Fructose-Induced Glycation End Products Promote Skin-Aging Phenotypes and Senescence Marker Expression in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
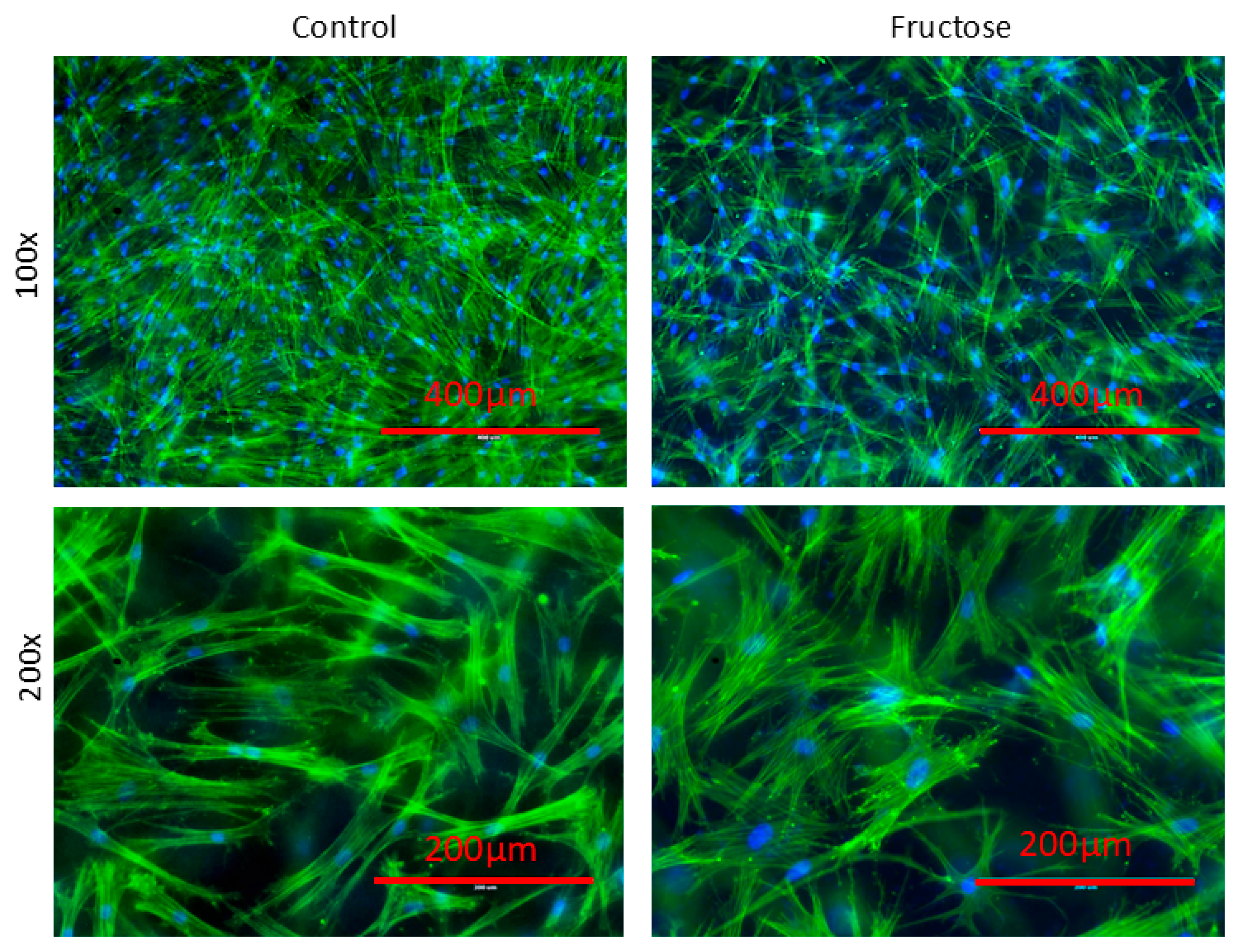
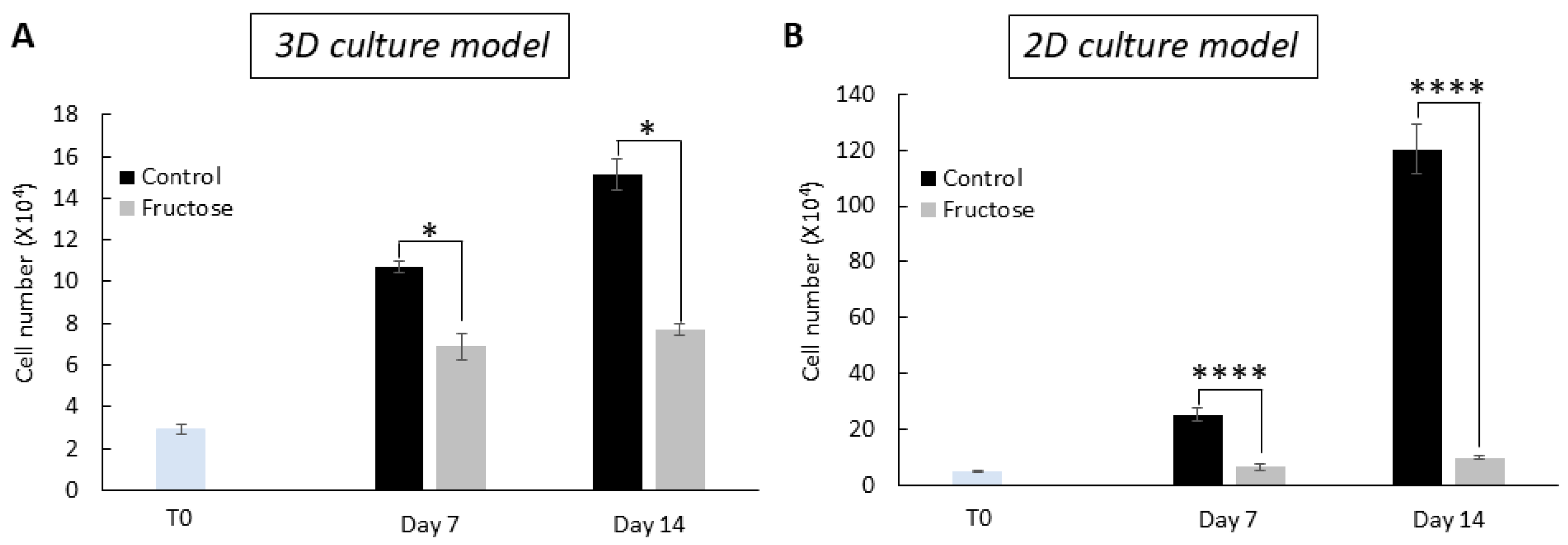
2.1. Human Dermal Fibroblasts Grown in the Presence of Fructose Showed Cellular Abnormalities
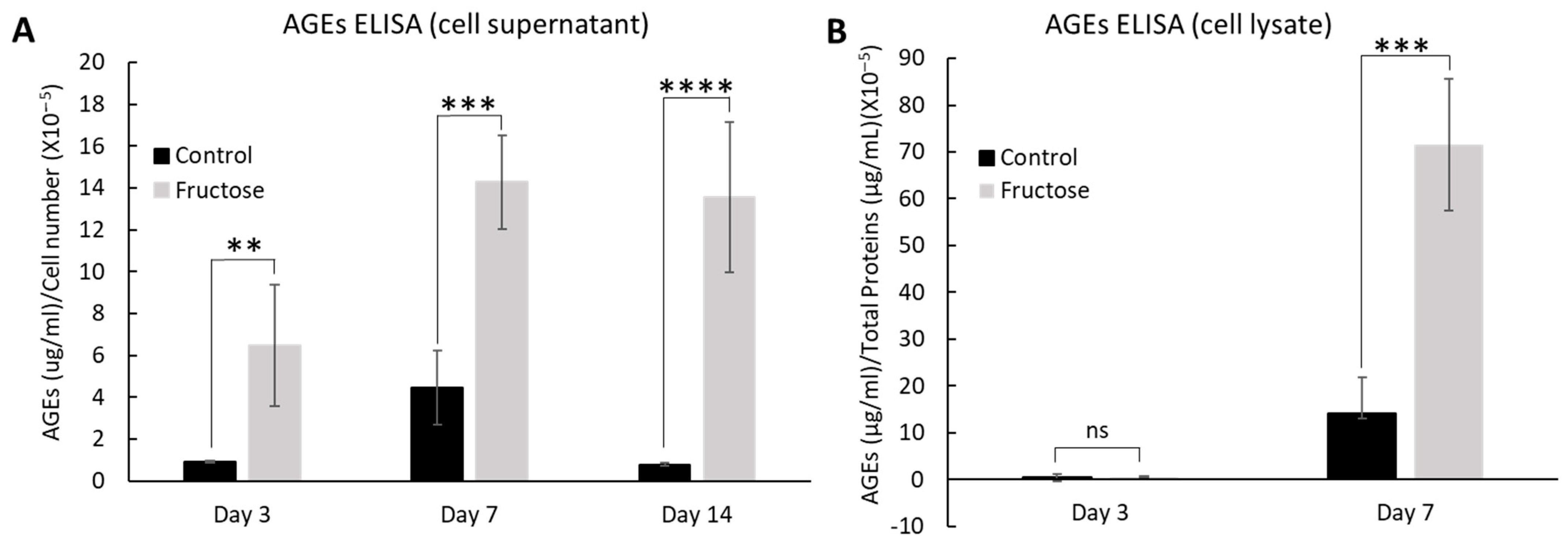
2.2. Fructose Increases the Production of Advanced Glycation End-Products
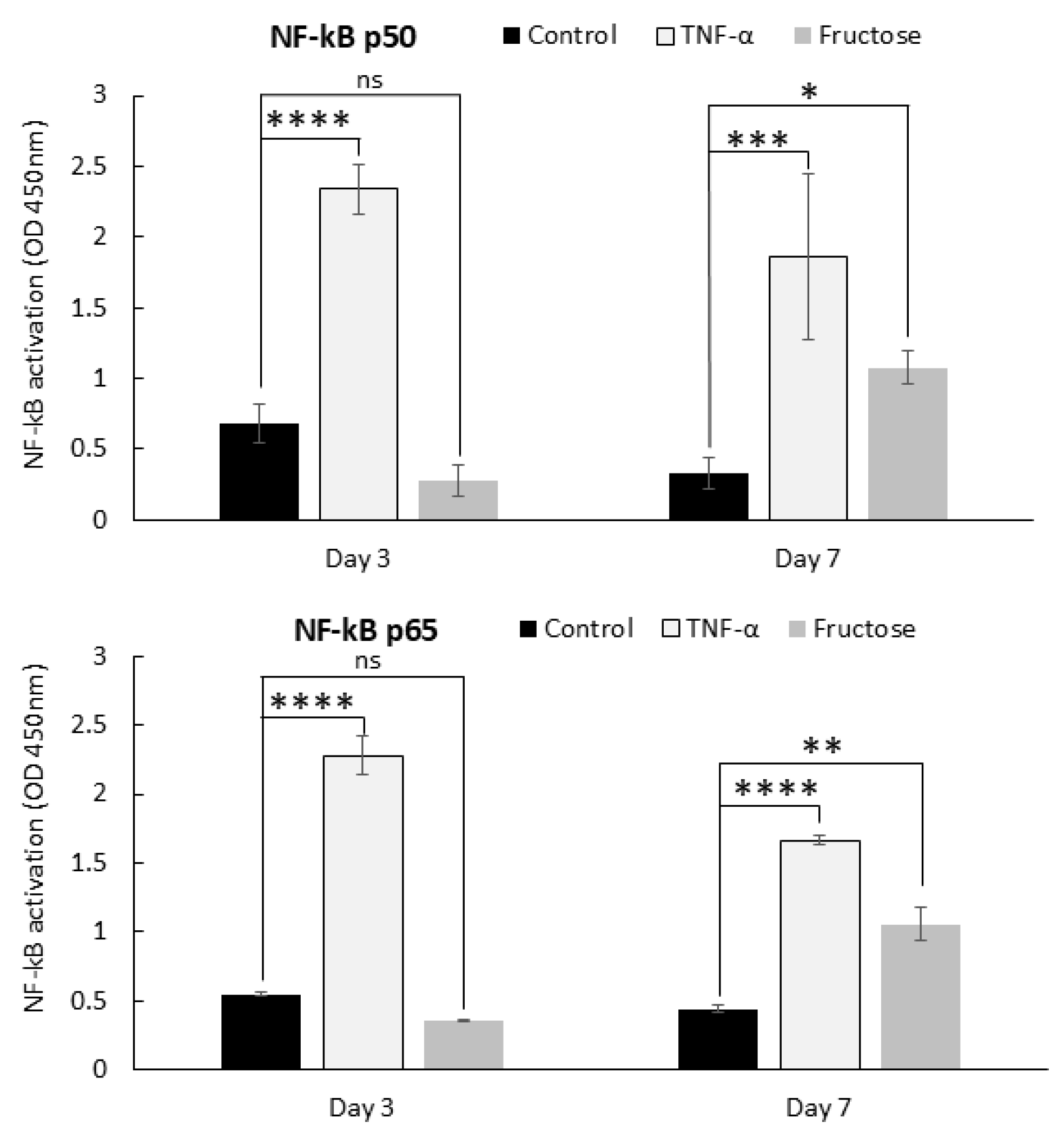
2.3. Advanced Glycation End-Products and Inflammation
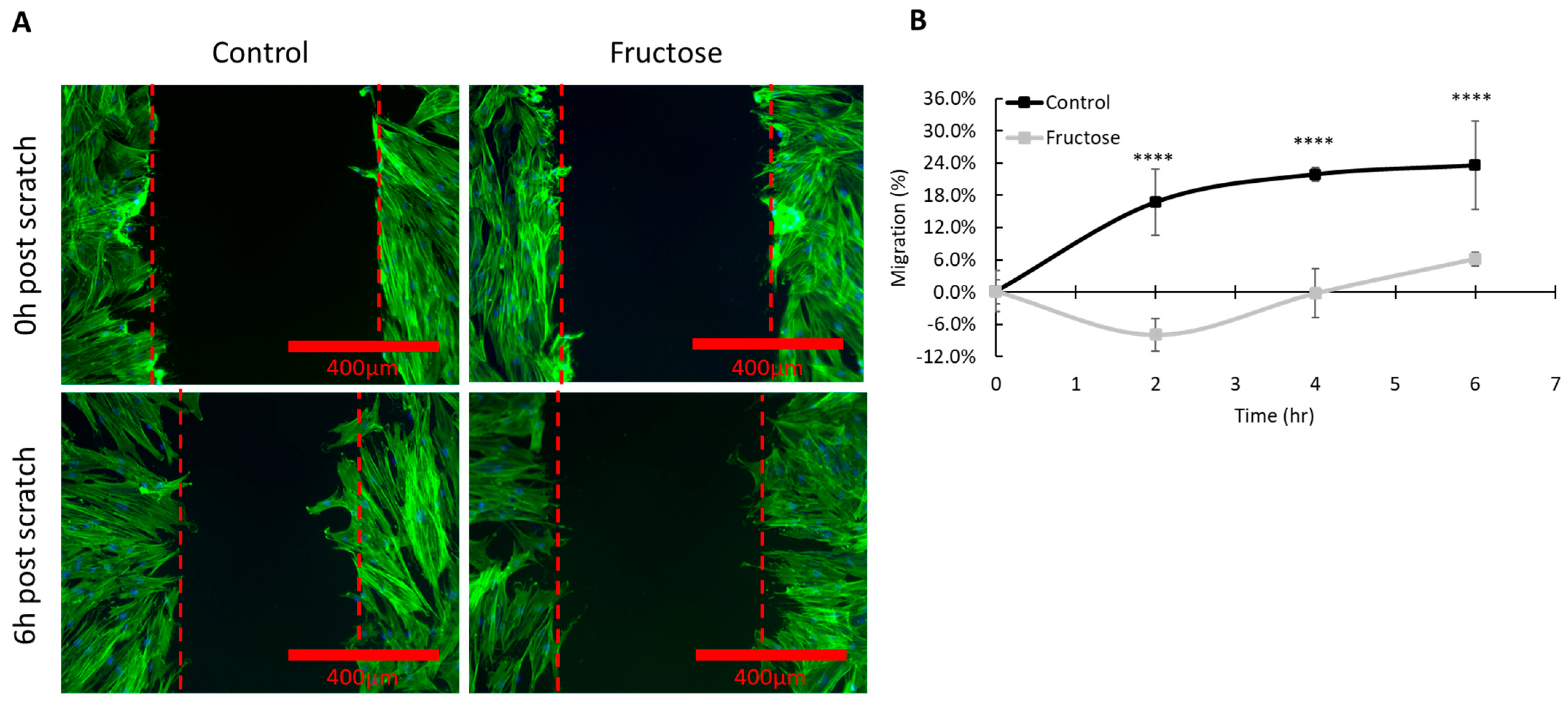
2.4. Elevated Level of Advanced Glycation End Products Delays Skin Wound Healing
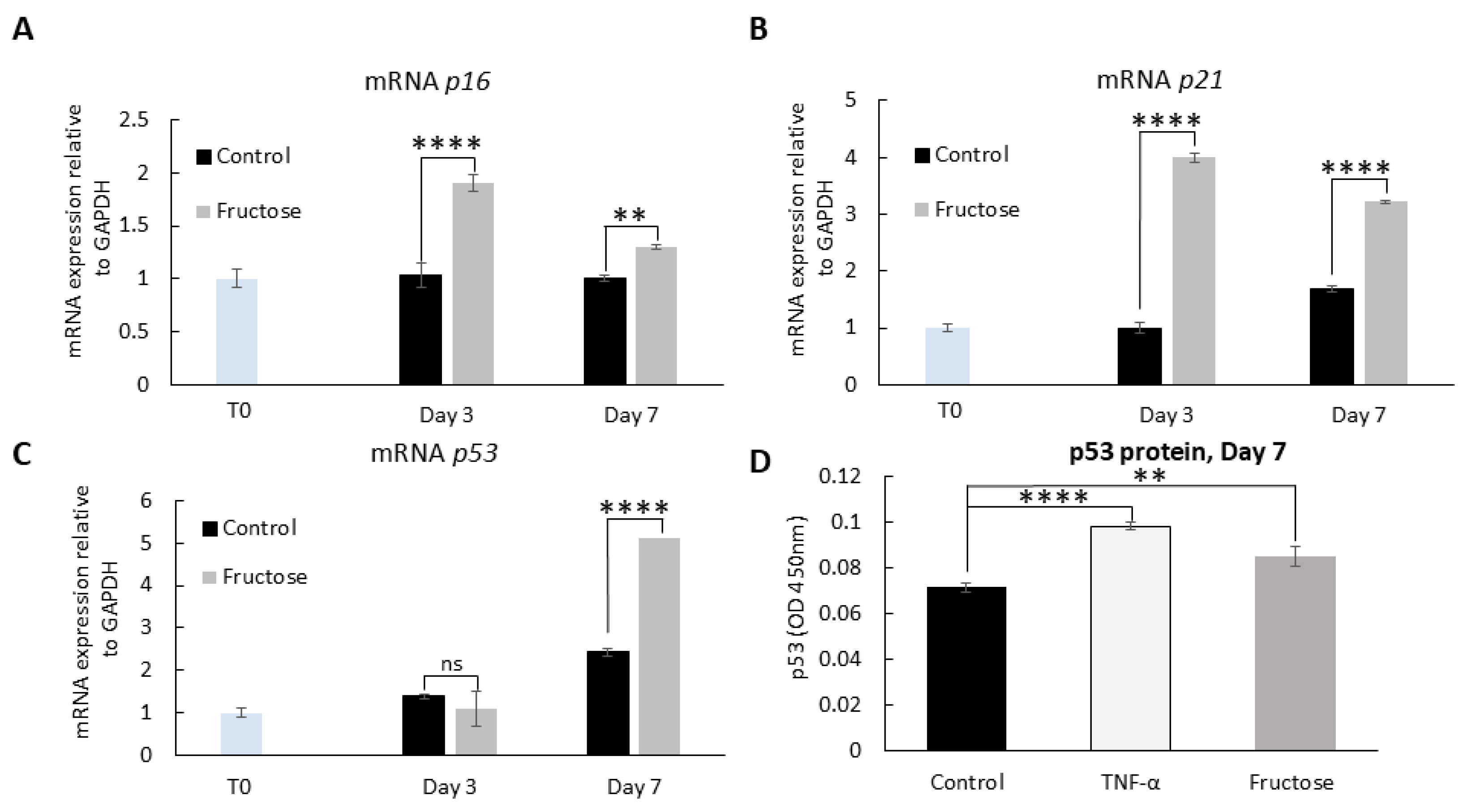
2.5. Advanced Glycation End Products and Cellular Senescence
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Fluorescence Imaging of Human Dermal Fibroblasts in Collagen Gel Matrix
4.3. Measurement of Advanced Glycation End Products in Protein Extracts and Culture Media
4.4. Inflammatory Cytokines-Chemokines Profiling
4.5. Measurement of Activated NF-κB Subunits p50 and p65
4.6. Cell Migration-Proliferation Assay
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Detection of p53 Protein
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puizina-Ivic, N. Skin aging. Acta Dermatovenerol. Alp. Panon. Adriat. 2008, 17, 47. [Google Scholar]
