Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats
Abstract
1. Introduction
2. Results
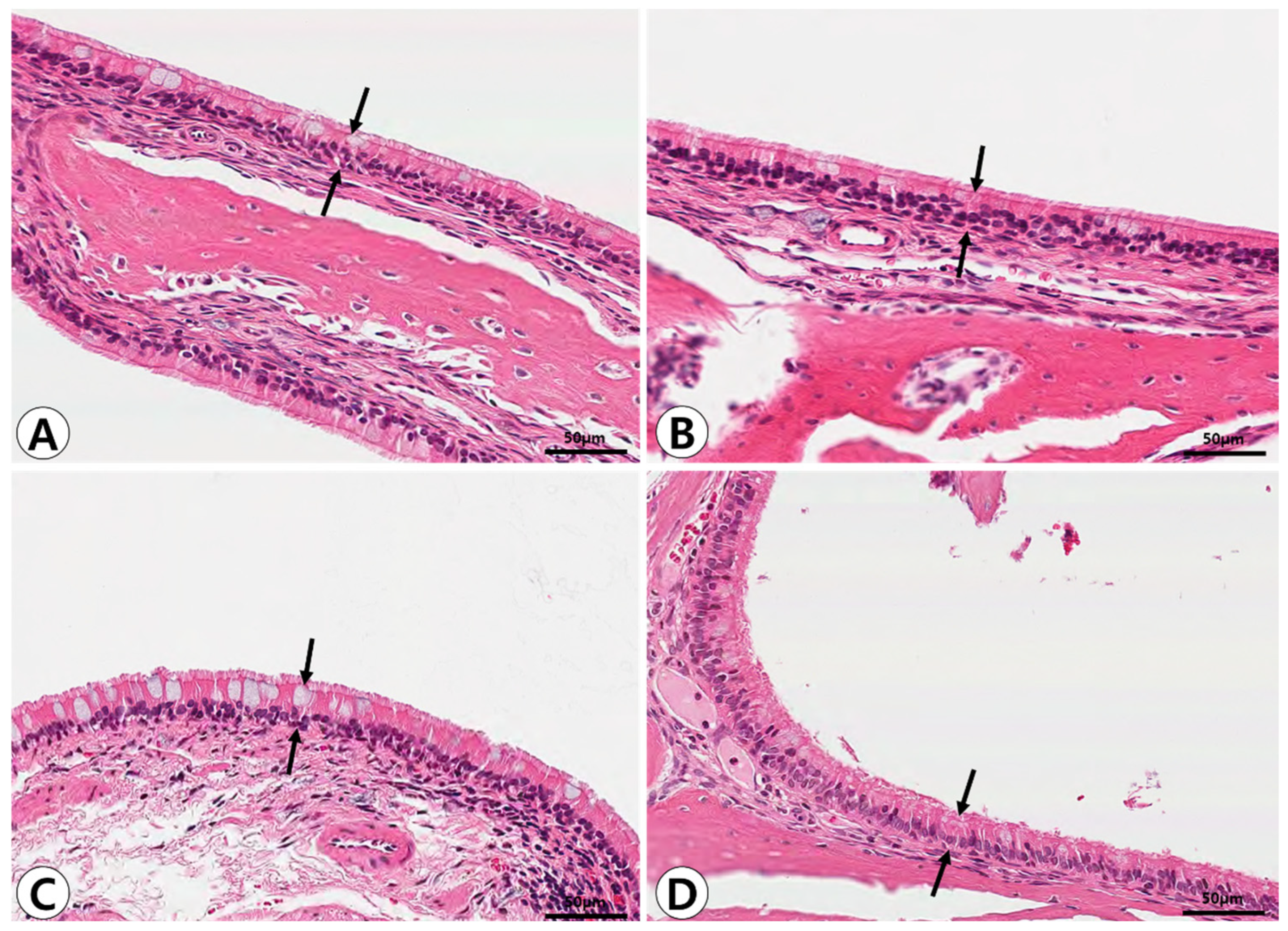
2.1. Histopathologic Analysis of Nasal and Paranasal Sinus Mucosa
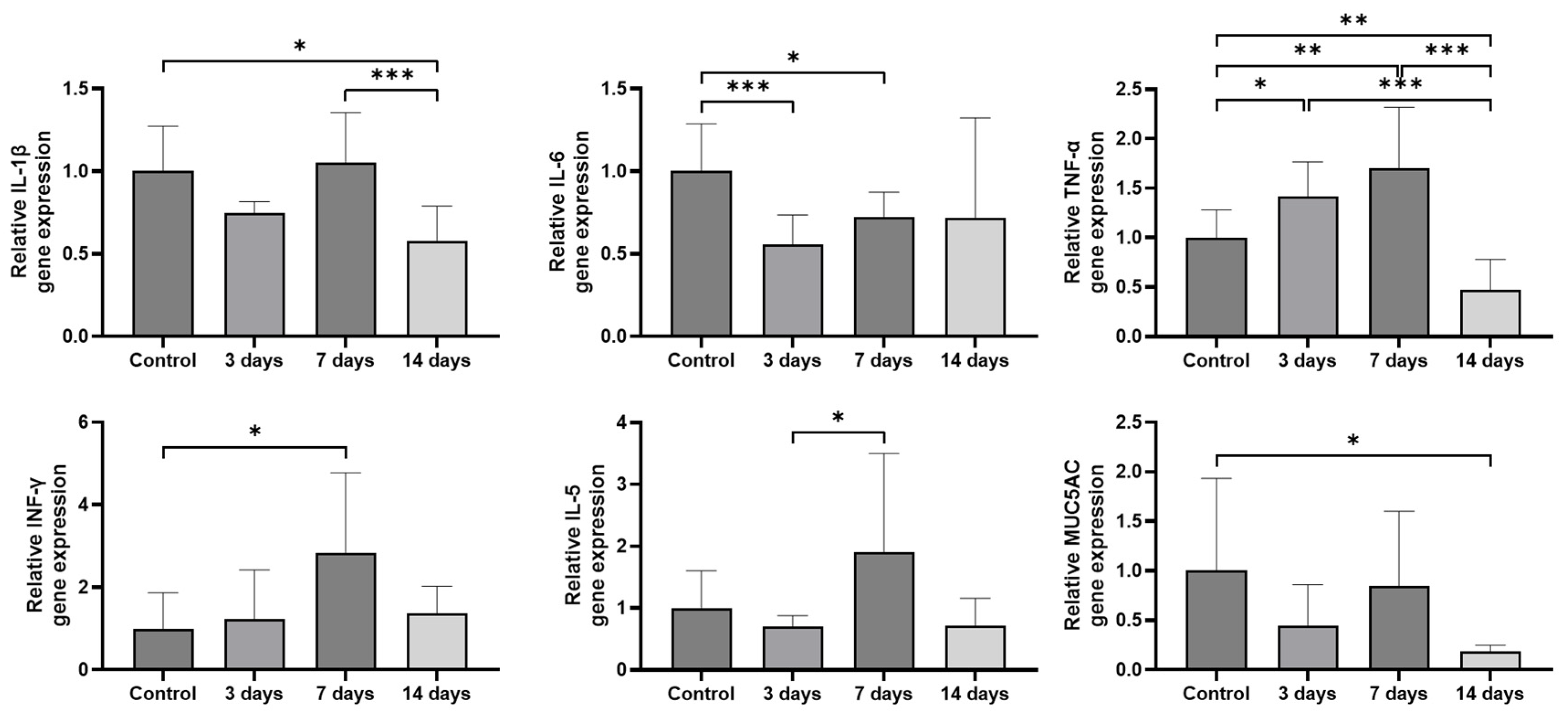
2.2. Expression of Inflammatory Cytokines and MUC5AC in the Sinonasal Mucosa
3. Discussion
4. Materials and Methods
4.1. Animals
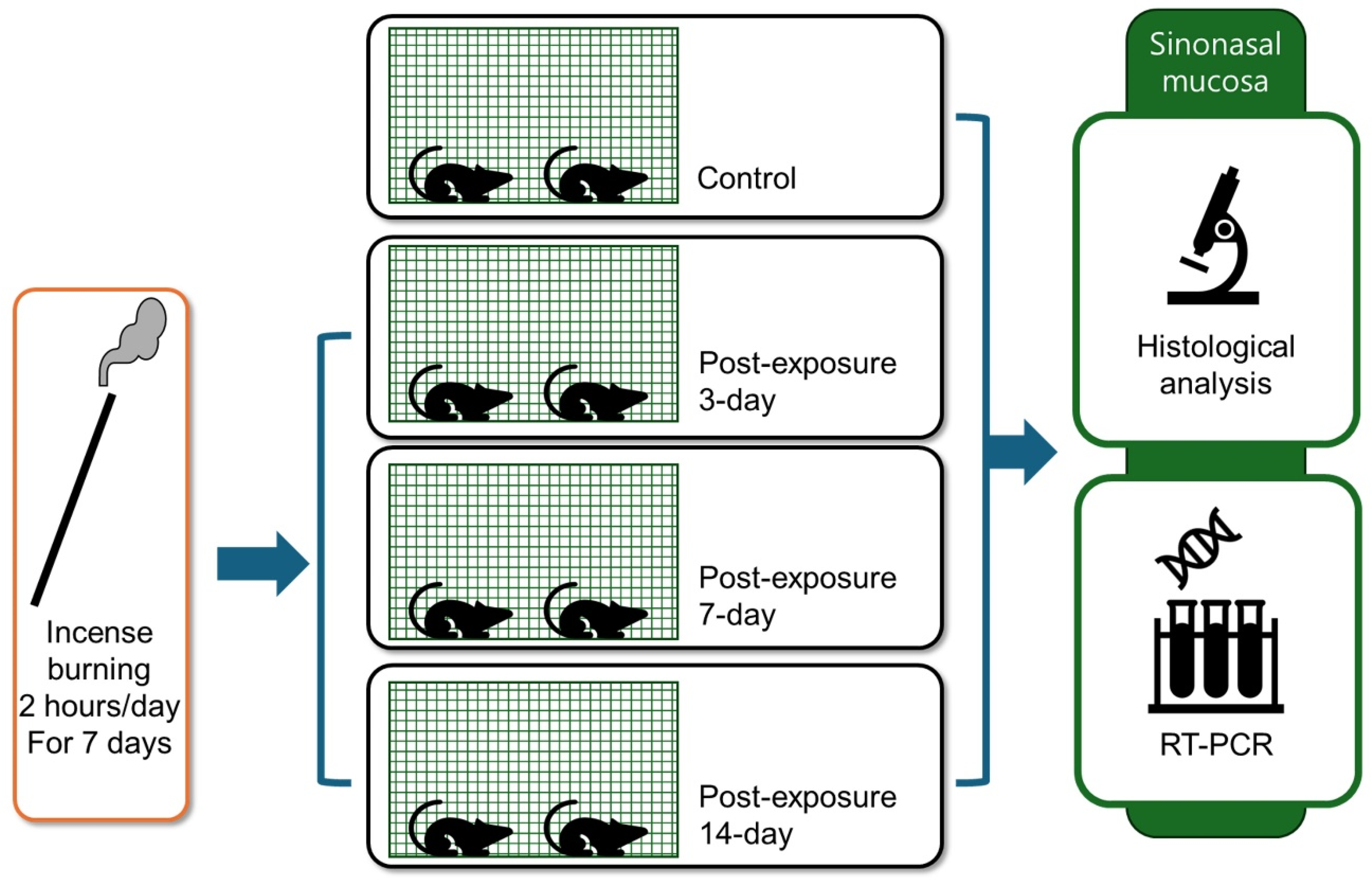
4.2. PM Exposure Model
4.3. Histopathologic Analysis
4.4. Analysis of Sinonasal Mucosa Using RT-PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Yu, Y.; Sun, Z.; Duan, J. A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence. Part. Fibre Toxicol. 2022, 19, 67. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Reed, W.; Maronpot, R.R.; Henriquez-Roldán, C.; Delgado-Chavez, R.; Calderón-Garcidueñas, A.; Dragustinovis, I.; Franco-Lira, M.; Aragón-Flores, M.; Solt, A.C.; et al. Brain inflammation and Alzheimer’s like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 2004, 32, 650–658. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B.; Bernstein, J.A. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef]
- Fisher, J.A.; Puett, R.C.; Hart, J.E.; Camargo, C.A., Jr.; Varraso, R.; Yanosky, J.D.; Laden, F. Particulate matter exposures and adult-onset asthma and COPD in the Nurses’ Health Study. Eur. Respir. J. 2016, 48, 921–924. [Google Scholar] [CrossRef]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Morales-Rubio, R.; Amador-Muñoz, O.; Rosas-Pérez, I.; Sánchez-Pérez, Y.; García-Cuéllar, C.; Segura-Medina, P.; Osornio-Vargas, Á.; De Vizcaya-Ruiz, A. PM2.5 induces airway hyperresponsiveness and inflammation via the AhR pathway in a sensitized Guinea pig asthma-like model. Toxicology 2022, 465, 153026. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Scheeren, R.A. Upper airway defense mechanisms. Paediatr. Respir. Rev. 2000, 1, 336–341. [Google Scholar]
