AD. |
| Marani et al. [38] | 2023 | 686 adult patients with AD (357 males and 329 females) | Men with AD were significantly more likely to have hypertriglyceridemia and HTN compared to women with AD. |
| Kiiski et al. [39] | 2023 | 124,038 patients with AD | People with severe AD were more likely to develop HTN (OR = 1.15) and atherosclerosis than those with mild AD. |
| Artime et al. [40] | 2022 | 1995 patients with AD | Two major comorbidities were associated with AD: arterial HTN (36%), and dyslipidemia (35%). |
| Yoo et al. [41] | 2022 | N0—32,086 healthy people N1—383 child-onset AD N2—440 adult-onset AD | Childhood-onset AD (SE = 1.69) had lower rates of comorbid T2DM, HTN, and dyslipidemia in comparison to those with adult-onset AD (SE = 2.39). |
| Arruda et al. [42] | 2023 | N0—1995 patients N1—187 patients with AD | The main comorbidity of the 187 patients with AD was HTN (10.2%). |
| Wu et al. [43] | 2021 | N0—397,380 healthy patients N1—132,460 patients with AD | The association between AD and HTN was stronger in patients with more severe AD. |
| Ivert et al. [44] | 2019 | N0—1,022,435 healthy patients N1—104,832 patients with AD | The incidence of HTN was higher in patients with severe AD compared to controls. |
| Cho et al. [45] | 2020 | 12,780 patients with AD | The association between AD and HTN was only significant in patients with severe AD. |
| Kok et al. [46] | 2019 | 10,077 patients with AD | HTN was significantly correlated with having moderate-to-severe AD. |
| Lee et al. [25] | 2014 | 47,351 participants | No significant correlation between HTN and adult AD. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| Obesity in Atopic Dermatitis | |||
| Jung et al. [47] | 2020 | N1—20 normal-weight patients with AD N2—obese patients with AD | Weight reduction was associated with significant improvement in AD symptoms. |
| Silverberg et al. [48] | 2011 | N0—778 healthy patients N1—389 patients with AD | There was a significant association between obesity in children and an increased risk of developing AD. |
| Kusunoki et al. [49] | 2008 | 50,086 questionnaires | A large waist was positively associated with both the prevalence and severity of AD. |
| Luo et al. [50] | 2013 | N0—532 healthy patients N1—266 atopic patients | Obesity and overweight were associated with an increased risk of allergic diseases, including AD. |
| Koutroulis et al. [51] | 2015 | 104 patients with AD | An increased BMI was associated with increased severity of AD in children older than two years. |
| Kilpelainen et al. [52] | 2016 | 10,667 students | AD increased linearly with BMI among women but not men |
| Silverberg et al. [53] | 2011 | 2090 participants | Obesity in adults was associated with an increased risk of AD. |
| Xie et al. [54] | 2017 | N0—2217 control patients N1—3515 atopic patients | Patients suffering from AD for more than one year had a higher BMI than healthy controls. |
| Drucker et al. [55] | 2019 | N0—11,211 control patients N1—2058 patients with AD | Maternal gestational weight gain was associated with an increased risk of AD in offspring. |
| Zhang et al. [56] | 2022 | 56,896 participants | Class I obesity (BMI 30–34.9 kg/m2) was positively associated with moderate-to-severe AD. |
| Ascott et al. [57] | 2020 | N0—849,722 healthy patients N1—1,441,746 patients with AD | People with AD had a slightly higher risk of being overweight or obese compared to people without AD. |
| Vehapoglu et al. [58] | 2021 | N0—429 healthy patients N1—278 atopic patients | Obese and overweight children were at an increased risk of developing AAD, including AD in comparison to children with normal weight. |
| Kim et al. [59] | 2018 | 703,869 participants (363,180 boys and 340,689 girls) | Obesity and overweight were associated with an increased risk of allergic diseases, including AD. |
| Lin et al. [60] | 2015 | N0—69,012 control patients N1—5676 patients with AD | Adolescents with both restricted fetal growth and a high BMI had a significantly increased risk of developing AD. |
| Harpsøe et al. [61] | 2012 | 38,874 children and their mothers | There was no association between maternal obesity or gestational weight gain and the risk of atopic eczema or hay fever. |
| Sybilski et al. [62] | 2013 | 4783 participants | There was not a significant association between BMI and the prevalence of AD in children and adults. |
| Saadeh et al. [63] | 2014 | 6733 schoolchildren | A high BMI was not significantly (p < 0.05) associated with atopic eczema. |
| Kries et al. [64] | 2001 | 9357 children | Atopic eczema was unrelated to children’s weight. |
| Yoo et al. [65] | 2009 | 717 adolescents | There was not a significant association between overweight and AD. |
| Vlaski et al. [66] | 2006 | 2926 young adolescents | There was no significant association between being overweight and atopic eczema. |
| Kajbaf et al. [67] | 2011 | 903 children | There was no significant association between obesity and atopic eczema. |
| Nicholas et al. [68] | 2022 | N0—8777 healthy patients N1—1834 atopic patients | AD was associated with lower height and a higher BMI in children. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| Hypertriglyceridemia in Atopic Dermatitis | |||
| Kim et al. [69] | 2021 | Subset I—248 patients Subset II—52,725 patients | Children with AD had significantly higher levels of TC and TG compared to children without AD. |
| Baek et al. [70] | 2020 | N0—179 controls N1—69 atopic patients | Patients with AD had significantly higher levels of TC and TG compared to children without AD. |