- Guinot, C.; Malvy, D.J.-M.; Ambroisine, L.; Latreille, J.; Mauger, E.; Tenenhaus, M.; Morizot, F.; Lopez, S.; Le Fur, I.; Tschachler, E. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch. Dermatol. 2002, 138, 1454–1460. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; LINskENs, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef]
- Michikawa, Y.; Mazzucchelli, F.; Bresolin, N.; Scarlato, G.; Attardi, G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science 1999, 286, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging. Mutat. Res./DNAging 1992, 275, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, Z.A. An attempt at a rational classification of theories of ageing. Biol. Rev. 1990, 65, 375–398. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermato-Endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef]
- Yamauchi, M.; Prisayanh, P.; Haque, Z.; Woodley, D.T. Collagen cross-linking in sun-exposed and unexposed sites of aged human skin. J. Investig. Dermatol. 1991, 97, 937–941. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Bian, X.; Li, B.; Yang, J.; Ma, K.; Sun, M.; Zhang, C.; Fu, X. Regenerative and protective effects of dMSC-sEVs on high-glucose-induced senescent fibroblasts by suppressing RAGE pathway and activating Smad pathway. Stem Cell Res. Ther. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.L.; Wu, Q.R.; Zeng, P.; Li, S.M.; Cai, Y.J.; Chen, S.Z.; Luo, X.S.; Kuang, S.J.; Rao, F.; Lai, Y.Y.; et al. Advanced glycation end products induce senescence of atrial myocytes and increase susceptibility of atrial fibrillation in diabetic mice. Aging Cell 2022, 21, e13734. [Google Scholar] [CrossRef] [PubMed]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars Burn. Heal. 2016, 2, 2059513116676828. [Google Scholar] [CrossRef]
- Levi, B.; Werman, M.J. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J. Nutr. 1998, 128, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Wolfrom, C.; Loriette, C.; Polini, G.; Delhotal, B.; Lemonnier, F.; Gautier, M. Comparative effects of glucose and fructose on growth and morphological aspects of cultured skin fibroblasts. Exp. Cell Res. 1983, 149, 535–546. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Bunn, H.F.; Higgins, P.J. Reaction of monosaccharides with proteins: Possible evolutionary significance. Science 1981, 213, 222–224. [Google Scholar] [CrossRef]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.D.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 2024, 33, e15065. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Airaksinen, K.J.; Salmela, P.I.; Linnaluoto, M.K.; Ikäheimo, M.J.; Ahola, K.; Ryhänen, L.J. Diminished arterial elasticity in diabetes: Association with fluorescent advanced glycosylation end products in collagen. Cardiovasc. Res. 1993, 27, 942–945. [Google Scholar] [CrossRef]
- Sueki, H.; Nozaki, S.; Numazawa, S.; Aoki, K.; Kuroiwa, Y.; Fujisawa, R. Effect of non-enzymatic glycosylation and heating on browning of human stratum corneum and nail. Dermatology 1991, 183, 197–202. [Google Scholar] [CrossRef]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef]
- Berge, U.; Behrens, J.; Rattan, S.I. Sugar-induced premature aging and altered differentiation in human epidermal keratinocytes. Ann. N. Y. Acad. Sci. 2007, 1100, 524–529. [Google Scholar] [CrossRef]
- Krasnova, L.; Wong, C.-H. Understanding the chemistry and biology of glycosylation with glycan synthesis. Annu. Rev. Biochem. 2016, 85, 599–630. [Google Scholar] [CrossRef]
- Buckingham, B.; Reiser, K. Relationship between the content of lysyl oxidase-dependent cross-links in skin collagen, nonenzymatic glycosylation, and long-term complications in type I diabetes mellitus. J. Clin. Investig. 1990, 86, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, C.; Danoux, L.; Pauly, G. Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. Br. J. Dermatol. 2001, 145, 10–18. [Google Scholar] [CrossRef]