- Lubner, R.J.; Rubel, K.; Chandra, R.K.; Turner, J.H.; Chowdhury, N.I. Particulate matter exposure is associated with increased inflammatory cytokines and eosinophils in chronic rhinosinusitis. Allergy 2024, 79, 1219–1229. [Google Scholar] [CrossRef]
- Naclerio, R.; Ansotegui, I.J.; Bousquet, J.; Canonica, G.W.; D’Amato, G.; Rosario, N.; Pawankar, R.; Peden, D.; Bergmann, K.-C.; Bielory, L.; et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: Impact of air pollution on patients with AR: Current knowledge and future strategies. World Allergy Organ. J. 2020, 13, 100106. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Ma, S.; Wang, K.; Lou, H.; Wang, Y.; Zhang, L.; Wang, C.; Akdis, C.A. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol. Res. 2020, 12, 56–71. [Google Scholar] [CrossRef]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guo, Z.; Zhang, R.; Deng, C.; Xu, J.; Dong, W.; Hong, Z.; Yu, H.; Situ, H.; Liu, C.; et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J. Appl. Toxicol. 2018, 38, 678–687. [Google Scholar] [CrossRef]
- Laza-Stanca, V.; Message, S.D.; Edwards, M.R.; Parker, H.L.; Zdrenghea, M.T.; Kebadze, T.; Kon, O.M.; Mallia, P.; Stanciu, L.A.; Johnston, S.L. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog. 2011, 7, e1002114. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Green, D.; Bellmeyer, A.; Baker, C.M.; Burgess, Z.; Rajamannan, N.; Christman, J.W.; Foiles, N.; Kamp, D.W.; Ghio, A.J.; et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Investig. 2007, 117, 2952–2961. [Google Scholar] [CrossRef]
- Bayram, H.; Devalia, J.L.; Sapsford, R.J.; Ohtoshi, T.; Miyabara, Y.; Sagai, M.; Davies, R.J. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1998, 18, 441–448. [Google Scholar] [CrossRef]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Hershey, G.K.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e2. [Google Scholar] [CrossRef]