| Agón-Banzo et al. [71] | 2019 | N0—105 healthy controls N1—134 children diagnosed with AD | Patients with AD had significantly higher levels of TG compared to healthy controls. |
| Seong et al. [72] | 2023 | 1617 Korean adolescents | Patients with AD had significantly higher levels of TG compared to healthy controls. |
| Li et al. [73] | 2017 | N0—15 healthy individuals N1—27 patients with AD | Patients with AD and S. aureus colonization had significantly lower levels of TG46:1, TG48:1, TG48:2, TG50:1, TG50:2, and TG50:3. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| HDL in Atopic Dermatitis | |||
| Schafer et al. [19] | 2003 | N0—1491 controls N1—46 patients with AD | The level of HDL was positively associated with AD in men. |
| Trieb et al. [74] | 2019 | N0—19 controls N1—20 patients with AD | HDL may play a role in the pathogenesis of low-grade inflammation in AD. |
| Lundin et al. [37] | 2023 | N0—1850 patients without AD N1—420 patients with AD | Young adolescents with AD had lower HDL levels compared to those without AD. |
| Kusunoki et al. [75] | 2011 | 654 children | Patients with AD had significantly lower HDL levels than healthy controls. |
| Leigh et al. [76] | 2021 | N0—1782 healthy controls N1—230 patients with AD | No significant difference in HDL-C levels between adolescents with AD and those without AD was noticed. |
| Author | Year | Population | Key Observation |
|---|---|---|---|
| Elevated Blood Sugar Levels in Atopic Dermatitis | |||
| Lee et al. [77] | 2023 | 36,692 individuals with AD | Adults with AD have a significantly increased risk of developing T2DM compared to adults without AD. |
| Hung et al. [78] | 2022 | N0—862,612 children without AD N1—312,329 children with AD | Children who have a parent with an autoimmune disease, such as T1DM, are at an increased risk of developing AD. |
| Lu et al. [79] | 2023 | N0—95,464 individuals without AD N1—21,399 individuals with AD | There is a shared genetic basis between AD and T1DM and T2DM. |
| Woo et al. [80] | 2023 | N0—2,643,602 patients without AD N1—38,391 patients with AD | Patients suffering from T2DM were characterized by a significantly elevated incidence of dementia in those with AD compared to those without AD. |
| Olesen et al. [81] | 2001 | N0—7683 patients without diabetes mellitus N1—817 patients with diabetes mellitus | An incidence of AD up to age 15 years with T1DM was about two-thirds of that among nondiabetic controls. |
| Lu et al. [82] | 2015 | N0—12,725 patients without T1DM N1—3386 patients with T1DM | The overall incidence rate of AD was significantly higher in patients with T1DM. |
| Kumar et al. [83] | 2009 | N0—680 total amount of children N1—231 children with AD | GDM was significantly associated with AD in term deliveries. |
| Karavanaki et al. [84] | 2008 | N0—150 control patients N1—127 patients with T1DM | Atopic eczema was more common in higher socioeconomic classes children suffering from T1DM. |
| Li et al. [85] | 2023 | N0—1,585,844 controls N1—396,461 patients with AD | There was no significant association between AD and T1DM. |
| Berg et al. [86] | 2023 | N0—45,656 controls N1—4111 patients with T1DM | No significant association between T1DM and AD was noted. |
| Stromberg et al. [87] | 1995 | N0—78 healthy patients N1—61 children with T1DM | The prevalence of atopic eczema was similar in children with T1DM and healthy controls. |
| Campanati et al. [88] | 2021 | 684 participants | There was no significant difference in the prevalence of T2DM between patients with different severities of AD. |
| Rosenbauer et al. [89] | 2003 | N0—1871 patients without T1DM N1—760 patients with T1DM | T1DM was significantly lower among children with AD compared to children without AD. |
| Stene et al. [90] | 2004 | N0—1668 controls N1—554 patients with T1DM | AD was associated with a lower risk of T1DM. |
| Thomsen et al. [91] | 2011 | N1—5289 patients with AD N2—143 patients with T1DM | The diabetic twin had a significantly lower risk of AD compared to the non-diabetic twin. |
| Cakir et al. [92] | 2008 | N0—100 healthy controls N1—52 children with T1DM | The prevalence of atopy, asthma, and atopic eczema was lower in children with T1DM compared to the control group. |
| Meerwaldt et al. [93] | 2002 | N0—777 control patients N1—188 patients with T1DM | Children with T1DM suffered less from atopic eczema than the control group. |
| Kim et al. [94] | 2015 | 18,066 participants | The prevalence of HTN, T2DM, and obesity was lower in subjects with AD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matwiejuk, M.; Myśliwiec, H.; Mikłosz, A.; Chabowski, A.; Flisiak, I. Is Atopic Dermatitis Associated with Systemic Metabolic Disturbances? A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5884. https://doi.org/10.3390/ijms26125884
Matwiejuk M, Myśliwiec H, Mikłosz A, Chabowski A, Flisiak I. Is Atopic Dermatitis Associated with Systemic Metabolic Disturbances? A Systematic Review. International Journal of Molecular Sciences. 2025; 26(12):5884. https://doi.org/10.3390/ijms26125884
Chicago/Turabian StyleMatwiejuk, Mateusz, Hanna Myśliwiec, Agnieszka Mikłosz, Adrian Chabowski, and Iwona Flisiak. 2025. "Is Atopic Dermatitis Associated with Systemic Metabolic Disturbances? A Systematic Review" International Journal of Molecular Sciences 26, no. 12: 5884. https://doi.org/10.3390/ijms26125884
APA StyleMatwiejuk, M., Myśliwiec, H., Mikłosz, A., Chabowski, A., & Flisiak, I. (2025). Is Atopic Dermatitis Associated with Systemic Metabolic Disturbances? A Systematic Review. International Journal of Molecular Sciences, 26(12), 5884. https://doi.org/10.3390/ijms26125884