- Corstjens, H.; Dicanio, D.; Muizzuddin, N.; Neven, A.; Sparacio, R.; Declercq, L.; Maes, D. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp. Gerontol. 2008, 43, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Reihsner, R.; Menzel, E. Two-dimensional stress-relaxation behavior of human skin as influenced by non-enzymatic glycation and the inhibitory agent aminoguanidine. J. Biomech. 1998, 31, 985–993. [Google Scholar] [CrossRef]
- Reddy, G.K. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp. Diabetes Res. 2004, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Koyama, H.; Tanaka, S.; Fukumoto, S.; Shinohara, K.; Shoji, T.; Emoto, M.; Tahara, H.; Kakiya, R.; Tabata, T. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 2008, 57, 1452–1457. [Google Scholar] [CrossRef]
- Kasas, S.; Wang, X.; Hirling, H.; Marsault, R.; Huni, B.; Yersin, A.; Regazzi, R.; Grenningloh, G.; Riederer, B.; Forro, L. Superficial and deep changes of cellular mechanical properties following cytoskeleton disassembly. Cell Motil. Cytoskelet. 2005, 62, 124–132. [Google Scholar] [CrossRef]
- Lambrechts, A.; Van Troys, M.; Ampe, C. The actin cytoskeleton in normal and pathological cell motility. Int. J. Biochem. Cell Biol. 2004, 36, 1890–1909. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.-W.; Koh, C.-G. Actin cytoskeleton dynamics and the cell division cycle. Int. J. Biochem. Cell Biol. 2010, 42, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Kueper, T.; Grune, T.; Prahl, S.; Lenz, H.; Welge, V.; Biernoth, T.; Vogt, Y.; Muhr, G.-M.; Gaemlich, A.; Jung, T. Vimentin is the specific target in skin glycation structural prerequisites, functional consequences, and role in skin aging. J. Biol. Chem. 2007, 282, 23427–23436. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshikawa, H.; Saruwatari, K.; Akazawa, Y.; Inoue, T.; Kuze, T.; Sayo, T.; Uchida, N.; Sugiyama, Y. The presence of Nε-(Carboxymethyl) lysine in the human epidermis. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 1246–1252. [Google Scholar] [CrossRef]
- Howard, E.W.; Benton, R.; Ahern-Moore, J.; Tomasek, J.J. Cellular contraction of collagen lattices is inhibited by nonenzymatic glycation. Exp. Cell Res. 1996, 228, 132–137. [Google Scholar] [CrossRef]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflamm. 2013, 2013, 403460. [Google Scholar] [CrossRef]