- Aalapati, S.; Ganapathy, S.; Manapuram, S.; Anumolu, G.; Prakya, B.M. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology 2014, 8, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Rönkkö, T.J.; Jalava, P.I.; Happo, M.S.; Kasurinen, S.; Sippula, O.; Leskinen, A.; Koponen, H.; Kuuspalo, K.; Ruusunen, J.; Väisänen, O.; et al. Emissions and atmospheric processes influences the chemical composition and toxicological properties of urban air particulate matter in Nanjing, China. Sci. Total Environ. 2018, 639, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Harari, S.; Martinelli, I.; Franchini, M. Effects on health of air pollution: A narrative review. Intern. Emerg. Med. 2015, 10, 657–662. [Google Scholar] [CrossRef]
- Jetter, J.J.; Guo, Z.; McBrian, J.A.; Flynn, M.R. Characterization of emissions from burning incense. Sci. Total Environ. 2002, 295, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Krishnaswamy, G.; Chi, D.S. Incense smoke: Clinical, structural and molecular effects on airway disease. Clin. Mol. Allergy 2008, 6, 3. [Google Scholar] [CrossRef]
- Lung, S.C.; Kao, M.C. Worshippers’ exposure to particulate matter in two temples in Taiwan. J. Air Waste Manag. Assoc. 2003, 53, 130–135. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kan-o, K.; Tatsuta, M.; Ishii, Y.; Ogawa, T.; Shinozaki, S.; Fukuyama, S.; Nakanishi, Y.; Matsumoto, K. Incense smoke-induced oxidative stress disrupts tight junctions and bronchial epithelial barrier integrity and induces airway hyperresponsiveness in mouse lungs. Sci. Rep. 2021, 11, 7222. [Google Scholar] [CrossRef]
- Lee, H.M.; Son, Y.S.; Kim, H.S.; Kim, J.Y.; Kim, S.H.; Lee, J.H.; Choi, S.-W.; Oh, S.-J.; Kong, S.-K.; Baek, M.J.; et al. Effects of particulate matter exposure on the Eustachian tube and middle ear mucosa of rats. Clin. Exp. Otorhinolaryngol. 2023, 16, 225–235. [Google Scholar] [CrossRef]