- Peterszegi, G.; Molinari, J.; Ravelojaona, V.; Robert, L. Effect of advanced glycation end-products on cell proliferation and cell death. Pathol. Biol. 2006, 54, 396–404. [Google Scholar] [CrossRef]
- Ravelojaona, V.; Robert, A.; Robert, L. Expression of senescence-associated β-galactosidase (SA-β-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch. Gerontol. Geriatr. 2009, 48, 151–154. [Google Scholar] [CrossRef]
- Sullivan, R. Contributions to senescence: Non-enzymatic glycosylation of proteins. Arch. Physiol. Biochem. 1996, 104, 797–806. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Howard III, T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
- Edelstein, D.; Brownlee, M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes 1992, 41, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.; Forbes, J.M.; Thomas, M.C.; Thallas, V.; Dean, R.G.; Burns, W.C.; Tikellis, C.; Ritchie, R.H.; Twigg, S.M.; Cooper, M.E. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ. Res. 2003, 92, 785–792. [Google Scholar] [CrossRef]
- Njikan, S.; Manning, A.J.; Ovechkina, Y.; Awasthi, D.; Parish, T. High content, high-throughput screening for small molecule inducers of NF-kappaB translocation. PLoS ONE 2018, 13, e0199966. [Google Scholar] [CrossRef]