- Wagner, J.G.; Van Dyken, S.J.; Wierenga, J.R.; Hotchkiss, J.A.; Harkema, J.R. Ozone exposure enhances endotoxin-induced mucous cell metaplasia in rat pulmonary airways. Toxicol. Sci. 2003, 74, 437–446. [Google Scholar] [CrossRef]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef]
- Ghio, A.J.; Kim, C.; Devlin, R.B. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am. J. Respir. Crit. Care Med. 2000, 162, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Blomberg, A.; Rudell, B.; Kelly, F.; Sandström, T.; Holgate, S.T.; Frew, A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999, 159, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M., Jr.; London, N.R., Jr.; Tharakan, A.; Surya, N.; Sussan, T.E.; Rao, X.; Lin, S.Y.; Toskala, E.; Rajagopalan, S.; Biswal, S. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2017, 57, 59–65. [Google Scholar] [CrossRef]
- Park, B.; London, N.R., Jr.; Tharakan, A.; Rengasamy, P.; Rajagopalan, S.; Biswal, S.; Pinto, J.M.; Ramanathan, M., Jr. Particulate matter air pollution exposure disrupts the Nrf2 pathway in sinonasal epithelium via epigenetic alterations in a murine model. Int. Forum Allergy Rhinol. 2022, 12, 1424–1427. [Google Scholar] [CrossRef]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Takeyama, K.; Dabbagh, K.; Lee, H.M.; Agustí, C.; Lausier, J.A.; Ueki, I.F.; Grattan, K.M. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. USA 1999, 96, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Park, S.Y.; Choi, K.; Hong, E.S.; Kim, S.; Kim, H.S. Air pollution and atopic dermatitis (AD): The impact of particulate matter (PM10) on an AD mouse-model. Int. J. Mol. Sci. 2020, 21, 6079. [Google Scholar] [CrossRef]
- Hussain, T.; Al-Attas, O.S.; Al-Daghri, N.M.; Mohammed, A.A.; De Rosas, E.; Ibrahim, S.; Vinodson, B.; Ansari, M.G.; Alam El-Din, K.I. Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol. Cell Biochem. 2014, 391, 127–136. [Google Scholar] [CrossRef]