- Pitz Hda, S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.; Affonso, R.C.; Fanan, S.; Trevisan, A.C.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rella, A.; Layman, D.; Dang, R.; Rafailovich, M.; Maidhof, R.; Pernodet, N. Fructose-Induced Glycation End Products Promote Skin-Aging Phenotypes and Senescence Marker Expression in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2025, 26, 6162. https://doi.org/10.3390/ijms26136162
Rella A, Layman D, Dang R, Rafailovich M, Maidhof R, Pernodet N. Fructose-Induced Glycation End Products Promote Skin-Aging Phenotypes and Senescence Marker Expression in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2025; 26(13):6162. https://doi.org/10.3390/ijms26136162
Chicago/Turabian StyleRella, Antonella, Dawn Layman, Rong Dang, Miriam Rafailovich, Robert Maidhof, and Nadine Pernodet. 2025. "Fructose-Induced Glycation End Products Promote Skin-Aging Phenotypes and Senescence Marker Expression in Human Dermal Fibroblasts" International Journal of Molecular Sciences 26, no. 13: 6162. https://doi.org/10.3390/ijms26136162
APA StyleRella, A., Layman, D., Dang, R., Rafailovich, M., Maidhof, R., & Pernodet, N. (2025). Fructose-Induced Glycation End Products Promote Skin-Aging Phenotypes and Senescence Marker Expression in Human Dermal Fibroblasts. International Journal of Molecular Sciences, 26(13), 6162. https://doi.org/10.3390/ijms26136162