- Choi, S.W.; Choi, S.; Kang, E.J.; Lee, H.M.; Oh, S.J.; Lee, I.W.; Kong, S.-K. Effects of cigarette smoke on Haemophilus influenzae-induced otitis media in a rat model. Sci. Rep. 2021, 11, 19729. [Google Scholar]



| Sequence (5′–3′) | Product Size (bp) | ||
|---|---|---|---|
| IL-1ß | Forward | GCAATGGTCGGGACATAGTTGA | 158 |
| Reverse | AGACCTGACTTGGCAGAGGA | ||
| IL-6 | Forward | ACCCCAACTTCCAATGCTCT | 135 |
| Reverse | GGTTTGCCGAGTAGACCTCA | ||
| TNF-a | Forward | ACCACGCTCTTCTGTCTACTG | 170 |
| Reverse | TGCTTGGTGGTTTGCTACGAC | ||
| INF-γ | Forward | GGCAAAAGGACGGTAACAC | 200 |
| Reverse | GTTGTTCACCTCGAACTTGG | ||
| IL-5 | Forward | CAATGAGACGATGAGGCTTC | 175 |
| Reverse | CACTTCTCTTTTTGTCCGTCAA | ||
| MUC5AC | Forward | ACTATGAGGTGCGACTGCTT | 158 |
| Reverse | CTTGTGGGATGTCACAGGAGT | ||
| GAPDH | Forward | GATGGTGAAGGTCGGTGTGA | 163 |
| Reverse | GAACTTGCCGTGGGTAGAG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, H.-H.; Park, J.-H.; Kim, H.-S.; Lee, H.M.; Kim, S.-D.; Mun, S.J.; Cho, K.-S. Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats. Int. J. Mol. Sci. 2025, 26, 5885. https://doi.org/10.3390/ijms26125885
Kwak H-H, Park J-H, Kim H-S, Lee HM, Kim S-D, Mun SJ, Cho K-S. Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats. International Journal of Molecular Sciences. 2025; 26(12):5885. https://doi.org/10.3390/ijms26125885
Chicago/Turabian StyleKwak, Hyun-Ho, Ji-Hwan Park, Hyang-Sook Kim, Hyun Min Lee, Sung-Dong Kim, Sue Jean Mun, and Kyu-Sup Cho. 2025. "Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats" International Journal of Molecular Sciences 26, no. 12: 5885. https://doi.org/10.3390/ijms26125885
APA StyleKwak, H.-H., Park, J.-H., Kim, H.-S., Lee, H. M., Kim, S.-D., Mun, S. J., & Cho, K.-S. (2025). Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats. International Journal of Molecular Sciences, 26(12), 5885. https://doi.org/10.3390/ijms26125885







